Abstract
De novo genetic variation is an important class of risk factors for autism spectrum disorder (ASD). Recently, whole exome sequencing of ASD families has identified a novel de novo missense mutation in the human dopamine (DA) transporter (hDAT) gene, which results in a Thr to Met substitution at site 356 (hDAT T356M). The dopamine transporter (DAT) is a presynaptic membrane protein that regulates dopaminergic tone in the central nervous system by mediating the high-affinity re-uptake of synaptically released DA, making it a crucial regulator of DA homeostasis. Here, we report the first functional, structural, and behavioral characterization of an ASD-associated de novo mutation in the hDAT. We demonstrate that the hDAT T356M displays anomalous function, characterized as a persistent reverse transport of DA (substrate efflux). Importantly, in the bacterial homolog leucine transporter, substitution of A289 (the homologous site to T356) with a Met promotes an outward-facing conformation upon substrate binding. In the substrate-bound state, an outward-facing transporter conformation is a required for substrate efflux. In Drosophila melanogaster, expression of hDAT T356M in DA neurons lacking Drosophila DAT leads to hyperlocomotion, a trait associated with DA dysfunction and ASD. Taken together, our findings demonstrate that alterations in DA homeostasis, mediated by aberrant DAT function, may confer risk for ASD and related neuropsychiatric conditions.
Keywords: dopamine, transporter, autism spectrum disorder, anomalous dopamine efflux, de novo mutation, Drosophila melanogaster
INTRODUCTION
Genetic factors have been implicated as important components in the etiology of autism spectrum disorder (ASD). It is now accepted that rare genetic variation affecting single nucleotides of protein-coding DNA, as well as rare genomic copy number variants (CNVs), are significant ASD risk factors1–4. In particular, increasing evidence suggests that de novo genetic variation is a risk factor in ASD and other neuropsychiatric diseases1, 3, 5–7. Several groups have conducted whole exome sequencing (WES) on ASD families, and collectively, these studies indicate that discrete de novo mutation (single nucleotide variation (SNV) or small indels) contribute to the overall genetic risk of ASD2, 8–10. Among these variations is the first ASD-associated, de novo mutation found in the human dopamine (DA) transporter (hDAT) gene (SLC6A3)8. This mutation results in a Thr to Met substitution at position 356 (hDAT T356M). The consequences of this de novo mutation and its impact on DA neurotransmission have yet to be elucidated.
The neurotransmitter DA plays an important role in the central nervous system by regulating a variety of functions, including motor activity, motivation, attention, and reward11–14. Disrupted DA function is implicated in a number of neuropsychiatric disorders, including bipolar disorder, schizophrenia, attention-deficit hyperactivity disorder (ADHD)15–17 and, more recently, ASD18–27. The dopamine transporter (DAT) is a presynaptic membrane protein that regulates DA neurotransmission via the high-affinity reuptake of synaptically released DA28. It is the major molecular target of cocaine, amphetamine (AMPH; Adderall™), and methylphenidate (Ritalin™)29–33. Due to DAT’s role in DA neurotransmission, SLC6A3 variants have been a focus of genetic association studies linking the etiology of brain disorders to dysregulated DA neurotransmission34, 35. Recent studies have identified a rare, inherited, functional missense SLC6A3 variant, hDAT A559V (rs28364997)36, 37, that has been associated with ADHD, which is commonly comorbid in ASD subjects38–41. These studies point to a contribution of DAT genetic variants in complex brain disorders.
While the role of DA in ADHD has been established42, DA’s role in ASD is poorly understood43. Many individuals with ASD exhibit co-occurrence of ADHD symptoms (~20–45%)44–47. The comorbid nature of ADHD with ASD points to dysregulation of common signaling pathways (e.g. DA) as a mechanism underlying these neuropsychiatric disorders48.
Here, we characterized the first ASD-identified, de novo mutation in hDAT by presenting structural, functional, and behavioral analysis of this de novo variant. These results implicate altered DA homeostasis as a potential liability in ASD risk.
METHODS
Subjects and clinical assessment
Subjects from this family included the proband, both parents and unaffected sibling, who were recruited by the Boston Autism Consortium as described previously8,49. Clinical assessment followed standard research criteria for ASD diagnosis. The proband was classified as having a comparatively “narrow” diagnosis (as opposed to a “broader ASD”) based on diagnostic algorithms from the Autism Diagnostic Interview Revised (ADI-R)50 using criteria described by Risi et. al51, and classification resulting from the diagnostic algorithm of the Autism Diagnostic Observational Session (ADOS)52. Proband IQ was assessed at age 5 years, 9 months using the Wechsler Preschool and Primary Scale of Intelligence (WPPSI; Wechsler, D. (1967)). The Social Responsiveness Scale (SRS; Western Psychological Services) was performed on both parents to index the presence and severity of broader autism phenotype traits, followed by medical and family history provided by the biological mother.
SLC6A3 T356M de novo discovery
Methodological details and validation of the de novo mutation are published8. Briefly, DNA derived from whole blood of both parents and the probands was subjected to whole exome sequence analysis. The T356M variant, identified as a heterozygote in the proband and absent in both parents, was experimentally validated and confirmed to be a de novo mutation that does not appear in the unaffected sibling.
Cell culture and transfection
The GFP-hDAT-pCIHygro expression vectors containing either hDAT or hDAT T356M sequence were generated, confirmed and transiently transfected into Chinese hamster ovary cells using FuGENE-6 (Promega, Fitchburg, WI, USA). Assays were conducted 24–48 hours post transfection.
Amperometry and patch clamp electrophysiology
hDAT and hDAT T356M cells were plated at a density of ~30,000 per 35-mm culture dish and experiments conducted as previously described53, 54.
[3H]DA uptake
hDAT and hDAT T356M cells were seeded (50,000 cells/well) into polyornithine coated, 24-well plates, 48 hours before assaying. Uptake kinetic assays were performed as described in the supplementary information of Rickhag et al.55 and in Rasmussen et al.56.
AMPH uptake
Plated hDAT and hDAT T356M cells were washed with KRH buffer and incubated for 5 min at 37°C with 10 nM AMPH. Cells were washed three times with ice-cold KRH. AMPH was quantified using a HPLC system previously described57.
Cell surface biotinylation and western blot
For cell surface biotinylation assays and Western blots, hDAT and hDAT T356M cells were cultured in 6-well plates and experiments conducted as in Mazei-Robinson et al.54.
Homology Modeling of hDAT and construction of the T356M simulation system
As the template, the homology model uses the known crystal structure for the cognate and homologous structure of the recent outward-open crystal structure of LeuT58. The substrate DA, two Na+ ions and a Cl− ion were positioned as described in Shan et al.59. To model the mutant hDAT construct with T356M, the mutation was introduced using the free energy perturbation (FEP) method60.
