Abstract
Objectives
To evaluate the incidence of and risk factors for hypertensive disorders in a cohort of HIV-infected pregnant women.
Methods
Hypertensive disorders (HD) including preeclampsia/eclampsia (PE/E) and pregnancy-induced hypertension, and risk factors were evaluated in a cohort of HIV-infected pregnant women from Latin America and the Caribbean enrolled between 2002-2009. Only pregnant women enrolled for the first time in the study and delivered at ≥ 20 weeks gestation were analyzed.
Results
HD were diagnosed in 73 (4.8%, 95%CI: 3.8%-6.0%) of 1513 patients; 35(47.9%) had PE/E. HD was significantly increased among women with a gestational age-adjusted body mass index (gBMI) ≥ 25 kg/m2 (OR=3.1; 95%CI: 1.9-5.0), hemoglobin (Hg) ≥11 g/dL at delivery (OR=2.1; 95%CI: 1.2-3.6) and age ≥35 years (OR=1.8; 95%CI: 1.1-3.2). PE/E was increased among women with a gBMI ≥25 kg/m2 (OR=3.0; 95%CI: 1.5-6.0) and Hg ≥11 g/dL at delivery (OR=2.8; 95%CI: 1.2-6.5). A previous history of PE/E increased the risk of PE/E 6.7 fold (95%CI: 1.8-25.5). HAART before conception was associated with PE/E (OR=2.3; 95%CI: 1.1-4.9)
Conclusions
HIV-infected women, with a previous history of PE/E, a gBMI ≥25 kg/m2, Hg at delivery ≥11 g/dL and in use of HAART before conception are at an increased risk of developing PE/E during pregnancy.
INTRODUCTION
The prevention of maternal to child transmission (PMTCT) of HIV has progressed from recommending caesarean section, formula feeding, and treatment with Zidovudine monotherapy1, to additionally, implementing the use of highly active antiretroviral therapy (HAART), which led to transmission rates as low as 1-2%2. In spite of this impressive result, the use of HAART during pregnancy has been related to adverse outcomes such as low birth weight, prematurity3, and an increased rate of gestational diabetes was observed comparing data before and after the introduction of HAART 4.
Hypertensive disorders during pregnancy are a major cause of morbidity and mortality for both mother and child worldwide, and are the number one cause of maternal mortality in some regions in Brazil5,6.
However, prevalence data on preeclampsia and eclampsia (PE/E) among HIV-infected women are discrepant. In PACTG 185, where pregnant women were treated predominantly with ZDV during pregnancy, PE was reported to be as low as 2% among 497 women studied7. In the USA, the rate of PE has remained stable, regardless of the use of HAART during pregnancy4. Other studies have suggested that HIV-infected pregnant women treated with HAART have an increased risk for PE and fetal death8,9. Wimalasundera et al (2002) showed that the rate of PE in HIV-infected women was not different from that in uninfected pregnant women (4.2% vs. 5.6%, respectively), but within the HIV-infected women, the rate of PE in those treated with mono or dual therapy was 0-1% compared to 11% among those treated with triple therapy8. A probable role of immune reconstitution was implicated in the pathogenesis of PE in women treated with HAART. Suy et al (2006)9 found higher rates of PE among HIV-infected women (11/100 deliveries) when compared to HIV-uninfected women (2.9/100 deliveries). They also demonstrated that the rate of PE among HIV-infected women increased from 0% to 11% in two periods studied, and that this increment was related to use of HAART, especially in those treated with HAART prior to pregnancy.
In Latin America, the prevalence of PE among HIV-infected women has been rarely studied. In one report from Brazil, the rate of PE was 0.8% in HIV-infected women (1/123) and 10% among 1708 HIV-negative women, where 78% of the first group was treated with HAART10.
As more HIV infected women are being put on HAART earlier in pregnancy it is important to understand the impact of HAART on hypertensive disorders during pregnancy. Our objectives were to determine the prevalence of and risk factors for hypertensive disorders and PE/E among HIV-infected pregnant women in Latin America and to evaluate the impact of HAART in the development of these complications.
METHODS
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) International Site Development Initiative (NISDI) and the Perinatal Longitudinal Study in Latin American Countries (LILAC) are two consecutive observational, prospective cohorts of HIV-infected pregnant women enrolled from 2002-2009. The main objectives of the NISDI Perinatal and LILAC Studies are to describe utilization of interventions for PMTCT of HIV, rates of mother-to-child infection, and to characterize adverse events associated with receipt of and exposure to ARVs11. The protocols were approved by the ethics committee review board in each clinical site enrolling subjects, the sponsoring institution (NICHD), and the data management and statistical center (Westat). Clinical, immunologic, and virologic characteristics of the women were assessed at enrollment, during pregnancy, at the time of hospital discharge after delivery, and at the 6–12 week postpartum visit. Maternal history of substance use during the index pregnancy was ascertained through patient interview at enrollment. Maternal clinical disease staging12 was performed at each study visit. Gestational age-adjusted maternal BMI (gBMI), an approach to correcting for the weight gain expected to occur during pregnancy, was calculated using a program produced by the Argentinean Ministry of Health13. Women who enrolled with a gBMI > 25 were considered overweight.
Our study population was restricted to women enrolled in the NISDI Perinatal or LILAC protocols for the first time (second pregnancies on-study excluded), prior to labor and delivery (L&D), and whose infant were born at ≥ 20 weeks gestation. Women with pre-existing nephropathy were also excluded. HAART was defined as the use of at least three drugs in two different classes. Other non-HAART regimens (mono, dual, triple NRTI) therapy was categorized as “other ARV”.
