Abstract
Oxidative stress is increased in systemic lupus erythematosus (SLE), and it contributes to immune system dysregulation, abnormal activation and processing of cell-death signals, autoantibody production and fatal comorbidities. Mitochondrial dysfunction in T cells promotes the release of highly diffusible inflammatory lipid hydroperoxides, which spread oxidative stress to other intracellular organelles and through the bloodstream. Oxidative modification of self antigens triggers autoimmunity, and the degree of such modification of serum proteins shows striking correlation with disease activity and organ damage in SLE. In T cells from patients with SLE and animal models of the disease, glutathione, the main intracellular antioxidant, is depleted and serine/threonine-protein kinase mTOR undergoes redox-dependent activation. In turn, reversal of glutathione depletion by application of its amino acid precursor, N-acetylcysteine, improves disease activity in lupus-prone mice; pilot studies in patients with SLE have yielded positive results that warrant further research. Blocking mTOR activation in T cells could conceivably provide a well-tolerated and inexpensive alternative approach to B-cell blockade and traditional immunosuppressive treatments. Nevertheless, compartmentalized oxidative stress in self-reactive T cells, B cells and phagocytic cells might serve to limit autoimmunity and its inhibition could be detrimental. Antioxidant therapy might also be useful in ameliorating damage caused by other treatments. This Review thus seeks to critically evaluate the complexity of oxidative stress and its relevance to the pathogenesis and treatment of SLE.
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune inflammatory disease of unknown aetiology, and for which approved therapies are inadequate. Current treatments rely on the use of cytotoxic, anti-proliferative, and anti-metabolite drugs as well as the depletion or inactivation of B cells (Supplementary Table 1).1 The pathogenesis of SLE is attributed to dysfunction of T cells, B cells, dendritic cells, macrophages and neutrophils, secondary to genetic and/or environmental factors.2 In broad terms, molecular mimicry (that is, crossreactivity between exogenous and self antigens), aberrant exposure or modification of endogenous antigens, accompanied by expression of autoantibodies, leads to the formation of pathogenic immune complexes that mediate tissue damage in SLE.3
Defined as an imbalance between the production and neutralization of reactive oxygen intermediates (ROI),4 and governed by a combination of inherent and environmental factors, oxidative stress is thought to underlie some of the aberrant exposure of and reaction to cell-death signals that characterizes SLE pathology. Abnormal activation and processing of cell-death signals by the immune system triggers the release of nuclear debris from apoptotic and necrotic cells and stimulates the production of antinuclear (for example, anti-Ro and anti-DNA) antibodies, with subsequent inflammation, organ damage and pathology.2–5 Excessive production and ineffective clearance of ROI are implicated in the development of SLE.
ROI mostly originate from mitochondria, and T cells from patients with SLE exhibit mitochondrial dysfunction, characterized by mitochondrial hyperpolarization (that is, elevated mitochondrial transmembrane potential [ΔΨm]) (Figure 1a).6–8 Increased production of ROI8 or diminished levels of reduced glutathione (an inverse marker of cellular oxidative toxicity) have been found in peripheral blood lymphocytes (PBL) from patients with SLE.6–10 Besides mitochondrial production, ROI can also be generated by NADPH oxidase (NOX) enzymes in phagocytes and, to a lesser extent, in endothelial cells, T cells and B cells,11–13 as well as by environmental oxidants. Genetic factors that compromise antioxidant defences against ROI14,15 or control endogenous ROI production by altering mitochondrial homeostasis15–17 have been linked to SLE pathology.18,19
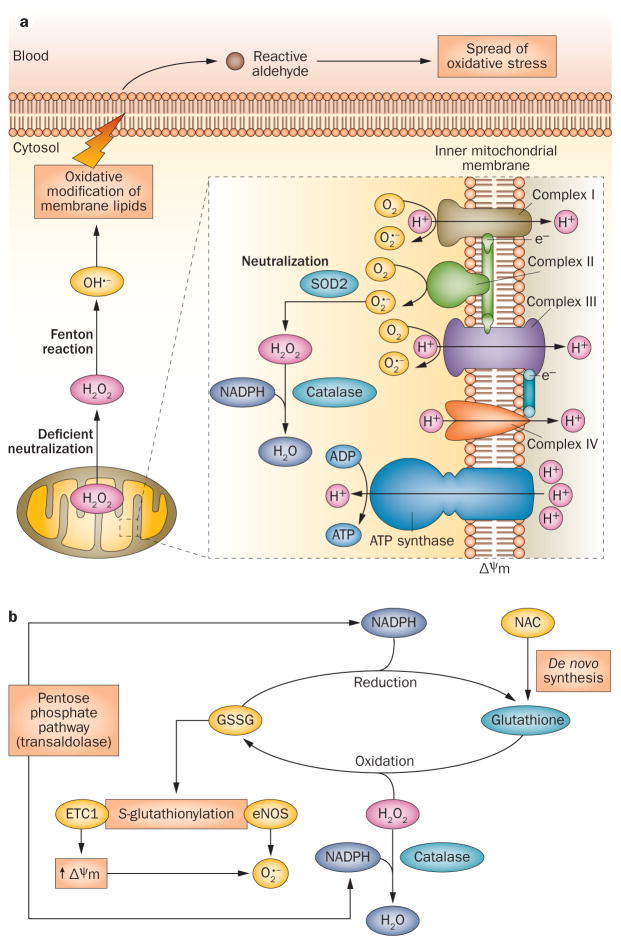
Figure 1.
Mitochondrial generation and systemic propagation of oxidative stress, and overview of redox balance mechanisms. a | Production of ROI and spread of oxidative stress. In all mammalian cells, ROI are generated by the ETC. Mitochondrial hyperpolarization, which occurs in T cells in SLE, increases this production by promoting transfer of electrons to molecular oxygen (generating O2 •−). O2 •− is not membrane permeable and is converted into H2O2 by SOD2 within the mitochondria. H2O2 is further neutralized into H2O by catalase at the expense of NADPH, but can also diffuse through membranes.25 Excess H2O2 is transformed into highly toxic OH•− through the Fenton reaction. OH•− damages lipids and other macromolecules in the immediate vicinity and generates diffusible and highly toxic lipid aldehydes, which spread oxidative stress from mitochondria to other intracellular organelles and through the bloodstream. b | Redox mechanisms that control oxidative stress. Glutathione metabolism regulates mitochondrial hyperpolarization, via S-glutathionylation of ETC complex 1, which increases production of O2•−. Reduced glutathione is regenerated at the expense of NADPH, which is primarily produced through the pentose phosphate pathway.4 Transaldolase activity, which is increased in T cells from patients with SLE,10 has been associated with depletion of NADPH and glutathione and with mitochondrial hyperpolarization.134 Abbreviations: ETC, electron transport chain; ROI, reactive oxygen intermediates; SLE, systemic lupus erythematosus; SOD2, superoxide dismutase [Mn], mitochondrial.
Once initiated, oxidative damage can be propagated via numerous molecular targets. Products of these cascades of oxidative modification are associated with disease activity, organ damage, and comorbidities in SLE. Protection against ROI is normally afforded by intracellular antioxidant systems that are primarily dependent on the availability of reduced glutathione (Figure 1b).4 Boosting this availability, as discussed in the section “NAC and rapamycin”, is a developmental strategy in SLE therapy and acts upstream of oxidative stress. Downstream pathways also represent potential points of intervention (Figure 2).
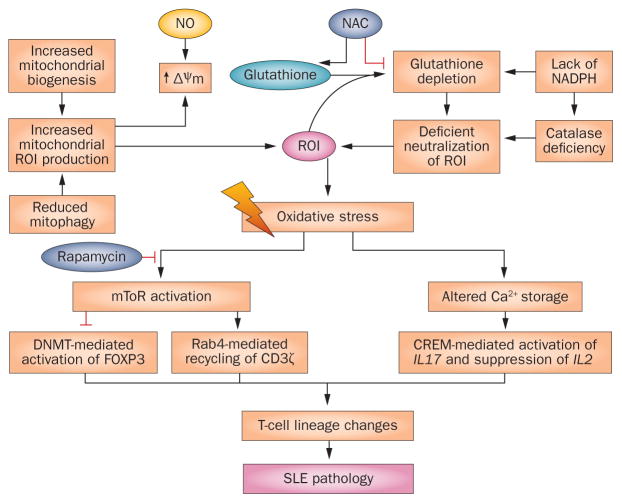
Figure 2.
Overview of molecular pathways of oxidative stress and potential points of intervention in T cells in SLE. Upstream of oxidative stress, exposure to NO and/or depletion of glutathione generates ROI, which cause MHP, mitochondrial biogenesis and oxidative stress. In addition to biogenesis, an increase in the number of T-cell mitochondria in SLE has also been attributed to reduced mitophagy.28 NADPH is generated by the pentose phosphate pathway and is required both to generate NO and to regenerate glutathione. The glutathione precursor NAC is thought to work upstream of oxidative stress by replenishing glutathione and preventing MHP. mTOR is a sensor of MHP but precisely how it is activated by oxidative stress, and thus how NAC prevents its activation, is currently unclear. Downstream of oxidative stress, mTOR activates Rab4A and associated endocytic recycling of CD3ζ.10 These changes alter intracellular signal transduction and T-cell lineage specification, causing contraction of TH1-cell, TREG-cell and CD8+ T-cell subsets, and expansion of TH2-cell, TH17-cell and CD4−CD8− T-cell subsets.34,89 Rapamycin prevents the activation of mTOR without affecting oxidative stress. Abbreviations: FcεRIγ, high affinity immunoglobulin ε receptor subunit β, γ chain; MHP, mitochondrial hyperpolarization; mTOR, serine/threonine-protein kinase mTOR; ROI, reactive oxygen intermediates; SLE, systemic lupus erythematosus; TREG cell, regulatory T cell.
