Abstract
Prostate cancer is a major health concern as it has the second highest incidence rate among cancers in men. Despite progress in tumor diagnostics and therapeutic approaches, prognosis for men with advanced disease remains poor. In this review we provide insight into the changes of the intermediary metabolism in normal prostate and prostate cancer. In contrast to normal cells, prostate cancer cells are reprogrammed for optimal energy-efficiency with a functional Krebs cycle and minimal apoptosis rates. A key element in this relationship is the uniquely high zinc level of normal prostate epithelial cells. Zinc is transported by the SLC30 and SLC39 families of zinc transporters. However, in prostate cancer the intracellular zinc content is remarkably reduced and expression levels of certain zinc transporters are altered. Here, we summarize the role of different zinc transporters in the development of prostate cancer.
Keywords: zinc, transport, prostate cancer, SLC30, SLC39
1. Introduction
It is already well known that zinc is a key regulatory factor in the intermediary metabolism of prostate cells and changes in zinc homeostasis contribute to the development and progression of prostate cancer (PCa). Intracellular zinc concentration is tightly regulated by two families of zinc transporters, the SLC30 and the SLC39 families. It has been demonstrated that during the development of prostate adenocarcinoma, the expression of certain zinc transporters is changed compared to their expression in the healthy state and in benign prostate hyperplasia. The purpose of this review is to present first the current state of knowledge about zinc in the intermediary metabolism in the prostate and then to review and discuss the association of different zinc transporters in PCa development.
2. The prostate gland
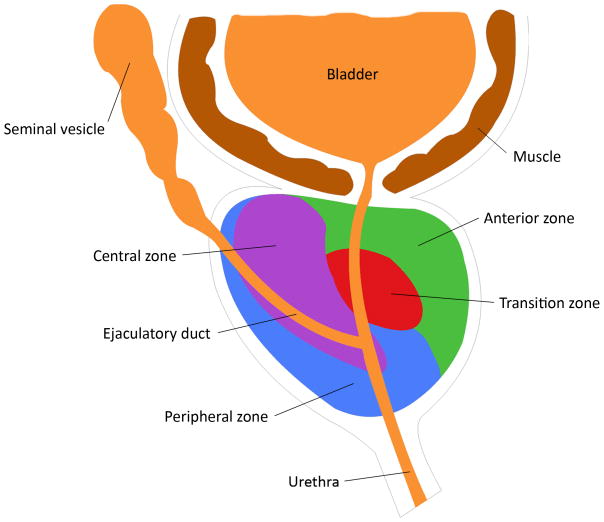
The prostate gland is an exocrine organ of the male reproductive system located in the pelvic cavity. It is enveloped by a thin fibromuscular layer and can be divided into four zones: the anterior zone, transition zone, central zone and peripheral zone (Fig. 1). Most benign prostate hyperplasia (BPH) develops in the transition zone and the central zone. The peripheral zone, which is located most dorsal and close to the rectum, constitutes about 70% of the prostatic volume. Most prostate carcinomas arise from the peripheral zone. Except for the anterior zone, the prostate consists of glandular tissue with a secretory pseudostratified columnar epithelium embedded within a fibromuscular stroma. The main function of the prostate is to secrete the prostatic fluid that is slightly acidic (pH of ~6.4) and forms 20% (by volume) of the semen. Prostate secretions contain several important factors including zinc, citrate and the PCa biomarker, prostate specific antigen (PSA). PSA is a glycoprotein secreted by prostate epithelial cells with the normal functional role of liquefying semen. PSA also enters the bloodstream and its elevation in the circulation is one indicator of the presence of prostate cancer. Circulating PSA level is proportional to the size of the prostate and can also be elevated in BPH.
Fig. 1. Zones of the prostate gland.
The prostate gland can be divided into four zones: the anterior zone, transition zone, central zone and peripheral zone out of which most prostate carcinomas arise.
3. Prostate cancer
PCa has the second highest incidence among men worldwide after lung cancer with a very high mortality rate for extracapsular disease. Although the incidence rates are the highest in the developed countries, mortality is significantly lower in the developed regions (Ferlay et al., 2010). For prevention, PSA is used as tumor marker for screening together with rectal exams. Since the start of PSA-based early detection, relative survival increased markedly, a phenomenon partly due to overdiagnosis (Neppl-Huber et al., 2011). Risk for PCa increases with age and is elevated in African-Americans (Rishi et al., 2003).