Double Electron Electron Resonance
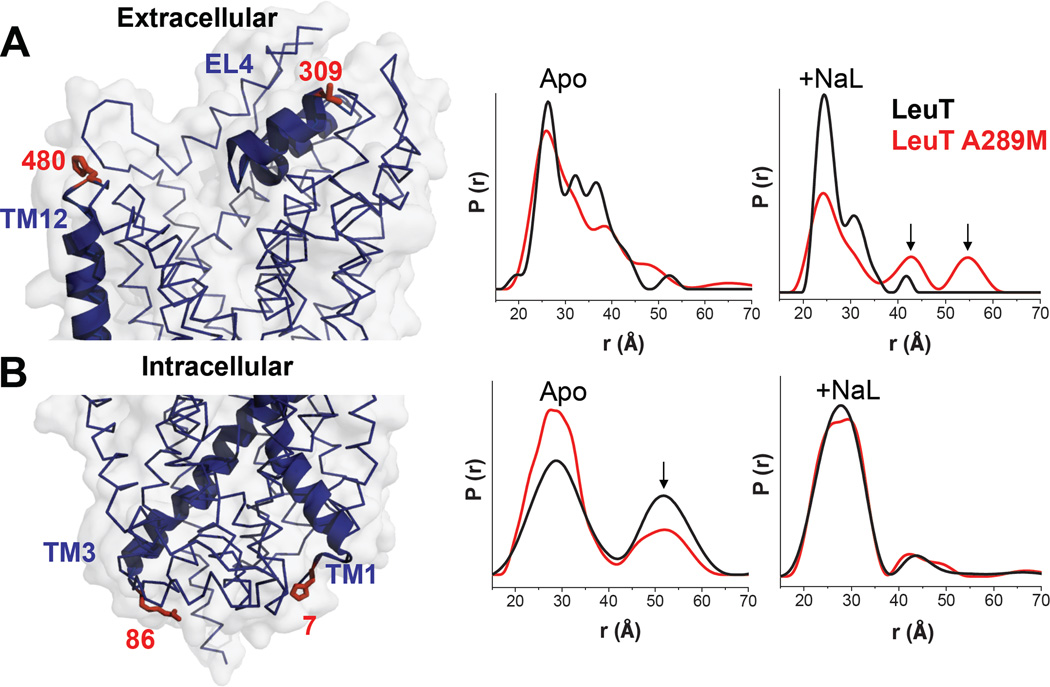
Cysteine residues were introduced using site directed mutagenesis into LeuT and LeuT A289M constructs61. Experiments were conducted as in Claxton et al.61. In Figure 4, Apo refers to ion Na+ and leucine-free transporter while the +NaL state was obtained by addition of 200 mM NaCl and 4-fold molar excess of Leu relative to LeuT. Double Electron Electron Resonance (DEER)62 was performed at 83K on a Bruker 580 pulsed EPR spectrometer operating at Q-band frequency using a standard 4-pulse sequence63. DEER echo decays were analyzed to obtain distance distributions64.
Figure 4. In LeuT, substitution of Ala289 with a Met supports an outward-open facing conformation.
Distance distributions of extracellular and intracellular spin labeled Cys pairs in LeuT reveal changes in the conformational equilibrium caused by mutating Ala289 to a Met. A) Left: extracellular reporter pairs (309–480) tagged on three-dimensional structure of LeuT. Right: distance of the extracellular reporter pair for LeuT (black) and A289M (red), in the Apo conformation (Apo) and in the presence of Na+ and Leu (+NaL). B) Left: intracellular reporter pairs (7–86) tagged on the three-dimensional structure of LeuT. Right: distance of the intracellular reporter pair for LeuT (black) and A289M (red), in the Apo conformation (Apo) and in the presence of Na+ and Leu (+NaL). The LeuT structure was obtained from PDB 2A65. The structures were generated using PyMOL.
Drosophila Genetics
Drosophila homozygotes for the DAT null allele DATfmn (dDAT KO)65 and flies harboring TH-Gal466 were outcrossed to a control line (Bloomington Indiana (BI) 6326) and selected by PCR or eye color. TH-GAL4 (Bl 8848) and M{vas-int.Dm}ZH-2A, M{3xP3-RFP.attP'} ZH-22A (Bl 24481) were obtained from the BI stock center and outcrossed to dDAT KO flies carrying the white (w1118) mutation (BI stock number 6236) for 5–10 generations. Transgenes (hDAT or hDAT T356M) were cloned into pBI-UASC67 and constructs were injected into embryos from M{vas-int.Dm}ZH-2A, M{3xP3-RFP.attP'}ZH-22A (Bl 24481). Initial potential transformants were isolated and selected.
Behavioral Analysis
Three days post eclosion male Drosophila were collected and placed into tubes with food for 72 hours. Locomotion was recorded by beam breaks and analyzed using equipment/software from Trikinetics (www.trikinetics.com). For the AMPH-induced locomotion, males were starved for six hours and then fed sucrose (5 mM) containing either AMPH (10 mM) or vehicle. Data were analyzed by One-way ANOVA followed by a Newman-Keuls Multiple Comparison Post-test.
