Abstract
A North Carolinian developed fatal coccidioidomycosis immediately after bilateral lung transplantation. The donor had previously traveled to Mexico, and the recipient had no travel history to an area where Coccidioides immitis is endemic. Immunosuppresive therapy of the transplant recipient likely reactivated latent Coccidioides infection in the donor lungs, leading to posttransplant coccidioidomycosis.
CASE REPORT
A 61-year-old North Carolina resident with chronic obstructive pulmonary disease and emphysema received a bilateral lung transplant. Subsequent immunosuppressive therapy included methylprednisone, azathioprine, and cyclosporine. During hospitalization, the patient also received cefuroxime, clindamycin, piperacillin-tazobactam, and acyclovir as prophylaxis, but no antifungal prophylaxis was initiated. Posttransplantation, the patient was poorly responsive and had complications, including coagulopathy, hypotension, and hypoxia, resulting in increased oxygen requirements. Laboratory findings were consistent with liver, pancreas, and kidney dysfunction, and the patient ultimately required hemodialysis. Chest X-ray images during the first postoperative week indicated bilateral diffuse pulmonary opacities consistent with edema or infection.
At 2 weeks posttransplantation, cerebrospinal fluid (CSF) was obtained. CSF analysis showed <1 red blood cell/mm3 and 1 total nucleated cell/mm3 (22% lymphocytes, 63% monocytes, 15% other cells). The CSF contained 32 mg of protein/dl (normal, 15 to 45 mg/dl). A glucose level determination was not performed. The Gram stain showed no polymorphonuclear leukocytes and no organisms. The CSF culture was reported as no growth at 7 days, and peripheral blood cultures were negative after 5 days of incubation. Bronchial washings from the donor lungs at the time of transplantation grew oxacillin-susceptible Staphylococcus aureus. Stenotrophomonas maltophilia and coagulase-negative Staphylococcus spp. were recovered from subsequent washings. The patient's chest X-ray images showed persistent bilateral infiltrates and worsening alveolar consolidation.
During the third week posttransplantation, chest X-ray images demonstrated extensive, progressive bilateral air space and interstitial opacities, as well as bilateral apical and lateral pleural thickening. In addition, a brain magnetic resonance image showed multiple areas of enhancement in the region of the gray-white matter junction thought to be consistent with a fungal infection. The patient had multiple line-drawn blood cultures positive with Candida parapsilosis. Further, a KOH preparation of the patient's bronchial washing showed budding yeast with pseudohyphae, and the Gram stain showed polymorphonuclear leukocytes and yeast. Interestingly, this culture not only grew Candida and S. maltophilia but also Coccidioides immitis. In less than 1 month postoperatively, the patient died, and an autopsy was performed.
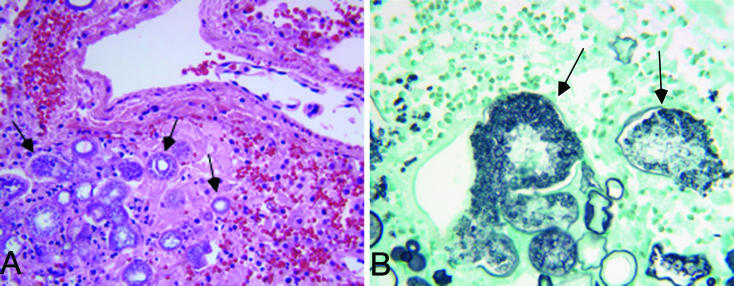
Anatomically, the patient had extensive pneumonia, multiple small abscesses in the spleen, and extensive acute fungal pericarditis and pyelonephritis. Hematoxylin and eosin and Gomori's methenamine silver (GMS) staining of lungs, pericardium, and spleen sections revealed numerous endosporulating spherules consistent with C. immitis (Fig. 1). In addition, cultures of the spleen and lungs grew C. immitis. GMS staining of the kidneys and pericardium showed extensive yeast with pseudohyphae, a finding consistent with Candida. Thus, the cause of death was likely disseminated coccidioidomycosis and candidiasis secondary to immunosuppressive therapy post-transplantation.
