Abstract
17β-Estradiol is a multi-active steroid that imparts neuroprotection via diverse mechanisms of action. However, its role as a neuroprotective agent after spinal cord injury (SCI), or the involvement of the estrogen receptor-alpha (ER-α) in locomotor recovery, is still a subject of much debate. In this study, we evaluated the effects of estradiol and of Tamoxifen (an estrogen receptor mixed agonist/antagonist) on locomotor recovery following SCI. To control estradiol cyclical variability, ovariectomized female rats received empty or estradiol filled implants, prior to a moderate contusion to the spinal cord. Estradiol improved locomotor function at 7, 14, 21, and 28 days post injury (DPI), when compared to control groups (measured with the BBB open field test). This effect was ER-α mediated, because functional recovery was blocked with an ER-α antagonist. We also observed that ER-α was up-regulated after SCI. Long-term treatment (28 DPI) with estradiol and Tamoxifen reduced the extent of the lesion cavity, an effect also mediated by ER-α. The antioxidant effects of estradiol were seen acutely at 2 DPI but not at 28 DPI, and this acute effect was not receptor mediated. Rats treated with Tamoxifen recovered some locomotor activity at 21 and 28 DPI, which could be related to the antioxidant protection seen at these time points. These results show that estradiol improves functional outcome, and these protective effects are mediated by the ER-α dependent and independent-mechanisms. Tamoxifen’s effects during late stages of SCI support the use of this drug as a long-term alternative treatment for this condition.
Keywords: Trauma, neuroprotection, Selective Estrogen Receptor Modulators, 17β-estradiol, locomotor recovery, estrogen receptor expression
1. Introduction
The estimated number of Americans that suffer from spinal cord injury (SCI) is 270,000 persons, with 12,000 new cases reported annually (National Spinal Cord Statistical Center, 2012). The main problem with SCI is that a cascade of independent events are initiated after the trauma; therefore, a combination therapy is required to better approach the progressive stages of this illness (Oudega et al., 2012). The devastating effects of SCI are associated with its immediate and secondary effects that result in cell degeneration at the trauma site and surrounding areas. Immediate damage that results from the direct physical impact includes hemorrhagic necrosis, excitotoxicity and the production of free radicals by the injured tissue (Bramlett and Dietrich, 2007). The secondary damage is a consequence mainly of an inflammatory response which involves apoptotic cell death, cyst formation and development of scar tissue (Hulsebosch CE, 2002). Therefore, a therapeutic strategy that addresses both stages is essential to target the condition and improve the outcome of SCI patients.
One of the clinical approaches to reduce neural damage after SCI is to curtail the inflammatory response by applying pharmacological doses of methylprednisolone. Although this treatment has been successful to inhibit secondary injury in animal models, it has controversial and questionable conclusions in clinical studies (Bydon et al., 2013). Development of multi-active compounds that target and/or block several of the detrimental cellular events triggered by the injury to the spinal cord are under intensive research.
Recent studies have explored the use of steroid hormones, like estradiol, which increase the viability of cells from the nervous system after a traumatic insult. In vitro and in vivo evidence shows that estradiol confers neuroprotection in different CNS pathologies and traumatic conditions including Alzheimer’s disease, ischemia/stroke, and traumatic brain injury (Amtul et al., 2010; Dhandapani and Brann, 2002; Dubal et al., 2006; Etgen et al., 2011; Rau et al., 2003; Soustiel et al., 2005). One of the mechanisms by which estradiol confers neuroprotection is by reducing apoptosis (Chaovipoch et al., 2006; Sribnick et al., 2006a) and inducing activation of anti-apoptotic, neurotrophic, and regeneration associated genes (Scott et al., 2012; Segarra and Lee, 2004). In addition, its steroidal structure (phenol hydroxyl ring) confers anti-inflammatory and antioxidant properties, reducing cellular toxicity and death (Behl et al., 1997; Sugioka et al., 1987; Winterle et al., 2001).
The role of estradiol on locomotor recovery after SCI is still controversial. Swartz et al. 2007 showed that exposure to estradiol at low (28.2 pg/mL) or high (72 pg/mL) doses did not improve locomotor recovery in injured female rats. Baker & Haggs in 2005 concluded that the level of estradiol at different stages of the estrous cycle did not affect the functional outcome after SCI. In contrast, Yune et al. 2004 demonstrated that injecting 17β-estradiol before or immediately after SCI improved locomotor function and reduced the lesion size. In addition, Sribnick et al. (2005; 2010) showed that injecting a supraphysiological dose of estradiol immediately and 24 hours after SCI reduced astrogliosis, reduced inflammation and decreased the extent of myelin loss by 2 days post-injury, an effect that persisted for 6 weeks after injury. To address these discrepancies, this study evaluated the effect of infusing constantly high physiological levels of estradiol to female rats before receiving a moderate contusion to the cord. Although this strategy could not be used in clinical practice, pretreatment of ovariectomized rats with estradiol controls the hormone’s cyclical variability. Moreover, the continuous infusion of a high dose of estradiol, instead of a single application, should increase the availability of this neuroprotective agent and might further stimulate the body’s neuroprotective response after SCI.
Neuroprotective effects of selective estrogen receptor modulators (SERMs) have also been reported (Don Carlos et al., 2009), without the complications that estradiol may generate, like the mitogenic effect on uterine and breast tissue. SERMs are compounds that interact with the estrogen receptors, producing estrogenic or antiestrogenic effects depending on the target tissue. Tamoxifen (TAM) is a SERM commonly used for the treatment of cancer in patients with tumors that test positive for the estrogen receptor, due to its antagonistic activity. Moreover, Tamoxifen exerts neuroprotection in amyotrophic lateral sclerosis, (Traynor et al., 2006), in ischemic brain injury (Dhandapani and Brann, 2002; Kimelberg et al., 2003; Mehta et al., 2003; Zhang et al., 2005) and acutely after SCI, when analyzed at 6 hours (Ismailoglu et al., 2010), 7 days (Tian et al., 2009) or 35 days post-injury (Guptarak et al., 2014). The hypothesis to be tested is that TAM exerts long-term neuroprotective effects in these cells after physical trauma to the adult spinal cord.
The current study assessed the neuroprotective effects of estradiol and Tamoxifen pretreatment on recovery after SCI. The following parameters were measured: (1) recovery of hindlimb motor function (assessed using the BBB open field test), (2) amount of spared tissue (determined with Luxol staining), and (3) oxidative stress (measured by superoxide production). Insight into the mechanism of action was obtained by blocking the estrogen receptor (ER)-α with the selective antagonist MPP Dihydrochloride (MPP) and investigating the temporal pattern of ER-α expression after trauma. Therefore, the hypothesis tested was that pretreatment followed by continuous infusion of physiological high levels of estradiol will exert acute and chronic neuroprotective effects in a contused spinal cord model and that ER mediates this effect.
2. Results
2.1 Estradiol plasma levels after silastic implants
Rats with Silastic implants containing 3 mg 17β-Estradiol-benzoate show that estradiol levels peaked at day 7, the time point when the SCI was performed (Table 1). Mean plasma levels of estradiol at the time of SCI were approximately 154 pg/mL and decrease gradually over time reaching 86.3 pg/mL by week 4 after Silastic tube implant. Control group (Silastic implant empty) had a mean low level of approximately 8 pg/mL (see Table 1) throughout the four weeks. In addition to plasma levels, verification of estradiol release was confirmed indirectly by changes in body weight (data not shown). All estradiol treated animals maintained their body weight after 4 weeks of estradiol administration at approximately 262 g ± 4.19 (p>0.05), while all non-treated control animals gained weight (approximately 46 g/animal in 4 weeks).
Table 1.
17β-Estradiol Plasma Levels (pg/ml) at different time points.
| Treatment | Day 0* (before Ovx) | Day 7 (day of SCI) | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|
| 3 mg 17β-Estradiol | 19.6 ± 1.6 | 153.9 ± 13.7 | 118.7 ± 9.1 | 103.6 ± 8.1 | 86.3 ± 8.6 |
| Control | 8.9 ± 0.2 | 8.6 ± 0.4 | 9.0 ± 0.3 | 7.7 ± 0.3 |
Estradiol plasma levels expressed as mean ± S.E.M. At least an n=11 per group.
Day 0 shows the mean of the rats before ovariectomy n=25.