RESULTS
T356M de novo DAT variant has impaired function
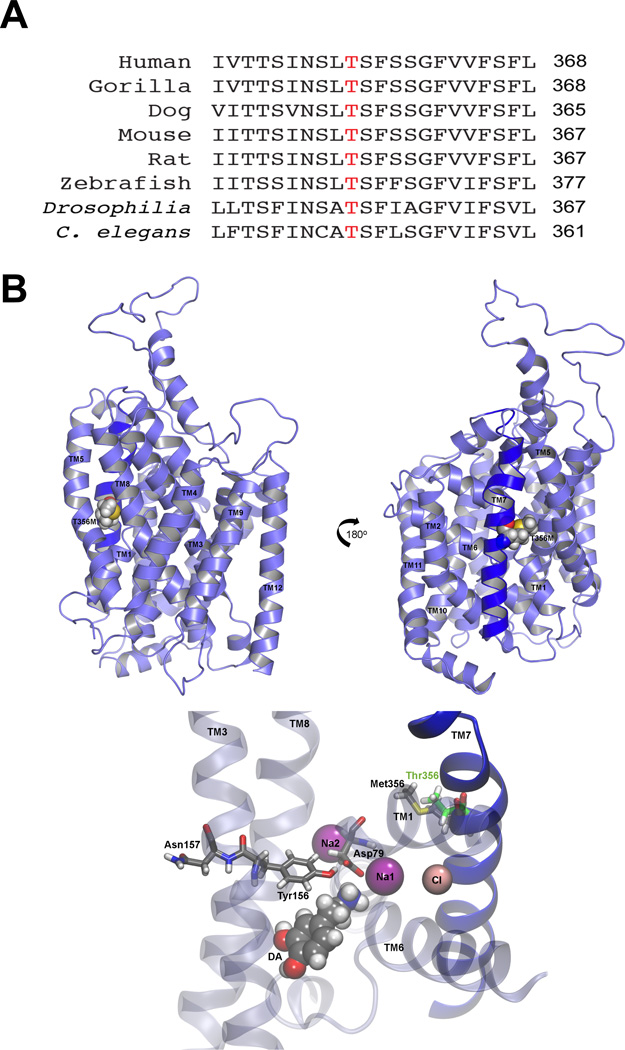
A recent study assessed the role of de novo variation in ASD by using WES in 175 ASD parent-child trios8. In this study, a de novo SLC6A3 variant was identified, resulting in a Thr to Met substitution at site 3568. Given the rarity of non-synonymous de novo events, it is not surprising that this mutation was absent in the ~1,000 unrelated ASD cases and controls8 and has not been deposited in 1,000 genomes68, dbSNP (build 137)69, or the NHLBI Exome Sequencing Project. The subject carried no other coding de novo mutations. The T356 is completely conserved across several species (Fig. 1A). Importantly, the T356 residue is located in the seventh transmembrane domain of hDAT and resides in a highly conserved region implicated in ion binding70. The molecular modeling of hDAT and in silico mutagenesis of T356 is shown in Figure 1B.
Figure 1. Cross-species conservation and in silico mutagenesis of T356.
A) Alignment of the DAT amino acid sequence across multiple species. Threonine 356 is represented in red. B) In an equilibrated three-dimensional homology model of hDAT, the T356M mutation is located on transmembrane domain 7 (TM7). Top: Schematic views representing a 180° rotation show T356M with respect to the TM helices. TM7 is shown in dark blue. Bottom: Critical residues that interact with DA79, 80 are shown, as well as the bound Na+ and Cl− ions. The methionine is rendered together at position 356 with the wild type threonine (green).
The subject harboring this de novo mutation is the elder male child of healthy nonconsanguineous Caucasian parents (proband has a healthy younger sibling). There is no immediate family history of ASD or related psychiatric conditions. The subject has a normal IQ (94) and has no history of seizures or other co-morbidites. By the age of 6 years-old, the proband was diagnosed with ASD (see supplemental material for full clinical reports).
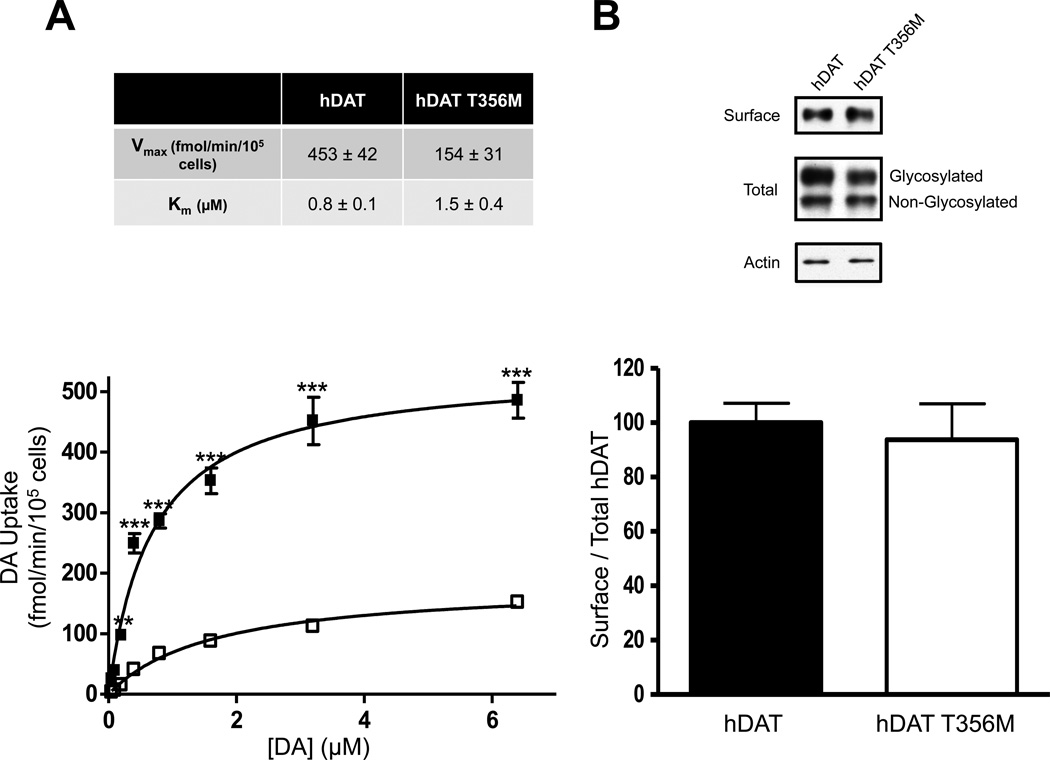
To evaluate whether the T356M variant may be a risk factor in the proband’s ASD, we compared the activities of hDAT and hDAT T356M in a heterologous expression system. We examined radioactive [3H]DA uptake and affinity. In hDAT T356M cells, the maximal velocity of DA influx (Vmax) was significantly reduced, whereas the apparent DA affinity (Km) of hDAT T356M was not significantly different from that of hDAT (Fig. 2A, top). A representative plot of DA uptake kinetics for hDAT and hDAT T356M is shown in Fig. 2A (bottom). The reduced [3H]DA transport capacity was not associated with a reduction in either total or DAT surface expression (Fig. 2B, top), as assessed by measuring changes in DAT proteins in the total and biotinylated fraction, respectively. The total fraction for hDAT and hDAT T356M contained both glycosylated and non-glycosylated forms of the DAT. Surface fractions were quantitated, normalized to total DAT (glycosylated), and expressed as a percent of hDAT (Fig. 2B; bottom). Furthermore, normalizing the total DAT fraction (glycosylated) to actin loading control yielded no significant differences between hDAT and hDAT T356M total expression (data are expressed as a percentage of hDAT; hDAT 100 ± 17.6% versus hDAT T356M 96.4 ± 13.7%; p ≥ 0.87 by Student’s t-test; n = 8–11).
Figure 2. Human dopamine transporter (hDAT) T356M has impaired function.
