Abstract
We cloned the Aspergillus fumigatus mannoprotein 2 (AFMP2) gene, which encodes a novel immunogenic protein (Afmp2p) of the antigenic mannoprotein superfamily, in A. fumigatus. Sequence analysis revealed that Afmp2p has 510 amino acid residues, with a predicted molecular mass of 51.5 kDa. Afmp2p has a putative N-terminal signal peptide, a putative C-terminal glycosylphosphatidylinositol membrane attachment signal sequence, and an upstream GAA cleavage site commonly used for cytoplasmic membrane attachment and implicated in fungal cell wall assembly. Upstream of the GAA cleavage site, Afmp2p contains a 302-amino-acid serine- and threonine-rich region as a site for potential O-glycosylation. Within this serine- and threonine-rich region, 13 repeats of ETSTPCE(T)n were observed. Western blot analysis of Afmp2p in A. fumigatus fungal cell lysate and culture supernatant and immunogold staining and electron microscopy showed that Afmp2p is predominantly secreted into the culture supernatant, whereas only minimal amounts can be detected in the cell lysate and cell wall. Finally, it was observed that patients with aspergilloma and invasive aspergillosis due to A. fumigatus develop a specific antibody response against recombinant Afmp2p. The abundance of Afmp2p in secreted form, its minimal cross-reactivity with Afmp1p, and the presence of an antibody response against Afmp2p in patients with A. fumigatus infections suggest that Afmp2p is a good candidate for complementing Afmp1p in serodiagnosis of A. fumigatus infections.
With advances in medicine such as the wider application of bone marrow and organ transplantation and use of potent cytotoxic and immunosuppressive agents, opportunistic pathogens, such as Aspergillus species, are increasingly reported as causes of infections in human beings. Aspergillus species seldom cause serious illnesses in immunocompetent hosts, except for mycetoma in patients with existing chronic lung diseases or traumatic inoculation with foreign bodies. However, invasive aspergillosis is one of the most important infectious causes of mortality in patients with hematological malignancies, bone marrow transplant recipients, and solid-organ transplant recipients, patients with chronic granulomatous disease, and patients with AIDS (7, 15). The mortality rate in patients with invasive aspergillosis with pulmonary involvement and persistent neutropenia is 95% (6).
The successful management of invasive aspergillosis is hampered by difficulties in establishing the microbiological diagnosis. The gold standard for diagnosis is a positive culture of Aspergillus and demonstration of mycelial invasion by histology in tissue biopsy specimens. However, the illness of these patients and the frequent presence of bleeding diathesis have often rendered tissue biopsy difficult. In 2001, we reported the cloning and characterization of a novel gene (AFMP1) which encodes a secretary galactomannoprotein (Afmp1p) in A. fumigatus, the commonest Aspergillus species causing aspergillosis in Western countries (13). Subsequently, in 2002, we reported the use of recombinant Afmp1p for serodiagnosis of A. fumigatus infections (4, 10). Recently, the homologous immunogenic protein (Aflmp1p) in A. flavus, the commonest Aspergillus species that causes aspergillosis in this locality and other parts of Asia, was also cloned (12). Furthermore, we have also used recombinant Afmp1p and Aflmp1p for detection of occult aspergillosis in patients with hemoptysis (5).
Although the use of recombinant Afmp1p for antibody detection had a very high sensitivity and specificity for serodiagnosis of aspergilloma caused by A. fumigatus, the sensitivities of antibody and antigen detection for patients with invasive aspergillosis caused by A. fumigatus were only 33 and 53%, respectively (4, 10). In this study, we report the cloning and characterization of AFMP2, a gene that encodes a homologous immunogenic protein (Afmp2p) in A. fumigatus. The potential of using this protein in combination with Afmp1p for serodiagnosis of aspergillosis is also discussed.