2.2 Locomotor Functional Recovery
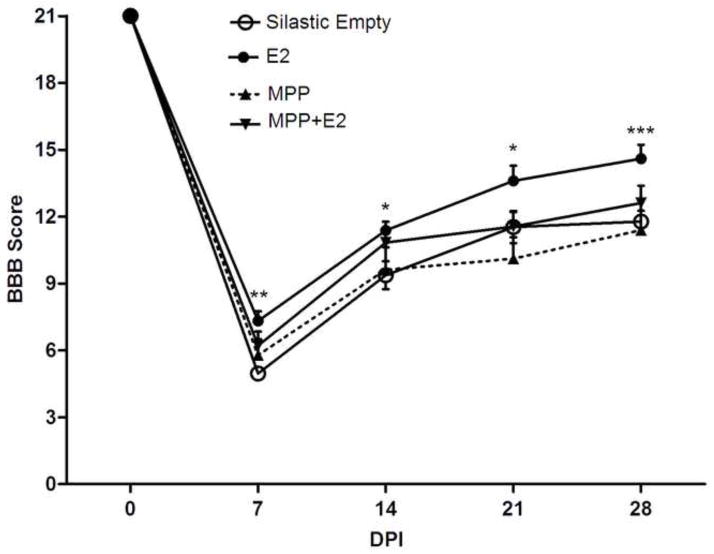
BBB locomotor score test was used to assess the recovery of locomotor function after SCI. This test evaluates spontaneous open field behavior, which assesses hindlimb function, coordination, and weight support, among other parameters (Basso et al., 1995). Rats pre-treated with estradiol (3 mg) showed an improvement in all three locomotor components after SCI according to a multiple repeated measures Two-Way ANOVA (F(3, 41)=5.06; p<0.005). The BBB locomotor score (Fig. 1) was significantly higher in estradiol treated rats than non-treated control group at 7**, 14*, 21* and 28*** days post-injury (DPI) (*p<0.05, **p<0.01, ***p<0.001). However, locomotion of control rats always remained below the experimental group and plateaued at a score of 10–11. Mean scores at 7 DPI (7.3 vs. 4.5), 14 DPI (11.4 vs. 9.4), 21 DPI (13.6 vs. 11.5), and 28 DPI (14.6 vs. 11.8) showed that estradiol treatment enhances and accelerates the onset of locomotor recovery. To determine if the effects observed after estradiol treatment were mediated by the estrogen receptor alpha (ER–α), a group of animals was treated with a selective ER-α antagonist, MPP dihydrochloride. In contrast to estradiol rats, the group pretreated with both estradiol and MPP showed no significant locomotor improvement in BBB scores throughout the 4 weeks (see Fig. 1). The group pre-treated with MPP dihydrochloride alone, scored similar to control group.
Fig. 1.
Estrogen receptor-α (ER-α) mediates the enhanced locomotor recovery observed with estradiol (E2) after spinal cord injury (SCI). Ovariectomized (OVX) rats pretreated with estradiol (3 mg silastic implants) demonstrated a better locomotor performance in the initial and later stages after SCI. BBB scores for estradiol treated rats were significantly higher at 7 (**p<0.01), 14 (*p<0.05), 21 (*p<0.05), and 28 (***p<0.001) days post injury (DPI) according to Repeated Measures Two-way ANOVA and Bonferroni post-hoc analyses. Data are the mean ± SEM (estradiol, n=17 and control Silastic Empty, n=14). Moreover, rats treated with estradiol + MPP dihydrochloride (an ER-α antagonist, n=7) showed lower locomotor scores than rats with estradiol throughout the 4 weeks after spinal cord injury. Rats treated with MPP dihydrochloride (n=7) scored similar to non-treated control group. Statistical analysis did not show significant difference between the control groups and rats treated with estradiol + MPP dihydrochloride (*p<0.05; **p<0.01; ***p<0.001).
2.3 Histological analysis
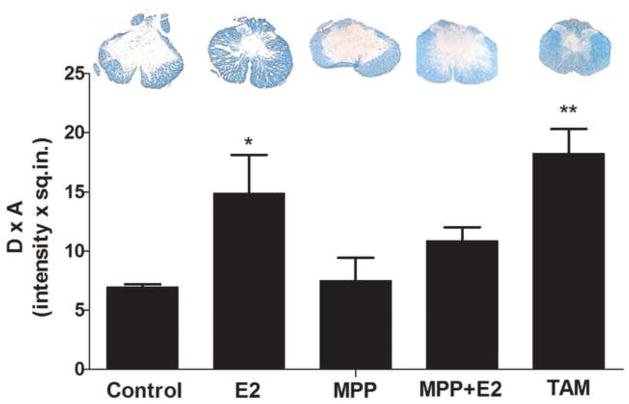
To determine if hormonal treatment altered the lesioned area after SCI, serial transverse sections through the lesion epicenter were stained with Luxol fast blue. As illustrated in Fig. 2, estradiol treated animals at 28 DPI showed a reduced lesion area compared to controls (*p<0.05) that resulted in a higher amount of spared tissue compared to MPP and non-treated animals (*p<0.05). Furthermore, the result of estradiol on spared tissue is receptor mediated because the effect was blocked in the group of animals treated with estradiol + MPP. Animals treated with TAM for 28 days also presented an increase in the amount of spared tissue, compared to controls (**p<0.01). Histological analysis of spinal cord sections did not show significant amount of spared tissue in regions rostral and caudal to the lesion epicenter, with the exception of TAM that produced an increase in white matter tissue rostral to the lesion epicenter (supplemental material 1 & 2).
Fig. 2.
Estradiol and Tamoxifen treatment promotes white matter sparring during SCI. A: The extent of the lesion cavity in treated and non-treated rats was performed by tissue staining with Luxol (SE-Silastic Empty; E2- Estradiol; MPP- MPP dihydrochloride [estrogen receptor alpha antagonist]; TAM- Tamoxifen). B: Densitometry analysis to determine the lesioned area was performed at 28 DPI using MCID, Imaging Research, Inc. Estradiol (E2) treated rats show a significant increase in white matter tissue at 28 DPI and the effect is mediated through the estrogen receptor alpha because the effect was blocked with MPP dihydrochloride. Rats treated with TAM show a significant white matter sparring at 28 DPI when compared to control, according to One-way ANOVA and Dunnet’s Multiple Comparison post-hoc (n=at least 3, *p<0.05; **p<0.01).
2.4 ER-α protein is Up-regulated After SCI
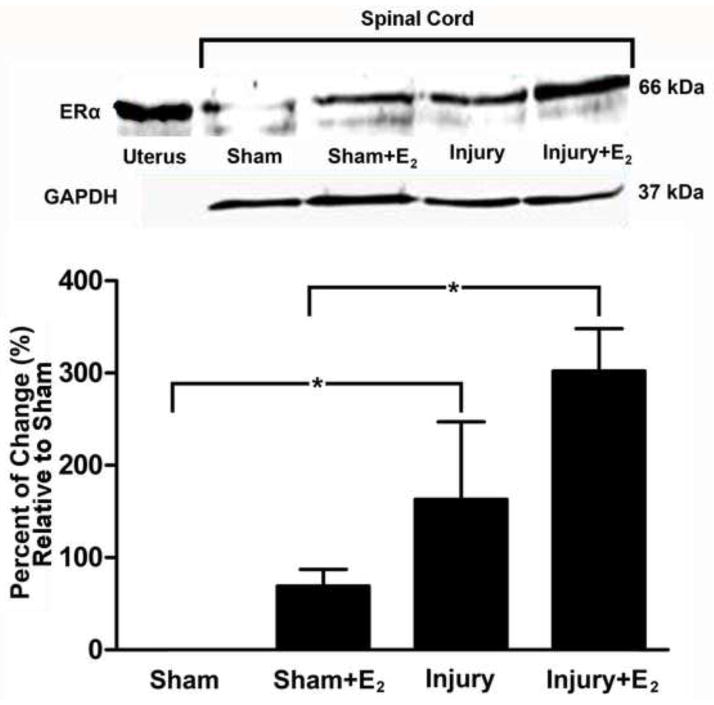
Western Blot studies were performed to assess changes in the expression of the estrogen receptor alpha (ER-α) after prolonged exposure to estradiol and after SCI. Immunoblots detected an immunoreactive band in the expected position of 66 kDa in samples obtained from the uterus (positive control) and the adult spinal cord (Fig. 3). Our data confirms the presence of ER-α in the spinal cord of adult sham animals (Top panel Fig. 3, lane 2) (Vanderhorst et al., 2005). ER-α expression did not change significantly after 3 weeks of estradiol exposure (Top panel Fig. 3, lane 3, which is equivalent to 14 days post laminectomy). In contrast, Western Blot results showed an increase in ER-α expression at 14 days post-injury (lane 4) compared to sham group (lane 2); that was statistically significant (Bottom panel Fig. 3B, p<0.005). This upregulation of ER-α was prominent in injured animals treated with estradiol (compare lane 5 with lane 2 or lane 3, Fig. 3, p<0.005). The increase of ER-α was maintained for up to 28 DPI (data not shown), compared to the level of this receptor in sham animals (p<0.05).
Fig. 3.