A) Top: Kinetic parameters (Vmax and Km) for hDAT and hDAT T356M (Vmax: p ≤ 0.005; by Student’s t-test; n = 3, in triplicate; Km: p ≥ 0.20; by Student’s t-test; n = 3, in triplicate). Bottom: Representative plot of [3H]DA uptake kinetics in hDAT (filled squares) or hDAT T356M (empty squares) cells (** = p ≤ 0.01, *** = p ≤ 0.001; by two-way ANOVA followed by Bonferroni posttest; n = 3, in triplicate). B) Representative immunoblots for biotinylated (surface) and total protein fractions from hDAT and hDAT T356M cells. Surface fractions were quantitated, normalized to total DAT (Glycosylated), and expressed as a percent of hDAT (p ≥ 0.05; by Student’s t-test; n = 8–11).
hDAT T356M displays ADE
Although hDAT T356M displays similar surface expression to that of hDAT, it demonstrates reduced ability to accumulate intracellular DA. One possibility is that constitutive DA efflux, here referred to as anomalous DA efflux (ADE), contributes to this reduced DA uptake in the hDAT T356M cells. This efflux would impede the intracellular accumulation of DA.
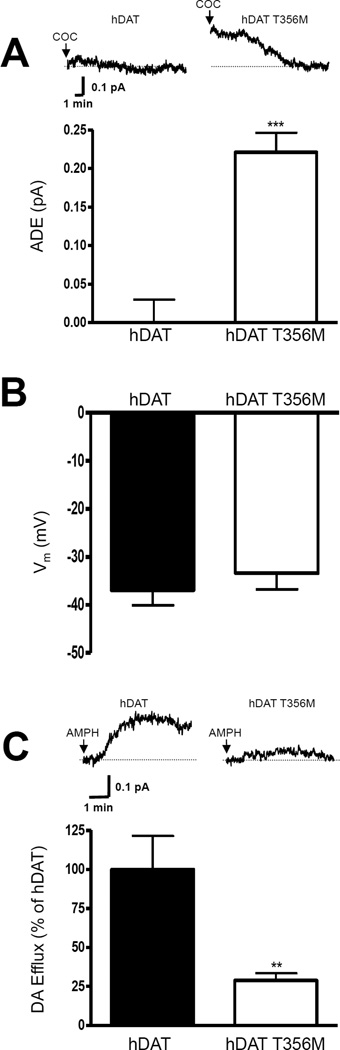
To determine whether hDAT T356M exhibits ADE, cells were whole cell patch clamped and perfused for 10 minutes with an internal solution containing 2 mM DA53. The electrode, in current clamp configuration, allows the cell to control its membrane voltage. In addition, this technique ensures that cells expressing either hDAT or hDAT T356M were equally loaded with DA. DA efflux was quantified through amperometry53. We have previously shown that, in the presence of ADE, cocaine decreases the amperometric signal through blockade of DAT54. In hDAT cells, amperometric currents were unaffected by application of cocaine (Fig 3A, top, hDAT, COC), indicating no ADE. In contrast, amperometric signals from hDAT T356M cells were significantly reduced by the application of cocaine (Fig. 3A, top, hDAT T356M, COC), revealing ADE. hDAT T356M-expressing cells displayed a significant increase in ADE, compared to hDAT transfected cells (Fig. 3A, bottom).
Figure 3. hDAT T356M exhibits robust ADE.
A) Top: representative amperometric currents recorded from hDAT and hDAT T356M cells. Arrows indicate application of 10 µM cocaine (COC). Bottom: quantitation of the cocaine-induced reduction in the amperometric current (ADE). Data are reported as maximal current (*** = p ≤ 0.001 by Student’s t-test; n = 4–5). B) De novo mutation in the hDAT gene causes DA dysfunction 25 hDAT T356M cells do not display altered resting membrane potential with respect to hDAT cells (p ≥ 0.05 by Student’s t-test, n = 9–25). C) Representative AMPH-induced amperometric currents recorded from hDAT and hDAT T356M cells. Arrows indicate application of 10 µM AMPH. Bottom: quantitation of AMPH-induced DA efflux. Data are represented as maximal current expressed as percent of the current recorded in hDAT cells (** = p ≤ 0.01 by Student’s t-test; n = 5–7).
Cell membrane depolarization has been shown to support DA efflux71. Figure 3B reveals that there is not a significant difference in resting membrane potential (measured in current clamp) between cells expressing hDAT or hDAT T356M. This indicates that differences in the function of hDAT and hDAT T356M are not due to resting membrane potential.
Next, we determined possible changes in the ability of AMPH to cause DA efflux by patch delivering DA into hDAT or hDAT T356M cells while recording DA efflux with amperometry. AMPH dose-response assays (measuring the peak of the amperometric current at different AMPH concentrations) revealed that hDAT T356M and hDAT cells have comparable AMPH EC50 (EC50; hDAT: 0.15 ± 0.05 µM; hDAT T356M: 0.16 ± 0.07 µM; n = 4; p ≥ 0.95 by Student’s t-test). Then, using a saturating AMPH concentration (10 µM) we determined DA efflux in either hDAT or hDAT T356M cells (Fig. 3C, top). AMPH-induced DA efflux was significantly reduced in hDAT T356M cells in comparison to hDAT cells (Fig. 3C). These results strongly suggest that ADE does not share common mechanisms with AMPH-induced DA efflux.
In LeuT, substitution of Ala289 with a Met promotes an outward-facing conformation
To investigate the structural consequences of the T356M de novo mutation in a DAT homolog with a known crystal structure, we analyzed changes in the conformational cycle of the leucine transporter (LeuT). We substituted A289 (the homologous amino acid to T356) with a Met (LeuT A289M). We measured distances between pairs of spin labels (r (Å)) and the distance distribution (P(r), the probability of a given distance between the two labels) monitoring the intraand extracellular gates by double electron electron resonance (DEER)72. First, we examined the pair 309/480 (Fig. 4A, left) that monitors the relative movement of the extracellular loop 4 (EL4) in LeuT. This loop obstructs the permeation pathway in the Apo conformation61, as indicated by the close proximity of the pair 309/480 (Fig. 4A, middle, Apo black line). Upon Na+ binding, the distance between EL4 and TM12 increases61, indicating opening of the extracellular vestibule and enabling substrate access (data not shown)61. Subsequent Leu binding resets the closed EL4 conformation in the occluded conformation of the transporter (Na+ and Leu bound in the vestibule) (Fig. 4A, right; +Na/L black line). The extracellular Apo state (Fig. 4A, middle; Apo; compare red and black lines) as well as Na+ bound state (data not shown) are similar in LeuT and LeuT A289M. Yet, LeuT A289M with Na+ and Leu bound in the vestibule has a destabilized bound structure with fluctuations on the extracellular side (Fig. 4A, right; +Na/L; compare red and black lines). The probability distribution in the Na/Leu bound state contains distinct populations of conformations that indicate fluctuations of LeuT A289M to a permeation pathway that has increased probability to be open to the outside, relative to LeuT (Fig. 4A, right; red line, arrows).