The primary outcome measure of interest was the diagnosis of any hypertensive disorder (HD) after 20 weeks of gestation. This included all cases of pregnancy induced hypertension (PIH) [defined as blood pressure persistently ≥140/90 mmHg without proteinuria and onset after first 20 weeks gestation with no hypertension prior to pregnancy], PE [defined as blood pressure persistently ≥140/90 with: proteinuria of ≥ 1+ by dipstick, on two occasions, and/or ≥ 300 mg protein in 24 hour urine collection], and eclampsia [defined as seizure during pregnancy in the absence of any underlying known etiology or without any known reason for seizure and no suspicion of epilepsy or trauma]. Previous history of hypertension (not associated with pregnancy), PIH, preeclampsia or eclampsia was obtained at enrollment. Gestational age at enrollment was estimated based on the date of the last menstrual period or any earlier ultrasound dating, if available. Analyses were also done in the subset of participants who experienced PE/E.
Statistical analysis
Descriptive statistics (frequencies) were used to describe the study population. Bivariate analyses (Fisher’s exact test) examined the association of hypertensive disorders (HD) and the subset of PE/E with variables assessed at the time of eligibility for the study.
All co-variates with alpha level of 0.2 or lower in bivariate analyses were considered candidates for inclusion in the adjusted models. Logistic regression modeling used forward stepwise, backward elimination. All analyses were conducted using the SAS statistical software, version 9.0 (SAS Institute Inc. Cary, NC).
RESULTS
Of 1548 women experiencing their first pregnancy on study, thirty-five were excluded; three were lost to follow-up, twenty-one enrolled at the time of L&D, six delivered at <20 weeks gestation, and five for pre-existing nephropathy, leaving 1513 women in the final study population (Figure 1).
Figure 1.
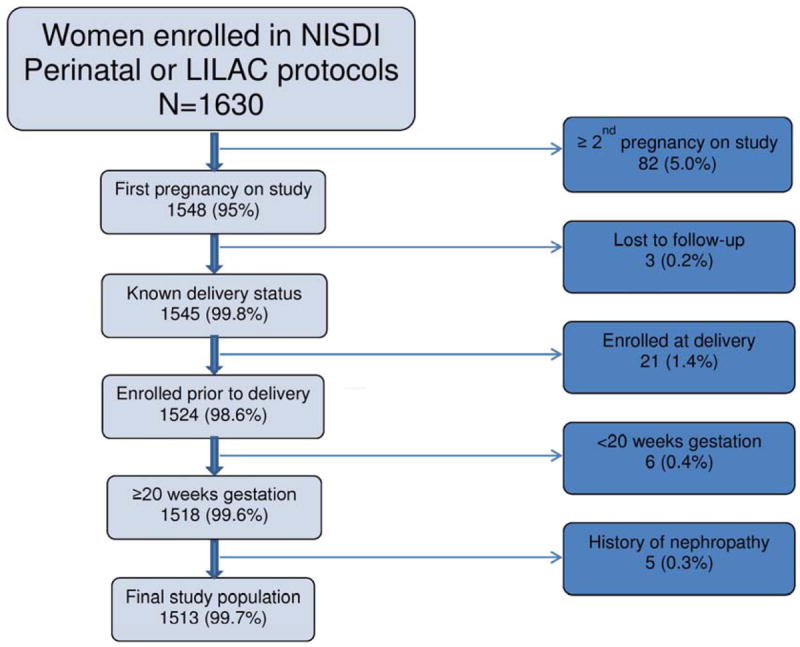
Derivation of Study Population
The majority of women were from Brazil (62.5%). Maternal age ranged from 20 to 34 years old for 77.7% of the participants, 72.9% were married or living with a partner, 56.2% enrolled in the cohort during the third trimester of pregnancy and 86.1% reported at least one previous pregnancy prior to study enrollment (Table 1).
Table 1.
Demographic and Clinical Characteristics of the Study Population
| Variable | Stratification | Total N (%)a | HDb by row stratification N (%) | Pc |
|---|---|---|---|---|
| Country of residence | Argentina | 377 (24.9) | 14 (3.7) | 0.04 |
| Brazil | 945 (62.5) | 49 (5.2) | ||
| Peru | 69 (4.5) | 2 (2.9) | ||
| Mexico | 42 (2.8) | 0 | ||
| Jamaica | 37 (2.4) | 2 (5.4) | ||
| Bahamas | 43 (2.8) | 6 (14.0) | ||
|
| ||||
| Maternal Age at enrollment (yrs) | <20 | 99 (6.6) | 2 (2.0) | 0.04 |
| 20-34 | 1176 (77.7) | 52 (4.4) | ||
| ≥ 35 | 238 (15.7) | 19 (8.0) | ||
|
| ||||
| Race | White | 703 (57.8) | 27 (3.8) | 0.21 |
| Black | 316 (26.0) | 20 (6.3) | ||
| Mestizo/other | 196 (13.7) | 13 (6.6) | ||
| Missing | 298 | 13 | ||
|
| ||||
| Maternal education | 0-6 | 473 (31.1) | 24 (5.1) | 0.49 |
| 7-12 | 958 (63.3) | 43 (4.5) | ||
| >12 | 82 (5.4) | 6 (7.3) | ||
|
| ||||
| Gainfully employed | Yes | 399 (26.4) | 27 (6.8) | 0.04 |
| No | 1114 (73.6) | 46 (4.1) | ||
|
| ||||
| Marital status | Married / partner | 1103 (72.9) | 61 (5.5) | 0.05 |
| Single/prev married | 410 (27.1) | 12 (2.9) | ||
|
| ||||
| Previous hx of PE / Ed | Yes | 20 (1.3) | 3 (15.0) | 0.07 |
| No | 1493 (98.7) | 70 (4.7) | ||
|
| ||||
| Gravida | 1 | 211 (13.9) | 12 (5.7) | 0.32 |
| >1 | 1302 (86.1) | 61 (4.7) | ||
|
| ||||
| Multiple gestation | Yes | 23 (1.5) | 2 (8.7) | 0.30 |
| No | 1478 (98.5) | 71 (4.8) | ||
| Missing | 12 | 0 | ||
|
| ||||