An A. fumigatus strain isolated from a bone marrow transplant recipient (UPN158) was used in this study. A 1-μl suspension of conidia obtained by flushing the surface of A. fumigatus colonies grown on Sabouraud agar at 37°C for 4 days was used to inoculate 25 ml of Czapek-Dox medium (Difco) in a 500-ml conical flask at 37°C in a gyratory shaker. A 2-day-old culture was harvested for genomic DNA extraction with the DNeasy Plant minikit (Qiagen, KJ Venlo, The Netherlands), and RNA extraction was done with the RNeasy Plant minikit (Qiagen), according to the manufacturer's instructions. cDNA was generated with the ThermoScript reverse transcription-PCR System (Invitrogen) according to the manufacturer's instructions.
By performing BLAST analysis against the A. fumigatus genome database with the National Center for Biotechnology Information server at the National Library of Medicine with the Afmp1p amino acid sequence, a homologous region other than AFMP1 (contig no. 4929, TIGR_5085) was found. The complete open reading frame of the region was determined with ORF Finder at the NCBI website and GENSCAN (http://genes.mit.edu/GENSCAN.html).
The complete open reading frame was amplified with the genomic DNA and cDNA of A. fumigatus as templates, with primers LPW378 (5′-ATGCGGTTCTCTGCGTTAACT-3′) and LPW379 (5′-TTACAGCAACAGTGCAAATGC-3′) (Invitrogen) designed from the sequence information of the A. fumigatus genome. The PCR mixture (100 μl) contained denatured A. fumigatus DNA, PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 2 mM MgCl2 and 0.01% gelatin), 200 μM each of the deoxynucleoside triphosphates, and 2.5 U of Taq polymerase (Perkin-Elmer Cetus). The sample was amplified for 40 cycles of 95°C for 1 min, 48°C for 1.5 min, and 72°C for 4 min, with a final extension at 72°C for 10 min in an automated thermal cycler (Perkin-Elmer Cetus, Gouda, The Netherlands). Both strands of the PCR products were sequenced twice with an ABI 377 automated sequencer according to the manufacturer's instructions (Perkin-Elmer), with the PCR primers LPW378 and LPW379 and additional sequencing primers LPW670 (5′-CTCGGGAATCACCTCGGC-3′) and LPW671 (5′-TGGAGGTTTCAGGAGGAGTA-3′).
To produce a fusion plasmid for protein purification, primers were used to amplify the AFMP2 gene from the cDNA of A. fumigatus. The sequence coding for amino acid residues 21 to 510 of Afmp2p was amplified and cloned into the BamHI and EcoRI sites of expression vector pGEX-2T in frame and downstream of the glutathione S-transferase (GST) coding sequence. The GST-Afmp2p fusion protein was expressed and purified with the GST Gene Fusion System (Amersham Biosciences, Buckinghamshire, United Kingdom) according to the manufacturer's instructions. Recombinant Afmp2p was harvested by thrombin digestion. Approximately 1 mg of purified protein was routinely obtained from 1 liter of Escherichia coli cells carrying the fusion plasmid.
To produce a polyclonal guinea pig antibody, 10 ml of mycelial sediment (approximately 100 mg), obtained from centrifugation of a 1-day-old culture of A. fumigatus, was washed three times in phosphate-buffered saline (PBS; 13.7 mM sodium chloride, 0.27 mM potassium chloride, 1 mM phosphate buffer [pH 7.4]) and suspended in PBS with 0.05% phenol at a turbidity of McFarland standard 3. An equal volume of complete Freund's adjuvant was mixed with 500 μl of mycelial suspension and injected intramuscularly into the thigh of a guinea pig. Incomplete Freund's adjuvant was used in subsequent immunizations, and a total of four inoculations were completed in 2 months.
To prepare specific antibodies against Afmp2p, 75 μg of purified Afmp2p recombinant protein (125 μl) was mixed with an equal part of complete Freund's adjuvant and injected into a rabbit subcutaneously and a guinea pig intramuscularly. Incomplete Freund's adjuvant was used in subsequent injections. Serum samples were taken 2 weeks after the third injection.