Estradiol treatment enhances the up-regulation of the ER-α 14 days after SCI. Top panel: The ER-α is up-regulated in injured non-treated animals after 14 DPI when compared to sham group (p<0.005), according to Western blot studies. The up-regulation is more robust in injured animals treated with estradiol (E2) (p<0.005). Uterus (Ut) tissue was used as positive control as seen in lane 1. No changes were observed with GAPDH at each time point and all data was standardized accordingly (n=3). Bottom panel: Densitometric analysis represents the mean ± SEM and the statistical testing was performed at a pre-set alpha of p<0.05 (B). Comparison was made between sham/control and estradiol treated animals using ANOVA followed by Dunnet’s multiple comparisons post-hoc test (*p<0.005).
2.5 Oxidative Stress after SCI
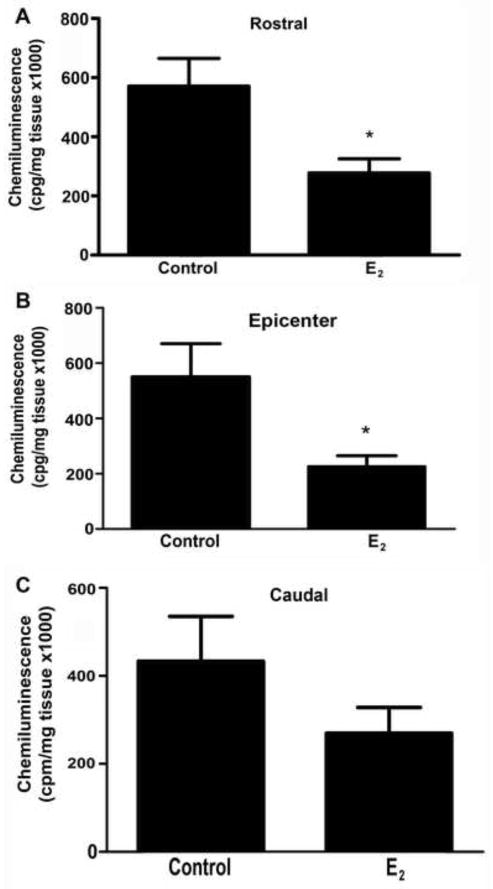
To evaluate a possible mechanism by which estradiol exerts neuroprotective effects, the amount of superoxide production was determined by lucigenin chemiluminescence at the lesion epicenter and in adjacent segments at 2 DPI and 28 DPI. Estradiol decreased superoxides at the lesion epicenter and at rostral segments 2 days after injury (*p<0.05) (Fig. 4). No significant change was seen at 28 days post injury (data not shown).
Fig. 4.
Determination of superoxide activity in spinal cord segments 2 days after injury. Superoxides from rostral (A), epicenter (B), and caudal (C) segments (5 mm each) of lesioned cord were determined by lucigenin chemiluminescence in the presence and absence of estradiol (E2). The values shown are the mean ± SEM (n=4/group) and significance evaluated with an unpaired two-tailed student t-test (*p < .05 vs control).
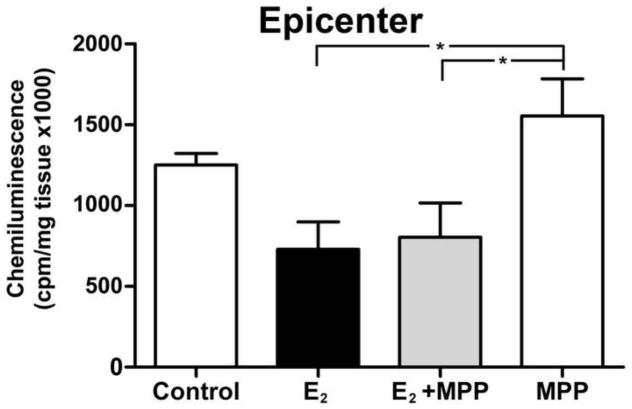
To determine if the antioxidant effect of estradiol at 2 DPI was mediated through the ER-α, a group of animals was treated with estradiol plus MPP and the reactive oxygen species (ROS) production after injury was evaluated. The amount of ROS present in the lesioned epicenter of estradiol treated animals was reduced compared to control (*p<0.05) at 2 DPI (Fig. 5). Animals receiving MPP hydrochloride alone behave similar to control non-treated group. Rats treated with estradiol plus MPP have similar antioxidant effect to that seen in rats treated with estradiol alone. Therefore, estradiol effect of reducing ROS production at 2 DPI was independent of the ER-α.
Fig. 5.
The effect of estradiol to reduce superoxide production at the lesion epicenter is not receptor mediated. Determination of superoxide activity in spinal cord tissue of estradiol + MPP treated rats 2 days after SCI. Epicenter and caudal segments of lesioned spinal cord 2 days after injury where analyzed for lucigenin chemiluminescence. Estradiol + MPP did not block the effect seen with estradiol (E2) alone. The values shown are the mean ± SEM (n=4/group and *p < .05 when compared to control).
2.6 Tamoxifen plasma levels: its effect in locomotor recovery and superoxide activity
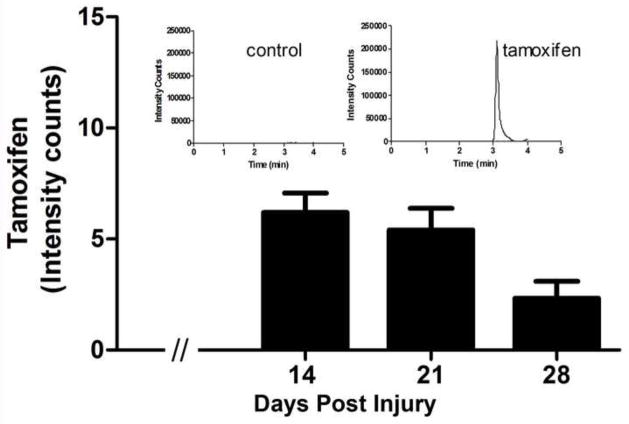
The continuous release of TAM by the commercial pellets was confirmed by the presence of the parent drug in serum samples analyzed through ultra performance liquid chromatography-tandem mass spectroscopy (UPLC-MS/MS). In conditions described in the experimental procedure section, TAM (Fig. 6) and the internal standard Raloxifene (supplement 3) exhibited excellent chromatography–mass spectroscopy baseline resolution. Results showed that there were no foreign peaks interfering with analysis and the internal standard showed a single peak corresponding to TAM present in the serum of rats treated with this drug (Fig. 6-inserts). Plasma from treated animals with TAM was evaluated weekly and the same results were obtained (Fig. 6). The levels of TAM in animals treated for 14 days was 6.2 ng/mL ± 0.87 (n=10), 5.44 ng/mL ± 0.99 (n=9) at 21 days and 2.33 ng/mL ± 0.29 (n=7) at 28 days. According to ANOVA analysis, these values were significantly different (p<0.001) from control-placebo samples because TAM was not quantified in the serum of these control animals, showing the absence of this drug (Fig. 6-insert).
Fig. 6.
Levels of Tamoxifen in the plasma of female rats after commercial pellet implantation. The ion chromatogram acquired by UPLC-MS/MS demonstrated the retention time for Tamoxifen (TAM) from serum samples obtained from treated and untreated animals (insert). Serum TAM levels measured by UPLC-MS/MS over time (histogram) demonstrated that TAM was present in serum of all treated groups and absent in control non-treated animals, according to ANOVA analysis (p<0.001). Levels decreased over time in the treated animals. (Data are the mean ± SEM, n=6).
Figure 7A shows improved locomotor recovery at 21 and 28 DPI with TAM treatment in the BBB open field test. Repeated measures Two-way ANOVA demonstrated that injured rats treated with TAM for four weeks showed improved functional locomotor recovery (F(1, 12)= 9.94; p<0.008), that was significant at 21 (p<0.005) and 28 DPI (p<0.005). The neuroprotective capacity of TAM after SCI was evaluated by the production of superoxides at the lesion epicenter and regions rostral or caudal to it. Lucigenin-induced chemiluminescence was used to determine oxidative stress from tissue extracted at 2 and 28 DPI. TAM reduced oxidative stress at the rostral and epicenter segments of the injured spinal cord at 28 DPI (Fig. 7B, C). This reduction correlated with improved locomotor function observed with the behavioral assay at 28 DPI and the histological observations with Luxol staining (Fig. 2) in animals treated with TAM. However, no changes in oxidative stress were observed in animals treated with TAM at 2 DPI (data not shown). In addition, when pellets with lower amounts of TAM were implanted in lesioned animals, no locomotor recovery or an effect in the formation of reactive oxygen species were observed (data not shown).
Fig. 7.