| Tobacco use during pregnancy | Yes | 372 (24.9) | 13 (3.5) | 0.20 |
| No | 1123 (75.1) | 58 (5.2) | ||
| Missing | 18 | 2 | ||
|
| ||||
| Alcohol use during pregnancy | Yes | 104 (9.1) | 5 (4.8) | 0.80 |
| No | 1041 (90.9) | 58 (5.2) | ||
| Missing | 368 | 10 | ||
|
| ||||
| gBMIe at enrollment | <20 | 245 (16.4) | 4 (1.6) | <0.01 |
| 20-24 | 761 (51.0) | 25 (3.3) | ||
| ≥25 | 487 (32.6) | 43 (8.8) | ||
| Missing | 20 | 1 | ||
|
| ||||
| Trimester at enrollment | 1st (1-12 wk) | 71 (4.7) | 7 (9.9) | 0.10 |
| 2nd (13-26 wk) | 591 (39.1) | 30 (5.1) | ||
| 3rd (27+ wk) | 851 (56.2) | 36 (4.2) | ||
|
| ||||
| Maternal Diabetes | Yes | 35 (2.3) | 0 | 0.41 |
| No | 1478 (97.7) | 73 (4.8) | ||
|
| ||||
| Creatinine at enrollment | ≤ 1 | 1466 (99.0) | 69 (4.7) | 0.14 |
| >1 | 14 (1.0) | 2 (14.3) | ||
| Missing | 33 | 2 | ||
|
| ||||
| Hemoglobin at enrollment | < 11 | 708 (47.7) | 26 (3.7) | 0.04 |
| ≥ 11 | 777 (52.3) | 47 (6.0) | ||
| Missing | 28 | 0 | ||
|
| ||||
| Hemoglobin at delivery | < 11 | 676 (45.4) | 21 (3.1) | <0.01 |
| ≥ 11 | 814 (54.6) | 52 (6.4) | ||
| Missing | 23 | 0 | ||
|
| ||||
| CD4 at enrollment | <200 | 210 (14.1) | 13 (6.2) | 0.64 |
| 200-499 | 762 (51.0) | 36 (4.7) | ||
| ≥500 | 520 (34.9) | 24 (4.6) | ||
| Missing | 21 | 0 | ||
|
| ||||
| CD4 prior to delivery | <200 | 180 (12.0) | 12 (6.7) | 0.21 |
| 200-499 | 693 (46.3) | 27 (3.9) | ||
| ≥500 | 625 (41.7) | 34 (5.4) | ||
| Missing | 15 | 0 | ||
|
| ||||
| VLf at enrollment | <1000 | 951(63.7) | 48 (5.0) | 0.93 |
| 1,000 to 10,000 | 277 (18.6) | 13 (4.7) | ||
| ≥ 10,000 | 265 (17.7) | 12 (4.5) | ||
| Missing | 20 | 0 | ||
|
| ||||
| VLf at delivery | <1000 | 1192 (79.4) | 62 (5.2) | 0.34 |
| 1,000 to 10,000 | 184 (12.3) | 5 (2.7) | ||
| ≥ 10,000 | 126 (8.3) | 6 (4.8) | ||
| Missing | 11 | 0 | ||
|
| ||||
| ARVg at conception | Yes | 310 (20.5) | 21 (6.8) | 0.08 |
| No | 1199 (79.5) | 52 (4.3) | ||
| Missing | 4 | |||
|
| ||||
| Type ARV at conception | Any PI/ HAART | 294 (19.5) | 21 (7.1) | 0.04 |
| Some ARV1 or None | 1251 (80.5) | 52 (4.3) | ||
| Missing | 4 | 0 | ||
|
| ||||
| ARV during 1st trimester | Any PI/ HAART | 362 (23.9) | 24 (6.6) | 0.07 |
| Some ARV2 or None | 1151 (76.1) | 49 (4.2) | ||
|
| ||||
| ARV during 2nd trimester | Any PI/HAART | 1072 (70.8) | 57 (5.3) | 0.18 |
| Some ARV3 or None | 441 (29.2) | 16 (3.6) | ||
|
| ||||
| ARV during 3rd trimester | Any PI/HAART | 1367 (90.4) | 69 (5.0) | 0.21 |
| Some ARV or None4 | 146 (9.6) | 4 (2.7) | ||
|
| ||||
| Delivery outcome | Stillbirth | 30 (2.0) | 2 (6.7) | 0.65 |
| Live birth | 1483 (98.0) | 71 (4.8) | ||
Subjects missing values are not included in percentages,
Hypertensive disorders (HD),
p value by Fisher’s exact testing,
pre-eclampsia/eclamspia (PE/E),
gestational age adjusted body mass index (gBMI),
Viral load (VL),
antiretroviral (ARV)
16 subjects on other ARV regimen
29 subjects on other ARV regimen
121 subjects on other ARV regimen
15 subjects not on any ARV
There were 73 cases of HD: 38 (52.1%) diagnosed with PIH and 35 (47.9%) diagnosed with PE/E for a cumulative prevalence for HD of 4.8% (95% CI: 3.8% - 6.0%) and 2.3% (95% CI: 1.7%-3.2%) for PE/E. Three women developed eclampsia, but none died. Among those diagnosed with PIH, 81.6% (31/38) had documented normal blood pressures at the time of enrollment prior to their diagnosis of hypertension.
On bivariate analyses, HD was significantly more frequent among women who were gainfully employed, married or living with a partner, gBMI ≥ 25 at enrollment, and with a higher hemoglobin value (≥ 11 g/dL) at enrollment and following delivery. While use of any antiretroviral therapy (ARV) at conception was not significantly associated with HD, being on HAART or any PI regimen at conception was associated with HD (p=.04) (Table 2).
