The explosive increase since the beginning of 1990s in the number of publications reporting PCR-based methods for detection or molecular typing of food-borne pathogens has attracted the attention of end-user laboratories.
However, the well recognized difficulties in reproducing published tests due to variation in performance of PCR thermal cyclers, in efficiency of different DNA polymerases, personnel and the presence of PCR inhibitors in the sample matrix can hamper implementation in laboratories, particularly those with extensive quality assurance programs.
Lack of reproducible methods often forces testing laboratories to spend substantial resources on adaptation of the published tests. It is thus necessary to have internationally validated, open-formula PCR-based methods available in which the target gene, performance characteristics and validation criteria are known (12) and which follow the ISO criteria for validation of alternative microbiological methods (2, 3). This issue has been partially addressed by a recent European research project (Food-PCR; www.pcr.dk) involving 35 laboratories (17).
A major drawback of most published PCRs, surprisingly even to date, is that they do not contain an internal amplification control (IAC). In contrast to a (external) positive control, an IAC is a nontarget DNA sequence present in the very same sample tube, which is coamplified simultaneously with the target sequence. In a PCR without an IAC, a negative response (no band or signal) could mean that there was no target sequence present in the reaction. But, it could also mean that the reaction was inhibited, due to malfunction of thermal cycler, incorrect PCR mixture, poor DNA polymerase activity, or not least the presence of inhibitory substances in the sample matrix (19). Conversely, in a PCR with an IAC, a control signal should always be produced even though there is no target sequence present. This can reveal failure of a PCR.
The European Standardization Committee (CEN), in collaboration with International Standard Organization (ISO) has proposed a general guideline for PCR testing of food-borne pathogens that requires presence of IAC in the reaction mixture (2). However, CEN has left the design of the IAC open.
The purpose of the present review is to discuss different IAC strategies and focus on advantages or drawbacks of each strategy. In addition, this is not intended to be a review of the existing literature in the classical sense, but rather a practical approach based on different hands-on experience with design and production of IAC. The readers are referred to relevant books or publications for precise details of the laboratory protocols.
WHICH AMPLIFICATION STRATEGY?
While some design approaches such as cloning require substantial technical skills, others can be done using basic PCR methodology. There are two main strategies for use of an IAC in a diagnostic PCR assay. Their difference lies in whether the IAC is to be used competitively or noncompetitively.
Competitive IAC.
By using the composite primer technique (23) the target and the IAC are amplified with one common set of primers and under the same conditions and in the same PCR tube. In this strategy, there is always some competition between target DNA and IAC, and the amount of IAC is critical to the detection limit. One has to consider that simultaneous amplification of two different DNA fragments flanked by the same primer sites can result in either inhibition or enhancement of one or both products depending on the molar ratio, the length, the sequence and the secondary structure of those DNA fragments.
The competition by IAC can, however, lower the amplification efficiency of PCR and thereby result in a lower detection limit. Thus, the most critical parameter to consider is the concentration of the IAC itself. The lowest reproducible IAC DNA concentration must be determined carefully; otherwise too many IAC DNAs will compete with the target DNA product and abolish the target signal. This will by itself cause a false-negative result. In addition, if used at high concentration, the IAC might not detect weak inhibition, which could cause false-negative results if the target is present in extremely low concentrations (21). The calculation of detection probability would provide an indication of the detection limit of the final PCR method in diagnostic samples (16, 19).
The second critical parameter is the size of the IAC. Increasing the size of one target relative to another should, in theory, drive the reaction kinetics towards the smaller target PCR product (22). However, some authors have mentioned that regardless of the size of IAC, competition with the target sequence in PCR could be observed; Brightwell et al. (5) and Abdulmawjood et al. (1) reported that IAC size of less than 500 bp does not influence the native PCR sensitivity. Nevertheless, one can recommend that the size of the IAC should be larger than the target sequence, to ensure the competitive edge of the latter.
Owing to competition, if the target DNA is amplified but the IAC is not, it is assumed that the target DNA is present in a proportionally greater amount. When this occurs, the positive result is valid because the IAC amplification is unnecessary. If neither the IAC nor the target DNA is amplified, it is assumed that inhibition of the PCR has occurred and the test for that sample is not valid. However, the drawback of this approach could be a lower detection limit due to the competition by IAC.