FIG. 1.
C. immitis spherules identified in the transplant recipient's lung tissue procured during autopsy. (A) Hematoxylin and eosin stain (magnification, ×400); (B) GMS stain (magnification, ×600).
The disease coccidioidomycosis is caused by the dimorphic fungus C. immitis, which is endemic in dry climates such as the southwestern United States, northern Mexico, and parts of Central and South America. In the soil, C. immitis exists in a mycelial state, and the hyphae develop into arthroconidia that are highly infectious and airborne. Inhaled arthroconidia are the source of human infection. Once in tissue, C. immitis arthroconidia develop into thick-walled spherules, each containing endospores (Fig. 1). Endospores are released when the spherule ruptures, and each endospore can develop into a new spherule, and thus the infection progresses (6).
In immunocompetent individuals, disseminated coccidioidomycosis is extremely rare. Infection with C. immitis is usually subclinical (∼60%) but may occasionally present as a nonspecific influenza-like syndrome, or less commonly, as pneumonia (9). It is estimated based on delayed dermal hypersensitivity testing that, in areas where this organism is endemic, one-third of the local population are infected with C. immitis (5). Populations that are at risk for developing serious complications from C. immitis infection include those with diabetes mellitus, pregnant women, and immunocompromised patients, such as those at the extremes of age, human immunodeficiency virus-infected individuals, and transplant recipients (12).
Coccidioidomycosis in transplant patients typically occurs as a result of either a primary infection posttransplantation in areas where this organism is endemic or reactivation of latent infection. The medical literature indicates that reactivation of latent C. immitis infection likely represents the majority of coccidioidomycosis posttransplant cases, primarily occurring in patients who have resided in or visited these areas (2, 3). In fact, some transplant centers in endemic areas test potential organ recipients for C. immitis serology and prophylactically treat those with a positive result or a past history of coccidioidal infection (1). The appearance of coccidioidomycosis due to reactivation typically occurs in 4 to 6 months posttransplant (10).
In addition, there are rare reports of transmission due to infected donor lungs, liver, and kidney (13, 14). Infection from donor lungs has been reported to develop in the immediate postoperative period (13). Since the patient reported here was hospitalized during the entire postoperative period and was a North Carolina resident, it is unlikely that C. immitis was acquired as a primary infection posttransplantation. Therefore, the patient's fatal coccidioidomycosis most likely developed due to reactivation of a latent infection, either provided by the patient's own latent infection or by the donor lungs. The recipient's lack of a travel history outside of North Carolina and the accelerated postoperative course of the coccidioidomycosis suggest that the donor lungs provided the coccidioidal infection. The donor was a 30-year-old North Carolina resident without a significant past medical or travel history. However, the donor had visited Mexico 2 years prior to organ donation.
The incidence of postoperative fungal infections in lung transplant recipients is reportedly 15 to 35% (8, 11). In this patient population, the most commonly isolated fungal organisms are Candida and Aspergillus spp. due to their ubiquity. In addition, in areas where these organisms are endemic, coccidioidomycosis occurs in 4 to 8% of transplant patients, generally occurring within the first postoperative year (2, 7). However, in areas where these organisms are not endemic, the diagnosis of coccidioidomycosis is often delayed due to a low index of suspicion (4). Therefore, for solid organ transplant recipients who reside in an area where this organism is endemic, who have a positive Coccidioides serology, or who are receiving a donor organ from a resident such an area, antifungal prophylaxis with an azole may be prudent (1, 2). It is also of great importance for areas where this organism is not endemic, where Coccidioides serology is not routinely performed, to establish the patient's travel history to areas where this organism is endemic in order to assess the relative risk of coccidioidomycosis due to latent reactivation. Further, as demonstrated in this case, previous travel history of the donor may also prove to be beneficial. Broadening the view of the patients that are at high risk for Coccidioides reactivation and applying appropriate prophylaxis may prevent the potentially fatal posttransplant complication of disseminated coccidioidomycosis.