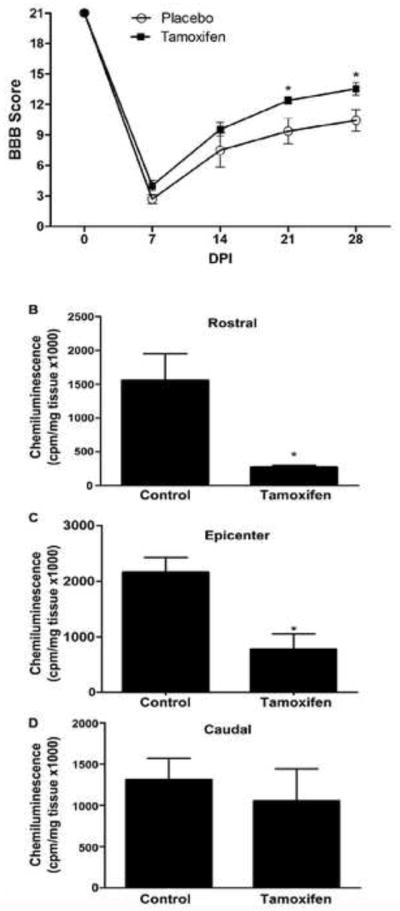
Tamoxifen enhances functional recovery at late stages of SCI. A: BBB scores from TAM treated animals were not significantly different from control scores at 7 and 14 days post injury (DPI). However, TAM induced a significant change at 21 and 28 DPI (*p<0.05) according to Repeated Measures Two-way ANOVA followed by Bonferroni post hoc test. Results are mean ± SEM (Tamoxifen, n=10 and placebo, n=4). B–D: Determination of superoxide activity in spinal cord segments 28 days after injury in TAM-treated rats. Superoxides from rostral (B), epicenter (C), and caudal (D) segments of lesioned cord were determined by lucigenin chemiluminescence. The values shown are the mean ± SEM (n=4) and significance evaluated with an unpaired two-tailed student t-test (*p < .05 vs control).
3. Discussion
Our findings illustrate that pretreatment, followed by a continuous infusion of estradiol at high doses has beneficial effects after SCI at the behavioral and anatomical levels. This is the first report that shows the role of ER-α in mediating the enhanced locomotor outcomes after SCI, and the results support previous studies that demonstrate the beneficial effects of estradiol in pathological conditions. Tamoxifen’s long-term effect on locomotor recovery, spared tissue and decrease in the formation of ROS indicate that this SERM could be considered as a drug that improves locomotor recovery at later stages of SCI.
The beneficial effect of estradiol on multiple pathologies and animal models has made this drug a potential candidate for the treatment of SCI (Arevalo et al., 2010; Garcia-Segura et al., 2001, 2009; Sribnick et al., 2011). In 2003 Sribnick and colleagues, proposed the use of estradiol for the treatment of SCI, and in 2004 Yune T.Y. et al., demonstrated that estradiol enhanced outcomes in locomotor function in relation to the expression of anti-apoptotic genes after a single injection to the injured cord. The outcomes in female mice after a compression SCI also showed a smaller sized injury and better motor functional recovery than in males (Farooque et al., 2006; Hauben et al., 2002). As seen in brain injury, these results suggest that endogenous estrogens are sufficient to confer protection after SCI. Supporting this view, Chaovipoch and colleagues (2006) used continuous infusion of estradiol after a crushed SCI model in post- and pre-menopausal rats. This estradiol therapy resulted in significant improvements at the anatomical, physiological, molecular, and behavioral levels at 14 DPI. The dose of estradiol used produces serum levels equivalent to the low physiological levels observed in the rat estrous cycle. Moreover, continuous administration of estradiol at low physiological doses (Samantaray et al., 2011) stimulates a greater neuroprotective response than that seen in normal cycling rats. These findings suggest that maintaining constant levels of estradiol promotes a greater neuroprotective effect, as well as some locomotor recovery, and these results are in contrast to some published studies (Baker and Hagg, 2005; Swartz et al., 2007). However, differences in the doses of estradiol used, regimen of infusion, and mode of administration have expanded the array of results reported (Elkabes and Nicot, 2014). The results presented here are the effects of constant high physiological levels of estradiol (average 115 pg/ml) after SCI, as confirmed by the plasma levels of the drug (Table 1). Estradiol plasma levels of control groups decreased to approximately 8 pg/ml, reflecting extra-gonadal estradiol production.
3.1 Effect of estradiol on locomotor recovery and role of ER-α
Estradiol protective effects after SCI were evident through the enhanced performance in locomotor function associated with the open field BBB test. These results were similar to those obtained by Chaovipoch and colleagues (2006), with a different injury model (complete crush injury at T8–T9). We observed that rats treated with estradiol, before the injury and continuously after the lesion, showed an improvement in the recovery of locomotor function (BBB) by the first week after injury, which continued over control rats until 28 DPI. Although others investigations of SCI in animals showed that estradiol reduces apoptotic cell death inflammation and myelin loss (Cuzzocrea et al., 2008; Ritz and Hausmann, 2008), little information exists on its antioxidant effects in SCI and the role of ER-α. MPP was used to assess the mechanism of action of estradiol, showing that the behavioral response observed was mediated through ER-α, whereas the immediate response was likely mediated through its antioxidant effects, independent of the ER-α. Previous studies using the non-selective antagonist ICI 182,780, demonstrated that estradiol’s beneficial effects on neuronal survival, decreased inflammation and gliotic response, are receptor dependent (Siriphorn et al., 2012). Our study supports these conclusions and extends these results to demonstrate the crucial role that ER-α plays in neuroprotection after SCI.
3.2 Effect of estradiol on spared tissue and expression profile of ER-α after SCI
Anatomical results support neuroprotective actions of estradiol by showing greater amount of spared tissue in female, as well as in male rats (Chaovipoch et al., 2006; Cuzzocrea et al., 2008; Kachadroka et al., 2010; Sribnick et al., 2005). Moreover, the experiments with the ER-α antagonist MPP demonstrated that the reduction of lesion cavity was mediated by ER-α. It is possible that ER-α activates transcription of anti-apoptotic genes (Yune et al., 2004, 2008) and non-ER-α mediated effects occur by a reduction in oxidative stress.
The increased expression of ER-α in the injured cord could be an endogenous response of the cells to interact with the available estradiol to protect the affected tissue. Similar results were reported previously in an excitotoxic model in culture (Sribnick et al., 2006b), in a model of brain ischemia (Miller et al., 2005) and in a traumatic cerebral contusion model (Li et al., 2011). Our results differ from other studies (Kachadroka et al., 2010; Lee et al., 2012), which did not detect any change in the levels of ER-α after SCI. The difference could be attributed to the type of injury model used, species, gender, hormone delivery system used or if the animals were pre- or post-treated with estradiol relative to the SCI (Elkabes and Nicot, 2014).
3.3 Effects of Tamoxifen treatment during SCI
Although estradiol therapy could be valuable in SCI as well as Alzheimer, Parkinson, TBI, and ischemic strokes, long-term therapy may be associated with an increased risk of cancer, deep vein thrombosis or pulmonary embolism (Breckwoldt and Karck, 2000; Cummings et al., 2002; Laliberté et al., 2011; Sare et al., 2008). In addition, some reports demonstrated that estradiol is neuroprotective in young adult female rats after middle cerebral artery occlusion but worsens ischemic brain injury in aged rats (Leon et al., 2012). Therefore, it is important to understand the mechanism of action of estradiol and develop drugs that target those pathways. Tamoxifen treatment was investigated with the intent of offering a safer alternative. Tamoxifen, one of the most well-known and studied SERMs, is neuroprotective in focal cerebral ischemia and reduces tissue edema after SCI (Tian et al., 2009). Moreover, Tamoxifen crosses the blood brain barrier and accumulates in the brain (Biegon et al., 1996) and hence could be an excellent neuroprotective agent in SCI.
Animal models of stab wound brain injury, irradiation brain injury and focal cerebral ischemia demonstrated that Tamxoxifen reduces the reactive gliotic response, microglial inflammatory response, infarct volume, increases the number of viable cells in cortex and striatum, and improves the neurobehavioral deficit scores (Barreto et al., 2009; Kimelberg et al., 2003; Liu et al., 2010; Zhang et al., 2005). Possible neuroprotective mechanisms mediated by Tamoxifen are blocking the formation of peroxynitrites (Osuka et al., 2001), scavenging reactive oxygen species (Zhang et al., 2007) and decreasing lipid peroxidation (Feng et al., 2004). Several groups reported that TAM treatment 30 minutes or 2 hours after SCI in male rats produced some locomotor recovery during the first two weeks and reduced the amount of TNF, IL-1β or GFAP positive cells (Guptarak et al., 2014; Ismailogh et al., 2010; Tian et al., 2009). Recently, similar results were reported when the SERM Raloxifene was administered to spinal cord injured rats (Ismailoglu et al., 2013). Unexpectedly, in our injury model TAM did not improve locomotor function during the first 2 weeks after SCI but it did so by the third and fourth week. Anatomical studies show that Tamoxifen promotes white matter sparring at 28 DPI. These results correlate with the locomotor improvement seen at 21 and 28 DPI in the BBB locomotor test and also with the reduction in ROS at 28 DPI but not during the acute phases (2 DPI) of SCI. Similar results were observed in focal cerebral ischemia, when the animals were treated with TAM and an antioxidant effect was detected (Zhang et al., 2007). Altogether these suggest the potential use of Tamoxifen during the chronic stages of SCI.
