Abstract
Recently, lenalidomide and low dose dexamethasone were found to result in superior overall survival compared to lenalidomide and high dose dexamethasone. The immune suppressive effects of dexamethasone can antagonize lenalidomide immunomodulatory activity and may explain this observation. We conducted a retrospective analysis to evaluate the single agent activity of lenalidomide in newly diagnosed myeloma. Records of patients with newly diagnosed symptomatic multiple myeloma treated with single agent lenalidomide at H. Lee Moffitt Cancer Center and Roswell Park Cancer Institute were reviewed. Data were collected on disease characteristics, demographics, and treatment outcomes. Responses were assessed as per the International Myeloma Working Group criteria. From March 2007 to July 2009, 17 patients with newly diagnosed multiple myeloma were treated with single agent lenalidomide at both institutions. The median age was 70 years (range 46–84 years). Lenalidomide was generally well tolerated and no grade 4 hematologic toxicities were noted. The overall response rate ( partial remission) to lenalidomide alone was 47% at a median follow-up of 7 months (range 1–26). This experience suggests that lenalidomide alone can induce an anti-myeloma effect in previously untreated patients who are considered poor candidates for concurrent dexamethasone.
Keywords: Lenalidomide, immune modulation, multiple myeloma
Background
Multiple myeloma is a malignant disorder of plasma cells that is characterized by infiltration of the bone marrow, production of monoclonal immunoglobulin, lytic bone disease, and an overall immune compromised state. It represents approximately 10% of all hematologic cancers and ~1% of all malignant disorders [1]. The annual incidence in the United States is approximately 19 000 patients with a median age at the time of diagnosis of 70 years [1]. Treatment is often initiated with symptom development or with developing evidence of end organ damage. Despite aggressive therapeutic approaches, including high dose chemotherapy and autologous stem cell rescue, multiple myeloma remains an incurable disorder, and, eventually, all patients relapse and succumb to disease related complications.
The last decade has brought renewed hope for myeloma patients, with several novel agents demonstrating impressive clinical responses, and, subsequently, securing the Food and Drug Administration's approval for myeloma therapy. These agents include the immunomodulatory (IMiD) thalidomide and lenalidomide, the first in class proteasome inhibitor bortezomib, and pegylated liposomal doxorubicin in the chemotherapeutic category. Although the availability of these drugs has already made an important impact on the survival of patients with multiple myeloma, the disease remains incurable. In the absence of a curative regimen or drug, the strategic use of available therapeutics is of high priority. Optimizing the use of a specific agent may help control the disease effectively and avoid unnecessary exposure to multiple agents that can be subsequently sequenced. High dose corticosteroids are effective for short-term disease control but lack the ability to maintain durable responses. Furthermore, they have substantial toxicity effects such as hyperglycemia, uncontrolled hypertension, depression, weight gain, and fluid retention, as well as immune suppression resulting in infectious complications. These side effects of high dose steroid therapy are particularly problematic in older adults with comorbid conditions. Despite these toxicities, corticosteroids remain an important ingredient in various combination regimens in multiple myeloma. The addition of corticosteroids to novel agents (lenalidomide, thalidomide, and bortezomib) has resulted in considerable improvement in efficacy [2–5]. Recognizing the significant morbidity associated with steroid addition in these regimens, our group has focused on the development of steroid independent regimens for patients with myeloma [6,7].
Lenalidomide is a novel immunomodulatory agent with the ability to induce an immune mediated response in patients with B-cell cancers [8,9]. Preclinical evaluation reveals that this immune mediated response is dependent upon proliferation and activation of T and natural killer (NK) cells that are mediated by lenalidomide and suppressed by steroids [10]. Several clinical observations are in line with these preclinical data. A retrospective analysis of the large clinical trial of lenalidomide in combination with dexamethasone (the MM-009 and the MM-010 trials) revealed improved progression free survival (PFS) among patients whose dexamethasone dose was reduced due to toxicity versus those patients who remained on high dose dexamethasone [11]. Similarly, the Eastern Cooperative Oncology Group (ECOG) study, E4A03, comparing lenalidomide and low dose dexamethasone to lenalidomide and high dose dexamethasone, demonstrated a better 1 and 2 year survival for the group that received a lower dose of dexamethasone [12]. Collectively, these observations suggest that the immune mediated anti-tumor effects of lenalidomide can be better harnessed when used alone (or independent of corticosteroids). In a single patient experience, we observed a significant anti-myeloma effect of lenalidomide alone, demonstrating the feasibility of such an approach [6].