Recombinant Afmp2p and Afmp1p samples were run on an sodium dodecyl sulfate-8% polyacrylamide gel and electroblotted onto a nitrocellulose membrane (Bio-Rad). The blot was cut into strips, and the Afmp2p and Afmp1p strips were incubated with preimmune guinea pig serum and sera of guinea pigs immunized with A. fumigatus, Afmp1p (13), and Afmp2p. Moreover, the Afmp2p strips were incubated with sera from patients with culture-documented A. fumigatus aspergilloma, acute myeloid leukemia patients with culture-documented A. fumigatus invasive aspergillosis, patients with A. flavus aspergilloma, patients with Candida albicans fungemia, patients with Cryptococcus neoformans fungemia, patients with Penicillium marneffei infection, or healthy blood donors. All guinea pig sera were diluted at 1:4,000, and human sera were diluted at 1:500. Antigen-antibody interaction was detected with the ECL fluorescence kit (Amersham Biosciences, Buckinghamshire, United Kingdom).
For Western blot analysis of Afmp2p in A. fumigatus fungal cell lysate and culture supernatant, whole-cell A. fumigatus cell lysate was prepared by sonication of A. fumigatus culture. The supernatant of the A. fumigatus culture was concentrated with Centricon YM-10 (Millipore) according to the manufacturer's instructions. Cell lysate and concentrated culture supernatant were run on a sodium dodecyl sulfate-8% polyacrylamide gel and electroblotted onto a nitrocellulose membrane (Bio-Rad). The blot was cut into strips, and the strips were incubated with preimmune guinea pig serum, serum from a guinea pig immunized with Afmp1p diluted at 1:4,000, and serum from a guinea pig immunized with Afmp2p diluted at 1:4,000. Antigen-antibody interaction was detected with the ECL fluorescence kit (Amersham Biosciences).
For immunogold staining and electron microscopic examination, A. fumigatus hyphae were harvested and washed twice in PBS. The hyphae were fixed in filtered PBS containing 4% (wt/vol) paraformaldehyde and 2% (vol/vol) glutaraldehyde for 30 min at room temperature, followed by dehydration in a graded series of ethanol and embedding in LR White (Sigma). Ultrathin sections were cut and mounted onto 200-mesh gold grids for immunostaining.
For immunostaining, sections were blocked for 20 min in 3% (wt/vol) bovine serum albumin fraction V (BSA) (Sigma). Serum from rabbits immunized with Afmp1p or Afmp2p (diluted 1:80 in PBS with 3% BSA) was added and incubated with the cell sections for 2 h, with a preimmune rabbit serum as the negative control. After being washed in PBS containing 0.1% Tween 20, the sections were incubated in 1% TBSA (20 mM Tris [pH 8.2], 1% BSA) containing 1:20-diluted goat anti-rabbit immunoglobulin G conjugated with gold particles 10 nm in diameter (Amersham Biosciences). After being washed in 1% TBSA, the sections were counterstained with uranyl acetate and lead citrate. Electron microscopy was performed with an EM 208S transmission electron microscope.
Based on the sequence information of the homologous region of Afmp1p obtained from BLAST analysis, the complete open reading frame (AFMP2) was amplified with the genomic DNA and cDNA of A. fumigatus. Bidirectional DNA sequencing revealed that the cDNA contained a single open reading frame encoding 510 amino acid residues with a predicted molecular mass of 51.5 kDa. The nucleotide sequence of this region of the genomic DNA was the same as that of the cDNA, implying that AFMP2 has no introns.
The nucleotide sequence of the AFMP2 gene has been deposited with GenBank under accession no. AY460182.
Protein sequence (inferred from the DNA sequence) analysis revealed that this protein has several features that are similar to Mp1p of P. marneffei, Afmp1p of A. fumigatus, Aflmp1p of A. flavus, and Anmp1p of Aspergillus nidulans. Similar to Mp1p, Afmp1p, Aflmp1p, and Anmp1p, this protein has a putative N-terminal signal peptide found in most secretory proteins. It also has a putative C-terminal glycosylphosphatidylinositol membrane attachment signal sequence and an upstream GAA cleavage site commonly used for cytoplasmic membrane attachment and implicated in fungal cell wall assembly. Upstream of the GAA cleavage site, Afmp2p contains a 302-amino-acid serine- and threonine-rich region as a site for potential O-glycosylation. Within this serine- and threonine-rich region, 13 repeats of ETSTPCE(T)n were observed. However, unlike Mp1p, which contains two potential N-glycosylation sites, Afmp2p, Afmp1p, Aflmp1p, and Anmp1p do not contain any potential N-glycosylation sites. After processing, Afmp2p should have 490 amino acid residues with a predicted molecular mass of 49.5 kDa. The only region of Afmp2p homologous to Mp1p, Afmp1p, Aflmp1p, and Anmp1p is a 151-amino-acid-residue region situated between the signal peptide and the serine- and threonine-rich region. The gene was named AFMP2 (for Aspergillus fumigatus mannoprotein 2), and the protein was named Afmp2p.