3.4 Effect of estradiol treatment on Reactive Oxygen Species (ROS) formation after SCI
17β-Estradiol is a multi-active steroid that exerts anti-inflammatory, anti-oxidant, and anti-apoptotic effects via genomic and non-genomic pathways in numerous CNS conditions (Cuzzocrea et al., 2008; Lee et al., 2012). We showed that estradiol improves locomotor function through ER-α, but other mechanisms could also be contributing to the overall improved outcomes. Accumulating evidence points toward increased oxidative stress and high ROS (superoxide, peroxynitrite, hydroxyl radical and hydrogen peroxide) levels as critical contributors to the damage developed after SCI (Xiong and Hall, 2009; Xu et al., 2005). We demonstrated that estradiol reduced total ROS in the lesioned and adjacent segments of the spinal cord 2 days after injury, an effect independent of ER-α and a response not seen at 28 DPI. It is possible that estradiol treatment could activate endogenous antioxidant mechanisms such as an increase in the expression of glutathione and SOD enzymes. In addition, the chemical structure of estradiol may act as a free radical scavenger (Prokai and Simpkins, 2007; Sugishita et al., 2003; Winterle et al., 2001), reducing the levels of ROS formed after trauma to the spinal cord. In general, reducing oxidative stress could increase cell viability by improving mitochondrial function, and reducing calpain-mediated cytoskeletal degradation, and neurodegeneration. Further studies are needed to better understand how estradiol or Tamoxifen reduces the amount of ROS after SCI.
3.5 Conclusion
In conclusion, our results are consistent with earlier findings (Sribnick et al., 2005, 2010; Yuen et al., 2004) and suggest that high constant levels of 17β-Estradiol offer acute and chronic neuroprotective effects by increasing the viability of neurons and glia after a lesion to the spinal cord. ER-α mediates the functional recovery and tissue sparing observed in this study, and the antioxidant effects in the acute phase after SCI may also contribute to the improved outcomes. These results support the idea that estradiol could indeed be a potential treatment after SCI and facilitate locomotor rehabilitation. However, the use of high physiological levels of estradiol for long periods of time may trigger unwanted mitogenic side-effects in specific tissues. Therefore, the use of Tamoxifen or other SERMs as neuroprotective agents after SCI should be further explored. Additional studies with post-injury hormone administration are warranted to investigate the neuroprotective properties of estrogenic and SERM agents to treat SCI.
4. Experimental Procedures
4.1 Animal and Housing
Adult female Sprague-Dawley rats (250 g) were purchased from Hilltop Lab Animal (Scottdale, PA). Rats were maintained in a 12:12-h light-dark cycle with lights on at 5:00am. Tap water and rat food chow (Harlan Teklad) were provided ad libitum. A period of 1 week was given for acclimation to the animal facilities.
4.2 Surgical Procedures
All animal procedures followed the National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Puerto Rico, Medical Sciences Campus. Rats were anesthetized with a cocktail of Ketamine/Xylazine/Acepromazine, 87.5/4.2/0.8 mg/kg, (Fort Dodge Animal Health, Fort Dodge, IO) administered intraperitoneal.
Ovariectomy
Bilateral ovariectomy (OVX) was performed under aseptic and sterile conditions, to eliminate gonadal estradiol and cyclical variability. Although this paradigm may at first appear not clinically relevant, it is necessary to establish the baseline to determine the role of estradiol on SCI. The ovaries were clamped and the oviducts were ligated, followed by removal of the ovaries, via a 1 cm incision on the dorsum of the animal. The muscle was then sutured and the wound closed with staples. Half of the animals received silastic implants with 3 mg estradiol as described by Febo et al. 2002, and the other half received empty silastic implants. Animals were allowed one week for recovery prior to experimental manipulations.
Spinal Cord Injury (SCI)
SCI was produced 7 days after ovariectomy to allow for recovery and to achieve steady levels of the hormonal/drug treatments (estradiol, Tamoxifen (TAM), control or MPP-dihydrochloride). A moderate contusion injury was induced using the NYU impactor device (Santiago et al., 2009) in at least five groups of animals containing: a) control-empty silastic implants, b) implants with 3 mg of estradiol, c) implants with 2 mg of MPP dihydrochloride (estrogen receptor α antagonist), d) silastic implants with estradiol and MPP, or e) 15 mg of TAM pellets. Briefly, the back of the animals were shaved and disinfected with iodine and alcohol. A longitudinal incision in the dorsal midline was made and the paravertebral muscles exposed. A laminectomy at T9–T10 level was done without disrupting the dura membrane. Clamps were used rostral and caudal of the laminectomy to stabilize the spine. A moderate SCI was produced using a 10 g rod (2.5 mm in diameter) dropped from a height of 12.5 mm. Control sham rats received only a laminectomy without damaging the dura matter. Muscles and skin were sutured in layers. Animals were housed one per cage after surgery. Post-operative care included administration of Cefazolin and Buprenex twice daily for seven and three days, respectively. In addition, their bladders were expressed until the rats regained normal bladder function.
4.3 Administration of Estradiol, MPP dihydrochloride and Tamoxifen (TAM)
17-β Estradiol 3-benzoate (Sigma-Aldrich, St. Louis, MO, USA) and MPP dihydrochloride (Tocris Biosciences, Ellisville, MO, USA) were administered in its solid form in Silastic tube implants (Dziuk and Cook, 1966). Silastic tube implants were prepared according to Legan and Karsch, 1975. Briefly, 5 mm Silastic tubes (1.47 mm internal diameter, 1.97 mm outside diameter; Dow Corning, distributed by Fisher Scientific, Cayey, Puerto Rico) were filled with 3 mg of estradiol benzoate or were left empty to use as control. These tubes were sealed at each end with toothpick wood and silicone adhesive sealant and stored in saline 3 hrs prior to use. Their floating ability confirmed the integrity of the tubing. According to Wise et al., 1981, these Silastic capsules release estradiol very rapidly within an hour of implantation and the levels of the hormone in the blood stabilizes by 24 hours. The same protocol was followed for the MPP dihydrochloride implants, which were filled with 2 mg of this antagonist to block ER-α. TAM was administered in pellets (Innovative Research, Sarasota, FL, USA) that released a total of 15 mg of this SERM for 30 days. Placebo pellets or empty Silastic tubes were used as controls (Jordan et al., 1991). Both, Silastic tube implants and pellets, were placed subcutaneously in the back of the neck in the midscapular region, immediately after ovariectomy.
4.4 Estradiol Measurement: Radioimmunoassay
Rat blood samples (300–500 μl) were obtained from the tail weekly, and placed in a 1.5 ml microtube. Samples were centrifuged at 2,655 g for 5 minutes (4°C) and plasma was extracted. Total plasma estradiol levels were determined using the Coat-Count RIA kit (TKE22 Diagnostic Product Corporation, Los Angeles, California, USA). A total of 20 animals were used for estradiol (n=10) and control group (n=10).
4.5 UPLC-MS/MS: Standard curve preparation
Levels of TAM in the rat’s plasma were evaluated with UPLC-MS/MS (ultra performance liquid chromatography-tandem mass spectroscopy) at the Institute of Forensic Sciences Institute, San Juan, PR. TAM stock solution was made at 100 μg/mL in methanol and standard curve was prepared in rat plasma (250 μL) from 0.5 ng/mL to 12.5 ng/mL. Internal standard (Raloxifene) stock solution was prepared at 1 mg/mL in methanol and then diluted in acetonitrile at 1.0 μg/mL for sample preparation procedure.
4.6 UPLC-MS/MS: Sample preparation for tamoxifen determination
Proteins from control and treated rat plasma samples (250 μL) were precipitated using 750 μL cold acetonitrile with internal standard (IS, Raloxifene 1.0 μg/mL) and incubated for 30 min at −20 °C. Samples were then centrifuged at 8,000 rpm for 10 min and 500 μL of supernatant containing the analyte and IS were transferred to new microcentrifuge tubes. Supernatant was dried under vacuum then reconstituted with 100 μL of 0.1 % formic acid in water and transferred to autosampler vials.
Sample analyses were performed using Acquity Ultra Performance Liquid Chromatography (UPLC) system equipped with a refrigerated autosampler from Waters (Milford, MA). A reversed phase chromatography was conducted using a BEH C18 (2.1 mm × 100 mm, 1.7 μm particle size). A gradient elution was performed from 60:40 to 60:40 of 0.1% formic acid in water: acetonitrile at 200 μL/min. Sample injection was 10 μL and total run time 4 minutes.