Oxidative stress contributes substantially to cardiovascular disease (CVD),20 which—alongside renal failure and infections—is a major cause of morbidity and mortality in SLE.21 Antioxidant therapy can improve cardiovascular outcomes in patients with end-stage renal disease.22 Although the evidence that oxidative stress is increased in SLE and that it contributes to immune dysregulation, organ damage, and fatal comorbidities is overwhelming, compartmentalized oxidative stress is necessary and its induction can be beneficial. Phagocytic cells use oxidative stress to eliminate pathogenic organisms, whereas cytotoxic drugs—used with the intent of eliminating autoreactive cells and thus restraining immunity—induce oxidative stress and cell death. Thus, where therapeutic oxidative stress is required, antioxidant therapies have a role in limiting the toxicity of immunosuppressant therapies.23
This Review, therefore, critically evaluates the complexity of oxidative stress in SLE. The molecular pathways that generate oxidative stress are examined, with a focus on mitochondrial dysfunction in T cells—as a source of ROI and of proinflammatory necrotic debris. Propagation of oxidative stress through the bloodstream by reactive aldehydes, and the roles of these pro-oxidant molecules with respect to SLE pathogenesis and the generation of autoreactivity to self-antigens, alterations in signal transduction and cytokine production are discussed. Subsequent injury to organs such as the kidney and the skin, and contributions to life-shortening comorbidities, such as atherosclerosis, thrombosis, and infections are outlined. Counterbalancing discussion of these pathogenic pathways, the potential benefits of compartmentalized oxidative stress, in killing of infectious organisms by phagocytic cells and limiting immune system activation, are also examined. Checkpoints of oxidative stress as regulators of pathogenesis, biomarkers of disease activity, and targets of treatment in SLE are evaluated.
Endogenous sources of oxidative stress
Increased ROI production in mitochondria
Increased production of ROI8 or diminished levels of reduced glutathione (Glu–Cys–Gly tripeptide), which is oxidized to glutathione disulphide (GSSG) by ROI and can thus serve as an inverse marker of redox toxicity, reveal cellular oxidative stress. In mitochondria— the main source of ROI—transfer of electrons to molecular oxygen during electron transport chain (ETC) activity generates ROI including superoxide anion, O2 •− (Figure 1a). ETC activity generates electrical energy, stored as ΔΨm, that generates ATP during oxidative phosphorylation.24 ΔΨm is regulated by oxidation–reduction equilibria of ROI, pyridine nucleotides (NADH–NAD+ and NADPH–NADP+) and glutathione–GSSG.4 Enabling maintenance of ΔΨm, the pentose phosphate pathway provides the NADPH required by catalase, glutathione reductase, and NO synthase (NOS) enzymes (Figure 1b).4 In a state of mitochondrial hyperpolarization, H+ ions are extruded from the mitochondrial matrix and cytochromes within the ETC become more reduced, promoting production of ROI and generating oxidative stress (Figure 1a).24
The most damaging ROI are the hydroxyl radical, OH•− and O2•−; the latter can be converted into relatively stable, nonradical hydrogen peroxide (H2O2) by superoxide dismutases and then into water by catalase. As OH•− cannot be eliminated without causing oxidative damage, its formation needs to be prevented by neutralizing the upstream sources O2 •− and H2O2. OH•− is formed in the presence of a metal ion through the Fenton reaction or triggered by UV light.25 Because of its short half-life (10−9 seconds at 37 °C),26 OH•− attacks molecules in the vicinity of its formation, resulting in immediate damage to proteins, nucleic acids, and lipids.25
Genetic data implicate disrupted mitochondrial homeostasis in SLE. In 2012, a mouse gene at Sle1c2 (a sub-locus of the major lupus susceptibility locus Sle1) was identified as oestrogen-related receptor γ, encoding an orphan nuclear receptor that regulates oxidative metabolism and mitochondrial function—reduced expression of the gene in T cells caused greater mitochondrial mass, as estimated by increased voltage-dependent anion channel protein content.15 Similarly, non-synonymous polymorphisms in human mitochondrial DNA encoding components of ETC complexes I and V have been associated with SLE.17 Furthermore, inactive alleles of UCP2, which encodes mitochondrial uncoupling protein 2—a protein that reduces oxidative stress—confer predisposition to several autoimmune diseases, including multiple sclerosis, rheumatoid arthritis (RA), SLE, granulomatosis with polyangiitis, Crohn disease, and ulcerative colitis.16 Increased production of ROI may also originate from the accumulation of mitochondria due to increased biogenesis27 and diminished turnover of mitochondria in lupus T cells.28 According to an evaluation of T cells from patients with SLE (n = 33) and from lupusprone mice, the latter effect is caused by T-cell depletion of dynamin-1-like protein (also known as dynamin-related protein 1, DRP1), an initiator of mitochondrial fission and mitophagy, as a consequence of overexpression of Ras-related protein Rab4A (encoded by RAB4A).28 Mitophagy, an autophagic process in which mitochondria are targeted for lysosomal degradation, is thought to help maintain functional and structural integrity.28,29
Extramitochondrial sources of oxidative stress
Besides ROI, redox signalling also involves reactive nitrogen intermediates (RNI), such as NO and peroxynitrite (ONOO•−, which is generated by the reaction of NO with O2•−). NO is synthesized from L-arginine by NOS, using NADPH as a cofactor.4 Functionally crucial cysteine residues of NOS are compromised by oxidative stress, which increases S-glutathionylation—attachment of glutathione to cysteine through thiol–disulphide exchange with GSSG.30 S-glutathionylation of specific cysteine residues in the reductase domain of the endothelial isoform of NOS (eNOS), which is expressed in T cells,27 switches production from NO to O2•−, generating oxidative stress.31
NOX enzymes are another immune-cell source of ROI. Macrophages and granulocytes express NOX2, which produces O2•− for the killing of phagocytosed bacteria.11 NOX2 is less clear as a source of ROI in T cells,11 but studies in mice have indicated that its expression might be induced by T-cell receptor (TCR) stimulation.12
Diminished antioxidant defences
Antioxidant defences are dependent upon de novo synthesis of glutathione and thioredoxin, both of which can be regenerated at the expense of NADPH (Figure 1b, Figure 2). NADPH itself is primarily produced through the metabolism of glucose via the pentose phosphate pathway.4 Besides factors that diminish the availability of these factors, loss of endogenous antioxidant enzyme activities also predispose to SLE. For example, female mice lacking nuclear factor erythroid 2-related factor 2 (Nrf2), a transcriptional activator of antioxidant and phase II drug metabolizing enzymes, have reduced life-span and increased lipid peroxidation, with development of anti-DNA autoantibodies, splenomegaly, mesangial deposits and massive granular deposits of IgG, IgM, and C3 along renal capillary walls, and glomerulonephritis.18 A 2010 study involving 362 patients with childhood-onset SLE and 379 controls linked a polymorphism of NRF2 to the risk of nephritis in SLE.32 Pharmacological stimulation of Nrf2 has subsequently been found to improve nephritis in lupus prone-mice.33 Similarly, genetic polymorphism of the detoxifying enzyme glutathione S-transferase has been linked to SLE.19
Mitochondrial dysfunction6 and glutathione depletion can be detected in T cells from patients with SLE.6,9,10,35 The oxidative stress evidenced by these findings can promote autoimmunity by modulating signal transduction and cytokine production within the originating T cells, and by triggering autoreactivity to self-antigens.
Environmental sources of oxidative stress
Autoantigenesis in SLE has been attributed, at least in part, to post-translational modification of self-antigens secondary to environmental triggers such as ultra-violet radiation, infection and chemical exposure.36 Autoantigens are created during cell death, typically through enzymatic cleavage, as with Ro,36,37 or generated by oxidative modification, as for oxidized DNA.38 Present in circulating immune complexes in patients with SLE,39 oxidized DNA is also found in necrotic debris, which is more pro-inflammatory than apoptotic debris.41 Indeed, in lupus-susceptible mice, systemic autoimmune disease is induced by dendritic cells that have captured necrotic but not apoptotic debris.42 Here we review how oxidative stress is triggered by environmental factors, such as viruses and UV, and facilitated by genetic factors that influence generation or neutralization of ROI.
Viral infection
Infectious agents, particularly viruses, have long been implicated in SLE pathogenesis: initial manifestations and disease flares of SLE resemble the febrile illness of viral infection; immune responses in SLE can be cross-reactive between viral and self-antigens; and viral nucleic acids are more prevalent in blood and tissue samples of patients with SLE than in healthy controls.42 Epstein–Barr virus is associated with autoimmune disorders, including SLE;38 furthermore, the immunodeficient state of patients with SLE is similar to that induced by infection with HIV with regard to involvement of endogenous retroviruses42 and other retroelements.43 CD4+ T cells from patients with SLE10 and from people infected by HIV-1 show activation of the HRES-1 locus containing RAB4A.44 As we discuss in the section “Altered signal transduction: mTOR and TCR”, redox-dependent over-expression of RAB4A mediates downregulation of T-cell surface glycoproteins CD4 and CD3ζ, changes that underlie defective T-cell receptor (TCR) signalling in both conditions (Figure 3).10,44
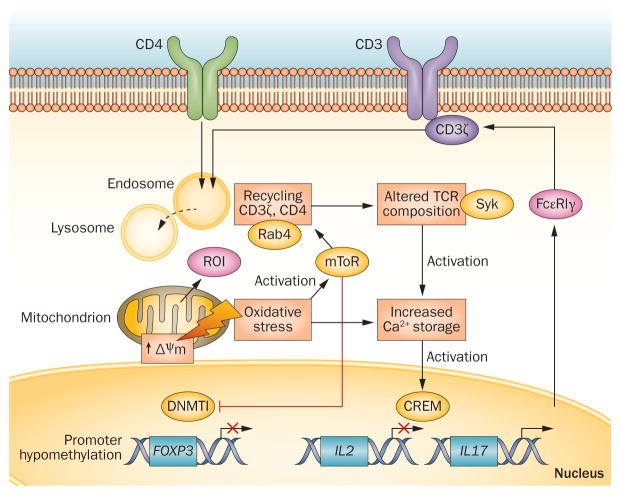
Figure 3.