To date, no prospective trial has explored the role of single agent lenalidomide in previously untreated multiple myeloma patients, and such data are lacking. To investigate the merit of such an approach, we reviewed the records of all patients with newly diagnosed multiple myeloma who were prescribed lenalidomide alone (without corticosteroids) as primary therapy at the H. Lee Moffitt Cancer Center and the Roswell Park Cancer Institute. This article summarizes our findings, and reports on the clinical efficacy of single agent lenalidomide as primary therapy in patients with previously untreated multiple myeloma.
Patients and methods
The institutional review boards of both institutions approved this study. All patients who had previously untreated and symptomatic multiple myeloma [13] and were treated with single agent lenalidomide as initial therapy (without corticosteroids) were eligible for this analysis. Data were collected on patient demographics (age, gender, ethnicity), disease characteristics (heavy and light chain type, cytogenetics, extent of bone marrow involvement, presence of lytic lesions), and clinical stage (determined by the international staging system as well as the Durie and Salmon staging system). Data were also collected on response outcome and the toxicities associated with treatment.
The most common reason to omit corticosteroids (dexamethasone) from the treatment regimen was treating physician discretion or patient preference. Lenalidomide was administered at 25 mg daily for 21 days of a 28-day cycle in all patients but one (who started at 15 mg due to coexistent renal dysfunction). Lenalidomide was continued until disease progression. Although low dose dexamethasone (40 mg orally weekly) was added to lenalidomide in the absence of response (≤stable disease) or at the time of progression, this article only reports on the response obtained with single agent lenalidomide therapy. Thromboprophylaxis (low dose aspirin or low dose warfarin) was given to all patients.
Response was assessed and recorded according to the uniform response criteria proposed by the International Myeloma Working Group [13]. Briefly, a complete remission (CR) is defined as complete disappearance of the monoclonal protein by immunofixation in the serum and urine as well as less than 5% bone marrow plasmacytosis. A stringent complete remission (sCR) refers to a complete remission where the serum free light chain ratio has normalized and with the absence of clonal plasma cells in the bone marrow biopsy. Partial remission (PR) is defined as greater than 50% improvement in the serum paraprotein and greater than 90% improvement in the urine paraprotein (or to less than 200 mg/24 h). Patients achieving greater than 90% reduction in the serum paraprotein and a decrease in the urine paraprotein to less than 100 mg/24 h are categorized as having a very good partial remission (VGPR). A minimal remission (MR) is defined as greater than 25% reduction (but less than 50% reduction) in the serum paraprotein. Progressive disease (PD) is defined as a 25% increase in the serum paraprotein. Stable disease is defined as a response not meeting any of the above criteria for MR or PD [13]. Progression free survival is defined as the time from diagnosis to death or progression. Overall survival is defined as the time from diagnosis to death.
Categorical data are summarized in frequencies, whereas continuous variables are summarized using means and standard deviations or medians and range when applicable. Survival data were estimated by the method of Kaplan and Meier.
Results
From March 2007 to July 2009, all patients (n = 17) with previously untreated multiple myeloma who were treated with single agent lenalidomide at either of the institutions were included in this report. Patient characteristics are summarized in Table I. The median age was 70 years (range 46–84 years), with a median β2-microglobulin of 2.9 mg/L (range 2.1–10.7 mg/L) and advanced stage disease noted in 13 patients. Except for one patient (who had a creatinine clearance of 49 mL/min), all patients were started on a standard dose of lenalidomide.
Table I.
Characteristics of patients treated with single agent lenalidomide.
| Characteristic | Number (%) |
|---|---|
| Median age (years) | 70 |
| Range | (46–84) |
| Gender | |
| Male | 10 (59%) |
| Heavy chain | |
| IgG | 10 (59%) |
| IgA | 5 (29%) |
| Light chain only | 2 (12%) |
| Light chain | |
| Lambda | 10 (59%) |
| Durie–Salmon stage | |
| IIA | 4 (24%) |
| IIIA | 13 (76%) |
| International staging system | |
| I | 11 (65%) |
| II | 6 (35%) |
| III | 2 (12%) |
| β2-Microglobulin (mg/L) | |
| Median (range) | 2.84 (2.13–10.7) |
| Patients with β2-microglobulin >5.5 mg/L | 1 (5%) |
| Hemoglobin (g/dL) | |
| Median (range) | 11.6 (8.2–15.8) |
| Patients with hemoglobin < 10 g/dL | 2 (11%) |
| Cytogenetics | |
| Deletion 13q | 4 (24%) |
| Deletion 17p | 1 (6%) |
| Not available | 10 (59%) |
| Lytic bone disease | 11 (65%) |
Toxicity
There were no reported grade 4 hematologic toxicities. Grade 3 neutropenia, anemia, and thrombocytopenia were observed in one, one, and three patients, respectively (Table II). There was no thromboembolic event reported. The lenalidomide dose was reduced in six patients primarily due to grade 3 hematologic toxicities (noted above) and in one patient for grade 2 non-hematologic toxicities (rash, diarrhea, and fatigue).