To produce recombinant Afmp2p protein, the AFMP2 sequence minus the signal peptide (codon 21 to 510) was amplified by PCR and cloned in frame with GST in expression plasmid pGEX-2T. The Afmp2p protein was expressed in E. coli and subsequently purified. The purified Afmp2p was separated on a sodium dodecyl sulfate gel followed by Coomassie blue staining. After purification, a single band was visible on the gel at about 95 kDa. The size of the protein compared to the expected size could be due to intensive folding of the protein as a result of cross-linking of cysteine residues and/or dimer formation.
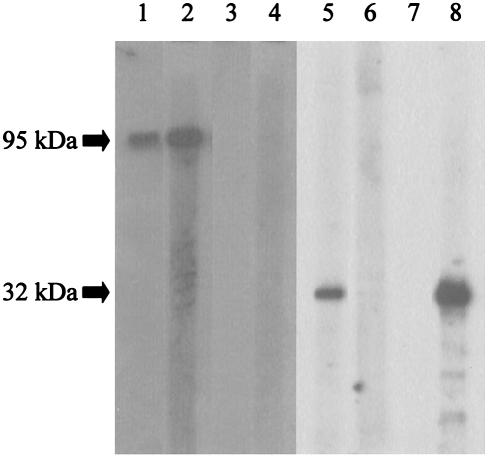
Western blot analysis showed that recombinant Afmp2p reacted strongly with serum from a guinea pig immunized with A. fumigatus (Fig. 1, lane 1) or Afmp2p (Fig. 1, lane 2), but not preimmune guinea pig serum (Fig. 1, lane 3) or Afmp1p (Fig. 1, lane 4). These results indicate that Afmp2p is highly immunogenic in guinea pigs, but there was minimal cross-reactivity between Afmp1p and Afmp2p.
FIG. 1.
Western blot analysis of purified Afmp2p and Afmp1p of A. fumigatus with guinea pig sera. When purified Afmp2p was used as the antigen, a strong antigen-antibody interaction was detected with serum from a guinea pig immunized with A. fumigatus (lane 1) or Afmp2p (lane 2), but not with preimmune guinea pig serum (lane 3) or serum from a guinea pig immunized with Afmp1p (lane 4). When purified Afmp1p was used as the antigen, a strong antigen-antibody interaction was detected with serum from a guinea pig immunized with A. fumigatus (lane 5) or Afmp1p (lane 8), but not with preimmune guinea pig serum (lane 7) or serum from a guinea pig immunized with Afmp2p (lane 6).
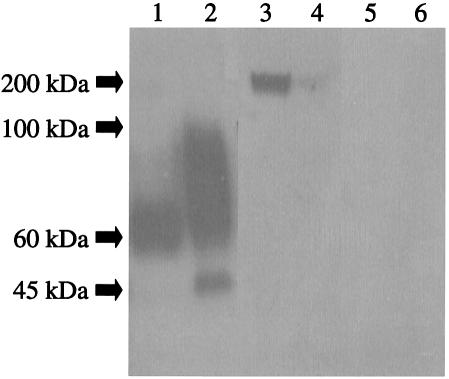
A band of about 60 kDa was detected when serum from a guinea pig immunized with Afmp1p reacted with concentrated culture supernatant (Fig. 2, lane 1). A band of about 45 kDa (probably due to core glycosylation) and a broad band of about 60 to 100 kDa (probably due to various forms of glycosylation) were detected when the serum reacted with sonicated fungal cell lysate (Fig. 2, lane 2). A band of about 200 kDa was detected when serum from a guinea pig immunized with Afmp2p reacted with concentrated culture supernatant (Fig. 2, lane 3), and a very faint band of the same size was detected when the serum reacted with sonicated fungal cell lysate (Fig. 2, lane 4). The apparently large size of the protein could be due to glycosylation, intensive folding of the protein as a result of cross-linking of cysteine residues, and/or multimerization of the protein.