Molecular targets of oxidative stress in T-cell signal transduction. The source of oxidative stress, ROI, primarily originate from mitochondria; small amounts can also be generated by NOX activity following TCR stimulation.11,12 MHP is induced by oxidative stress and activates mTOR, which in turn promotes T-cell activation via Rab4A-mediated downregulation of the TCR component CD3ζ10 and increased calcium flux.92 Activated mTOR inhibits DNMT1; subsequent promoter hypomethylation suppresses the transcription of FOXP3, which is required for TREG-cell development. Oxidative stress and associated changes to calcium storage also activate CREM, which suppresses IL-2 and enhances IL-17 promoter activity; these changes result in TH1-to-TH17 skewing in the T-cell compartment, as shown in Figure 4. Generation of ROI and MHP are depicted in Figure 1. Abbreviations: CREM, cAMP response element modulator; DNMT1, DNA methyltransferase 1; MHP, mitochondrial hyperpolarization; mTOR, serine/threonine-protein kinase mTOR; NAC, N-acetylcysteine; NOX, NADPH oxidase; ROI, reactive oxygen intermediates; TCR, T-cell receptor; TREG cell, regulatory T cell; TH1, type 1 T helper (cell); TH17, type 17 T helper (cell).
One potential pathogenic mechanism by which oxidative stress seems to combine with viral infection to induce SLE involves inactivation of anti-retroviral defences. DNA reverse-transcribed from endogenous retroviruses is metabolized by 3′ repair exonuclease 1 (TREX1), and inactivating mutations of TREX1 have been documented in a rare form of SLE, termed chilblain lupus.43 TREX1 also degrades HIV-derived DNA and might protect from viral infections.45 Translocating from the endoplasmic reticulum to the nucleus upon oxidative stress,43 TREX1 activity is inhibited by cyclopurine deoxynucleoside photoproducts generated by UV-induced ROI.46 Thus, oxidative stress might lead to a functional TREX1 deficiency and persistence of viral DNA that contributes to immune-complex formation in SLE.42
Bacterial infection
Oxidative stress contributes to the destruction of organisms in phagocytic cells: ROI, generated by the respiratory burst with the involvement of NOX2, participate in elimination of bacteria by neutrophils. Leftover DNA from failure to clear bacteria, as a result of defective ROI generation, is thought to chronically stimulate the innate immune system and trigger SLE.47 Conversely, however, ROI also induce formation of neutrophil extracellular traps (NETs) involved in NETosis, a specialized form of cell death that is implicated in aberrant exposure of antigens and SLE pathogenesis (roles of neutrophils in SLE have been reviewed in this journal48).49,50
Chronic granulomatous disease (CGD)—associated with recurrent bacterial infections and most commonly caused by NOX2 deficiency in phagocytic cells—has been linked with lupus. 10 cases of discoid lupus and 2 cases of SLE were reported in a national registry of 368 patients with CGD; the association with discoid lupus was significant only in those with X chromosome-linked recessive CGD, and not in autosomal chromosome-linked recessive CGD (19/219 versus 1/71; P <0.01).51 Supporting a link between defective neutrophil ROI generation and SLE, Nox2 deficiency exacerbated lupus disease activity in MRL/lpr mice, through inhibiting NET formation and NETosis).52 Paradoxically, however, NETosis seems to be increased and associated with endothelial damage in patients with SLE;50 factors that underlie this increase, and whether the process is represented by the MRL/lpr mouse model, remain unknown.
Besides the link with CGD, other genetic data implicate NOX2 defects in SLE. A hypoactive C242T polymorphism in CYBB, encoding the NOX2 subunit cytochrome b-245 heavy chain has been associated with SLE in a Chinese population.53 Similarly, polymorphism of NCF2, encoding a 67-kilodalton activating cytosolic subunit of NOX2, has been associated with SLE in a Chinese cohort,54 and a loss-of-function His389Gln mutation of NCF2 has been linked to SLE in US patients.55 NOX2 deficiency in the context of T-cell dysfunction is discussed in the next section.
Oxidative stress and T-cell dysfunction
We have discussed how excessive oxidative stress can arise in patients with SLE, and how it can lead to persistence of bacterial and viral antigens. ROI, originally regarded only as toxic by-products of aerobic existence, are now recognized as essential modulators of various signal-transduction pathways, including epigenetic regulation of gene transcription,56 production of regulatory microRNA,57 control of mRNA translation58 and protein folding,59 as well as degradation and recycling of proteins and organelles via autophagy.60 In accordance with these diverse functions, oxidative stress seems to mediate T-cell dysfunction in SLE at multiple levels. Such T-cell defects result in aberrant immune responses and, in concert with the products of oxidative autoantigenesis, are thought to elicit the inflammatory pathology and comorbidities of SLE.
Lineage and cytokine shifts
As discussed under “Bacterial infection”, NOX2 deficiency is implicated in defective clearance of pathogenic antigens, but it is also implicated in altered T-cell responses in SLE. Activation of purified naive CD4+ T cells from Nox2-deficient mice led to augmented levels of IFN-γ and diminished IL-4 production, in comparison with wild-type cells.61 An increased expression ratio of the TH1 cell-specific T-box transcription factor TBX21 (also known as T-bet) over the TH2 cell-specific trans-acting T-cell-specific transcription factor GATA-3 in the absence of Nox2 is consistent with skewing of naive T-cell development to a TH1-cell response.61,62 T cells from patients with SLE and animal models of the disease, however, exhibit defective production of TH1 cytokines IL-22 and IFN-γ,63 and increased production of TH2 cytokines IL-462–65 and IL-10,8,66 favouring B-cell activation and ANA production.67 Thus, the shifts in cytokine expression patterns that occur in SLE are consistent with promotion of IL-4 production and TH2-cell development by oxidative stress.68 In support of the influence of oxidative status on T-cell development, glutathione depletion— that is, diminished antioxidant defences—also favours TH1-to-TH2 polarization of T-cell development. 37 Oxidative stress is associated with increased mitochondrial biogenesis and Ca2+ storage,69 which can enhance the activity of calmodulin-dependent kinase IV and of cAMP response element modulator (CREM), thus suppressing expression of IL-2 and promoting that of IL-17 in lupus T cells (Figure 4). Therefore, mechanisms by which oxidative stress induces such T-cell alterations are discussed throughout this section.
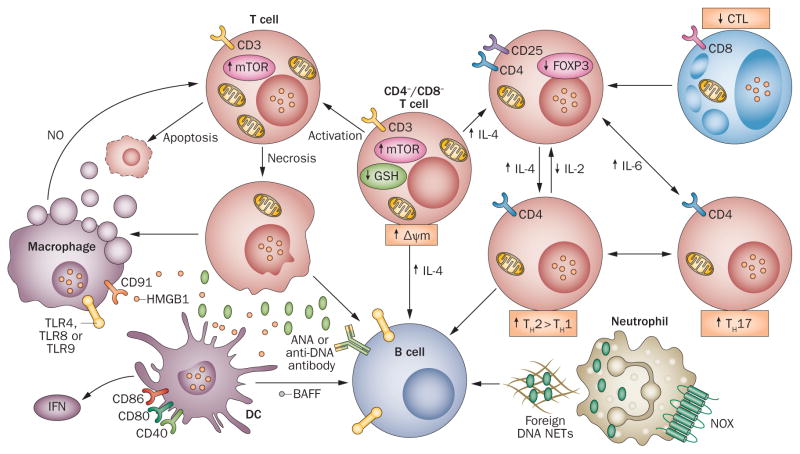
Figure 4.
Consequences of compartmentalized oxidative stress in T cells and phagocytic cells for the proinflammatory intercellular signalling network in SLE. Oxidative stress is proposed to originate in CD4−CD8− T cells, which are implicated in orchestrating dysfunction of the immune system in SLE. MHP, accumulation of mitochondria, and oxidative stress activate mTOR; in CD4−CD8− T cells production of IL-17 and IL-4 is consequently increased, thus stimulating B cells and causing contraction of the CD4+CD25+FOXP3+ TREG-cell subset. mTOR activation also inhibits the development of TH1 cells and CD8+ T cells. Following activation, necrosis-prone T cells, marked by increased mitochondrial mass, release oxidized DNA and HMGB1, which stimulate B cells, macrophages, and DCs.5 In turn, macrophages and DCs produce NO, which stimulates MHP in T cells and production of BAFF, which further activates B cells and the production of ANA. GSH depletion and oxidative stress thus favour TH1 to TH2 polarization in the development of CD4+ T cells. Neutrophils extrude foreign DNA in NETs (by NETosis) that stimulate B cells via TLRs. NOX2-dependent oxidative stress facilitates destruction of infectious organisms in phagocytic cells, potentially limiting NETosis and TLR-mediated stimulation of B cells. Mitochondria are depicted in T cells that exhibit mitochondrial dysfunction. Abbreviations: ANA, antinuclear antibodies; BAFF, B cell activating factor (also known as BLyS and as TNF ligand superfamily, member 13b); DC, dendritic cell; GSH, glutathione; HMGB1, high mobility group protein-1; MHP, mitochondrial hyperpolarization; NET, neutrophil extracellular trap; NOX, NADPH oxidase; TH, helper T (cell); TLR, Toll-like receptor; TREG cell, regulatory T cell.
Regulation of gene transcription
Epigenetic mechanisms—DNA methylation, methyl and acetyl modification of histones, ADP-ribosylation and activity of non-coding RNA—are implicated in SLE.70 Hypomethylation of DNA in T cells contributes to pathogenesis in patients with SLE67 and in lupusprone mice.72 Changes to histone acetylation status and the activity of transcription factors also govern altered transcription patterns in SLE. These factors combine to result in altered T-cell signalling and skewing of lineage development in SLE.