Table II.
Incidence of hematologic toxicity observed during lenalidomide-alone therapy (n = 16).
| Toxicity | Grade 2 (n) | Grade 3 (n) |
|---|---|---|
| Anemia | 5 (31%) | 1 (6%) |
| Thrombocytopenia | 2 (12%) | 3 (19%) |
| Neutropenia | 5 (31%) | 1 (6%) |
Response
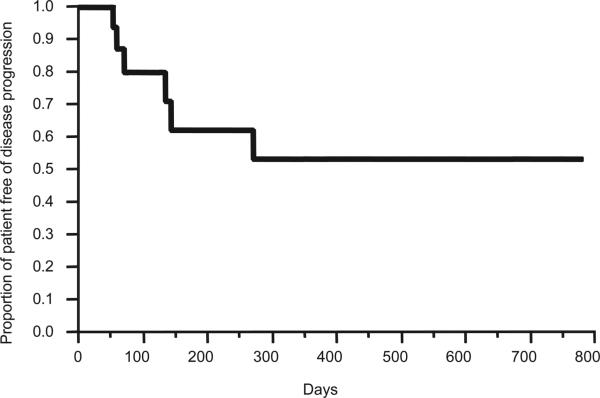
The overall response rate (complete and partial remission) was 47%. Three patients achieved an unconfirmed CR (confirmatory bone marrow biopsy was not done, and two patients had normalization of the serum free light chain assay), two patients had a VGPR, three patients had a PR, five patients had an MR, and four patients had stable disease as their best response to date. Thus, evidence of anti-myeloma clinical activity (≥ minimal response, MR) was noted in 76.5% of the patients. The median time to first response was 50 days (range 28–98 days) and median time to best response was 69 days (range 30–591 days). At a median follow-up of 7 months (range 1–26), one patient died of PD (despite the addition of dexamethasone and subsequent therapies) (Figure 1). At the time of this report, six patients required the addition of dexamethasone for progression of the disease, and three of these patients progressed after the addition of dexamethasone to lenalidomide. It is to be noted that the data reported in this article are reflective of single agent lenalidomide.
Figure 1.
Progression free survival curve of previously untreated multiple myeloma patients treated with single agent lenalidomide.
Discussion
Lenalidomide has several proposed mechanisms of action in myeloma: a direct anti-tumor effect as well as an immunomodulatory effect with resultant generation of an anti-tumor immune response. The traditional approach has relied on its combination with high doses of dexamethasone to induce a clinical response. Based on the evolving preclinical and clinical data on its immune dependent mechanisms [8,10,14–17], its combination with dexamethasone seems counterintuitive. Our observation is unique, as it reports for the first time the clinical efficacy of lenalidomide alone in treatment naive myeloma patients. While lenalidomide has been previously investigated as a single agent in relapsed or relapsed/ refractory myeloma, the response observed was significantly lower (25% of patients achieving minimal response and better) [18]. This observation is consistent with our hypothesis that ‘healthy’ components of an immune response (such as T and NK cells) are critical in mediating lenalidomide's activity, and that its use in heavily pre-treated patients with more significant immune deficits as a result of prior chemotherapy may not yield optimal effects. This observation also differentiates lenalidomide from other novel agents such as thalidomide and bortezomib, whose response rate is not significantly different in previously untreated versus previously treated myeloma patients [19–21].
Dexamethasone is usually added to lenalidomide therapy, and this is thought not only to improve the clinical response rate but also to hasten anti-myeloma effects [22,23]. However, recent clinical investigations such as reported by Rajkumar et al. of the ECOG E4A03 trial [12] suggest that decreasing the dexamethasone dose in a lenalidomide combination can actually improve clinical outcome. We note that the anti-myeloma effect of lenalidomide in our series was reasonably favorable, with a median time to first response at 50 days. Our observation, albeit in a limited number of patients, not only reassures that lenalidomide alone can induce significant clinical responses, but also shows that the induction of anti-tumor effects can be observed in a reasonably acceptable time frame.