FIG. 2.
Western blot analysis of Afmp1p and Afmp2p in sonicated fungal cell lysate and concentrated culture supernatant of A. fumigatus with guinea pig sera. A band of about 60 kDa was detected when serum from a guinea pig immunized with Afmp1p reacted with concentrated culture supernatant (lane 1). A band of about 45 kDa (probably due to core glycosylation) and a broad band of about 60 to 100 kDa (probably due to various forms of glycosylation) were detected when the serum reacted with sonicated fungal cell lysate (lane 2). A band of about 200 kDa was detected when serum from a guinea pig immunized with Afmp2p reacted with concentrated culture supernatant (lane 3), and a very faint band of the same size was detected when the serum reacted with sonicated fungal cell lysate (lane 4). No bands were detected with preimmune guinea pig serum (lanes 5 and 6).
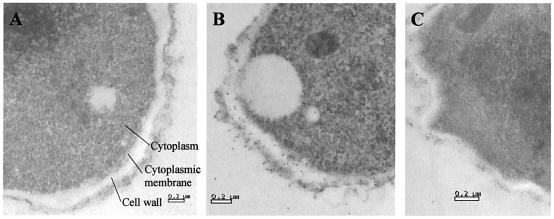
Electron micrographs demonstrated that Afmp2p was present in the cell wall of A. fumigatus hyphae (Fig. 3C), while preimmune rabbit serum showed no staining (Fig. 3A). However, the amount of Afmp2p was less than that of Afmp1p (Fig. 3B) in the fungal cell wall.
FIG. 3.
Immunoelectron microscopy of A. fumigatus hyphae stained with preimmune rabbit serum (A), rabbit anti-Afmp1p antibody (B), and rabbit anti-Afmp2p antibody (C). Magnification, ×28,000.
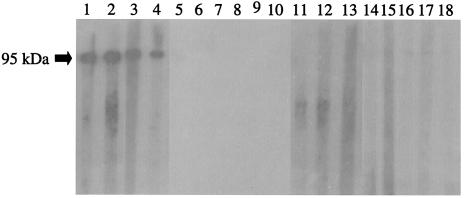
Western blot analysis showed that recombinant Afmp2p reacted specifically with sera from patients with aspergilloma or invasive aspergillosis (Fig. 4, lanes 1 to 4). No specific reaction was seen between Afmp2p and sera from either healthy blood donors (Fig. 4, lanes 5 to 10), patients with A. flavus aspergilloma (Fig. 4, lanes 11 and 12), patients with documented Candida albicans fungemia (Fig. 4, lanes 13 and 14), patients with documented Cryptococcus neoformans fungemia (Fig. 4, lanes 15 and 16), or patients with documented P. marneffei infections (Fig. 4, lanes 17 and 18).
FIG. 4.
Western blot analysis of purified Afmp2p of A. fumigatus with human sera. Strong antigen-antibody interaction was detected with the serum from two patients with aspergilloma (lanes 1 and 2) and two patients with invasive aspergillosis (lanes 3 and 4). Controls were sera from healthy blood donors (lanes 5 to 10) and from patients infected with A. flavus (lanes 11 and 12), Candida albicans (lanes 13 and 14), Cryptococcus neoformans (lanes 15 and 16), and P. marneffei (lanes 17 and 18).