We then examined the pair 7/86 (Fig. 4B, left), to determine the distance between the N-terminus and intracellular loop 1 (IL1) and to monitor fluctuation dynamics on the intracellular side72. This is necessary to describe changes in the population of transporters with an inward facing conformation72. In the LeuT background, distance distributions between spin73 or fluorescent probes74 in the Apo state are bimodal, reflecting the equilibrium of this intracellular gate between closed and open conformations (Fig. 4B, middle; Apo; black lines). Introduction of the A289M leads to a shift in the equilibrium to favor the closed conformation side (Fig. 4B, middle; Apo; compare red and black lines, arrow). Na+ binding does not alter the equilibrium between the two conformations (data not shown), while Na+ and Leu binding resets this shift to LeuT-like conformations (Fig. 4B, middle; +Na/L; compare red and black lines).
Our results demonstrate that, because of the A289M, the presence of substrate and Na+ fails to completely close the extracellular pathway as in LeuT, inducing fluctuations on the extracellular side. These fluctuations to an open-to-the-outside permeation pathway persist, possibly enabling substrate release. This is in contrast to LeuT, where substrate binding closes the extracellular permeation pathway.
Drosophila expressing hDAT T356M in DA neurons are hyperactive
Locomotion is an elemental behavior regulated across species, including Drosophila melanogaster, by DA75–77. Thus, locomotion in flies offers a powerful model for elucidating the behavioral impact of ADE associated with hDAT T356M.
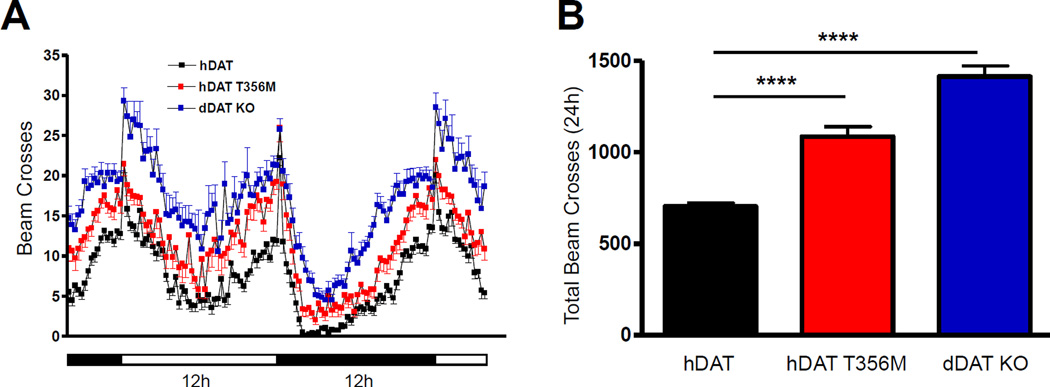
We expressed hDAT or hDAT T356M in flies homozygous for the Drosophila DAT null allele, DATfmn (dDAT KO)65, by using the Gal4/UAS system to express a single copy of hDAT or hDAT T356M in a dDATfmn mutant background, selectively in DA neurons78. To generate the transgenic flies, we used phiC31 based integration, which leads to the expression of comparable levels of mRNA for the relevant transgenes (hDAT or hDAT T356M). Locomotion was quantitated by beam crossing detection over a >24 hour period (data binned in 15 minute intervals) during both the light (horizontal white bar) and dark (horizontal black bar) cycle. While dDAT KO are hyperactive65, flies expressing hDAT in DA neurons have reduced locomotion as compared to dDAT KO, demonstrating the behavioral significance of our approach (Fig. 5A, compare hDAT to dDAT KO).
Figure 5. Expression of hDAT T356M in Drosophila leads to hyperactivity.
hDAT or hDAT T356M was expressed in DA neurons of dDAT KO flies. A) Locomotor activity was assayed over 32 hours during the light (horizontal white bars) or dark (horizontal black bars) cycle. Flies expressing hDAT T356M (red squares), as well as dDAT KO flies (blue squares), were hyperactive throughout the 32 hour period with respect to flies expressing wild type hDAT (black squares) (n = 32; beam breaks binned in 15 minute intervals). B) Quantitation of total beam crosses over 24 hours for hDAT, hDAT T356M, and dDAT KO flies (**** = p ≤ 0.0001; n = 32).
We hypothesized that flies harboring the hDAT T356M would be hyperactive with respect to hDAT expressing flies due to an increase in extracellular DA promoted by ADE. This is shown in Figure 5A. Figure 5B shows total (24 hour) locomotor activity in the different fly lines. Total activity (24 hours) of hDAT T356M and dDAT KO flies is significantly higher than hDAT flies (Fig. 5B).
hDAT T356M cells display compromised AMPH-induced DA efflux (Fig. 3C). This suggests a reduced ability of AMPH to increase locomotion in flies expressing hDAT T356M. Changes in locomotion were determined upon AMPH or vehicle exposure (15 minutes) and calculated as beam crosses. We observed no significant increase in locomotion in hDAT T356M flies when exposed to AMPH as compared to vehicle control (hDAT T356M (vehicle) 9.7 ± 0.7 beam breaks versus hDAT T356M (AMPH) 12.7 ± 1.6 beam breaks; n = 24; p ≥ 0.05). This is in contrast to flies expressing hDAT, where AMPH induced a significant increase in locomotion (hDAT (vehicle) 6.2 ± 0.9 beam breaks versus hDAT (AMPH) 18.2 ± 1.0 beam breaks; n = 24; p ≤ 0.001). Moreover, in the dDAT KO flies, similar to the hDAT T356M flies, AMPH failed to induce a significant increase in locomotion (data not shown).