PCa arises from malignant transformation of prostate cells. The most frequent cancer type, which accounts for about 80% of all prostate cancer cases, is adenocarcinoma, which develops from the secretory epithelial cells of the peripheral zone. Usually, PCa has a slow growth rate, but has the potential for invasion of neighboring organs such as the seminal vesicles, urinary bladder or rectum. PCa cells disseminate via the bloodstream and form metastases mostly in lymph nodes and bone.
Two systems are used to grade prostate cancer in patients. The TNM cancer staging system evaluates cancers based on the size of the primary tumor (T), the involvement of the lymph nodes (N) and metastasis formation (M). The Gleason Grading System assesses tissue architecture and cellular appearance and gives scores between 1 and 5, which are added to give a composite Gleason Sum Score. Higher scores predict more aggressive tumor growth. These grading systems are used to define a prognosis and select a therapy that is a combination of radiotherapy, chemotherapy, surgery and hormone therapy.
4. Zinc in human health
Zinc is an essential trace element that is ubiquitous in the human body. It plays an important role as a co-factor of many enzymes and is an essential structural component of zinc-finger motifs in many proteins, such as transcription factors and intracellular signaling enzymes (Murakami and Hirano, 2008). Zinc also regulates membrane ion channels and transporters, is involved in neurotransmission in certain cell types, and suppresses apoptotic pathways by regulating caspase activity (Truong-Tran et al., 2001). In human physiology, zinc is most important for RNA and DNA synthesis and metabolism, and consequently plays a major role in cell division. Hence, zinc is important for tissue repair, wound healing, normal growth and development and normal functioning of the immune system, particularly in the context of nonspecific and acquired immunity (Shankar and Prasad, 1998). The human body contains 2–4 g of zinc, with highest concentrations in the prostate and certain parts of the eye. The blood plasma concentration of zinc is 15μM, of which 85% is bound to carbonic anhydrase in erythrocytes, with minor fractions bound to leukocytes, albumin or globulins (Dennes et al., 1962). The free zinc concentration of plasma and interstitial fluid, a plasma filtrate, is ~200 nM (Magneson et al., 1987). Although the total intracellular zinc content is 0.1–0.5 mM, most is bound tightly to intracellular metalloproteins, mainly metallothionein and other metalloenzymes. Only small amounts are attached loosely to amino acids or citrate, or are sequestered in intracellular vesicles. The free cytosolic zinc concentration is in the pM to fM range (Franklin et al., 2005b).
5. The prostate gland and zinc
The prostate gland accumulates the highest zinc and citrate levels in the body. The highest levels are observed in the intramitochondrial compartment within secretory epithelial cells of the peripheral zone. Whereas other soft tissues contain 0.200 nmoles zinc/g wet weight on average, zinc levels in the peripheral prostate are 3–10 fold higher (3 umoles/g wet weight). Associated with the high zinc levels, citrate levels in the peripheral prostate are 30–50 fold higher than found in other tissues (0.150–0.450 umoles/g wet weight vs. 13 umoles/g wet weight) (Franklin et al., 2005b). As mentioned earlier, only a very small portion of intracellular zinc is bound loosely and serves as reactive zinc pool in mammalian cells. In prostate cells, a significant amount of zinc is bound to the abundantly present citrate thereby increasing the mobile reactive pool.