DNA hypomethylation
Two pathways by which oxidative stress might lead to DNA hypomethylation in SLE have emerged; the first involves downregulated activity of DNA (cytosine-5)-methyltransferase 1 (DNMT1). A 2012 study using CD4+ T cells from patients with active SLE identified oxidative stress as a cause of demethylation of CD70, with consequent overexpression of CD70 antigen, and provided insights into the signalling events involved. Production of ONOO•− increased the nitration of protein kinase Cδ— preventing its phosphorylation—and in turn inhibited ERK-dependent activation of DNMT1.72 The alternative mechanism of hypomethylation involves restricted availability of S-adenosylmethionine (SAM), which serves as a methyl donor for methylation of DNA and histones, as a consequence of lack of glutathione.73
T-cell DNA hypomethylation, howsoever caused, promotes inflammation. DNA is preferentially methylated at CpG dinucleotide islands, inhibiting binding of the proinflammatory transcription factor nuclear factor κB (NFκB); hypomethylation thus promotes NFκB signalling. 74 FoxP3, conversely, is an anti-inflammatory transcription factor whose expression in mouse regulatory T (TREG) cells is maintained by serine/threonine-protein kinase mTOR-sensitive methylation of the Foxp3 promoter, and is thus diminished in the absence of methylation.75
Histone modifications
Histone acetyltransferases (HATs) acetylate specific lysine residues on N-terminal tails of core histones, thereby uncoiling the DNA and increasing accessibility to transcription factors, whereas histone deacetylases (HDACs) generally repress gene transcription.56 Histone acetylation status is affected by oxidative stress; reversing the consequent changes to gene transcription is emerging as a potential therapeutic approach in SLE. Oxidative stress causes, for example, proteolytic degradation of the HDAC NAD-dependent protein deacetylase sirtuin-1.76 Importantly for the relevance of such pathways in SLE, pharmacological reversal of histone deacetylation through HDAC inhibition improves lupus disease activity in mice.77 Several potential new HDAC inhibitors are under investigation for a range of purposes; for example, the endogenous compound β-hydroxybutyrate has substantial antioxidant properties in mice via inducing the expression of Sod2 and catalase.78 Another natural compound, sulphurophane—found at high levels in broccoli—inhibits HDAC when coupled to cysteine or N-acetylcysteine (NAC).79
Transcription factor activation
As mentioned above, NFκB is a focal point in the signal transduction cascades that mediate inflammatory cues from antigen receptors on T cells and B cells,80 and from Toll-like receptors (TLRs) on cells of the innate immune system.47 Besides hypomethylation-driven activation of NFκB-sensitive genes, other oxidation-induced alterations in transcription factor programmes are implicated in SLE, some following the identification of lupus susceptibility genes. Among these pathways,81 oxidative stress modulates activation of the Janus kinase–signal transducer and activator of transcription (JAK–STAT) cascade.82 Activating phosphorylation of STAT3 is induced in human PBLs by oxidative stress,83 and in mice, oxidative-stress induced mitochondrial translocation of Stat3 provides feedback regulation of ROI production by modulating ETC activity.84 Expression of STAT3 is increased both in T cells from patients with SLE85 and in B cells from mouse models of the disease.86 STAT3-dependent signals have key roles in the differentiation of human type 17 T helper (TH17) cells,87 which are thought to mediate nephritis in patients with SLE via the production of IL-17.2 Similarly, STAT4 expression88 and the development of TH1 cells are responsive to oxidative stress via mTOR,89 a sensor of mitochondrial dysfunction.10,90 Redox-controlled activation of mTOR— discussed further in the next section—thus represents a key mechanism in T-cell lineage specification and potentially in SLE pathogenesis (Figure 4).
Altered signal transduction: mTOR and TCR
As we have mentioned, T cells from patients with SLE exhibit persistent mitochondrial hyperpolarization and oxidative stress,6 and insights into how ROI can alter T-cell functions is accumulating. Physiological TCR signal transduction requires T-cell surface glycoprotein CD3ζ chain, which is susceptible to ROI-induced degradation91 by endocytic recycling to lysosomes.10 Consequent to this degradation, CD3ζ is replaced by γ chains of the high affinity immunoglobulin ε receptor subunit β (FcεRIγ), which facilitate the recruitment of Syk and flux of Ca2+ upon TCR activation.2 mTOR, which serves as a sensor of ΔΨm90 and is markedly activated in T cells from patients with SLE,7,10,34 seems to mediate the loss of CD3ζ. In six10 and nine92 patients with SLE effectively treated by mTOR blockade with rapamycin, inhibition of mTOR normalized T-cell Ca2+ flux, without influencing mitochondrial hyperpolarization. In turn, mTOR was found to activate the small GTPase Rab4A, which regulates endocytic recycling and causes lysosomal degradation of CD4 and CD3ζ in T cells from patients with SLE.10 The relative availability of CD3ζ and FcεRIγ for TCR signal transduction in SLE is also influenced by altered phosphorylation, as discussed in a proximate section of this manuscript, whereas therapeutic targeting of mTOR activation is discussed under “NAC and rapamycin”.
Upstream of mTOR
Alterations in mitochondrial and endocytic recycling gene expression patterns have been detected, and validated at protein and mechanistic levels, in naive T cells from patients with SLE.10 Among these changes, increased expression of the mitochondrial proteins voltage-dependent anion-selective channel protein 1 and superoxide dismutase10 is consistent with increased mitochondrial mass69 and oxidative stress in T cells in SLE.8 Also overexpressed and likely to act upstream of mitochondrial hyperpolarization in SLE is the pentose phosphate pathway enzyme transaldolase (TAL),10 which is a metabolic regulator of Δψm.93 Expression of NOS-interacting protein (NOSIP), which inhibits activity of eNOS and NO production, is reduced in T cells in SLE. Expression of neither TAL nor NOSIP is affected by rapamycin treatment.10
Altered phosphorylation
Experiments using primary human T cells from healthy donors have shown that oxidative stress stimulates expression of serine/threonine-protein phosphatase 2A (PP2A) catalytic subunit α isoform by diminishing DNMT1 activity and thus causing demethylation of the PPP2CA promoter.94 PP2A dephosphorylates ETS-related transcription factor Elf-1 at Thr231; increased PP2A activity reduces binding of Elf-1 to promoters in two of its target genes, those encoding CD3ζ and FcεRIγ, thus reducing the expression of the former and increasing the expression of the latter.95 By facilitating ‘exchange’ of these TCR signalling components, PP2A promotes TH2-polarization of T-cell lineage development in SLE.2
Increased PP2A activity as a consequence of oxidative stress seems to also skew TH1-cell cytokine production, away from IL-2 expression toward increased production of IL-17. PP2A also dephosphorylates cyclic AMP-responsive element-binding protein 1 (CREB1) and reduces its binding to the IL-2 promoter.2 Oxidative stress also contributes to diminished IL-2 production through enhanced Ca2+ signalling. In particular, increased mitochondrial biogenesis and accumulation of mitochondria allow for greater Ca2+ storage30 that may enhance the activity of calmodulin-dependent kinase IV and CREM, which in tandem suppress IL-2 and promote IL-17 expression in lupus T cells.2
Oxidative autoantigenesis
In addition to causing altered T-cell signalling and development, oxidative stress is implicated in SLE pathology via the oxidative modification of self antigens, leading to autoimmunity. Evidence for the propagation of oxidative autoantigenesis through the circulation, and how this process triggers inflammation, organ damage and comorbidities in SLE is discussed in this section. Oxidative autoantigenic processes relevant to SLE include: the accumulation of oxidized HDL cholesterol (oxHDL) in the blood; oxidation of β2 glycoprotein I (β2GPI);96 modification of polyunsaturated fatty acids and generation of autoantibodies crossreactive with DNA;97 and UV light-triggered autoantigenicity of Ro.98
Propagation of oxidative stress
Lipid peroxidation in mitochondrial, lysosomal and cell membranes by ROI generates reactive aldehydes including malondialdehyde and 4-hydroxy-2-nonenal (HNE), which can ‘spread’ oxidative damage through the circulation. Products of these cascades of oxidative modification can be detected in the circulation of patients with SLE, and include advanced glycation end products (AGE)99 and oxHDL.100 Modification of serum albumin by HNE reflects oxidative stress101 and its extent correlates with disease activity in SLE.102
Autoantigenesis and inflammation
The production of hallmark SLE autoantibodies can be triggered by oxidative stress. UV light, for example, triggers the autoantigenicity of Ro, which is displayed in cell surface blebs during apoptosis,98 and the triggering role of oxidative stress in this process is evidenced by the stimulation of Ro immunogenicity through direct treatment with HNE.103,104 Anti-DNA antibodies form in response to stimulation of the B-cell receptor and TLRs by DNA, particularly oxidized DNA,39 which is released from apoptotic and necrotic cells and in NETs.50 HNE also oxidizes β2GPI, increasing its immunogenicity via a TH1 cell-mediated immunological mechanism and thus promoting the production of anti-β2GPI antibodies and the development of antiphospholipid syndrome (APS), which represents a substantial comorbidity in SLE.96 In the MRL/lpr mouse model of SLE, the reactive aldehyde 4-oxo-2-nonenal (ONE) was identified as a source of autoantigenic epitopes and autoimmunity.97 Bovine serum albumin became crossreactive with sera from the mice after it was incubated with peroxidized polyunsaturated fatty acids; anti-ONE reactivity was detected and a subset of anti-DNA antibodies in the mice was found to be crossreactive with ONE-specific epitopes.97
Comorbidities and organ damage
In addition to spreading inflammation through the bloodstream, mediators of oxidative stress contribute to damage in various organ systems. Free thiol–containing β2GPI protects endothelial cells from oxidative stress–induced cell death104 and facilitates the phagocytosis of phosphatidylserine-coated apoptotic particles by macrophages; 105 thus, oxidation of β2GPI promotes not only thrombosis but also endothelium damage, the accumulation of apoptotic bodies and accelerated atherosclerosis. CVD is associated with renal disease both in the general population and in patients with SLE.106
Renal disease
Elevated malondialdehyde concentrations and diminished vitamin C levels indicate oxidative stress in the plasma of patients with lupus nephritis,107 in whom oxidative stress has been identified as a major contributor to organ damage.33,108,109 Reflected by a diminished glutathione:GSSG ratio in the kidneys of the female B/W mouse model of SLE,111 oxidative stress may inflict renal damage via production of TNF by infiltrating macrophages. In NZB/NZW mice with IFNα-induced lupus nephritis, TNF blockade reduced oxidative stress and renal pathology.111 Although the treatment stabilized nephritis and markedly prolonged survival, autoantibody production and systemic immune activation were not inhibited, suggesting that TNF might be a tissue-specific mediator of oxidative stress-associated organ damage.111
Oxidative stress is also reported to contribute to hypertension and proteinuria in diseased kidneys in mice with experimental lupus nephritis.112 As renal disease is a major contributor to cardiovascular disease, oxidative stress can be expected to also underlie CVD,20 which is a major cause of morbidity and mortality in SLE along with renal failure and infections.21 Indeed, IgG antibodies to oxidized phospholipids both elicit atherosclerosis and predispose to nephritis in ApoE−/ −Fas−/ − mice.113
Cardiovascular disease
The accumulation of oxHDL in the blood is associated with accelerated artherosclerosis in patients with SLE,100 who have 5–8-fold increased age-adjusted incidence of CVD.114 Malondialdehyde-modified oxidized low-density lipoproteins (oxLDL) are implicated in CVD in general,115 and especially in SLE-associated CVD.114 HDL, but not oxHDL, protects against the atherogenic effects of oxLDL.100 Hypertension116,117 and coronary artery disease have also been associated with oxidative stress in patients with SLE.117 As noted in the therapeutic section, NAC was found to improve cardiovascular outcomes in patients with end-stage renal disease.22 Oxidative stress in myeloid cells promotes NETosis and contributes to endothelial cell damage in patients with SLE.50
Cutaneous disease
In relation to skin damage, exposure of keratinocytes to UV light triggers exposure of autoantigenic Ro in apoptotic blebs.98 Thus, via induction of oxidative stress, UV mediates development of photosensitive rash, a common organ manifestation in SLE.