In this study, we describe the cloning and characterization of AFMP2, which encodes a secreted antigenic protein, Afmp2p, in A. fumigatus. This protein showed significant homologies with similar secreted antigenic proteins in A. fumigatus (Afmp1p), A. flavus (Aflmp1p), A. nidulans (Anmp1p), and P. marneffei, a dimorphic fungus phylogenetically closely related to Aspergillus (Mp1p) (1, 11-14). We propose to name this family of proteins the antigenic mannoprotein superfamily. In contrast to Afmp1p, most Afmp2p is probably secreted rather than present in the fungal cell wall. Afmp1p is abundantly present in the cell lysate (Fig. 2, lane 2), cell wall (Fig. 3B), and culture supernatant (Fig. 2, lane 1) of A. fumigatus. On the other hand, with the same amounts of cell lysate and culture supernatant and in similar histological sections, Afmp2p is barely present in the cell lysate (Fig. 2, lane 4) and cell wall (Fig. 3C) of the fungus. On the other hand, it is abundantly present in the culture supernatant (Fig. 2, lane 3). These results are in line with those obtained from in silico analysis of the serine- and threonine-rich region in Afmp2p.
In 1986, Rogers et al. discovered that the amino acid sequences of 10 proteins with intracellular half-lives of less than 2 h contained one or more stretches of amino acids rich in proline (P), glutamic acid (E), serine (S), and threonine (T) (PEST-rich sequences) (9). Subsequently, numerous experiments have shown that these PEST regions serve as proteolytic signals and are associated with degradation of the corresponding proteins (8). In the analysis of Afmp2p, it was observed that there were 13 repeats of ETSTPCE(T)n in the serine- and threonine- rich region. With the PEST-FIND program (http://emb1.bcc.univie.ac.at/embnet/tools/bio/PESTfind/), the score for this region was +32.27, which is highly suggestive of a PEST-rich region (by definition, a score above zero denotes a possible PEST region, but a value greater than +5 sparks real interest) (8). This PEST-rich region may explain the relatively low levels of Afmp2p detected in the cell wall and cell lysate of A. fumigatus. The presence of Afmp2p predominantly in the culture supernatant suggests that the protein is mainly in the secreted form and a potential candidate for both antigen and antibody detection in A. fumigatus infections.
The sensitivities of serological tests for diagnosis of aspergillosis due to A. fumigatus may be improved by using a combination of recombinant Afmp1p and recombinant Afmp2p. In our previous study, we showed that the sensitivity of a recombinant Afmp1p-based enzyme-linked immunosorbent assay for serodiagnosis of invasive aspergillosis due to A. fumigatus was only about 33% (4). With a combination of Afmp1p detection and Afmp1p antibody detection, the sensitivity can be improved to about 87% (10). Since there was no cross-reactivity between Afmp1p and Afmp2p in animal experiments, as shown by a lack of antigen-antibody reaction when recombinant Afmp2p reacted with serum obtained from a guinea pig immunized with Afmp1p or when recombinant Afmp1p reacted with serum obtained from a guinea pig immunized with Afmp2p, we speculate that there would probably be minimal cross-reactivity between the two proteins in humans. Using a combination of the two proteins to coat enzyme-linked immunosorbent assay plates may improve the sensitivity of the corresponding assays.
Cloning of genes encoding the homologous proteins of the antigenic mannoprotein superfamily in related pathogenic fungi is important for serodiagnosis of the corresponding infections. We have shown clearly that Mp1p-based serological tests are useful for serodiagnosis of penicilliosis marneffei, and Afmp1p-, Aflmp1p-, and probably Afmp2p-based serological tests are useful for serodiagnosis of aspergilloma, invasive aspergillosis, and occult Aspergillus infections in patients with existing parenchymal lung diseases due to the corresponding fungi (2-5, 10). However, it has also been shown that there is minimal cross-reactivity among these proteins of the antigenic mannoprotein superfamily. Since about 28% of the invasive mold diseases in our bone marrow transplant recipients were caused by Aspergillus species other than A. fumigatus and A. flavus, such as A. niger and A. terreus (15), and it is probable that similar percentages of aspergilloma and occult Aspergillus infections are also due to Aspergillus species other than A. fumigatus and A. flavus, the development of serological tests against other Aspergillus species will increase the detection of the corresponding infections.
Acknowledgments
This work was partly supported by the AIDS Trust Fund (MSS 083), a Research Grant Council grant, and the University Development Fund, Hong Kong.