DISCUSSION
Alterations in DA tone underlie multiple neuropsychiatric disorders, including bipolar disorder, schizophrenia, and ADHD15–17. With respect to ADHD, altered DA signaling, including changes in DAT function, may contribute to the cognitive and hyperactive traits of the disorder53, 54. ASD, like ADHD, is phenotypically and etiologically complex. However, there is mounting evidence that risk for ASD resides, at least in part, in dopaminergic factors. Variants in DA receptor (DRD) sub-type genes, including DRD1, DRD3, and DRD4, have been associated with increased risk for ASD21 as well as with specific phenotypic behavior within ASD. These include repetitive or stereotyped behaviors22–24, oppositional defiant disorder, and separation anxiety disorder24. Male children carrying four tandem repeats in the promoter region of the MAOA gene (the gene product responsible for degrading amine neurotransmitters, including DA) showed elevated risk for developing ASD27. It must be noted that none of these genes are significant in genome-wide association studies of ASD. However, in positron emission tomography (PET) studies in adults with ASD, DAT binding was significantly elevated in the orbitofrontal cortex18.
Genes harboring de novo events are highly significant for understanding the etiology of ASD8. This is not surprising since rate of reproduction is typically low in individuals with autism. Consequently, genetic variants would be subject to negative selection1. This suggests that the biological networks identified from these de novo events, and the broader pathways they function within, are candidate risk factors for ASD. In this study, we examine the functional, structural, and behavioral consequences of the first identified de novo hDAT missense variant associated with ASD.
Our amperometric recordings demonstrate that the de novo hDAT T356M mutation confers cocaine sensitive ADE. We also show that ADE does not share common mechanisms with AMPH-induced DA efflux since hDAT T356M has an impaired AMPH response. It is intriguing to speculate that anomalous transporter-mediated neurotransmitter efflux may be an unappreciated source of risk for mental illness, especially in disorders associated with altered DA signaling. It is possible that ADE, driven by DAT variants or variants in other genes in the DAT regulatory network (such as DRD subtypes), could impact risk for ASD. A similar point for ADE has been argued previously, in the context of ADHD, for the hDAT variant A559V, in its functional identification54 and original characterization53.
The question remains as to how the hDAT T356M de novo mutation perturbs transporter structure to trigger ADE. Since the crystal structure of hDAT is unavailable, we analyzed changes in the conformational cycle of the hDAT bacterial homolog, LeuT. In LeuT A289M, we measured the distances between pairs of spin labels monitoring the intra- and extracellular gates by DEER. The spin labels monitoring the extracellular gate clearly show that in LeuT A289M, in contrast to LeuT, the presence of Na+ and leucine promotes a permeation pathway unoccluded to the outside. In terms of transporter function, it is difficult to draw parallels between hDAT T356M and LeuT A289M. Nevertheless, it is compelling to speculate that the mechanism by which the substrate promotes an outward open conformation in LeuT A289M, could also support the ability of hDAT T356M to promote ADE when cytoplasmic DA is available. This would suggest that the mechanism of ADE for hDAT T356M is distinct from that of hDAT A559V, which is a result of a tonic activation of DRD2 and the downstream kinase CaMKII53. Thus, there may be multiple mechanistic routes to promote hDAT-mediated ADE, and yet ADE might support the comorbid nature of ASD with ADHD.
In an in vivo context, hDAT T356M may alter extracellular DA levels and, as a consequence, increase locomotion65. We selectively expressed hDAT T356M specifically in DA neurons of dDAT KO flies. Drosophila expressing hDAT T356M exhibited prominent hyperactivity as compared to Drosophila expressing hDAT. In addition, AMPH has an impaired ability to increase locomotion in hDAT T356M and dDAT KO flies. This might stem from the decreased ability of AMPH to cause DA efflux in hDAT T356M cells.
Here, we report novel and profound functional abnormalities associated with the hDAT de novo mutation T356M, resulting in enhancement of non-vesicular, DAT-dependent DA release, referred to as ADE. Our data raise the possibility that ADE could impact the risk for ASD.
Supplementary Material
Acknowledgements
We gratefully acknowledge investigators in the NIH ARRA Autism Sequencing Consortium: MJ Daly, RA Gibbs, E Boerwinkle, JD Buxbaum, EH Cook, B Devlin, ET Lim, BM Neale, K Roeder, A Sabo, GD Schellenberg, C Stevens and JS Sutcliffe; and programmatic support and contribution to the AASC project by T Lehner (NIMH), P Bender (NIMH) and A Felsenfeld (NHGRI). This work was supported by NSF Graduate Research Fellowship DGE0909667 and F31 DA 035535-01 (PJH), U54-GM087519 (SS and HSM), P50 HD055751 (EHC), DA13975 and P01 DA12408 (AG and UG), and MH089482 (JSS).
Abbreviations
- ASD
Autism spectrum disorder
- 5-hydroxytryptamine, DA
dopamine
- hDAT
human dopamine transporter
- AMPH
amphetamine
Footnotes
See acknowledgments.
Competing interests: The authors declare no competing interests.
REFERENCES
- 1.Devlin B, Scherer SW. Genetic architecture in autism spectrum disorder. Current opinion in genetics & development. 2012;22(3):229–237. doi: 10.1016/j.gde.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485(7397):237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70(5):863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466(7304):368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70(5):886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455(7215):919–923. doi: 10.1038/nature07458. [DOI] [PubMed] [Google Scholar]
- 8.Neale BM, Kou Y, Liu L, Ma'ayan A, Samocha KE, Sabo A, et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485(7397):242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485(7397):246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iossifov I, Ronemus M, Levy D, Wang Z, Hakker I, Rosenbaum J, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012;74(2):285–299. doi: 10.1016/j.neuron.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjorklund A, Dunnett SB. Fifty years of dopamine research. Trends in neurosciences. 2007;30(5):185–187. doi: 10.1016/j.tins.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends in pharmacological sciences. 1993;14(2):43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- 13.Carlsson A. Perspectives on the discovery of central monoaminergic neurotransmission. Annual review of neuroscience. 1987;10:19–40. doi: 10.1146/annurev.ne.10.030187.000315. [DOI] [PubMed] [Google Scholar]