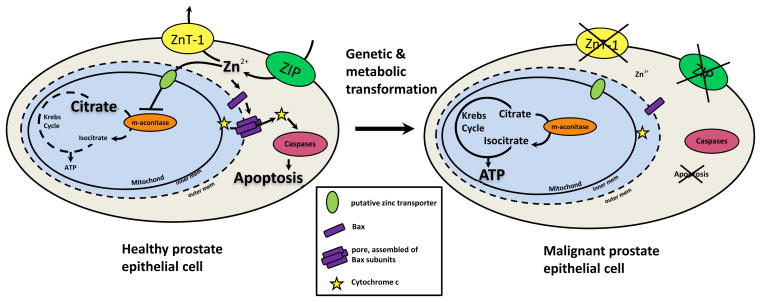
What is the physiologic significance of the accumulation of zinc and citrate in the prostate? Zinc inhibits the mitochondrial (m-) aconitase, an enzyme that catalyzes the first step of the Krebs cycle, the oxidation of citrate to isocitrate. Hence, citrate accumulates in a prostatic reservoir and is secreted into the prostatic fluid. This inhibitory effect of zinc is present in all mammalian cells, but is more efficient in prostate cells. Also, this effect is evoked only by free zinc ions and is not observed against the cytosolic (c-) aconitase. However, zinc is a competitive inhibitor of m-aconitase against citrate and inhibition occurs only with citrate as substrate (Costello et al., 2000; Costello et al., 1997). In non-prostate cells under aerobic conditions, zinc levels are not high enough to inhibit m-aconitase and the Krebs cycle serves to meet the major energy needs. Whereas citrate is an intermediary product of the Krebs cycle in aerobic cells, citrate is an end-product in prostate cells. This truncation of the Krebs cycle diminishes the major cellular energy supply and is lethal to aerobic cells, but not to prostate cells, which is a unique feature of prostatic epithelial cell differentiation and bioenergetically extremely costly. Another function of the high zinc accumulation is inhibition of proliferation and induction of apoptosis (Feng et al., 2002). Zinc exhibits direct actions on the mitochondria, leading to cytochrome c release followed by caspase activation inducing apoptosis (Franklin and Costello, 2007). This effect is mediated by Bax residing in the outer mitochondrial membrane. Zinc facilitates Bax-associated pore formation as well as increased recruitment of cytosolic Bax (Feng et al., 2008). Liang et al. established that zinc induces cell cycle arrest in the G2/M phase (Liang et al., 1999).
The high zinc and citrate levels are characteristic of healthy prostate cells in the peripheral zone. In BPH the levels are even slightly elevated, whereas in PCa zinc and citrate levels are reduced from normal, sometimes at undetectable levels. Zinc depletion is correlated with Gleason score and more pronounced with aggressive disease (Cortesi et al., 2010). Reduced zinc levels have two main effects on prostate cells: a metabolic and a growth effect. The inhibitory effects on m-aconitase are abolished when zinc levels are decreased, consequently allowing citrate oxidation and the production of ATP. As a result, in contrast to healthy citrate-producing, energy-inefficient cells, malignant cells become energy-efficient (Fig. 2). The possibility that m-aconitase enzyme levels or expression are altered during malignant transformation was ruled out; enzyme levels are the same, but their activity was increased (Singh et al., 2006). The growth effect of low intracellular zinc levels results from the elimination of the pro-apoptotic effect of zinc. Together with the increased energy production, the growth effect promotes proliferation of malignant cells (Costello et al., 2004). However, the effects of zinc are cell-specific and dependent on concentration. The question of why and how zinc can have opposite effects in different cell types still remains to be elucidated (Franklin and Costello, 2009).
Figure 2. The effects of zinc in healthy and malignant prostate epithelial cells.
In normal prostate Zn2+ enters epithelial cells via ZIP transporters and leaves via ZnTs (ZnT-1 is representative). Crossing the outer mitochondrial membrane through pores and the inner membrane via a putative zinc transporter, zinc inhibits m-aconitase which prevents oxidation of citrate in the Krebs cycle. Furthermore, zinc also induces Bax to form pores in the outer mitochondrial membrane through which cytochrome c is released into the cytosol. Cytochrome c then interacts with caspases to cause apoptosis. Hence, healthy prostate epithelial cells accumulate citrate, and they are energy-inefficient cells with a growth restriction. After a genetic and metabolic transformation, ZIP transporters are silenced resulting in reduced zinc influx and lowers intracellular zinc content. The Krebs cycle is not truncated any longer resulting in much higher ATP yield. In addition, the pro-apoptotic effect of zinc is abolished. Thus, malignant cells are transformed into energy-efficient, proliferative cells.