Clinical approaches and implications
Potential biomarker of disease activity
No biomarkers of oxidative stress are yet in routine clinical use, but among several candidates it can be hoped that such a test will emerge in the near future. Increased modification of serum albumin by HNE correlates with disease activity in patients with SLE.102 Urinary levels of F2 isoprostane, a derivative of lipid peroxidation, are associated with disease activity.118 Oxidized β2GPI is highly specific for the detection of APS in the setting of thrombosis.119 As mentioned in the section on comorbidities, oxHDL is associated with atherosclerosis in SLE,100 and oxLDL is a biomarker of CVD.115
ROI production is detectable in the nephritic kidneys of lupus-prone mice33 and in the circulation of patients with lupus nephritis.108 Serum ONOO•− levels positively correlated with disease activity in patients with nephritis. 120 Oxidized phospholipids, such as 1-palmitoyl-2-arachidonoyl- sn-3-glycero-phosphorylcholine, and a series of products derived from them, accumulate in atherosclerotic lesions and are serum markers of oxidative stress in inflammatory diseases, including SLE.121 Activation of mTOR (Figures 2 and 3), which is a sensor of mitochondrial hyperpolarization and oxidative stress, has been found to correlate with disease activity and, most importantly, to precede flares in patients with SLE.34
Target for treatment
Therapeutic targeting of oxidative stress includes preventative measures—against exogenous triggers such as UV light and endogenous sources such as mitochondria —as well as stimulation of antioxidant mechanisms. Some interventions are more straightforward than others; photo-resistant clothing and application of sun-screen with protection factor >50, for example, can be used to block UV light. Potential antioxidant therapies include NAC, rapamycin and dietary nutrients such as vitamins (as discussed later in this section), as well as conjugated linoleic acid (CLA). In MRL/lpr mice, CLA increased glutathione synthesis and thus reversed oxidative stress and lupus disease activity, by enhancing the expression of glutamate–cysteine ligase.122
As we have mentioned, effective antioxidant treatment might have a role not only in the therapeutic reversal of redox-mediated signalling defects, but also in mitigating the toxicity of immunosuppressive therapies. Indeed, conventional treatment modalities are directed to suppress immune responses, and do so partly by promoting the death of activated and rapidly proliferating self-reactive B cells and T cells. Unwanted consequences can ensue; alkylating agents such as cyclophosphamide, anti-metabolites (such as mycophenolic acid, 6-mercaptopurin, azathioprine and methotrexate) and corticosteroids (such as prednisone) also elicit toxicity and cell death in the bone marrow and liver through oxidative stress.1
NAC and rapamycin
As discussed in the section “Altered signal transduction: mTOR and TCR”, mTOR is activated by relative depletion of glutathione, the supply of which can be boosted by supplementation with its precursor, NAC, and is diminished by oxidative stress. Acting as a sensor of ΔΨm, mTOR governs T-cell signalling events implicated in the pathogenesis of SLE. As well as by ensuring an adequate supply of glutathione, mTOR activation can also be prevented by the bacterial-derived antifungal compound rapamycin.
In (NZB × NZW) F1 lupus-prone mice, NAC treatment prevented the decline of glutathione:GSSG ratios, reduced autoantibody production and development of nephritis, and prolonged survival.110 On the basis of this finding, and that of depleted glutathione in PBL from patients with SLE,6 we initiated a 3-month phase I–phase II double-blind placebo-controlled randomized pilot study of NAC in 36 patients with SLE, followed by a 1-month washout period, to investigate its safety, tolerability, and metabolic, immunological and therapeutic impact.7 Although a dose of 1.2 g per day was ineffective, NAC was found to be safe, and doses of 2.4 g per day and 4.8 g per day were effective in reversing glutathione depletion and improving disease activity and fatigue. Blocking activation of mTOR and promoting expansion of CD4+CD25+FOXP3+ T-cell subsets, possibly through preventing T-cell activation-induced methylation and silencing of the FOXP3 promoter,75 NAC treatment was able to inhibit anti-DNA antibody production.7 Indeed, NAC reversed the expansion of CD4−CD8− T cells, which exhibited the most prominent mTOR activation before treatment with NAC7 and had been deemed responsible for promoting anti-DNA autoantibody production by B cells.67 Whereas this proof-of-concept study has clearly shown that NAC can improve disease activity over 3 months in patients with SLE,7 confirmation in larger cohorts and with longer treatment duration is warranted. Nevertheless, on the basis of its preliminary safety profile and given that it selectively blocks mTOR in T cells, NAC might prove to provide an inexpensive alternative therapy in SLE, and potentially constitutes a synergistic approach to that of B-cell blockade.1 Good tolerability of NAC might be partly attributable to the improvements in fatigue that it elicited.7 Benefit in SLE might also result from NAC’s reported ability to upregulate HDL-cholesterol,123 and to reduce the incidence of cardiovascular events, as shown in patients with renal failure.22 Specific formulations of NAC are approved for use in acetaminophen overdose, contrast nephropathy and cystic fibrosis.
Similar to NAC, rapamycin (used clinically in preventing transplant rejection) also elicits mTOR blockade, although it does so downstream of oxidative stress (Figure 2).10 A study of 2 mg per day rapamycin in nine patients with SLE refractory to conventional therapy reported robust clinical improvement,92 confirmation of which is now being sought in an ongoing prospective phase II clinical trial, in which dosages are titrated to a plasma concentration of 6–15 ng/ml.34 Interim analysis of data from 24 patients with SLE treated with NAC, and 14 treated with rapamycin,34 found that in those treated with rapamycin (in whom baseline disease activity was greater), the treatment diminished disease activity and induced the expansion of TREG cells, which might contribute to the therapeutic mechanism.35 The mechanisms of action of NAC and rapamycin are currently being investigated in animal models, and prospective in-human combination studies of NAC and rapamycin are being planned.
Antioxidant nutrients
Several epidemiological studies have assessed the role of antioxidant nutrient intake in SLE. In a large prospective health survey in 184,643 US women, dietary intake of antioxidant nutrients—vitamins A, C, and E and α-carotene, β-carotene, cryptoxanthin, lycopene, lutein, or zeaxanthin— from foods and supplements was not associated with a decreased risk of developing RA or SLE.124 These findings suggest that antioxidants are generally safe but do not offer protection against SLE, possibly due to their inability to regulate intracellular signalling within the immune system. This theory is supported by the results of a double-blind, placebo-controlled 3-month pilot study of 500 mg vitamin C and 800 IU vitamin E daily in 39 patients with SLE, a regimen that reduced plasma levels of malondialdehyde without affecting endothelial function or disease activity.125 The lack of therapeutic efficacy might relate to insufficient dosing and/or to the inability of vitamin C to augment intracellular glutathione concentrations in T cells, a problem that makes this approach unlikely to succeed.126
Benefits of compartmentalized oxidative stress
Compartmentalized oxidative stress and apoptotic death induced by cytotoxic drugs in highly proliferative autoreactive B cells and T cells is clearly beneficial for patients with SLE; 10-year survival has improved from 50% to 90% since the introduction of cytotoxic and anti-metabolitedrugs. 127–130 Nevertheless, this approach can come at a price; the primary cause of death in patients with SLE is infection,21 secondary to bone-marrow toxicity and immunosuppression.