Electrospray ionization tandem mass spectrometry: UPLC was coupled to a TQD (triple quadrupole) mass spectrometer from Waters (Milford, MA). Tamoxifen and Raloxifene (internal standard) ionization was optimized in positive mode ESI+ and detection was performed in multiple reaction monitoring (MRM). The cone voltage was optimized for both analyte and internal standard at 45 V and 50 V, respectively. Meanwhile, the desolvation temperature was 350 °C and source temperature was 100 °C. Cone and desolvation gas flow were 20 and 800 L/hr, respectively. Precursor ions were fragmented by collision at 29 eV for Tamoxifen and 33 eV for Raloxifene with a cell pressure of approximately 2.9 × 10−3 mbar argon. MRM data were acquired in single function for the precursor ions (Tamoxifen: 372 → 72 and raloxifene: 474 → 112). MRM data was acquired using MassLynx 4.1 software and then processed with the TargetLynx application supplied by the manufacturer.
4.7 Locomotor Function and Behavioral Assessment
The BBB Open Field Locomotor Scale was used to assess hindlimb function during the rats open field walking test, as described by Basso and colleagues (1995). Pre-trained rats with scores of 21 were tested for locomotor deficits at 7, 14, 21, and 28 days after SCI. Rats were placed independently in a plastic pool for 4 min and two examiners, blinded to the treatment of each animal, evaluated hindlimb movements, stepping and coordination. Right and left hindlimbs were scored individually and reported as the average score for each animal at each time point. Repeated Measures Two-way ANOVA followed by Bonferroni post hoc test was used to demonstrate significant differences in locomotor activity between control-group versus estradiol, MPP and estradiol + MPP treated animals at different time points after SCI.
4.8 Histological analysis
To examine the lesioned area, serial transverse sections through the lesion epicenter from control, estradiol, MPP, MPP + estradiol and Tamoxifen-treated rats were stained with Luxol fast blue as previously reported (Santiago et al., 2009). Briefly, three to five sections per animal (containing regions rostral and caudal to the lesion epicenter) were dehydrated in 95% ethanol and placed directly into Luxol Fast Blue (in 95% ethanol) overnight at 37°C. Excess stain was rinsed off with 95% ethyl alcohol followed by a rinse in distilled water. Subsequently, the sections were differentiated in a 0.05% lithium carbonate solution for 4 minutes followed by 4 minutes in 70% ethyl alcohol. After a rinse in distilled water, the sections were differentiated in 95% ethyl alcohol for 5 minutes, followed by 2 washes in 100% alcohol for 5 min. Subsequently, the sections were rinsed twice in histoclear (National Diagnostic, Atlanta, Georgia) and coverslipped using Permount® (Fisher) as a mounting solution. The sections were visualized using a Zeiss Axioscope Microscope. Photomicrographs were captured with a Sony Progressive 3CDD camera. The stained spinal cord sections were morphometrically analyzed by measuring the total emptied area standardized over the total tissue area. Briefly, the outer border of the spinal cord section was identified to delineate the site of the spinal cord. Then, the amount of white matter spared tissue (stained with Luxol) and the lesion cavity (hollow region) was delineated. The sections were morphometrically analyzed using MCID software (Imaging Research, Inc. Ontario, Canada) to calculate the area of white matter spared tissue and area of the cavity using density per unit area (density/area). One-way ANOVA followed by Dunnett’s Multiple Comparison Test was used to demonstrate statistical difference between estradiol treated rats and control (*p<0.05) and Tamoxifen treated rats and control group at 28 DPI.
4.9 Protein Extraction and Western blot Analysis
Protein lysates from animals’ spinal cord were performed using Q proteome cell compartment kit (QIAGEN, USA). Briefly, 0.5 cm section of the spinal cord encompassing the lesion epicenter was washed in ice-cold PBS buffer. Tissue was transferred to a 2 ml microcentrifuge tube containing extraction buffer CE1 supplemented with protease inhibitor cocktail (PIC) and homogenized using a plastic pellet pestle. The tissue suspension was then transferred to QIA-shredder homogenizer and centrifuged at 510 g for 2 min at 4°C. The supernatant was transferred to a clean tube, then 1.5 ml of CE1 supplemented with PIC was added. Samples were incubated on a shaker of 10 min at 4°C and then centrifuged at 4,000 g for 10 min at 4°C. The cytosolic proteins in the supernatant fraction were transferred to a fresh microcentrifuge tube and stored at −80°C until use.
Immunoblot studies were performed to identify temporal changes of the ER-α protein after SCI with and without estradiol treatment. Briefly, 25 μg of protein (amount needed to be within the linear range of ER-α detection) diluted at a 1:1 ratio with 2X lamelli buffer were boiled for 5 min and separated in a 10% poly-acrylamide SDS gel. Before transferring the proteins from the gel to a nitrocellulose membrane, the membrane and blotting papers were cut according to the gel size and soaked for 5 min in Towbin transfer buffer (25 mM Tris, 192 mM glycine, 20% MeOH, pH 8.3). Proteins were transferred overnight for 18–22 hrs at a constant 35 mV while immersed in Towbin Transfer buffer (4°C). To asses for adequate transfer of proteins, the membrane was stained with 0.1% Poncieu S (made in 1% glacial acetic acid) and then washed in PBS 1X. The membrane was blocked with Blotto (3% dry skim milk in Tris-HCl pH 7.5, 150 mM NaCl, 0.05% Tween-20) for 1 hr on the shaker at room temperature (RT). The membrane was then incubated in primary antibody (1:800, Anti- ER-α C1355 Upstate) in sealed plastic bags overnight at 4°C. The primary antibody was washed with blotto blocking buffer (3 x for 10 min each) and the membrane incubated with secondary HRP-conjugated mouse anti-rabbit (1:2,500; SIGMA-Aldrich, St. Louis, MO) for 2 hr at RT. Secondary antibody was rinsed with 2 washes of blocking buffer (10 min each) and 2 washes of TBS (10 min each). The membrane was exposed using Super Signal West Dura Extended duration Kit for 1 minute according to the manufacturer’s instruction (Pierce, USA) before exposure and development in a Versa Max gel doc imaging system using Quantity One software (Bio-Rad Laboratories, Hercules, CA). The membrane was also probed for GAPDH with rabbit anti-rat GAPDH (1:5000; Sigma-Aldrich, St. Louis, MO, USA) for loading control.
4.10 Determination of Superoxide Production
To determine if estradiol and TAM alters oxidative stress after SCI, we evaluated the lucigenin-induced chemiluminescence (Crespo et al., 2008). Individual rostral, epicenter, and caudal segments (5 mm each) of the lesioned spinal cord were placed in scintillation vials that contained 2 mL of Krebs’ bicarbonate solution buffer (118 mM NaCl, 2.5 mM CaCl2, 5 mM KCl, 1.1 mM MgSO4, 25 mM NaHCO3, 1.2 mM KH2PO4, and 10 mM glucose; pH 7.4). The addition of 5 μM lucigenin to each vial initiated the reaction and chemiluminescence was assessed in the dark, using a Beckman Scintillation Counter (LS 9800, Fullerton, California). Samples were monitored for 5 minutes at 30-second intervals. Superoxide release was expressed as chemiluminescence units per minute (cpm) per mg of dry tissue. T-test analysis was used to demonstrate statistical difference between estradiol treated rats and the control group.
4.11 Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM). The Mead’s resource equation was used to determine the sample size in each experiment for a good estimate of errors (Mead, 1988). BBB locomotor scores were analyzed using a Repeated measures Two-way ANOVA followed by Bonferroni post-hoc comparison test (Nieuwenhuis et al., 2011). A relative risk estimate of the data was used to determine the effect of estradiol on functional locomotor recovery at different time points after SCI. Unpaired two-tailed student t-test was performed to compare groups (control versus treatment) at one time-point, as in the oxidative stress experiments. For Western Blot analysis, ANOVA and Dunnet’s post hoc test was used to compare all groups with sham control group. One-way ANOVA was used followed by Dunnett’s Multiple Comparison post hoc test for the Histological studies. Differences were considered to be significant when the alpha value was less than 0.05. GraphPad’s InStat3 and Prism (GraphPad Software, San Diego, CA, USA) were used to perform all graphs and statistical analysis.
Supplementary Material
Highlights.
Estradiol and tamoxifen treatment improved locomotor recovery after trauma.
Estrogen receptor-α mediates functional recovery after spinal cord injury.
Spinal cord injury induces estrogen receptor alpha expression.
Long-term treatment with estradiol and tamoxifen reduced the lesion cavity.
Some antioxidant effect was observed with estradiol and tamoxifen.