Table 2.
Bivariate Analyses of all Hypertensive disorders (HD) and Preeclampsia and Eclampsia (PE/E) by covariates of interest
| Covariates | Strata | HD N=73 (%) | No HD N=1440 (%) | P1 | PE/E N=35 (%) | No PE/E N=1478 (%) | P1 |
|---|---|---|---|---|---|---|---|
| Maternal age | <20 | 2 (2.0) | 97 (98.0) | 0.04 | 1 (1.0) | 98 (99.0) | 0.09 |
| 20-34 | 52 (4.4) | 1124 (95.6) | 24 (2.0) | 1152 (98.0) | |||
| ≥35 | 19 (8.0) | 219 (92.0) | 10 (4.2) | 228 (95.8) | |||
|
| |||||||
| Gainfully employed | Yes | 27 (6.8) | 372 (93.2) | 0.04 | 13 (3.2) | 386 (96.7) | 0.17 |
| No | 46 (4.1) | 1068 (95.9) | 22 (2.0) | 1092 (98.0) | |||
|
| |||||||
| Marital status2 | Married | 61 (5.5) | 1042 (94.5) | 0.05 | 27 (2.5) | 1076 (97.5) | 0.57 |
| Single | 12 (2.9) | 398 (97.1) | 8 (2.0) | 402 (98.0) | |||
|
| |||||||
| Previous history of PE/E | Yes | 3 (15.0) | 17 (85.0) | 0.07 | 3 (15.0) | 17 (85.0) | 0.01 |
| No | 70 (4.7) | 1423 (95.3) | 32 (2.1) | 1461 (97.9) | |||
|
| |||||||
| Gestational age adjusted BMI at enrollment (gBMI) | <20 | 4 (1.6) | 241 (98.4) | < 0.01 | 2 (0.8) | 243 (99.2) | <0.01 |
| 20-24 | 25 (3.3) | 736 (96.7) | 12 (1.6) | 749 (98.4) | |||
| ≥25 | 43 (8.8) | 444 (91.2) | 20 (4.1) | 467 (95.9) | |||
|
| |||||||
| Trimester3 at enrollment | 1st | 7 (9.9) | 64 (90.1) | 0.10 | 4 (5.6) | 67 (94.4) | 0.12 |
| 2nd | 30 (5.1) | 561 (94.9) | 15 (2.5) | 576 (97.5) | |||
| 3rd | 36 (4.2) | 815 (95.8) | 16 (1.9) | 835 (98.1) | |||
|
| |||||||
| Creatinine at Enrollment | ≤ 1 | 69 (4.7) | 1397 (95.3) | 0.14 | 33 (2.2) | 1433 (97.8) | 0.28 |
| >1 | 2 (14.3) | 12 (85.7) | 1 (7.1) | 13 (92.9) | |||
|
| |||||||
| Hemoglobin at enrollment (g/dL) | <11 | 26 (3.7) | 682 (96.3) | 0.04 | 10 (1.4) | 698 (98.6) | 0.03 |
| ≥11 | 47 (6.1) | 730 (93.9) | 25 (3.2) | 752 (96.8) | |||
|
| |||||||
| Hemoglobin after delivery (g/dL) | <11 | 21 (3.1) | 655 (96.9) | <0.01 | 8 (1.2) | 668 (98.8) | 0.01 |
| ≥11 | 52 (6.4) | 762 (93.6) | 27 (3.3) | 787 (96.7) | |||
|
| |||||||
| Type of ART4 at conception | HAART | 21 (7.1) | 273 (92.9) | 0.04 | 13 (4.4) | 281 (95.6) | <0.01 |
| No HAART | 52 (4.3) | 1167 (95.7) | 22 (1.8) | 1197 (98.2) | |||
|
| |||||||
| ART: 1st trimester | HAART | 24 (6.6) | 338 (93.4) | 0.07 | 13 (3.6) | 349 (96.4) | 0.07 |
| No HAART | 49 (4.2) | 1102 (95.7) | 22 (1.9) | 1129 (98.1) | |||
P value obtained using Fisher’s exact testing,
Marital status divided into Married(includes living with partner) and Single (includes divorced and widowed),
Trimester of pregnancy defined as follows: 1st (1-12 weeks gestation), 2nd (13-26 weeks gestation), 3rd (27+weeks gestation),
Type of antiretroviral therapy (ART) divided into: HAART (including use of any PI) and no HAART (includes any mono, dual, triple therapy and no therapy)
All covariates with p<.2 considered in logistic regression modeling
On bivariate analyses, the association of PE/E with risk factors was similar to HD; hemoglobin at delivery (≥ 11 g/dL), gBMI ≥ 25 kg/m2 at enrollment and a previous history of PE/E were all statistically significant factors for PE/E. We did not observe an association with employment or maternal age (Table 2). Being on any HAART or any PI at conception or during the 1st or 2nd trimester were all associated with increased risk of PE/E.
Logistic regression models were run using all variables with p values ≤ 0.2 which included: maternal age, employment, marital status, previous history of PE/E, gBMI, trimester of enrollment, ARV at conception, type of ART at conception, ART during 1st and 2nd trimesters (different models for each time period of ART use), and hemoglobin at delivery. Additional models were run forcing CD4 and viral load (to adjust for disease severity) to remain in the model. In the final logistic regression model, HD was significantly associated with women enrolled with a gestational age adjusted (gBMI) ≥ 25 kg/m2 (OR= 3.1; 95% CI: 1.9-5.0), maternal age ≥ 35years (OR=1.8; 95% CI: 1.1-3.2), and Hg ≥11 g/dl at delivery (OR=2.1; 95% CI: 1.2-3.6). Similarly, PE/E was significantly increased among women enrolled with a gBMI ≥ 25 kg/m2 (OR=3.0; 95% CI: 1.5-6.0) and Hg ≥11 g/dl at delivery (OR=2.8; 95% CI: 1.2-6.5). A history of PE/E in previous pregnancies was an independent risk factor increasing the risk of PE/E 6.7 times (95% CI: 1.8-25.5) compared to those without a history of PE/E (Table 3). Being on HAART at conception was associated with a PE/E in adjusted models (OR=2.3; 95% CI: 1.1-4.9). Forcing CD4 and viral load (at enrollment or at the time of delivery) into the model had no impact on the final models.