6. The SLC30 and SLC39 zinc transporter families
Intracellular zinc homeostasis is tightly regulated by zinc transporters at the plasma and intracellular membranes. The two main families of zinc transporters are the SLC39 (Zrt- and Irt-like proteins, ZIP) and SLC30 (ZnT) zinc transporters. These families exhibit opposite functions in cellular zinc transport. Whereas SLC39 proteins increase intracellular cytoplasmic zinc levels by taking up zinc from extracellular fluid or intracellular vesicles, SLC30 proteins decrease intracellular cytoplasmic zinc levels by transporting zinc out of the cells or into intracellular vesicles (Eide, 2004; Liuzzi and Cousins, 2004; Palmiter and Huang, 2004).
The SLC39 family can be divided into four subfamilies: subfamily I containing fungal and plant sequences, subfamily II consisting of insect, nematode and mammalian sequences, and the gufA and LIV-1 (LZT) subfamilies. There are currently 14 identified mammalian proteins. Most SLC39 proteins have eight transmembrane domains (TMD) with extracellular or intravesicular amino and carboxy termini. TMDs IV and V are highly conserved and form the aqueous pore allowing passage of cations. The LZT subfamily contains a metalloprotease motif (HEXPHE), situated in TMD V, which might confer another function separate from zinc transport (Taylor and Nicholson, 2003). With the exception of ZIP7 (encoded by SLC39A7), which is located in the membrane of the Golgi apparatus, all SLC39 proteins are located at the plasma membrane (Murakami and Hirano, 2008).
The SLC30 family contains three subfamilies: subfamily I, containing prokaryotic members, while subfamilies II and III contain eukaryotic and prokaryotic members. The mammalian SLC30 family contains 10 members. Most SLC30 proteins have six TMDs and both amino and carboxy termini are cytoplasmic. Between TMDs IV and V, ZnT proteins have a long intracellular histidine-rich loop, which has been implicated as ion-binding domain (Murgia et al., 1999). Some SLC30 proteins form homo- or hetero-oligomers. ZnT1 (encoded by SLC30A1) is the only ZnT protein located at the plasma membrane, reflecting its role as primary regulator of cellular zinc efflux (Suzuki et al., 2005). All other SLC30 proteins are located at the membrane of intracellular compartments.
7. Alteration of zinc transporters in prostate cancer
As discussed above, high zinc levels are essential for prostate health, and the zinc content in cancerous tissue is significantly lowered or negligible. Hence, it is important to investigate the mechanisms by which prostate epithelial cells accumulate remarkably high zinc levels, and what transport/buffering elements are compromised in prostate cancer. There are several zinc transporters that are likely to be affected and involved in the development of PCa.
ZIP1 protein, encoded by SLC39A1, was found to be expressed at the basolateral membrane of prostate epithelia, but it is absent in the stromal tissue. In prostate epithelial cells, ZIP1 is postulated to be the major zinc uptake transporter involved in extraction of zinc from the blood. SLC39A1 expression was found to be markedly decreased or absent in PCa as compared to normal prostate or BPH tissue (Franklin et al., 2005a; Johnson et al., 2010).
In the transgenic adenocarcinoma of the mouse prostate (TRAMP) model of PCa, citrate and zinc levels were decreased and ZIP1 protein was lost in TRAMP tumors (Costello et al., 2011). Furthermore, the protein expression of ZIP1 was lower in RWPE2 human tumorigenic prostate cells when compared to RWPE1 non-tumorigenic counterparts (Huang et al., 2006). Interestingly, ZIP1 is expressed in LNCaP and PC-3, malignant cell lines derived from metastatic PCa tissue, demonstrating that the absence of ZIP1 expression in the malignant glands in situ is not due to the deletion or fatal mutation of the gene (Franklin et al., 2005a; Franklin et al., 2003). In these cells, ZIP1 was shown to be the major zinc uptake pathway. Furthermore, a strong correlation was shown between zinc levels and ZIP1 expression in the prostate. During PCa development, both the decrease of ZIP1 expression and the zinc depletion occur at early stages of the disease.
In healthy prostate tissue, ZIP2 is located in the apical membrane of epithelial cells, in contrast to the stroma which is devoid of this protein. Based on its location ZIP2 is hypothesized to be involved in maintaining cellular zinc homeostasis by reabsorbing zinc from the prostatic fluid (Desouki et al., 2007). It was demonstrated that the transport of zinc by ZIP2 is independent of energy, membrane potential and Na+. In contrast, transport activity is enhanced by increasing pH (inhibition occurs at pH below 7.0) or by HCO3− (Gaither and Eide, 2000). Recent studies have revealed that ZIP2 is downregulated in prostate cancer, whereas it is abundantly present in normal prostate epithelial cells and in BPH (Desouki et al., 2007). Furthermore, ZIP2 expression was found to be markedly lower in African-American patients, who generally have a higher predisposition to develop PCa compared to age-matched white individuals. In addition, ZIP2 levels are more decreased in tissue samples from African-Americans compared to Gleason grade-matched samples from white males (Rishi et al., 2003).