Small-molecule therapies in SLE (whether alkylating, such as cyclophosphamide and busulphan, or anti-metabolite, such as azathiprone, mycophenolate and methotrexate; Supplementary Table 1) are all anti-proliferative, as are steroid hormones such as prednisone, whereas the mechanism of the anti-malarial agent hydroxychloroquine in SLE seems to involve lysosomal acidification and regulation of autophagy. Alleviating the toxic effects of such drugs is a potential use for antioxidant therapy such as NAC in SLE, whether or not such therapy proves efficacious against the disease activity in its own right. In a trial of 600 mg NAC versus placebo in 182 patients with interstitial lung disease, for example, concomitant NAC therapy significantly reduced the myelotoxic effects of prednisone and azathioprine. 23 Among questions that remain to be answered in this regard is the extent of NAC’s protection against the bone-marrow toxicity of azathioprine and prednisone when used in SLE.1
Protection against the development of SLE might be conferred by compartmentalized oxidative stress, but any potential benefits of inducing such stress are likely to be outweighed by associated comorbidities. A protective effect of NOX2 against SLE, as documented in patients with CGD51 and in MRL/lpr mice, might be mediated through inhibition of NETosis, which involves oxidation-dependent destruction of pathogenic bacteria.52 Reduced capacity to generate oxidative stress in NOX2-deficient neutrophils causes the persistence of bacteria and pre-disposition to lupus,13,51 possibly through stimulation of the innate immune system by bacterial DNA.131 However, any protective effect of inducing such NOX-mediated oxidative stress is likely to be limited. Overall, NOX activity seems rather to have a pathogenic role in promoting CVD, as indicated by its increased expression along with increased production of ROI in and around atherosclerotic plaques in patients with SLE.132 Likewise, NOX contributes to atherosclerosis in mice; strains lacking p47phox,133 an essential subunit of NOX2, have diminished disease.133 These findings indicate the complexity of oxidative stress with respect to comorbidities in SLE.
Conclusions
Overwhelming evidence of increased oxidative stress in SLE that correlates with disease activity and mediates organ damage has been found both in patients with SLE6,8–10,101 and in lupus-prone mice.111,123 Oxidative stress is a trigger of inflammation,4 atherosclerosis,100 CVD,115 nephritis33,108 and APS.96 Oxidative stress leads to activation of mTOR, which skews lineage specification in T cells.7,10,34 Reduced capacity to generate oxidative stress can cause the persistence of bacteria and stimulation of the innate immune system by bacterial DNA.131 Deciphering the roles of compartmentalized oxidative stress in T cells and phagocytic cells in SLE can be expected to provide further insights into the disease pathogenesis. Dietary intake of antioxidant vitamins does not influence the risk of SLE in the general population124 or improve outcomes;125 nevertheless, reversal of glutathione depletion reduces disease activity both in lupus-prone mice110,122 and patients with SLE.7 Better characterization of the molecular and cellular sources of oxidative stress that are relevant to SLE will facilitate the development of strategies to minimize and/or reverse the effects of exposure to them and might lead to useful biomarkers of disease activity and response to therapy. We also need to determine the mechanism of mTOR blockade by NAC. Meanwhile, future controlled clinical studies should: evaluate the combination of NAC with rapamycin in terms of potential synergy in efficacy and safety of T-cell inactivation; assess the safety and efficacy of combining NAC with B-cell inhibitors, such as belimumab; and test the protective effect of NAC on bone-marrow and liver toxicity of anti-proliferative drugs, such as cyclophosphamide, azathioprine and mycophenolate. Leveraging beneficial processes of oxidative stress, and suppressing damaging pathways, might thereby ameliorate the effects of chronic therapy, reduce disease activity and improve quality of life for patients with SLE.
Key points.
Oxidative stress—generated through multiple mechanisms in a cell-type-specific manner—is a substantial contributor to disease pathogenesis, organ damage and comorbidities in patients with systemic lupus erythematosus (SLE)
Pathways of oxidative pathogenesis, such as oxidative modification of self antigens and T-cell dysfunction, have been identified
Organ systems in which the clinical importance of oxidative damage in SLE has been recognized include the cardiovascular and renal systems and the skin
Biomarkers of oxidative stress correlate directly with disease activity in SLE
Depletion of glutathione (reflecting oxidative stress) might have a pathogenic role; its reversal by N-acetylcysteine seems to have therapeutic benefit in mouse models and patients with SLE
Review criteria.
The following review criteria have been used to identify English-language original and review articles in PubMed and SCOPUS: key words: “oxidative stress”, “antioxidants”, “nephritis”, “atherosclerosis”, “antiphospholipid syndrome”, “autoimmunity”, and “lupus”; published between 1966 and August 2013. References were included according to the author’s opinion of their relevance to the subject.
Acknowledgments
This work was supported in part by grants AI 048,079, AI 072,648, and AT004332 from the National Institutes of Health, the Alliance for Lupus Research, and the Central New York Community Foundation. The author is grateful to Mariana Kaplan (University of Michigan) and Mark Shlomchik (University of Pittsburgh) for helpful discussions and to Paul Phillips (State University of New York) for continued encouragement and support. Due to space limitations, important discoveries of oxidative stress research in SLE may have only been referenced through reviews.
Footnotes
Supplementary information is linked to the online version of the paper at www.nature.com/nrrheum.
Competing interests
The author declares no competing interests.
References
- 1.Francis L, Perl A. Pharmacotherapy of systemic lupus erythematosus. Expert Opin Pharmacother. 2009;10:1481–1494. doi: 10.1517/14656560902971003. [DOI] [PubMed] [Google Scholar]
- 2.Tsokos GC. Systemic Lupus Erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 3.Perl A. Systems biology of lupus: Mapping the impact of genomic and environmental factors on gene expression signatures, cellular signaling, metabolic pathways, hormonal and cytokine imbalance, and selecting targets for treatment. Autoimmunity. 2010;43:32–47. doi: 10.3109/08916930903374774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perl A, Hanczko R, Telarico T, Oaks Z, Landas S. Oxidative stress, inflammation and carcinogenesis are controlled through the pentose phosphate pathway by transaldolase. Trends Mol Med. 2011;7:395–403. doi: 10.1016/j.molmed.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T cell life, death, and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gergely PJ, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai ZW, et al. N-acetylcysteine reduces disease activity by blocking mTOR in T cells of lupus patients. Arthritis Rheum. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gergely PJ, et al. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah D, Aggarwal A, Bhatnagar A, Kiran R, Wanchu A. Association between T lymphocyte sub-sets apoptosis and peripheral blood mononuclear cells oxidative stress in systemic lupus erythematosus. Free Rad Res. 2011;45:559–567. doi: 10.3109/10715762.2011.555765. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez DR, et al. Activation of mTOR controls the loss of TCR in lupus T cells through HRES-1/Rab4-regulated lysosomal degradation. J Immunol. 2009;182:2063–2073. doi: 10.4049/jimmunol.0803600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 12.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–827. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 13.Segal BH, Grimm MJ, Khan AN, Han W, Blackwell TS. Regulation of innate immunity by NADPH oxidase. Free Rad Biol Med. 2012;53:72–80. doi: 10.1016/j.freeradbiomed.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper GS, Makris SL, Nietert PJ, Jinot J. Evidence of autoimmune-related effects of trichloroethylene exposure from studies in mice and humans. Environ Health Persp. 2009;117:696–702. doi: 10.1289/ehp.11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry DJ, et al. Murine lupus susceptibility locus Sle1c2 mediates CD4+ T cell activation and maps to estrogen-related receptor γ. J Immunol. 2012;189:793–803. doi: 10.4049/jimmunol.1200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu X, et al. Association of UCP2 -866 G/A polymorphism with chronic inflammatory diseases. Genes Immun. 2009;10:601–605. doi: 10.1038/gene.2009.29. [DOI] [PubMed] [Google Scholar]
- 17.Vyshkina T, et al. Association of common mitochondrial DNA variants with multiple sclerosis and systemic lupus erythematosus. Clin Immunol. 2008;129:31–35. doi: 10.1016/j.clim.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoh K, et al. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 2001;60:1343–1353. doi: 10.1046/j.1523-1755.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 19.Fraser PA, et al. Glutathione S-transferase M null homozygosity and risk of systemic lupus erythematosus associated with sun exposure: a possible gene-environment interaction for autoimmunity. J Rheumatol. 2003;30:276–282. [PubMed] [Google Scholar]
- 20.Lee R, Margaritis M, Channon KM, Antoniades C. Evaluating oxidative stress in human cardiovascular disease: Methodological aspects and considerations. Curr Med Chem. 2012;19:2504–2520. doi: 10.2174/092986712800493057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trager J, Ward MM. Mortality and causes of death in systemic lupus erythematosus. Curr Opin Rheumatol. 2001;13:345–351. doi: 10.1097/00002281-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure. Circulation. 2003;107:992–995. doi: 10.1161/01.cir.0000050628.11305.30. [DOI] [PubMed] [Google Scholar]
- 23.Demedts M, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 24.Skulachev VP. Mitochondrial physiology and pathology; concepts of programmed death of organelles, cells and organisms. Mol Asp Med. 1999;20:139–140. doi: 10.1016/s0098-2997(99)00008-4. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Meth Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 26.Cui K, Luo X, Xu K, Ven Murthy MR. Role of oxidative stress in neurodegeneration: Recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:771–799. doi: 10.1016/j.pnpbp.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Nagy G, Koncz A, Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J Immunol. 2003;171:5188–5197. doi: 10.4049/jimmunol.171.10.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caza TN, et al. HRES-1/RAB4-mediated depletion of DRP1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Ann Rheum Dis. doi: 10.1136/annrheumdis-2013-203794. http://ard.bmj.com/content/early/2013/07/29/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed]