Table 3.
Final Logistic Regression Model*
| Co-variates | Final model-HD OR (95%CI) | Final Model-PE/E OR (95%CI) |
|---|---|---|
|
| ||
| gBMI: | 3.1 (1.9-5.0) | 3.0 (1.5-6.0) |
| ≥25 kg/m2 vs. < 25 kg/m2 | ||
|
| ||
| Hg at L&D: | 2.1 (1.2-3.6) | 2.8 (1.2-6.5) |
| ≥11 g/dl vs. < 11 g/dl | ||
|
| ||
| Maternal age: | 1.8 (1.1-3.2) | NA |
| ≥35 yr. vs. < 35 yr. | ||
|
| ||
| ARV type at conception: | NA | 2.3 (1.1-4.9) |
| HAART vs non HAART | ||
|
| ||
| Previous history of PE/E: | NA | 6.7 (1.8-25.5) |
| Yes vs. No | ||
HD – Hypertensive Disorders; PE/E – Preeclampsia/Eclampsia; NA – Not applicable
Forcing CD4 and viral load at enrollment or at L&D had no impact on the final models
DISCUSSION
In this study of HIV-infected pregnant women, we found the prevalence of HD and PE/E was 4.8% and 2.3%, respectively. These percentages are similar to the prevalence of HD and PE/E found among pregnant women described in some Latin America countries14,15. Data from the World Health Organization (WHO) Antenatal Care Trial, which included more than 39,000 pregnant women (with unknown HIV status) from Argentina, Cuba, Saudi Arabia and Thailand described PIH in 7.0% and PE/E in 2.2%14. In Brazil, a study with almost 5000 pregnant women with unknown HIV status, enrolled between 1991-1995, showed that 3.5% develop PIH and 2.3% develop PE/E15.
In this cohort, some risk factors for PE and HD were the same described for HIV uninfected population such as older maternal age16,17, a previous history of PE/E18,19, and being overweight20,21. Several abnormalities associated with the development of PE such as lower plasma volume, activation of complement system, hyperferritinemia, insulin resistance and metabolic syndrome are also more prevalent among overweight or obese uninfected patients22-26. Futhermore, overweight has been associated with oxidative stress consisting of an imbalance of lipid metabolism and an inflammatory state that is also associated with PE27. As almost 50% of the patients in this study were enrolled during the 3rd trimester of pregnancy we cannot disregard the fact that the increased weight can also be related to fluid retention. In general, reduction of weight in an overweight or obese women prior to conception has been shown to reduce PE and improve other health outcomes28,29 and should be also emphasized to HIV-infected women.
Higher hemoglobin among patients with PE/E and HD is expected and explained by the hemoconcentration caused by failure to increase the plasma volume during pregnancy, which is characteristic of PE. Usually, pregnant women start the expansion of plasma volume around the 7th week of gestation and this reaches a plateau around 32 weeks30. During pregnancy, uninfected women increase their plasma volume by almost 45% but this increase is 50% lower for those with PE/E31,32. Failure to increase the plasma volume during pregnancy leads to hemoconcentration, a higher vascular resistance with a consequently decrease in utero placental flow and higher odds for perinatal complications33.
The role of HIV infection in the development of preeclampsia is controversial. Wimalasundera et al. found a lower rate of PE among untreated HIV-infected women 8. when compared to uninfected women or HIV-infected on treatment. More recently, Kalumba et al34 described a lower rate of HIV-infection among women with pre-eclampsia when compared with women without pre-eclampsia. Several studies show no difference in the rates of PE between HIV-uninfected and HIV-infected women treated with HAART35,36 or even a lower rate of PE among HIV-infected treated when compared to uninfected women10.
We observed that the use of HAART at conception was a risk factor for PE/E. This is in agreement with two other studies which showed that the use of HAART prior to pregnancy increase the odds for hypertension and PE 9,37. Lack of stratification with the use of HAART in relation to pregnancy could explain the discrepancy among studies. Some authors suggest that immune restoration, secondary to the use of HAART could be involved in the development of PE8. It has been shown that low levels of retinol are correlated with PE and it was suggested that a mechanisms of HAART-induced PE could be a reduction of serum retinol concentrations due to hepatotoxicity38,39. HIV-infected pregnant women using HAART present a shift towards a Th1 cytokine production while, in a healthy pregnancy, a Th2 response is predominantly observed40-41. Also a Th1 immune response is observed in PE42. It is possible that HIV-infected pregnant women on HAART, especially those with long exposure to therapy, would have a blunted shift of Th2 cytokines increasing the predisposition to eclampsia.
The current pathophysiological mechanism of preeclampsia involves poor placental perfusion and the excessive release of inflammatory factors that damage the mother’s vascular endothelial cells, resulting in systemic hypertension and endothelial dysfunction43. In addition, regarding HIV-infected patients, some ART has been associated with endothelial dysfunction and CVD44. Nevertheless, Torriani et al (2008)45, comparing three different randomized ARV regimens among HIV-infected naïve individuals, in order to evaluate the effect of these ARV on endothelial function, noticed rapid improvement of endothelial function after ARV administration. Savvidou et al (2011) evaluated the uteroplacental circulation by Doppler ultrasound of HIV-infected and non-infected pregnant women in the first trimester of gestation – a probable early biomarker to predict PE –, with the aim to assess the degree of placental invasion. They found normal placental perfusion among HIV-infected women, with uncomplicated pregnancies, receiving and not receiving ARV regimen. Although, these authors state that the majority of women presented CD4 cell count > 250cells/mm3 and, therefore, they could not exclude problems of uteroplacental perfusion in women with a compromised immune system46.