Acknowledgments
Special thanks to Yvonne Torres for her support during the radioimmunoassay experiment and Nildris Cruz for her unconditional help with the oxidative stress analysis. Thanks to Luz C. Arocho, Odrick Rosas and the staff of the Animal Resource Center at the UPR-MSC, for their surgical and technical support. Special thanks to the Institute of Forensic Sciences for the standardization and analysis of tamoxifen in the samples. Finally, our appreciation to Dr. Anne Etgen for her editorial support, critiques, suggestions and recommendations in the preparation of the manuscript. This research project is in partial fulfillment of Mrs. Laurivette Mosquera and Ms. Jennifer Colón doctoral dissertations. This study was supported by COBRE (P20-GM103642), MBRS-RISE Program (R25-GM068138), MBRS-SCORE (S06-GM008224), RCMI Program (G12RR03051) and the Division of Biomedical Sciences of the UPR School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amtul Z, Wang L, Westaway D, Rozmahel RF. Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer’s disease. Neuroscience. 2010;169:781–786. doi: 10.1016/j.neuroscience.2010.05.031. [DOI] [PubMed] [Google Scholar]
- Arevalo MA, Santos-Galindo M, Bellini MJ, Azcoitia I, Garcia-Segura LM. Actions of estrogens on glial cells: Implications for neuroprotection. Biochimic et Biophys Acta. 2010;1800:1106–1112. doi: 10.1016/j.bbagen.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Baker KA, Hagg T. An adult rat spinal cord contusion model of sensory axon degeneration: The estrous cycle or a preconditioning lesion do not affect outcome. J Neurotrauma. 2005;22:415–428. doi: 10.1089/neu.2005.22.415. [DOI] [PubMed] [Google Scholar]
- Barreto G, Santos-Galindo M, Diz-Chaves Y, Pernía O, Carrero P, Azcoitia I, Garcia-Segura LM. Selective Estrogen Receptor Modulators Decrease Reactive Astrogliosis in the Injured Brain: Effects of Aging and Prolonged Depletion of Ovarian Hormones. Endocrinology. 2009;150:5010–5015. doi: 10.1210/en.2009-0352. [DOI] [PubMed] [Google Scholar]
- Basso DM, Battie MS, Bregnaham JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Behl C, Skutella T, Lezoualc’h F, Post A, Widmann M, Newton CJ, Holsboer F. Neuroprotection against oxidative stress by estrogens: structure-activity relationship. Mol Pharmacol. 1997;51:535–541. [PubMed] [Google Scholar]
- Biegon A, Brewster M, Degani H, Pop E, Somjen D, Kaye AM. A permanently charged tamoxifen derivative displays anticancer activity and improved tissue selectivity in rodents. Cancer Res. 1996;56:4328–31. [PubMed] [Google Scholar]
- Bramlet HM, Dietrich WD. Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog Brain Res. 2007;161:125–141. doi: 10.1016/S0079-6123(06)61009-1. [DOI] [PubMed] [Google Scholar]
- Breckwoldt M, Karck U. Tamoxifen for breast cancer prevention. Exp Clin Endocrinol Diabetes. 2000;108:243–246. doi: 10.1055/s-2000-7751. [DOI] [PubMed] [Google Scholar]
- Bydon M, Lin J, Macki M, Gokaslan ZL, Bydon A. The current role of steroids in acute spinal cord injury. World Neurosurgery. 2013:1–7. doi: 10.1016/j.wneu.2013.02.062. [DOI] [PubMed] [Google Scholar]
- Chaovipoch P, Bozak KA, Gernhold LM, West EJ, Chongthammakun S, Foyd CL. 173-Estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- Crespo MJ, Zalacaín J, Dunbar DC, Cruz N, Arocho L. Cardiac oxidative stress is elevated at the onset of dilated cardiomyopathy in streptozotocin-diabetic rats. J Cardiovasc Pharmacol Ther. 2008;13:64–71. doi: 10.1177/1074248407307854. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Duong T, Kenyon E, Cauley JA, Whitehead M, Krueger KA. Serum estradiol level and risk of breast cancer during treatment with raloxifene. JAMA. 2002;287:216–220. doi: 10.1001/jama.287.2.216. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Esposito E, Di Paola R, Muia C, Crisafulli C, Peli A, Bramanti P, Chaudry IH. Effect of 17[beta]-estradiol on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock. 2008;29:362–371. doi: 10.1097/shk.0b013e31814545dc. [DOI] [PubMed] [Google Scholar]
- Dhandapani KM, Brann DW. Protective Effects of Estrogen and Selective Estrogen Receptor Modulators in the brain. Biology of Reproduction. 2002;67:1379–1385. doi: 10.1095/biolreprod.102.003848. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, Azcoitia I, Garcia-Segura LM. Neuroprotective actions of selective estrogen receptor modulators. Psychoneuroendocrinology. 2009;34S1:113–122. doi: 10.1016/j.psyneuen.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (Ers) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–8. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- Dziuk PJ, Cook B. Passage of steroids through silicone rubber. Endocrinology. 1966;78:208–211. doi: 10.1210/endo-78-1-208. [DOI] [PubMed] [Google Scholar]
- Elkabes S, Nicot AB. Sex steroids and neuroprotection in spinal cord injury: A review of preclinical investigations. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.01.008. http://dx.doi.org/10.1016/j.expneurol.2014.01.008. [DOI] [PubMed]
- Etgen AM, Jover-Mengual T, Zukin RS. Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: Translational implications. Front Neuroendocrinol. 2011;32:336–352. doi: 10.1016/j.yfrne.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooque M, Suo Z, Arnold PM, Wulser MJ, Chou CT, Vancura RW, Fowler S, Festoff BW. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord. 2006;44:182–187. doi: 10.1038/sj.sc.3101816. [DOI] [PubMed] [Google Scholar]
- Febo M, Jimenez-Rivera CA, Segarra AC. Estrogen and opioids interact to modulate the locomotor response to cocaine in the female rat. Brain Res. 2002;943:151–61. doi: 10.1016/s0006-8993(02)02748-8. [DOI] [PubMed] [Google Scholar]
- Feng Y, Fratkins JD, LeBlanc MH. Treatment with tamoxifen reduces hypoxic-ischemic brain injury in neonatal rats. Eur J Pharmacol. 2004;484:65–74. doi: 10.1016/j.ejphar.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Azcoita I, DonCarlos LL. Neuroprotection by estradiol. Prog In Neurobiol. 2001;63:29–60. doi: 10.1016/s0301-0082(00)00025-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Balthazart J. Steroids and neuroprotection: New advances. Front Neuroendrocrinol. 2009;30:5–9. doi: 10.1016/j.yfrne.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guptarak J, Wiktorowicz JE, Sadygov RG, Zivadinovic D, Paulucci-Holthauzen AA, Vergara L, Nesic O. The cáncer drug tamoxifen: a potential therapeutic treatment for spinal cord injury. J Neurotrauma. 2014;31:268–283. doi: 10.1089/neu.2013.3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauben E, Mizrahi T, Agranov E, Schwartz M. Sexual dimorphism in the spontaneous recovery from spinal cord injury: a gender gap in beneficiary autoimmunity? Eur J Neurosci. 2002;16:1731–1740. doi: 10.1046/j.1460-9568.2002.02241.x. [DOI] [PubMed] [Google Scholar]
- Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;26:238–55. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- Ismailoglu O, Oral B, Gorgulu A, Sutcu R, Demir N. Neuroprotective effects of tamoxifen on experimental spinal cord injury in rats. J of Clinical Neuroscience. 2010;17:1306–1310. doi: 10.1016/j.jocn.2010.01.049. [DOI] [PubMed] [Google Scholar]
- Ismailoglu O, Oral B, Sutcu R, Kara Y, Tomruk O, Demir N. Neuroprotective effects of raloxifene on experimental spinal cord injury in rats. Am J Med Sci. 2013;345:39–44. doi: 10.1097/MAJ.0b013e3182522651. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Lababidi MK, Langan-Fahey S. Supression of mouse mammary tumorigenesis by long-term tamoxifen therapy. J Natl Cancer Inst. 1991;83:492–496. doi: 10.1093/jnci/83.7.492. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Jin Y, Charniga C, Feustel PJ. Neuroprotective activity of tamoxifen in permanent focal ischemia. J Neurosurg. 2003;99:138–42. doi: 10.3171/jns.2003.99.1.0138. [DOI] [PubMed] [Google Scholar]
- Laliberté F, Dea K, Sheng-Duh M, Kahler KH, Rolli M, Lefebvre P. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause. 2011;18:1052–1059. doi: 10.1097/gme.0b013e3182175e5c. [DOI] [PubMed] [Google Scholar]
- Lee JY, Choi SY, Oh TH, Yune TY. 17b-Estradiol inhibits apoptotic cell death of oligodendrocytes by inhibiting RhoA-JNK3 activation after spinal cord injury. Endocrinology. 2012;153:3815–3827. doi: 10.1210/en.2012-1068. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ. Modulation of pituitary responsiveness to luteinizing hormone-releasing factor during the estrous cycle of the rat. Endocrinol. 1975;93:571–575. doi: 10.1210/endo-96-3-571. [DOI] [PubMed] [Google Scholar]