- 29.Caza TN, Talaber G, Perl A. Metabolic regulation of organelle homeostasis in lupus T cells. Clin Immunol. 2012;144:200–213. doi: 10.1016/j.clim.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crabtree MJ, Brixey R, Batchelor H, Hale AB, Channon KM. Integrated redox sensor and effector functions for tetrahydrobiopterin- and glutathionylation-dependent endothelial nitric-oxide synthase uncoupling. J Biol Chem. 2013;288:561–569. doi: 10.1074/jbc.M112.415992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CA, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordova EJ, Velazquez-Cruz R, Centeno F, Baca V, Orozco L. The NRF2 gene variant, –653G/A, is associated with nephritis in childhood-onset systemic lupus erythematosus. Lupus. 2010;19:1237–1242. doi: 10.1177/0961203310367917. [DOI] [PubMed] [Google Scholar]
- 33.Tsai PY, et al. Antroquinonol differentially modulates T cell activity and reduces interleukin-18 production, but enhances Nrf2 activation, in murine accelerated severe lupus nephritis. Arthritis Rheum. 2012;64:232–242. doi: 10.1002/art.33328. [DOI] [PubMed] [Google Scholar]
- 34.Lai Z, et al. mTOR activation triggers IL-4 production and necrotic death of double-negative T cells in patients with systemic lupus erythematosus. J Immunol. 2013;35:2236–2246. doi: 10.4049/jimmunol.1301005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle HA, Mamula MJ. Autoantigenesis: the evolution of protein modifications in autoimmune disease. Curr Opin Immunol. 2012;24:112–118. doi: 10.1016/j.coi.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med. 1999;190:815–826. doi: 10.1084/jem.190.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraternale A, et al. GSH and analogs in antiviral therapy. Mol Aspects Med. 2009;30:99–110. doi: 10.1016/j.mam.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 38.James JJ, et al. An increased prevalence of Epstein–Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100:3019–3026. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lunec J, Herbert K, Blount S, Griffiths HR, Emery P. 8-Hydroxydeoxyguanosine. A marker of oxidative DNA damage in systemic lupus erythematosus. FEBS Lett. 1994;348:131–138. doi: 10.1016/0014-5793(94)00583-4. [DOI] [PubMed] [Google Scholar]
- 40.Sauter B, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma L, et al. Systemic autoimmune disease induced by dendritic cells that have captured necrotic but not apoptotic cells in susceptible mouse strains. Eur J Immunol. 2005;35:3364–3375. doi: 10.1002/eji.200535192. [DOI] [PubMed] [Google Scholar]
- 42.Perl A, Fernandez D, Telarico T, Phillips PE. Endogenous retroviral pathogenesis in lupus. Curr Opin Rheumatol. 2010;22:483–492. doi: 10.1097/BOR.0b013e32833c6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131:873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Nagy G, et al. Regulation of CD4 expression via recycling by HRES-1/RAB4 controls susceptibility to HIV infection. J Biol Chem. 2006;281:34574–34591. doi: 10.1074/jbc.M606301200. [DOI] [PubMed] [Google Scholar]
- 45.Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11:1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuraoka I, et al. Oxygen free radical damage to DNA: Translesion synthesis by human DNA polymerase eta and resistance to exonuclease action at cyclopurine deoxynucleoside residues. J Biol Chem. 2001;276:49283–49288. doi: 10.1074/jbc.M107779200. [DOI] [PubMed] [Google Scholar]
- 47.Pisetsky DS. The origin and properties of extracellular DNA: From PAMP to DAMP. Clin Immunol. 2012;144:32–40. doi: 10.1016/j.clim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaplan M. Neutrophils in the pathogenesis and manifestations of SLE. Nat Rev Rheumatol. 2011:691–699. doi: 10.1038/nrrheum.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurien BT, Hensley K, Bachmann M, Scofield RH. Oxidatively modified autoantigens in autoimmune diseases. Free Radic Biol Med. 2006;41:549–556. doi: 10.1016/j.freeradbiomed.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 50.Villanueva E, et al. netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winkelstein JA, et al. Chronic granulomatous disease: report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Campbell AM, Kashgarian M, Shlomchik MJ. NADPH oxidase inhibits the pathogenesis of systemic lupus erythematosus. Sci Transl Med. 2012;4:157ra141. doi: 10.1126/scitranslmed.3004801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang FY, Xie XW, Ling GH, Liu FY. Endothelial nitric oxide synthase and nicotinamide adenosine dinucleotide phosphate oxidase p22phox gene (C242T) polymorphisms and systemic lupus erythematosus in a Chinese Population. Lupus. 2010;19:192–196. doi: 10.1177/0961203309348980. [DOI] [PubMed] [Google Scholar]
- 54.Yu B, et al. The association between single-nucleotide polymorphisms of NCF2 and systemic lupus erythematosus in Chinese mainland population. Clin Rheumatol. 2011;30:521–527. doi: 10.1007/s10067-010-1567-3. [DOI] [PubMed] [Google Scholar]
- 55.Jacob CO, et al. Lupus-associated causal mutation in neutrophil cytosolic factor 2 (NCF2) brings unique insights to the structure and function of NADPH oxidase. Proc Natl Acad Sci USA. 2012;109:E59–E67. doi: 10.1073/pnas.1113251108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cyr AR, Domann FE. The redox basis of epigenetic modifications: From mechanisms to functional consequences. Antiox Redox Signal. 2011;15:551–589. doi: 10.1089/ars.2010.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tang H, et al. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 2012;26:4710–4721. doi: 10.1096/fj.11-197467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gerashchenko MV, Lobanov AV, Gladyshev VN. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proc Natl Acad Sci USA. 2012;109:17394–17399. doi: 10.1073/pnas.1120799109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margittai E, Sitia R. Oxidative protein folding in the secretory pathway and redox signaling across compartments and cells. Traffic. 2011;12:1–8. doi: 10.1111/j.1600-0854.2010.01108.x. [DOI] [PubMed] [Google Scholar]
- 60.Caza TN, Talaber G, Perl A. Metabolic regulation of organelle homeostasis in lupus T cells. Clin Immunol. 2012;144:200–213. doi: 10.1016/j.clim.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shatynski KE, Chen H, Kwon J, Williams MS. Decreased STAT5 phosphorylation and GATA-3 expression in NOX2-deficient T cells: Role in T helper development. Eur J Immunol. 2012;42:3202–3211. doi: 10.1002/eji.201242659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sieling PA, et al. CD1c-reactive, TH2 cytokine producing T-cells in human autoimmune disease. FASEB J. 1998;12:A1091. [Google Scholar]
- 63.Tsokos GC, et al. Deficient gamma-interferon production in patients with systemic lupus erythematosus. Arthritis Rheum. 1986;29:1210–1215. doi: 10.1002/art.1780291005. [DOI] [PubMed] [Google Scholar]
- 64.Sieling PA, et al. human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J Immunol. 2000;165:5338–5344. doi: 10.4049/jimmunol.165.9.5338. [DOI] [PubMed] [Google Scholar]
- 65.Dean GS, Anand A, Blofeld A, Isenberg DA, Lydyard PM. Characterization of CD3+CD4−CD8− (double negative) T cells in patients with systemic lupus erythematosus: Production of IL-4. Lupus. 2002;11:501–507. doi: 10.1191/0961203302lu234oa. [DOI] [PubMed] [Google Scholar]
- 66.Georgescu L, Vakkalanka RK, Elkon KB, Crow MK. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J Clin Invest. 1997;100:2622–2633. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shivakumar S, Tsokos GC, Datta SK. T cell receptor α/β expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989;143:103–112. [PubMed] [Google Scholar]
- 68.Wu Z, MacPhee IAM, Oliveira DBG. Reactive oxygen species in the initiation of IL-4 driven autoimmunity as a potential therapeutic target. Curr Pharm Design. 2004;10:899–913. doi: 10.2174/1381612043452875. [DOI] [PubMed] [Google Scholar]
- 69.Nagy G, Barcza M, Gonchoroff N, Phillips PE, Perl A. Nitric oxide-dependent mitochondrial biogenesis generates Ca2+ signaling profile of lupus T cells. J Immunol. 2004;173:3676–3683. doi: 10.4049/jimmunol.173.6.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Richardson B, et al. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990;33:1665–1673. doi: 10.1002/art.1780331109. [DOI] [PubMed] [Google Scholar]
- 71.Sawalha AH, et al. Defective T-cell ERK signaling induces interferon-regulated gene expression and overexpression of methylation-sensitive genes similar to lupus patients. Genes Immun. 2008;9:368–378. doi: 10.1038/gene.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gorelik GJ, Yarlagadda S, Richardson BC. Protein kinase Cδ oxidation contributes to ERK inactivation in lupus T cells. Arthritis Rheum. 2012;64:2964–2974. doi: 10.1002/art.34503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anstee QM, Day CP. S-adenosylmethionine (SAMe) therapy in liver disease: A review of current evidence and clinical utility. J Hepatol. 2012;57:1097–1109. doi: 10.1016/j.jhep.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 74.Imagawa K, et al. The epigenetic effect of glucosamine and a nuclear factor-κ B (NF-κB) inhibitor on primary human chondrocytes— Implications for osteoarthritis. Biochem Biophys Res Commun. 2011;405:362–367. doi: 10.1016/j.bbrc.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomasoni R, et al. Rapamycin-sensitive signals control TCR/CD28-driven Ifng, Il4 and Foxp3 transcription and promoter region methylation. Eur J Immunol. 2011;41:2086–2096. doi: 10.1002/eji.201041130. [DOI] [PubMed] [Google Scholar]
- 76.Yao H, Rahman I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem Pharmacol. 2012;84:1332–1339. doi: 10.1016/j.bcp.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111:539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimazu T, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2012;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 80.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor-κB. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Vaughn SE, Kottyan LC, Munroe ME, Harley JB. Genetic susceptibility to lupus: the biological basis of genetic risk found in B cell signaling pathways. J Leuk Biol. 2012;92:577–591. doi: 10.1189/jlb.0212095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol Cell Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 83.Carballo M, et al. Oxidative stress triggers STAT3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J Biol Chem. 1999;274:17580–17586. doi: 10.1074/jbc.274.25.17580. [DOI] [PubMed] [Google Scholar]