Among the strengths of this study are the large size of the cohort, enrollment of study participants across several countries in Latin America and the Caribbean and the prospective collection of data with a very low rate of loss to follow-up. One of the limitations of this study is that we did not have information regarding adherence to ART and, also, we did not collect data on the duration of ART prior to the current pregnancy.
Strategies to prevent HD among HIV-infected women should include preconception counseling to high risk women who are planning a pregnancy. It would also be interesting to compare risk of HD between women on treatment longer before pregnancy with normalization of CD4+T-cell counts with those who started HAART during pregnancy but have already a lower CD4+ T-cell count.
The findings of this study showing a significant association of BMI ≥ 25, a history of PE/E in previous pregnancies and hemoconcentration with PE are consistent with the literature regarding risk factors for PE and HD in uninfected pregnant women. Being on HAART at conception was associated with a 2.3 times risk of developing PE/E during pregnancy.
Acknowledgments
We would like to thank all the patients who agreed to take part on this study
Funding: Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland, USA (NICHD) Contract # N01-HD-3-3345 (2002-2007) and by NICHD Contract # HHSN267200800001C (NICHD Control #: N01-HD-8-0001) (2007-2012).
NICHD was involved in all aspects of study design, data analysis and interpretation, and manuscript writing/editing.
NICHD International Site Development Initiative: Perinatal/LILAC Protocol
Principal investigators, co-principal investigators, study coordinators, coordinating center representatives, and NICHD staff include: Argentina: Buenos Aires: Marcelo H. Losso, Irene Foradori, Alejandro Hakim, Erica Stankievich, Silvina Ivalo (Hospital General de Agudos José María Ramos Mejía); Brazil: Belo Horizonte: Jorge A. Pinto, Victor H. Melo, Fabiana Kakehasi, Beatriz M. Andrade (Universidade Federal de Minas Gerais); Caxias do Sul: Rosa Dea Sperhacke, Nicole Golin, Sílvia Mariani Costamilan (Universidade de Caxias do Sul/ Serviço Municipal de Infectologia); Nova Iguacu: Jose Pilotto, Luis Eduardo Fernandes, Gisely Falco (Hospital Geral Nova de Iguacu – HIV Family Care Clinic); Porto Alegre: Rosa Dea Sperhacke, Breno Riegel Santos, Rita de Cassia Alves Lira (Universidade de Caxias do Sul/Hospital Conceição); Rosa Dea Sperhacke, Mario Ferreira Peixoto, Elizabete Teles (Universidade de Caxias do Sul/Hospital Fêmina); Regis Kreitchmann, Luis Carlos Ribeiro, Fabrizio Motta, Debora Fernandes Coelho (Irmandade da Santa Casa de Misericordia de Porto Alegre); Ribeirão Preto: Marisa M. Mussi-Pinhata, Geraldo Duarte, Adriana A. Tiraboschi Bárbaro, Conrado Milani Coutinho, Fabiana Rezende Amaral, Anderson Sanches de Melo (Hospital das Clínicas da Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo); Rio de Janeiro: Ricardo Hugo S. Oliveira, Elizabeth S. Machado, Maria C. Chermont Sapia (Instituto de Puericultura e Pediatria Martagão Gesteira); Esau Custodio Joao, Leon Claude Sidi, Maria Leticia Santos Cruz, Maria Isabel Gouvêa, Mariza Curto Saavedra, Clarisse Bressan, Fernanda Cavalcanti A. Jundi (Hospital dos Servidores do Estado); São Paulo: Regina Celia de Menezes Succi, Prescilla Chow (Escola Paulista de Medicina- Universidade Federal de São Paulo); Peru: Lima: Jorge O. Alarcón Villaverde (Instituto de Medicina Tropical “Daniel Alcides Carrión”- Sección de Epidemiología, UNMSM), Carlos Velásquez Vásquez (Instituto Nacional Materno Perinatal), César Gutiérrez Villafuerte (Instituto de Medicina Tropical “Daniel Alcides Carrión”- Sección de Epidemiología, UNMSM); Data Management and Statistical Center: Yolanda Bertucci, Rachel Cohen, Laura Freimanis Hance, René Gonin, D. Robert Harris, Roslyn Hennessey, James Korelitz, Margot Krauss, Sue Li, Karen Megazzini, Orlando Ortega, Sharon Sothern de Sanchez, Sonia K. Stoszek, Qilu Yu (Westat, Rockville, MD, USA); NICHD: George K. Siberry, Rohan Hazra, Lynne M. Mofenson, Jennifer S. Read, Heather Watts (Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the US National Institutes of Health or the Department of Health and Human Services.