Another member of the SLC39 transport protein family associated with PCa is ZIP3, encoded by SLC39A3. Like ZIP2, ZIP3 is localized at the apical plasma membrane of prostate epithelial cells and might have a similar function: reabsorbing zinc from the prostate fluid. Together with ZIP2, protein expression of ZIP3 is also down-regulated in PCa (Desouki et al., 2007). It was also shown that protein expression changes with zinc status, whereas mRNA expression is not affected, suggesting post-translational regulation of this protein (Wang et al., 2004). In response to zinc, ZIP3 was redistributed to a significant extent from the plasma membrane to the lysosomal compartment in the tumorigenic RWPE2 cell line compared to the RWPE1 non-tumorigenic prostate cell line, suggesting increased degradation of this protein (Huang et al., 2006).
Zip4 is localized at the apical membrane of mouse enterocytes, and mutations of the human SLC39A4 gene encoding ZIP4 are found to be closely associated with the rare inherited condition acrodermatitis enteropathica that is known to result from defective intestinal absorption of zinc (Schmitt et al., 2009). Nevertheless, ZIP4 is also present in prostate epithelial cells (Chen et al., 2011). Recently, it was reported that mRNA as well as protein expression of SLC39A4 is decreased in PCa compared to BPH. However, no correlation was found between cancer grade and the level of ZIP4 expression (Chen et al., 2011). This highlights the fact that zinc and citrate changes occur early in development of malignancy and persist through disease progression. Furthermore, an inhibitory effect on proliferation and invasion has been demonstrated in cancer cells in vitro if the transporter was present (Chen et al., 2011).
Much less is known about the potential involvement of the zinc efflux transporters, the SLC30 family, in the malignant transformation of the prostate gland. The expression of SLC30A1 mRNA encoding ZnT-1 was found to be either decreased (Hasumi et al., 2003) or unchanged ((Beck et al., 2004), Freeman, Suzuki and Hediger, unpublished data) in prostate cancer samples compared to BPH tissues. In RWPE-1, an immortalized, non-tumorigenic prostate cell line, ZnT-1 was also less expressed compared to the widely used metastatic PCa lines PC-3, and DU-145 (Albrecht et al., 2008). The mRNA level of SLC30A4, encoding ZnT-4, was also decreased in PCa compared to normal prostate tissue samples (Beck et al., 2004; Henshall et al., 2003), but an increase was observed when data obtained from PCa samples were compared to BPH samples (Freeman, Suzuki and Hediger, unpublished data). ZnT-1 levels are observed to be normal in BPH and decreased in cancer (Beck et al., 2004; Hasumi et al., 2003). However, our unpublished observation shows that ZnT-1 levels increased ~7-fold at the mRNA level. ZnT-4 is located at the plasma membrane as well as in intracellular vesicles (Henshall et al., 2003). These data suggest that ZnT-4 expression changes in association with prostate tissue abnormalities independent of prostate zinc content. Furthermore, SLC30A5 mRNA level, encoding ZnT-5, was increased in PCa compared to BPH samples (see Table 1; Freeman, Suzuki and Hediger, unpublished data).
Table 1.