REFERENCES
- 1.Cao, L., C. M. Chan, C. Lee, S. S. Wong, and K. Y. Yuen. 1998. MP1 encodes an abundant and highly antigenic cell wall mannoprotein in the pathogenic fungus Penicillium marneffei. Infect. Immun. 66:966-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao, L., D. L. Chen, C. Lee, C. M. Chan, K. M. Chan, N. Vanittanakom, D. N. Tsang, and K. Y. Yuen. 1998. Detection of specific antibodies to an antigenic mannoprotein for diagnosis of Penicillium marneffei penicilliosis. J. Clin. Microbiol. 36:3028-3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao, L., K. M. Chan, D. Chen, N. Vanittanakom, C. Lee, C. M. Chan, T. Sirisanthana, D. N. Tsang, and K. Y. Yuen. 1999. Detection of cell wall mannoprotein Mp1p in culture supernatants of Penicillium marneffei and in sera of penicilliosis patients. J. Clin. Microbiol. 37:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan, C. M., P. C. Woo, A. S. Leung, S. K. Lau, X. Y. Che, L. Cao, and K. Y. Yuen. 2002. Detection of antibodies specific to an antigenic cell wall galactomannoprotein for serodiagnosis of Aspergillus fumigatus aspergillosis. J. Clin. Microbiol. 40:2041-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu, C. M., P. C. Woo, K. T. Chong, W. S. Leung, V. L. Chan, and K. Y. Yuen. 2004. Association of presence of Aspergillus antibodies with hemoptysis in patients with old tuberculosis or bronchiectasis but no radiologically visible mycetoma. J. Clin. Microbiol. 42:665-669. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Denning, D. W., and D. A. Stevens. 1990. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev. Infect. Dis. 12:1147-1201. [DOI] [PubMed] [Google Scholar]
- 7.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rechsteiner, M., and S. W. Rogers. 1996. PEST sequences and regulation by proteolysis. Trends Biochem. Sci. 21:267-271. [PubMed] [Google Scholar]
- 9.Rogers, S., R. Wells, and M. Rechsteiner. 1986. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234:364-368. [DOI] [PubMed] [Google Scholar]
- 10.Woo, P. C., C. M. Chan, A. S. Leung, S. K. Lau, X. Y. Che, S. S. Wong, L. Cao, and K. Y. Yuen. 2002. Detection of cell wall galactomannoprotein Afmp1p in culture supernatants of Aspergillus fumigatus and in sera of aspergillosis patients. J. Clin. Microbiol. 40:4382-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woo, P. C., H. Zhen, J. J. Cai, J. Yu, S. K. Lau, J. Wang, J. L. Teng, S. S. Wong, R. H. Tse, R. Chen, H. Yang, B. Liu, and K. Y. Yuen. 2003. The mitochondrial genome of the thermal dimorphic fungus Penicillium marneffei is more closely related to those of molds than yeasts. FEBS Lett. 555:469-477. [DOI] [PubMed] [Google Scholar]
- 12.Woo, P. C., K. T. Chong, A. S. Leung, S. S. Wong, S. K. Lau, and K. Y. Yuen. 2003. AFLMP1 encodes an antigenic cell wall protein in Aspergillus flavus. J. Clin. Microbiol. 41:845-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuen, K. Y., C. M. Chan, K. M. Chan, P. C. Woo, X. Y. Che, A. S. Leung, and L. Cao. 2001. Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis. J. Clin. Microbiol. 39:3830-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuen, K. Y., G. Pascal, S. S. Wong, P. Glaser, P. C. Woo, F. Kunst, J. J. Cai, E. Y. Cheung, C. Medigue, and A. Danchin. 2003. Exploring the Penicillium marneffei genome. Arch. Microbiol. 179:339-353. [DOI] [PubMed] [Google Scholar]
- 15.Yuen, K. Y., P. C. Woo, M. S. Ip, R. H. Liang, E. K. Chiu, H. Siau, P. L. Ho, F. F. Chen, and T. K. Chan. 1997. Stage-specific manifestation of mold infections in bone marrow transplant recipients: risk factors and clinical significance of positive concentrated smears. Clin. Infect. Dis. 25:37-42. [DOI] [PubMed] [Google Scholar]