References
- 1.Connor EM, SPerling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, et al. Reduction of maternal–infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 2.Coovadia H. Antiretroviral agents – how best to protect infants from HIV and save their mothers from AIDS. N Engl J Med. 2004;351:289–292. doi: 10.1056/NEJMe048128. [DOI] [PubMed] [Google Scholar]
- 3.Thorne C, Patel D, Newell ML. Increased risk of adverse pregnancy outcomes in HIV-infected women treated with highly active antiretroviral therapy in Europe. AIDS. 2004;18:2337–2339. doi: 10.1097/00002030-200411190-00019. [DOI] [PubMed] [Google Scholar]
- 4.Kourtis AP, Bansil P, McPheeters M, Meikle SF, Posner SF, Jamieson DJ. Hospitalizations of pregnant HIV-infected women in the USA prior to and during the era of HAART, 1994-2003. AIDS. 2006;20:1823–1831. doi: 10.1097/01.aids.0000244201.11006.1c. [DOI] [PubMed] [Google Scholar]
- 5.Kale PL, Costa AJL. Maternal deaths in the city of Rio de Janeiro, Brazil, 2000-2003. J Health Popul Nutr. 2009;27:794–801. doi: 10.3329/jhpn.v27i6.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega CEP, Kahhale S, Zugaib M. Maternal Mortality due to arterial hypertension in São Paulo City (1995-1999) Clinics. 2007;62:679–84. doi: 10.1590/s1807-59322007000600004. [DOI] [PubMed] [Google Scholar]
- 7.Lambert JS, Watts DH, Mofenson L, Stiehm ER, Harris DR, Bethel J, et al. Risk factors for preterm birth, low birth weight, and intrauterine growth retardation in infants born to HIV-infected pregnant women receiving zidovudine. Pediatric AIDS Clinical Trials Group 185 Team. AIDS. 2000;14:1389–99. doi: 10.1097/00002030-200007070-00012. [DOI] [PubMed] [Google Scholar]
- 8.Wimalasundera RC, Larbalestier N, Smith JH, de Ruiter A, McG Thom SA, Hughes AD, et al. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet. 2002;360:1152–4. doi: 10.1016/s0140-6736(02)11195-0. [DOI] [PubMed] [Google Scholar]
- 9.Suy A, Martínez E, Coll O, Lonca M, Palacio M, de Lazzari E, et al. Increased risk of pre-eclampsia and fetal death in HIV-infected pregnant women receiving highly active antiretroviral therapy. AIDS. 2006;20:59–66. doi: 10.1097/01.aids.0000198090.70325.bd. [DOI] [PubMed] [Google Scholar]
- 10.Mattar R, Amed AM, Lindsey PC, Sass N, Daher S. Preeclampasia and HIV infection. Eur J Obstet Gynecol Reprod Biol. 2004;117:240–241. doi: 10.1016/j.ejogrb.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Read JS, Cahn P, Losso M, Pinto J, Joao E, Duarte G, et al. Management of human immunodeficiency virus-infected pregnant women at Latin American and Caribbean sites. Obstet Gynecol. 2007;109:1358–1367. doi: 10.1097/01.AOG.0000265211.76196.ac. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41(RR-17):1–19. [PubMed] [Google Scholar]
- 13.Ministry of Health Argentina. Program to calculate Adjusted BMI during pregnancy” and copy the link, after the link you would write. “last accessed on {date}” http://www.msal.gov.ar/promin/
- 14.Villar J, Carroli G, Wojdyla D, Abalos E, Giordano D, Ba’aqeel H, et al. Pre-eclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am J Obstet Gynecol. 2006;194:921–31. doi: 10.1016/j.ajog.2005.10.813. [DOI] [PubMed] [Google Scholar]
- 15.Gaio DS, Schmidt MI, Duncan BB, Nucci LB, Matos MC, Branchtein L. Hypertensive disorders in pregnancy: frequency and associated factors in a cohort of Brazilian women. Hypertens Pregnancy. 2001;20:269–81. doi: 10.1081/PRG-100107829. [DOI] [PubMed] [Google Scholar]
- 16.Sultana R, Chen XK, Lee C, Hader J. Outcomes in multiple gestation pregnancies among Canadian women age 35 years and older. Healthc Q. 2011;14:22–4. doi: 10.12927/hcq.2011.22646. [DOI] [PubMed] [Google Scholar]
- 17.Lamminpaa R, Vehvilainen-Julkunen K, Gissler M, Heinonen S. Preeclampsia complicated by advanced maternal age: a registry-based study on primaparous women in Finland 1997-2008. BMC Pregnancy Childbirth. 2012;12:47. doi: 10.1186/1471-2393-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganesh KS, Unnikrishnan B, Nagaraj K, Jayaram S. Determinants of Pre-eclampsia: A case-contrtol study in a District Hospital in South India. Indian J Community Med. 2010;35:502–5. doi: 10.4103/0970-0218.74360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman-Juarez W, Avila-Esparza M, Contretas-Solia RE, Levario-Carrillo M. Factors associated with gestational hypertension and preeclampsia. Ginecol Obster Mex. 2012;80:461–6. [PubMed] [Google Scholar]
- 20.Dennedy MC, Avalos G, O’Reilly MW, O’Sullivan EP, Dunne FP. The impact of maternal obesity on gestational outcomes. Ir Med J. 2012;105:23–5. [PubMed] [Google Scholar]
- 21.Mandal D, Manda S, Rakshi A, Dey RP, Biswas SC, Banerjee A. Maternal obesity and pregnancy outcome: a prospective analysis. J Assoc Physicians India. 2011;59:486–9. [PubMed] [Google Scholar]
- 22.Aardenburg R, Spaanderman ME, Ekhart TH, van Eijndhoven HW, van der Heijden OW, Peeters LL. Low plasma volume following pregnancy complicated by pre-ecalmpsia predisposes for hypertensive disease in a next pregnancy. BJOG. 2003;110:1001–6. [PubMed] [Google Scholar]
- 23.Lynch AM, Eckel RH, Murphy JR, Gibbs RS, West NA, Giclas PC, et al. Prepregnancy obesity and complement system activation in early pregnancy and the susequent development of preeclampsia. Am J Obstet Gynecol. 2012;206:428.e1–8. doi: 10.1016/j.ajog.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raman L, Pawashe AB, Yasodhara P. Hyperferritinemia in pregnancy induced hypertension and eclampsia. J Postgrad Med. 1992;38:65–7. [PubMed] [Google Scholar]