Acknowledgments
We thank Jun Shen and Charles Jennette for expert analysis of autopsy findings and for assistance in locating donor information.
REFERENCES
- 1.Blair, J. E., D. D. Douglas, and D. C. Mulligan. 2003. Early results of targeted prophylaxis for coccidioidomycosis in patients undergoing orthotopic liver transplantation within an endemic area. Transplant. Infect. Dis. 5:3-8. [DOI] [PubMed] [Google Scholar]
- 2.Blair, J. E., and J. L. Logan. 2001. Coccidioidomycosis in solid organ transplantation. Clin. Infect. Dis. 33:1536-1544. [DOI] [PubMed] [Google Scholar]
- 3.Cha, J. M., S. Jung, H. S. Bahng, C. M. Lim, D. J. Han, J. H. Woo, and Y. Koh. 2000. Multi-organ failure caused by reactivated coccidioidomycosis without dissemination in a patient with renal transplantation. Respirology 5:87-90. [DOI] [PubMed] [Google Scholar]
- 4.Desai, S. A., O. A. Minai, S. M. Gordon, B. O'Neil, H. P. Wiedemann, and A. C. Arroliga. 2001. Coccidioidomycosis in non-endemic areas: a case series. Respir. Med. 95:305-309. [DOI] [PubMed] [Google Scholar]
- 5.Dodge, R. R., M. D. Lebowitz, R. Barbee, and B. Burrows. 1985. Estimates of Coccidioides immitis infection by skin test reactivity in an endemic community. Am. J. Public Health 75:863-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galgiani, J. 2000. Coccidioides immitis, p. 2746-2757. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, vol. 2. Churchill Livingstone, New York, N.Y. [Google Scholar]
- 7.Hall, K. A., G. K. Sethi, L. J. Rosado, J. D. Martinez, C. L. Huston, and J. G. Copeland. 1993. Coccidioidomycosis and heart transplantation. J. Heart Lung Transplant. 12:525-526. [PubMed] [Google Scholar]
- 8.Kanj, S. S., K. Welty-Wolf, J. Madden, V. Tapson, M. A. Baz, R. D. Davis, and J. R. Perfect. 1996. Fungal infections in lung and heart-lung transplant recipients: report of 9 cases and review of the literature. Medicine 75:142-156. [DOI] [PubMed] [Google Scholar]
- 9.Kirkland, T. N., and J. Fierer. 1996. Coccidioidomycosis: a reemerging infectious disease. Emerg. Infect. Dis. 2:192-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logan, J. L., J. E. Blair, and J. N. Galgiani. 2001. Coccidioidomycosis complicating solid organ transplantation. Semin. Respir. Infect. 16:251-256. [DOI] [PubMed] [Google Scholar]
- 11.Lortholary, O., and B. Dupont. 1997. Antifungal prophylaxis during neutropenia and immunodeficiency. Clin. Microbiol. Rev. 10:477-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens, D. A. 1995. Coccidioidomycosis. N. Engl. J. Med. 332:1077-1082. [DOI] [PubMed] [Google Scholar]
- 13.Tripathy, U., G. L. Yung, J. M. Kriett, P. A. Thistlethwaite, D. P. Kapelanski, and S. W. Jamieson. 2002. Donor transfer of pulmonary coccidioidomycosis in lung transplantation. Ann. Thorac. Surg. 73:306-308. [DOI] [PubMed] [Google Scholar]
- 14.Wright, P. W., D. Pappagianis, M. Wilson, A. Louro, S. A. Moser, K. Komatsu, and P. G. Pappas. 2003. Donor-related coccidioidomycosis in organ transplant recipients. Clin. Infect. Dis. 37:1265-1269. [DOI] [PubMed] [Google Scholar]