- 84.Wegrzyn J, et al. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–797. doi: 10.1126/science.1164551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harada T, et al. Increased expression of STAT3 in SLE T cells contributes to enhanced chemokine-mediated cell migration. Autoimmunity. 2007;40:1–8. doi: 10.1080/08916930601095148. [DOI] [PubMed] [Google Scholar]
- 86.Wu T, et al. Shared signaling networks active in B cells isolated from genetically distinct mouse models of lupus. J Clin Invest. 2007;117:2186–2196. doi: 10.1172/JCI30398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Beaucoudrey L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chiang PH, et al. mechanistic insights into impaired dendritic cell function by rapamycin: inhibition of Jak2/Stat4 signaling pathway. J Immunol. 2004;172:1355–1363. doi: 10.4049/jimmunol.172.3.1355. [DOI] [PubMed] [Google Scholar]
- 89.Delgoffe GM, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–304. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci USA. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nambiar MP, et al. Oxidative stress is involved in the heat stress-induced downregulation of TCR ζ chain expression and TCR/CD3-mediated [Ca2+]i response in human T-lymphocytes. Cell Immunol. 2002;215:151–161. doi: 10.1016/s0008-8749(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 92.Fernandez D, Bonilla E, Mirza N, Perl A. Rapamycin reduces disease activity and normalizes T-cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Banki K, Hutter E, Gonchoroff N, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 94.Sunahori K, Juang YT, Tsokos GC. Methylation status of CpG islands flanking a cAMP response element motif on the protein phosphatase 2Acα promoter determines CREB binding and activity. J Immunol. 2009;182:1500–1508. doi: 10.4049/jimmunol.182.3.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Juang YT, et al. PP2A dephosphorylates Elf-1 and determines the expression of CD3ζ and FcRγ in human systemic lupus erythematosus T cells. J Immunol. 2008;181:3658–3664. doi: 10.4049/jimmunol.181.5.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Passam FH, Giannakopoulos B, Mirarabshahi P, Krilis SA. Molecular pathophysiology of the antiphospholipid syndrome: The role of oxidative post-translational modification of β 2 glycoprotein I. J Thromb Haemost. 2011;9:275–282. doi: 10.1111/j.1538-7836.2011.04301.x. [DOI] [PubMed] [Google Scholar]
- 97.Otaki N, et al. Identification of a lipid peroxidation product as the source of oxidation-specific epitopes recognized by anti-DNA autoantibodies. J Biol Chem. 2010;285:33834–33842. doi: 10.1074/jbc.M110.165175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nienhuis HLA, et al. AGE and their receptor RAGE in systemic autoimmune diseases: An inflammation propagating factor contributing to accelerated atherosclerosis. Autoimmunity. 2009;42:302–304. doi: 10.1080/08916930902831746. [DOI] [PubMed] [Google Scholar]
- 100.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE—mechanisms and management. Nat Rev Rheumatol. 2012;8:214–223. doi: 10.1038/nrrheum.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Khatoon F, Moinuddin, Alam K, Ali A. Physicochemical and immunological studies on 4-hydroxynonenal modified HSA: Implications of protein damage by lipid peroxidation products in the etiopathogenesis of SLE. Hum Immunol. 2012;73:1132–1139. doi: 10.1016/j.humimm.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Wang G, Pierangeli SS, Papalardo E, Ansari GAS, Khan MF. Markers of oxidative and nitrosative stress in systemic lupus erythematosus: Correlation with disease activity. Arthritis Rheum. 2010;62:2064–2072. doi: 10.1002/art.27442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Scofield RH, et al. Modification of lupus-associated 60-kDa Ro protein with the lipid oxidation product 4-hydroxy-2-nonenal increases antigenicity and facilitates epitope spreading. Free Rad Biol Med. 2005;38:719–728. doi: 10.1016/j.freeradbiomed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 104.Ioannou Y, et al. Naturally occurring free thiols within β2-glycoprotein I in vivo: nitrosylation, redox modification by endothelial cells, and regulation of oxidative stress-induced cell injury. Blood. 2010;116:1961–1970. doi: 10.1182/blood-2009-04-215335. [DOI] [PubMed] [Google Scholar]
- 105.Balasubramanian K, Schroit AJ. Characterization of phosphatidylserine-dependent β-2-glycoprotein I macrophage Interactions: implications for apoptotic cell clearance by phagocytes. J Biol Chem. 1998;273:29272–29277. doi: 10.1074/jbc.273.44.29272. [DOI] [PubMed] [Google Scholar]
- 106.Sinicato NA, da Silva Cardoso PA, Appenzeller S. Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev. 2013;9:15–19. doi: 10.2174/157340313805076304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dwivedi J, Sarkar PD. Study of homocysteine, lipoprotein (a), lipid profile with oxidative stress in nephrotic syndrome and lupus nephritis. Res J Pharm Biol Chem Sci. 2010;1:670–679. [Google Scholar]
- 108.Moroni G, et al. Oxidative stress and homocysteine metabolism in patients with lupus nephritis. Lupus. 2010;19:65–72. doi: 10.1177/0961203309346906. [DOI] [PubMed] [Google Scholar]
- 109.Shao X, et al. Inducible expression of kallikrein in renal tubular cells protects mice against spontaneous lupus nephritis. Arthritis Rheum. 2013;65:780–791. doi: 10.1002/art.37798. [DOI] [PubMed] [Google Scholar]
- 110.Suwannaroj S, Lagoo A, Keisler D, McMurray RW. Antioxidants suppress mortality in the female NZB x NZW F1 mouse model of systemic lupus erythematosus (SLE) Lupus. 2001;10:258–265. doi: 10.1191/096120301680416940. [DOI] [PubMed] [Google Scholar]
- 111.Bethunaickan R, et al. Anti-tumor necrosis factor α treatment of interferon- α-induced murine lupus nephritis reduces the renal macrophage response but does not alter glomerular immune complex formation. Arthritis Rheum. 2012;64:3399–3408. doi: 10.1002/art.34553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mathis KW, et al. Oxidative stress promotes hypertension and albuminuria during the autoimmune disease systemic lupus erythematosus. Hypertension. 2012;59:673–679. doi: 10.1161/HYPERTENSIONAHA.111.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feng X, et al. ApoE−/ −Fas−/ − C57BL/6 mice: a novel murine model simultaneously exhibits lupus nephritis, atherosclerosis, and osteopenia. J Lipid Res. 2007;48:794–805. doi: 10.1194/jlr.M600512-JLR200. [DOI] [PubMed] [Google Scholar]
- 114.Manzi S, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 115.Viigimaa M, et al. Malondialdehyde-modified low-density lipoproteins as biomarker for atherosclerosis. Blood Press. 2010;19:164–168. doi: 10.3109/08037051.2010.484158. [DOI] [PubMed] [Google Scholar]
- 116.Ryan MJ. The pathophysiology of hypertension in systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1258–R1267. doi: 10.1152/ajpregu.90864.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yilmaz S, et al. Association between serum total antioxidant status and coronary microvascular functions in patients with SLE. Echocardiography. 2012;29:1218–1223. doi: 10.1111/j.1540-8175.2012.01797.x. [DOI] [PubMed] [Google Scholar]
- 118.Avalos I, et al. Oxidative stress in systemic lupus erythematosus: Relationship to disease activity and symptoms. Lupus. 2007;16:195–200. doi: 10.1177/0961203306075802. [DOI] [PubMed] [Google Scholar]
- 119.Ioannou Y, et al. Novel assays of thrombogenic pathogenicity in the antiphospholipid syndrome based on the detection of molecular oxidative modification of the major autoantigen β2-glycoprotein I. Arthritis Rheum. 2011;63:2774–2782. doi: 10.1002/art.30383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gilkeson G, et al. Correlation of serum measures of nitric oxide production with lupus disease activity. J Rheumatol. 1999;26:318–324. [PubMed] [Google Scholar]
- 121.Leitinger N. The role of phospholipid oxidation products in inflammatory and autoimmune diseases: evidence from animal models and in humans. Subcell Biochem. 2008;49:325–350. doi: 10.1007/978-1-4020-8830-8_12. [DOI] [PubMed] [Google Scholar]
- 122.Bergamo P, Maurano F, Rossi M. Phase 2 enzyme induction by conjugated linoleic acid improves lupus-associated oxidative stress. Free Rad Biol Med. 2007;43:71–79. doi: 10.1016/j.freeradbiomed.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 123.Kinscherf R, et al. Cholesterol levels linked to abnormal plasma thiol concentrations and thiol/disulfide redox status in hyperlipidemic subjects. Free Rad Biol Med. 2003;35:1286–1292. doi: 10.1016/j.freeradbiomed.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 124.Costenbader KH, Kang JH, Karlson EW. Antioxidant intake and risks of rheumatoid arthritis and systemic lupus erythematosus in women. Am J Epidemiol. 2010;172:205–216. doi: 10.1093/aje/kwq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tam LS, et al. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: A double blind, placebo controlled pilot study. J Rheumatol. 2005;32:275–282. [PubMed] [Google Scholar]
- 126.Puskas F, Gergely P, Banki K, Perl A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 2000;14:1352–1361. doi: 10.1096/fj.14.10.1352. [DOI] [PubMed] [Google Scholar]
- 127.Cervera R, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82:299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 128.Montero AJ, Jassem J. Cellular redox pathways as a therapeutic target in the treatment of cancer. Drugs. 2011;71:1385–1396. doi: 10.2165/11592590-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 129.Hiepe F, et al. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7:170–178. doi: 10.1038/nrrheum.2011.1. [DOI] [PubMed] [Google Scholar]
- 130.Engel P, Gomez-Puerta JA, Ramos-Casals M, Lozano F, Bosch X. Therapeutic targeting of B cells for rheumatic autoimmune diseases. Pharmacol Rev. 2011;63:127–156. doi: 10.1124/pr.109.002006. [DOI] [PubMed] [Google Scholar]
- 131.Pisetsky DS, Vrabie IA. Antibodies to DNA: Infection or genetics? Lupus. 2009;18:1176–1180. doi: 10.1177/0961203309106492. [DOI] [PubMed] [Google Scholar]
- 132.Sorescu D, et al. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 133.Barry-Lane PA, et al. p47phox is required for atherosclerotic lesion progression in ApoE−/ − mice. J Clin Invest. 2001;108:1513–1522. doi: 10.1172/JCI11927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A. Glutathione levels and sensitivity to apoptosis are regulated by changes in transaldolase expression. J Biol Chem. 1996;271:32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]