We note that this study analysis is limited by the small size and the retrospective nature of the observation. Also, while some toxicity data are available, comprehensive data on all toxicities are not available due to the retrospective nature of the study. Therefore, while our clinical observations are interesting, further validation is warranted through formal clinical investigations. We have therefore initiated a phase II trial to investigate the role of single agent lenalidomide as primary therapy for patients with multiple myeloma. Results from this study will further affirm the role of lenalidomide monotherapy in previously untreated myeloma and allow us to design new strategies for optimal sequencing of novel agents. Planned correlative studies will investigate the effect of lenalidomide on the immune microenvironment of myeloma patients.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Blood E, Vesole D, et al. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: a clinical trial coordinated by the Eastern Cooperative Oncology Group. J Clin Oncol. 2006;24:431–436. doi: 10.1200/JCO.2005.03.0221. [DOI] [PubMed] [Google Scholar]
- 4.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 5.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 6.Chanan-Khan A, Miller KC, Musial L, et al. Bortezomib in combination with pegylated liposomal doxorubicin and thalidomide is an effective steroid independent salvage regimen for patients with relapsed or refractory multiple myeloma: results of a phase II clinical trial. Leuk Lymphoma. 2009;50:1096–1101. doi: 10.1080/10428190902912460. [DOI] [PubMed] [Google Scholar]
- 7.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 8.Chanan-Khan AA, Cheson BD. Lenalidomide for the treatment of B-cell malignancies. J Clin Oncol. 2008;26:1544–1552. doi: 10.1200/JCO.2007.14.5367. [DOI] [PubMed] [Google Scholar]
- 9.Ferrajoli A, Lee BN, Schlette EJ, et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer PH, Gandhi AK, Zhang L-H, et al. Opposing effects of dexamethasone on lenalidomide activity in multiple myeloma: additive/synergistic effects on anti-proliferative activity on myeloma cells and antagonistic effects on immune function. Blood. 2008;112(Suppl. 1):953. (Abstract 2761) [Google Scholar]
- 11.Miguel JFS, Dimopoulos M, Weber D, et al. Dexamethasone dose adjustments seem to result in better efficacy and improved tolerability in patients with relapsed/refractory multiple myeloma who are treated with lenalidomide/dexamethasone (MM009/010 sub-analysis). Blood. 2007;110(Suppl. 1):796a. (Abstract 2712) [Google Scholar]
- 12.Rajkumar S, Jacobus S, Callander N, et al. A randomized trial of lenalidomide plus high-dose dexamethasone (RD) versus lenalidomide plus low-dose dexamethasone (Rd) in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology Group. Blood. 2007;110(Suppl. 1):31a. (Abstract 74) [Google Scholar]
- 13.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 14.Chanan-Khan AA, Whitworth A, Bangia N, Porter CW, Lee K. Lenalidomide-associated tumor flare reaction is manageable in patients with chronic lymphocytic leukemia. J Clin Oncol. 2008;26:4851–4852. doi: 10.1200/JCO.2008.18.2857. author reply 4852–4853. [DOI] [PubMed] [Google Scholar]
- 15.Corral LG, Kaplan G. Immunomodulation by thalidomide and thalidomide analogues. Ann Rheum Dis. 1999;58(Suppl. 1):I107–I113. doi: 10.1136/ard.58.2008.i107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies FE, Raje N, Hideshima T, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi AK, Rogovitz A, Lopez-Girona A, et al. Stimulation of T cells by lenalidomide involves putative lenalidomide binding proteins CD3-epsilon-associated protein and GDP-mannose pyrophosphorylase a. Blood. 2008;112(Suppl. 1):903. (Abstract 2606) [Google Scholar]
- 18.Richardson PG, Blood E, Mitsiades CS, et al. A randomized phase 2 study of lenalidomide therapy for patients with relapsed or relapsed and refractory multiple myeloma. Blood. 2006;108:3458–3464. doi: 10.1182/blood-2006-04-015909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajkumar SV, Gertz MA, Lacy MQ, et al. Thalidomide as initial therapy for early-stage myeloma. Leukemia. 2003;17:775–779. doi: 10.1038/sj.leu.2402866. [DOI] [PubMed] [Google Scholar]
- 20.Weber D, Rankin K, Gavino M, Delasalle K, Alexanian R. Thalidomide alone or with dexamethasone for previously untreated multiple myeloma. J Clin Oncol. 2003;21:16–19. doi: 10.1200/JCO.2003.03.139. [DOI] [PubMed] [Google Scholar]
- 21.Richardson PG, Xie W, Mitsiades C, et al. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009;27:3518–3525. doi: 10.1200/JCO.2008.18.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82:1179–1184. doi: 10.4065/82.10.1179. [DOI] [PubMed] [Google Scholar]
- 23.Niesvizky R, Jayabalan DS, Christos PJ, et al. BiRD (Biaxin [clarithromycin]/Revlimid [lenalidomide]/dexamethasone) combination therapy results in high complete- and overall-response rates in treatment-naive symptomatic multiple myeloma. Blood. 2008;111:1101–1109. doi: 10.1182/blood-2007-05-090258. [DOI] [PubMed] [Google Scholar]