- 25.Lu J, Zhao YY, Qiao J, Zhang HJ, Ge L, Wei Y. A follow-up study of women with a history of severe preeclampsia: relationship between metabolic syndrome and PE. Chin Med J (Engl) 2011;124:775–9. [PubMed] [Google Scholar]
- 26.Hauth JC, Clifton RG, Roberts JM, Myatt L, Spong CY, Leveno KJ, et al. Maternal insulin resistance and preeclampsia. Am J Obstet Gynecol. 2011;201:327.e1–6. doi: 10.1016/j.ajog.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zavalza-Gomez AB. Obesity and oxidative stress: a direct link to preeclampsia? Arch Gynecol Obstet. 2011;283:415–22. doi: 10.1007/s00404-010-1753-1. [DOI] [PubMed] [Google Scholar]
- 28.Thangaratinam S, Rogozinska E, Jolly K, Glinkowski S, Roseboom T, Tomlinson JW, et al. Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ. 2012;344:e2088. doi: 10.1136/bmj.e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostello D, Jen Chang J, Allen J, Luehr L, Shyken J, Leet T. Recurrent preeclampsia: the effect of weight change between pregnancies. Obster Gynecol. 2012;116:667–72. doi: 10.1097/AOG.0b013e3181ed74ea. [DOI] [PubMed] [Google Scholar]
- 30.Bridges EJ, Womble S, Wallace M, McCartney J. Hemodynamic monitoring in high-risk obstetrics patients, I: Expected hemodynamic changes in pregnancy. Crit Care Nurse. 2003;23:53–62. [PubMed] [Google Scholar]
- 31.Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14:601–12. [PubMed] [Google Scholar]
- 32.Silver HM, Seebeck M, Carlson R. Comparison of total blood volume in normal, preeclamptic, and nonproteinuric gestational hypertensive pregnancy by simultaneous measurement of red blood cell and plasma volumes. Am J Obstet Gynecol. 1998;179:87–93. doi: 10.1016/s0002-9378(98)70255-8. [DOI] [PubMed] [Google Scholar]
- 33.Yang JM, Yang YC, Wang KG. Central and peripheral hemodynamics in severe preeclampsia. Acta Obstet Gynecol Scand. 1996;75:120–6. doi: 10.3109/00016349609033302. [DOI] [PubMed] [Google Scholar]
- 34.Kalumba VMS, Moodley J, Naidoo TD. Is the prevalence of pre-eclampsia affected by HIV/AIDS? A retrospective case–control study. Cardiovascular J of Africa. 2013;24:24–27. doi: 10.5830/CVJA-2012-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of Preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol. 2008;198:7–22. doi: 10.1016/j.ajog.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Boyajian T, Shah PS, Murphy KE. Pregnant women receiving HAART: a matched cohort study. J Obstet Gynaecol Can. 2012;34:136–141. doi: 10.1016/S1701-2163(16)35156-8. [DOI] [PubMed] [Google Scholar]
- 37.Parekh N, Ribaudo H, Souda S, Chen J, Mmalane M, Powis K, et al. Risk factors for very preterm delivery and delivery of very-small-for-gestational-age infants among HIV-exposed and HIV-unexposed infants in Botswana. Int J Gynaecol Obstet. 2011;115:20–5. doi: 10.1016/j.ijgo.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C, Williams MA, Sanchez SE, King IB, Ware-Jauregui S, Larrabure G, et al. Plasma concentrations of carotenoids, retinol, and tocopherols in preeclamptic and normotensive pregnant women. Am J Epidemiol. 2001;153:572–80. doi: 10.1093/aje/153.6.572. [DOI] [PubMed] [Google Scholar]
- 39.Mawson AR. Effects of antiretroviral therapy on occurence of pre-eclampsia. Lancet. 2003;361:347–8. doi: 10.1016/S0140-6736(03)12359-8. [DOI] [PubMed] [Google Scholar]
- 40.Fiore S, Newell ML, Trabattoni D, Thorne C, Gray L, Savasi V, et al. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. J Reprod Immunol. 2006;70:143–50. doi: 10.1016/j.jri.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Jonsson Y, Matthiesen L, Berg G, Emerudh J, Nieminen K, Ekerfelt C. Indications of an altered immune balance in pre-eclampsia: a decrease in in vitro secretion of IL-5 and IL-10 from blood mononuclear cells an in blood basophil counts compared with normal pregnancy. J Reprod Immunol. 2005;66:69–84. doi: 10.1016/j.jri.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Laresgoiti-Servitje E, Gomez-Lopez N, Olson DM. An immunological insight into the origins of pre-eclampsia. Hum Reprod Update. 2010;16:510–24. doi: 10.1093/humupd/dmq007. [DOI] [PubMed] [Google Scholar]
- 43.Fei X, Hongxiang Z, Qi C, Daozhen C. Maternal plasma levels of endothelial dysfunction mediators including AM, CGRP, sICAM-1 and tHcy in pre-eclampsia. Adv Clin Exp Med. 2012;21:573–9. [PubMed] [Google Scholar]
- 44.Gupta SK, Shen C, Moe SM, Kamendulis LM, Goldman M, Dubé MP. Worsening endothelial function with efavirenz compared to protease inhibitors: a 12-month prospective study. PLoS One. 2012;7:e45716. doi: 10.1371/journal.pone.0045716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savvidou MD, Samuel MI, Akolekar R, Poulton M, Nicolaides KH. First trimester maternal uterine Artery Doppler examination in HIV-positive women. HIV Med. 2011;12:632–36. doi: 10.1111/j.1468-1293.2011.00930.x. [DOI] [PubMed] [Google Scholar]
- 46.Torriani FJ, Komarow L, Parker RA, Cotter BR, Currier JS, Dubé MP, et al. Endothelial function in HIV-infected antiretroviral naïve subjects before and after starting potent antiretroviral therapy: AIDS Clinical Trials Group Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]