- 14.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Annals of the New York Academy of Sciences. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeman P, Niznik HB. Dopamine receptors and transporters in Parkinson's disease and schizophrenia. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1990;4(10):2737–2744. doi: 10.1096/fasebj.4.10.2197154. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, et al. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Archives of general psychiatry. 2007;64(8):932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- 17.Cousins DA, Butts K, Young AH. The role of dopamine in bipolar disorder. Bipolar disorders. 2009;11(8):787–806. doi: 10.1111/j.1399-5618.2009.00760.x. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Archives of general psychiatry. 2010;67(1):59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- 19.Anderson BM, Schnetz-Boutaud N, Bartlett J, Wright HH, Abramson RK, Cuccaro ML, et al. Examination of association to autism of common genetic variationin genes related to dopamine. Autism research : official journal of the International Society for Autism Research. 2008;1(6):364–369. doi: 10.1002/aur.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gadow KD, Roohi J, DeVincent CJ, Hatchwell E. Association of ADHD, tics, and anxiety with dopamine transporter (DAT1) genotype in autism spectrum disorder. Journal of child psychology and psychiatry, and allied disciplines. 2008;49(12):1331–1338. doi: 10.1111/j.1469-7610.2008.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hettinger JA, Liu X, Schwartz CE, Michaelis RC, Holden JJ. A DRD1 haplotype is associated with risk for autism spectrum disorders in male-only affected sib-pair families. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2008;147B(5):628–636. doi: 10.1002/ajmg.b.30655. [DOI] [PubMed] [Google Scholar]
- 22.Staal WG, de Krom M, de Jonge MV. Brief report: the dopamine-3-receptor gene (DRD3) is associated with specific repetitive behavior in autism spectrum disorder (ASD) Journal of autism and developmental disorders. 2012;42(5):885–888. doi: 10.1007/s10803-011-1312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Krom M, Staal WG, Ophoff RA, Hendriks J, Buitelaar J, Franke B, et al. A common variant in DRD3 receptor is associated with autism spectrum disorder. Biological psychiatry. 2009;65(7):625–630. doi: 10.1016/j.biopsych.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 24.Gadow KD, Devincent CJ, Olvet DM, Pisarevskaya V, Hatchwell E. Association of DRD4 polymorphism with severity of oppositional defiant disorder, separation anxiety disorder and repetitive behaviors in children with autism spectrum disorder. The European journal of neuroscience. 2010;32(6):1058–1065. doi: 10.1111/j.1460-9568.2010.07382.x. [DOI] [PubMed] [Google Scholar]
- 25.Makkonen I, Kokki H, Kuikka J, Turpeinen U, Riikonen R. Effects of fluoxetine treatment on striatal dopamine transporter binding and cerebrospinal fluid insulin-like growth factor-1 in children with autism. Neuropediatrics. 2011;42(5):207–209. doi: 10.1055/s-0031-1291242. [DOI] [PubMed] [Google Scholar]
- 26.Nieminen-von Wendt TS, Metsahonkala L, Kulomaki TA, Aalto S, Autti TH, Vanhala R, et al. Increased presynaptic dopamine function in Asperger syndrome. Neuroreport. 2004;15(5):757–760. doi: 10.1097/00001756-200404090-00003. [DOI] [PubMed] [Google Scholar]
- 27.Tassone F, Qi L, Zhang W, Hansen RL, Pessah IN, Hertz-Picciotto I. MAOA, DBH and SLC6A4 variants in CHARGE: a case-control study of autism spectrum disorders. Autism research : official journal of the International Society for Autism Research. 2011;4(4):250–261. doi: 10.1002/aur.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, et al. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacological reviews. 2011;63(3):585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- 29.Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, et al. Amphetamine induces dopamine efflux through a dopamine transporter channel. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(9):3495–3500. doi: 10.1073/pnas.0407737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khoshbouei H, Sen N, Guptaroy B, Johnson L, Lund D, Gnegy ME, et al. N-terminal phosphorylation of the dopamine transporter is required for amphetamine-induced efflux. PLoS biology. 2004;2(3):E78. doi: 10.1371/journal.pbio.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379(6566):606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 32.Jones SR, Gainetdinov RR, Wightman RM, Caron MG. Mechanisms of amphetamine action revealed in mice lacking the dopamine transporter. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(6):1979–1986. doi: 10.1523/JNEUROSCI.18-06-01979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: a review. Progress in neurobiology. 2005;75(6):406–433. doi: 10.1016/j.pneurobio.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Kurian MA, Zhen J, Cheng SY, Li Y, Mordekar SR, Jardine P, et al. Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile parkinsonism-dystonia. The Journal of clinical investigation. 2009;119(6):1595–1603. doi: 10.1172/JCI39060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, et al. Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet neurology. 2011;10(1):54–62. doi: 10.1016/S1474-4422(10)70269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grunhage F, Schulze TG, Muller DJ, Lanczik M, Franzek E, Albus M, et al. Systematic screening for DNA sequence variation in the coding region of the human dopamine transporter gene (DAT1) Molecular psychiatry. 2000;5(3):275–282. doi: 10.1038/sj.mp.4000711. [DOI] [PubMed] [Google Scholar]
- 37.Mazei-Robison MS, Couch RS, Shelton RC, Stein MA, Blakely RD. Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology. 2005;49(6):724–736. doi: 10.1016/j.neuropharm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Ronald A, Simonoff E, Kuntsi J, Asherson P, Plomin R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. Journal of child psychology and psychiatry, and allied disciplines. 2008;49(5):535–542. doi: 10.1111/j.1469-7610.2007.01857.x. [DOI] [PubMed] [Google Scholar]
- 39.Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. Journal of autism and developmental disorders. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- 40.Roman T, Rohde LA, Hutz MH. Polymorphisms of the dopamine transporter gene: influence on response to methylphenidate in attention deficit-hyperactivity disorder. American journal of pharmacogenomics : genomics-related research in drug development and clinical practice. 2004;4(2):83–92. doi: 10.2165/00129785-200404020-00003. [DOI] [PubMed] [Google Scholar]
- 41.de Bruin EI, de Nijs PF, Verheij F, Hartman CA, Ferdinand RF. Multiple complex developmental disorder delineated from PDD-NOS. Journal of autism and developmental disorders. 2007;37(6):1181–1191. doi: 10.1007/s10803-006-0261-4. [DOI] [PubMed] [Google Scholar]
- 42.Nemoda Z, Szekely A, Sasvari-Szekely M. Psychopathological aspects of dopaminergic gene polymorphisms in adolescence and young adulthood. Neuroscience and biobehavioral reviews. 2011;35(8):1665–1686. doi: 10.1016/j.neubiorev.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. Journal of neurodevelopmental disorders. 2012;4(1):19. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein S, Schwebach AJ. The comorbidity of Pervasive Developmental Disorder and Attention Deficit Hyperactivity Disorder: results of a retrospective chart review. Journal of autism and developmental disorders. 2004;34(3):329–339. doi: 10.1023/b:jadd.0000029554.46570.68. [DOI] [PubMed] [Google Scholar]
- 45.Yoshida Y, Uchiyama T. The clinical necessity for assessing Attention Deficit/Hyperactivity Disorder (AD/HD) symptoms in children with high-functioning Pervasive Developmental Disorder (PDD) European child & adolescent psychiatry. 2004;13(5):307–314. doi: 10.1007/s00787-004-0391-1. [DOI] [PubMed] [Google Scholar]
- 46.Gadow KD, DeVincent CJ, Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. Journal of autism and developmental disorders. 2006;36(2):271–283. doi: 10.1007/s10803-005-0060-3. [DOI] [PubMed] [Google Scholar]