Expression of different zinc transporters in benign prostate hyperplasia (BPH) and prostate carcinoma (PCa) compared to normal prostate tissue.;
| Transporter | BPH | PCa |
|---|---|---|
| SLC39A1 | ➡(Franklin et al., 2005a; Johnson et al., 2010) | ⬇(Costello et al., 2011; Franklin et al., 2005a; Johnson et al., 2010) |
| SLC39A2 | ➡(Desouki et al., 2007) | ⬇(Desouki et al., 2007; Rishi et al., 2003) |
| SLC39A3 | ➡(Desouki et al., 2007) | ⬇(Desouki et al., 2007) |
| SLC39A4 | ⬇(Chen et al., 2011) | |
| SLC39A6 | ⬆#. * | |
| SLC39A7 | ⬆# | |
| SLC39A8 | ⬆# | |
| SLC39A14 | ⬇ # | |
| SLC30A1 | ➡(Hasumi et al., 2003) |
 (Hasumi et al., 2003) (Hasumi et al., 2003) |
| ➡(Beck et al., 2004), * | ||
| SLC30A4 | ⬇(Beck et al., 2004) |
 (Beck et al., 2004; Henshall et al., 2003) (Beck et al., 2004; Henshall et al., 2003) |
| ⬆* | ||
| SLC30A5 | ⬆* | |
| SLC30A7 | ⬆# | |
| SLC30A9 | ⬆# |
Freeman, Suzuki and Hediger, unpublished data. (In these studies, the difference in the expression of zinc transporters in normal prostate epithelial tissue, benign prostate hyperplasia (BPH) and prostate adenocarcinoma (PCa) were compared using quantitative PCR).
Anderle, expression data analysis of dataset GSE17951 obtained at http://www.ncbi.nlm.nih.gov/geo/
Expression data analysis of publicly available data obtained at http://www.ncbi.nlm.nih.gov/geo/ through accession number GSE17951 showed that the expression of SLC39A6, SLC39A7, SLC39A8, SLC30A5, SLC30A7 and SLC30A9 was increased whereas SLC39A14 was decreased in PCa (Jia et al., 2011; Wang et al., 2010).
8. Outlook
As discussed above, the zinc content of the prostate is decreased drastically in PCa. This leads to the assumption that restoration of high zinc levels would reverse the malignant phenotype of the cells and/or induce apoptosis. Zinc levels can be restored by either increasing circulating levels of zinc, increasing the uptake from the circulation or decrease zinc efflux from cells. The simplest approach is to enhance zinc bioavailability by increasing dietary intake, but results from these studies have been inconsistent and have described preventive, no effect, or even harmful effects (Costello et al., 2005). Another approach is to alter expression of zinc transporters by hormone treatment. It has been shown that testosterone and prolactin can increase expression of ZIP1 (Costello et al., 1999), but up-regulation of other alternative zinc uptake transporters should also be considered. Although the zinc transporter field has evolved significantly in the past several years, important questions remain to be answered through approaches like development of tissue-specific targeting of transporters, and specific chemical transporter activation. In summary, the central role of the decreased zinc content of the prostate gland in the course of PCa development is well established. Although the involvement of several zinc transporters is implicated, the relevant regulatory signals in tumor development and the underlying mechanisms remain to be elucidated. Further experiments are also needed to improve pharmacologic targeting of zinc transporters.
References
- Albrecht AL, Somji S, Sens MA, Sens DA, Garrett SH. Zinc transporter mRNA expression in the RWPE-1 human prostate epithelial cell line. Biometals. 2008;21 (4):405–416. doi: 10.1007/s10534-007-9129-0. [DOI] [PubMed] [Google Scholar]
- Beck FW, Prasad AS, Butler CE, Sakr WA, Kucuk O, Sarkar FH. Differential expression of hZnT-4 in human prostate tissues. Prostate. 2004;58 (4):374–381. doi: 10.1002/pros.10344. [DOI] [PubMed] [Google Scholar]
- Chen QG, Zhang Z, Yang Q, Shan GY, Yu XY, Kong CZ. The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol. 2011 doi: 10.1016/j.urolonc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Cortesi M, Fridman E, Volkov A, Shilstein S, Chechik R, Breskin A, Vartsky D, Raviv G, Ramon J. New prospective for non-invasive detection, grading, size evaluation, and tumor location of prostate cancer. Prostate. 2010;70 (15):1701–1708. doi: 10.1002/pros.21205. [DOI] [PubMed] [Google Scholar]
- Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7 (2):111–117. doi: 10.1038/sj.pcan.4500712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB, Feng P, Tan M, Bagasra O. Zinc and prostate cancer: a critical scientific, medical, and public interest issue (United States) Cancer Causes Control. 2005;16 (8):901–915. doi: 10.1007/s10552-005-2367-y. [DOI] [PubMed] [Google Scholar]
- Costello LC, Franklin RB, Liu Y, Kennedy MC. Zinc causes a shift toward citrate at equilibrium of the m-aconitase reaction of prostate mitochondria. J Inorg Biochem. 2000;78 (2):161–165. doi: 10.1016/s0162-0134(99)00225-1. [DOI] [PubMed] [Google Scholar]
- Costello LC, Franklin RB, Zou J, Feng P, Bok R, Mark GS, Kurhanewicz J. Human prostate cancer ZIP1/zinc/citrate genetic/metabolic relationship in the TRAMP prostate cancer animal model. Cancer Biol Ther. 2011;12(12) doi: 10.4161/cbt.12.12.18367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997;272 (46):28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- Costello LC, Liu Y, Zou J, Franklin RB. Evidence for a zinc uptake transporter in human prostate cancer cells which is regulated by prolactin and testosterone. J Biol Chem. 1999;274 (25):17499–17504. doi: 10.1074/jbc.274.25.17499. [DOI] [PubMed] [Google Scholar]
- Dennes E, Tupper R, Wormall A. Studies on zinc in blood. Transport of zinc and incorporation of zinc in leucocytes. Biochem J. 1962;82:466–476. doi: 10.1042/bj0820466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC. hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer. 2007;6:37. doi: 10.1186/1476-4598-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide DJ. The SLC39 family of metal ion transporters. Pflugers Arch. 2004;447 (5):796–800. doi: 10.1007/s00424-003-1074-3. [DOI] [PubMed] [Google Scholar]
- Feng P, Li T, Guan Z, Franklin RB, Costello LC. The involvement of Bax in zinc-induced mitochondrial apoptogenesis in malignant prostate cells. Mol Cancer. 2008;7:25. doi: 10.1186/1476-4598-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52 (4):311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127 (12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463 (2):211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RB, Costello LC. The important role of the apoptotic effects of zinc in the development of cancers. J Cell Biochem. 2009;106 (5):750–757. doi: 10.1002/jcb.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC. hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer. 2005a;4:32. doi: 10.1186/1476-4598-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RB, Ma J, Zou J, Guan Z, Kukoyi BI, Feng P, Costello LC. Human ZIP1 is a major zinc uptake transporter for the accumulation of zinc in prostate cells. J Inorg Biochem. 2003;96 (2–3):435–442. doi: 10.1016/s0162-0134(03)00249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RB, Milon B, Feng P, Costello LC. Zinc and zinc transporters in normal prostate and the pathogenesis of prostate cancer. Front Biosci. 2005b;10:2230–2239. doi: 10.2741/1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither LA, Eide DJ. Functional expression of the human hZIP2 zinc transporter. J Biol Chem. 2000;275 (8):5560–5564. doi: 10.1074/jbc.275.8.5560. [DOI] [PubMed] [Google Scholar]
- Hasumi M, Suzuki K, Matsui H, Koike H, Ito K, Yamanaka H. Regulation of metallothionein and zinc transporter expression in human prostate cancer cells and tissues. Cancer Lett. 2003;200 (2):187–195. doi: 10.1016/s0304-3835(03)00441-5. [DOI] [PubMed] [Google Scholar]
- Henshall SM, Afar DE, Rasiah KK, Horvath LG, Gish K, Caras I, Ramakrishnan V, Wong M, Jeffry U, Kench JG, Quinn DI, Turner JJ, Delprado W, Lee CS, Golovsky D, Brenner PC, O’Neill GF, Kooner R, Stricker PD, Grygiel JJ, Mack DH, Sutherland RL. Expression of the zinc transporter ZnT4 is decreased in the progression from early prostate disease to invasive prostate cancer. Oncogene. 2003;22 (38):6005–6012. doi: 10.1038/sj.onc.1206797. [DOI] [PubMed] [Google Scholar]