- Leon RL, Li X, Huber JD, Rosen CL. Worsened Outcomes from Middle Cerebral Artery Occlusion in Aged Rats Receiving 17β-Estradiol. Neuroendocrinol. 2012;153:3386–3393. doi: 10.1210/en.2011-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Li, Bao Y, Zhao M. 17Beta-estradiol attenuates programmed cell death in cortical pericontusional zone following traumatic brain injury via upregulation of ERalpha and inhibition of caspase-3 activation. Neurochem Internat. 2011;58:126–133. doi: 10.1016/j.neuint.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Liu J, Tian D, Li Z, Qu W, Zhan Y, Xie M, Yu Z, Wang W, Wu G. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010;1316:101–111. doi: 10.1016/j.brainres.2009.12.055. [DOI] [PubMed] [Google Scholar]
- Mead R. Designing useful experiments (Part IV) in The design of experiments. Cambridge, New York: Cambridge University Press; 1988. p. 587. [Google Scholar]
- Mehta SH, Dhandapani KM, De Sevilla LM, Clinton Webb R, Mahesh VB, Brann DW. Tamoxifen, a Selective Estrogen Receptor Modulator, Reduces Ischemic Damage Caused by Middle Cerebral Artery Occlusion in the Ovariectomized Female Rat. Reproductive Neuroendocrinology. 2003;77:44–50. doi: 10.1159/000068332. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Suzanne ZR, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- National Spinal Cord Statistical Center. Spinal cord injury facts and figures at a glance. J Spinal Cord Med. 2012;35:480–481. doi: 10.1179/1079026812Z.000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in Neuroscience: a problem of significance. Nat Neurosc. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Osuka K, Feustel P, Mongin A, Tranmer BI, Kimelberg HK. Tamoxifen inhibits nitrotyrosine formation after reversible middle cerebral artery occlusion in the rat. J of Neurochem. 2001;76:1842–1850. doi: 10.1046/j.1471-4159.2001.00198.x. [DOI] [PubMed] [Google Scholar]
- Oudega M, Bradbury EJ, Ramer MS. Combination therapies. Handb Clin Neurol. 2012;109:617–636. doi: 10.1016/B978-0-444-52137-8.00038-3. [DOI] [PubMed] [Google Scholar]
- Prokai L, Simpkins JW. Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds. Pharmacol Ther. 2007;114:1–12. doi: 10.1016/j.pharmthera.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J Neurosci. 2003;23:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Research. 2008;1203:177–88. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Smith JA, Das A, Matzelle DD, Varma AK, Ray SK, Banik NL. Low dose estrogen prevents neuronal degeneration and microglial reactivity in an acute model of spinal cord injury: effect of dosing, route of administration, and therapy delay. Neurochem Res. 2011;36:1809–1816. doi: 10.1007/s11064-011-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago JM, Rosas O, Torrado AI, González MM, Kalyan-Masih PO, Miranda JD. Molecular, Anatomical, Physiological and Behavioral studies of rats treated with Buprenorphine. J Neurotrauma. 2009;26:1783–1793. doi: 10.1089/neu.2007.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031–2041. doi: 10.1093/eurheartj/ehn299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott E, Zhang QG, Wang R, Vadlamudi R. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012;33:85–104. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra AC, Lee SJ. Neuroprotective effects of estrogen. In: Legato M, editor. Principles of Gender-Specific Medicine. Ch 11. Elsevier Science; San Diego, CA: 2004. pp. 96–103. [Google Scholar]
- Siriphorn A, Dunham KA, Chompoopong S, Floyd CL. Postinjury Administration of 17β-Estradiol Induces Protection in the Gray and White Matter with Associated Functional Recovery after Cervical Spinal Cord Injury in Male Rats. J Comp Neurol. 2012;520:2630–2646. doi: 10.1002/cne.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soustiel JF, Palzur E, Nevo O, Thaler I, Vlodavsky E. Neuroprotective Anti-Apoptosis Effect of Estrogens in Traumatic Brain Injury. J Neurotrauma. 2005;22:345–352. doi: 10.1089/neu.2005.22.345. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosc Res. 2006a;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Banik NL. Estrogen prevents glutamate-induced apoptosis in C6 glioma cells by a receptor-mediated mechanism. Neusoscience. 2006b;137:197–209. doi: 10.1016/j.neuroscience.2005.08.074. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL. Post-Injury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosc Res. 2011;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Ray SK, Banik NL. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann NY Acad Sci. 2003;993:125–133. doi: 10.1111/j.1749-6632.2003.tb07521.x. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen Attenuated Markers of Inflammation and Decreased Lesion Volume in Acute Spinal Cord Injury in Rats. J Neurosc Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosc Res. 2010;88:1738–50. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugioka K, Shimosegawa Y, Nakano M. Estrogens as natural antioxidants of membrane phospholipid peroxidation. FEBS Lett. 1987;210:37–39. doi: 10.1016/0014-5793(87)81293-0. [DOI] [PubMed] [Google Scholar]
- Sugishita K, Li F, Su Z, Barry WH. Anti-oxidant effects of estrogen reduce [Ca2+]i during metabolic inhibition. J Mol Cell Cardiol. 2003;35:331–336. doi: 10.1016/s0022-2828(03)00017-8. [DOI] [PubMed] [Google Scholar]
- Swartz KR, Fee DB, Joy KM, Roberts KN, Sun S, Scheff NN, Wilson ME, Scheff SW. Gender differences in spinal cord injury are not estrogen-dependent. J Neurotrauma. 2007;24:473–480. doi: 10.1089/neu.2006.0167. [DOI] [PubMed] [Google Scholar]
- Tian DS, Liu JL, Xie MJ, Zhan Y, Qu WS, Yu ZY, Tang ZP, Pan DJ, Wang W. Tamoxifen attenuates inflammatory-mediated damage and improves functional outcomes after spinal cord injury in rats. J of Neurochem. 2009;109:1658–1667. doi: 10.1111/j.1471-4159.2009.06077.x. [DOI] [PubMed] [Google Scholar]
- Traynor BJ, Bruijn L, Conwit R, Beal F, O’Neill G, Fagan SC, Cudkowicz ME. Neuroprotective agents for clinical trials in ALS: a systematic assessment. Neurology. 2006;67:20–7. doi: 10.1212/01.wnl.0000223353.34006.54. [DOI] [PubMed] [Google Scholar]
- Vanderhorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and –beta immunoreactive neurons in the brainstem and spinal cord of male and females mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol. 2005;488:152–179. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- Winterle JS, Mill T, Harris T, Goldbeck RA. Absolute kinetic characterization of 17β-estradiol as a radical-scavenging, Antioxidant synergist. Arch Biochem Biophis. 2001;392:233–244. doi: 10.1006/abbi.2001.2431. [DOI] [PubMed] [Google Scholar]
- Wise PM, Camp-Grossman P, Barraclough CA. Effects of estradiol and progesterone on plasma gonadotropins, prolactin, and LHRH in specific brain areas of ovariectomized rats. Biol Reprod. 1981;24:820–830. doi: 10.1095/biolreprod24.4.820. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hall ED. Pharmacological evidence for a role of peroxynitrite in the pathophysiology of spinal cord injury. Exp Neurol. 2009;216:105–114. doi: 10.1016/j.expneurol.2008.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Chi L, Xu R, Ke Y, Luo C, Cai J, Qiu M, Gozal D, Liu R. Increased production of reactive oxygen species contributes to motor neuron death in a compression mouse model of spinal cord injury. Spinal Cord. 2005;43:204–213. doi: 10.1038/sj.sc.3101674. [DOI] [PubMed] [Google Scholar]
- Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, Markelonis GJ, Oh TH. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
- Yune TY, Park HG, Lee JY, Oh TH. Estrogen-induced Bcl-2 expression after spinal cord injury is mediated through phosphoinositide-3-kinase/akt-dependent CREB activation. J Neurotrauma. 2008;25:1121–1131. doi: 10.1089/neu.2008.0544. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jin Y, Behr MJ, Feustel PJ, Morrison JP, Kimelberg HK. Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp Neurol. 2005;196:41–46. doi: 10.1016/j.expneurol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Milatovic D, Aschner M, Feustel PJ, Kimelberg HK. Neuroprotection by tamoxifen in focal cerebral ischemia is not mediated by an agonist action at estrogen receptors but is associated with antioxidant activity. Exp Neurology. 2007;204:819–827. doi: 10.1016/j.expneurol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.