- 47.Sturm H, Fernell E, Gillberg C. Autism spectrum disorders in children with normal intellectual levels: associated impairments and subgroups. Developmental medicine and child neurology. 2004;46(7):444–447. doi: 10.1017/s0012162204000738. [DOI] [PubMed] [Google Scholar]
- 48.Di Martino A, Zuo XN, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, et al. Shared and Distinct Intrinsic Functional Network Centrality in Autism and Attention-Deficit/Hyperactivity Disorder. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolfson W. Boston Autism Consortium searches for genetic clues to autism's puzzle. Chemistry & biology. 2007;14(2):117–118. doi: 10.1016/j.chembiol.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 50.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 51.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(9):1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- 52.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 53.Bowton E, Saunders C, Erreger K, Sakrikar D, Matthies HJ, Sen N, et al. Dysregulation of dopamine transporters via dopamine D2 autoreceptors triggers anomalous dopamine efflux associated with attention-deficit hyperactivity disorder. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(17):6048–6057. doi: 10.1523/JNEUROSCI.5094-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, et al. Anomalous dopamine release associated with a human dopamine transporter coding variant. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(28):7040–7046. doi: 10.1523/JNEUROSCI.0473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rickhag M, Hansen FH, Sorensen G, Strandfelt KN, Andresen B, Gotfryd K, et al. A C-terminal PDZ domain-binding sequence is required for striatal distribution of the dopamine transporter. Nature communications. 2013;4:1580. doi: 10.1038/ncomms2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rasmussen TN, Plenge P, Bay T, Egebjerg J, Gether U. A single nucleotide polymorphism in the human serotonin transporter introduces a new site for N-linked glycosylation. Neuropharmacology. 2009;57(3):287–294. doi: 10.1016/j.neuropharm.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 57.Siuta MA, Robertson SD, Kocalis H, Saunders C, Gresch PJ, Khatri V, et al. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS biology. 2010;8(6):e1000393. doi: 10.1371/journal.pbio.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481(7382):469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shan J, Javitch JA, Shi L, Weinstein H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PloS one. 2011;6(1):e16350. doi: 10.1371/journal.pone.0016350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guptaroy B, Zhang M, Bowton E, Binda F, Shi L, Weinstein H, et al. A juxtamembrane mutation in the N terminus of the dopamine transporter induces preference for an inward-facing conformation. Molecular pharmacology. 2009;75(3):514–524. doi: 10.1124/mol.108.048744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Claxton DP, Quick M, Shi L, de Carvalho FD, Weinstein H, Javitch JA, et al. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nature structural & molecular biology. 2010;17(7):822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jeschke G, Polyhach Y. Distance measurements on spin-labelled biomacromolecules by pulsed electron paramagnetic resonance. Physical chemistry chemical physics : PCCP. 2007;9(16):1895–1910. doi: 10.1039/b614920k. [DOI] [PubMed] [Google Scholar]
- 63.Zou P, McHaourab HS. Increased sensitivity and extended range of distance measurements in spin-labeled membrane proteins: Q-band double electron-electron resonance and nanoscale bilayers. Biophysical journal. 2010;98(6):L18–L20. doi: 10.1016/j.bpj.2009.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeschke G, Koch A, Jonas U, Godt A. Direct conversion of EPR dipolar time evolution data to distance distributions. J Magn Reson. 2002;155(1):72–82. doi: 10.1006/jmre.2001.2498. [DOI] [PubMed] [Google Scholar]
- 65.Kume K, Kume S, Park SK, Hirsh J, Jackson FR. Dopamine is a regulator of arousal in the fruit fly. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(32):7377–7384. doi: 10.1523/JNEUROSCI.2048-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Friggi-Grelin F, Coulom H, Meller M, Gomez D, Hirsh J, Birman S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. Journal of neurobiology. 2003;54(4):618–627. doi: 10.1002/neu.10185. [DOI] [PubMed] [Google Scholar]
- 67.Wang JW, Beck ES, McCabe BD. A modular toolset for recombination transgenesis and neurogenetic analysis of Drosophila. PloS one. 2012;7(7):e42102. doi: 10.1371/journal.pone.0042102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM, Handsaker RE, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491(7422):56–65. doi: 10.1038/nature11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic acids research. 2001;29(1):308–311. doi: 10.1093/nar/29.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437(7056):215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 71.Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A. Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. The Journal of biological chemistry. 2003;278(14):12070–12077. doi: 10.1074/jbc.M212815200. [DOI] [PubMed] [Google Scholar]
- 72.McHaourab HS, Steed PR, Kazmier K. Toward the fourth dimension of membrane protein structure: insight into dynamics from spin-labeling EPR spectroscopy. Structure. 2011;19(11):1549–1561. doi: 10.1016/j.str.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McHaourab HS, Lin YL, Spiller BW. Crystal structure of an activated variant of small heat shock protein Hsp16.5. Biochemistry. 2012;51(25):5105–5112. doi: 10.1021/bi300525x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao Y, Terry DS, Shi L, Quick M, Weinstein H, Blanchard SC, et al. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2011;474(7349):109–113. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wicker-Thomas C, Hamann M. Interaction of dopamine, female pheromones, locomotion and sex behavior in Drosophila melanogaster. Journal of insect physiology. 2008;54(10–11):1423–1431. doi: 10.1016/j.jinsphys.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Pendleton RG, Rasheed A, Sardina T, Tully T, Hillman R. Effects of tyrosine hydroxylase mutants on locomotor activity in Drosophila: a study in functional genomics. Behavior genetics. 2002;32(2):89–94. doi: 10.1023/a:1015279221600. [DOI] [PubMed] [Google Scholar]
- 77.Pizzo AB, Karam CS, Zhang Y, Yano H, Freyberg RJ, Karam DS, et al. The membrane raft protein Flotillin-1 is essential in dopamine neurons for amphetamine-induced behavior in Drosophila. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Matthies HJ, Broadie K. Techniques to dissect cellular and subcellular function in the Drosophila nervous system. Methods in cell biology. 2003;71:195–265. doi: 10.1016/s0091-679x(03)01011-2. [DOI] [PubMed] [Google Scholar]
- 79.Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K, et al. The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nature neuroscience. 2008;11(7):780–789. doi: 10.1038/nn.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bisgaard H, Larsen MA, Mazier S, Beuming T, Newman AH, Weinstein H, et al. The binding sites for benztropines and dopamine in the dopamine transporter overlap. Neuropharmacology. 2011;60(1):182–190. doi: 10.1016/j.neuropharm.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.