- Huang L, Kirschke CP, Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: a possible role in prostate cancer progression. Cancer Cell Int. 2006;6:10. doi: 10.1186/1475-2867-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Wang Y, Sawyers A, Yao H, Rahmatpanah F, Xia XQ, Xu Q, Pio R, Turan T, Koziol JA, Goodison S, Carpenter P, Wang-Rodriguez J, Simoneau A, Meyskens F, Sutton M, Lernhardt W, Beach T, Monforte J, McClelland M, Mercola D. Diagnosis of prostate cancer using differentially expressed genes in stroma. Cancer Res. 2011;71 (7):2476–2487. doi: 10.1158/0008-5472.CAN-10-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Kanak MA, Kajdacsy-Balla A, Pestaner JP, Bagasra O. Differential zinc accumulation and expression of human zinc transporter 1 (hZIP1) in prostate glands. Methods. 2010;52 (4):316–321. doi: 10.1016/j.ymeth.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Liang JY, Liu YY, Zou J, Franklin RB, Costello LC, Feng P. Inhibitory effect of zinc on human prostatic carcinoma cell growth. Prostate. 1999;40 (3):200–207. doi: 10.1002/(sici)1097-0045(19990801)40:3<200::aid-pros8>3.0.co;2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liuzzi JP, Cousins RJ. Mammalian zinc transporters. Annu Rev Nutr. 2004;24:151–172. doi: 10.1146/annurev.nutr.24.012003.132402. [DOI] [PubMed] [Google Scholar]
- Magneson GR, Puvathingal JM, Ray WJ., Jr The concentrations of free Mg2+ and free Zn2+ in equine blood plasma. J Biol Chem. 1987;262 (23):11140–11148. [PubMed] [Google Scholar]
- Murakami M, Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99 (8):1515–1522. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia C, Vespignani I, Cerase J, Nobili F, Perozzi G. Cloning, expression, and vesicular localization of zinc transporter Dri 27/ZnT4 in intestinal tissue and cells. Am J Physiol. 1999;277 (6 Pt 1):G1231–1239. doi: 10.1152/ajpgi.1999.277.6.G1231. [DOI] [PubMed] [Google Scholar]
- Neppl-Huber C, Zappa M, Coebergh JW, Rapiti E, Rachtan J, Holleczek B, Rosso S, Aareleid T, Brenner H, Gondos A. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: additional diagnoses and avoided deaths. Ann Oncol. 2011 doi: 10.1093/annonc/mdr414. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Huang L. Efflux and compartmentalization of zinc by members of the SLC30 family of solute carriers. Pflugers Arch. 2004;447 (5):744–751. doi: 10.1007/s00424-003-1070-7. [DOI] [PubMed] [Google Scholar]
- Rishi I, Baidouri H, Abbasi JA, Bullard-Dillard R, Kajdacsy-Balla A, Pestaner JP, Skacel M, Tubbs R, Bagasra O. Prostate cancer in African American men is associated with downregulation of zinc transporters. Appl Immunohistochem Mol Morphol. 2003;11 (3):253–260. doi: 10.1097/00129039-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Schmitt S, Kury S, Giraud M, Dreno B, Kharfi M, Bezieau S. An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum Mutat. 2009;30 (6):926–933. doi: 10.1002/humu.20988. [DOI] [PubMed] [Google Scholar]
- Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68 (2 Suppl):447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- Singh KK, Desouki MM, Franklin RB, Costello LC. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol Cancer. 2006;5:14. doi: 10.1186/1476-4598-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Ishihara K, Migaki H, Nagao M, Yamaguchi-Iwai Y, Kambe T. Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J Biol Chem. 2005;280 (35):30956–30962. doi: 10.1074/jbc.M506902200. [DOI] [PubMed] [Google Scholar]
- Taylor KM, Nicholson RI. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim Biophys Acta. 2003;1611 (1–2):16–30. doi: 10.1016/s0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- Truong-Tran AQ, Carter J, Ruffin RE, Zalewski PD. The role of zinc in caspase activation and apoptotic cell death. Biometals. 2001;14 (3–4):315–330. doi: 10.1023/a:1012993017026. [DOI] [PubMed] [Google Scholar]
- Wang F, Dufner-Beattie J, Kim BE, Petris MJ, Andrews G, Eide DJ. Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J Biol Chem. 2004;279 (23):24631–24639. doi: 10.1074/jbc.M400680200. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xia XQ, Jia Z, Sawyers A, Yao H, Wang-Rodriquez J, Mercola D, McClelland M. In silico estimates of tissue components in surgical samples based on expression profiling data. Cancer Res. 2010;70 (16):6448–6455. doi: 10.1158/0008-5472.CAN-10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]