Noncompetitive IAC.
Here, the target and IAC are amplified using a different primer set for each. This requires a PCR in which two reactions with different kinetics proceed simultaneously. The kinetics of each reaction are not influenced by a competition for the primers. The IAC primer set targets a synthetic DNA (e.g., IAC plasmid DNA) or another gene (e.g., encoding rRNA), which is present in any microorganism and in higher copy number than the principal target gene. Of course, if no target bacteria are present, there will be no amplicon from IAC. In this approach, PCR amplification of the IAC must be limited by a controlled concentration of the IAC- specific primers in order to limit the competition of the target- and the IAC-specific reaction for oligonucleotides and DNA polymerase (Fig. 1). The disadvantage is that amplification of noncompetitive sequences may not accurately reflect amplification of the primary target due to differences in the primer sequences. Therefore nucleotide composition and size of the IAC have to be carefully considered.
FIG. 1.
In a noncompetitive PCR, amplification of the IAC could be limited by a controlled concentration of the IAC-specific primers, in order to limit the competition of the target- and the IAC-specific reaction for oligonucleotides and DNA polymerase.
Taking these criteria into account, using IAC in a noncompetitive PCR requires the development of two PCRs, optimized to work by the same PCR conditions, which may become subefficient for one or both reactions. One way to overcome this problem is to optimize the assay only for the target and let the IAC reaction follow that; optimization of the IAC part of the PCR is not senso stricto part of the assay development, as long as the IAC amplicon is detected.
In addition, it is important to limit the production of IAC amplicon by keeping the concentration of its primers to a suboptimal (minimal) level. The main advantage of this method is that it can be used for many different assays in the same laboratory. The most popular approach is use of primers specific to conserved sequences of 16S and 23S ribosomal DNA. Another approach is to add, to the PCR mix, a microorganism, which is usually not found in the sample type to be tested. The latter approach could also be done in competitive PCR, but with addition of IAC plasmid in the vector microorganism. However, few end-use laboratories have permission to work with recombinant microorganisms.
PRODUCTION OF IAC
The simplest approach is to produce PCR products, which differ in size and hence can be easily visualized separately from the native product by an agarose gel electrophoresis. The most common approach is the composite primer technique, using the same primer as for the target DNA (23). This can be achieved by addition of a completely nonrelevant DNA to the PCR mixture, e.g., a fish virus for a PCR-based detection of Salmonella enterica in pig fecal samples or chicken rinse water samples (Fig. 2) (11, 15).
FIG. 2.
Illustration of the composite primer technique, where the same primer set is used to amplify by PCR both the target (Salmonella enterica) and the nonrelevant chimeric DNA (a fish virus) spiked in the PCR mixture. A specific hydrolysis (TaqMan) probe is used in the inner region of the chimeric DNA to distinguish the PCR signal of the target and internal amplification control (IAC) in a real-time PCR assay for Salmonella (11).
Another approach is production of a modified target IAC (so-called mimic) by deleting, inserting or modifying sequences between the recognition primer sites (1, 8, 18, 22). Via PCR mutagenesis it is possible to exchange only a short stretch of DNA, whose sequence differs from the target probe only in a few nucleotides. Based on this difference, a hybridization probe can be designed which detects the IAC specifically. These methods may also be applied to construction of IAC for quantitative PCR. Since many of these methods require a cloning step, it is strongly recommended to check any new chimeric sequence for the possible presence of undesired mutational changes. Therefore, it would be necessary to determine the sequence of the IAC, before design of hybridization probes, e.g., for use in fluorescence-based real-time PCR assays. In addition, the IAC amplicon size will be smaller than the target amplicon, which may favor the amplification of the IAC over that of the target amplicon.
However, this method is rather time-consuming and labor-intensive, because of the digestion, ligation, cloning, and purification steps.
In order to have the chance of similar amplification conditions between target and IAC DNA, both sequences should not be too heterologous. Modifying distinct nucleotides of DNA often requires complicated PCR mutagenesis methodology (7). Such IACs are useful when sequence-dependent detection (hybridization) methods are applied for the confirmation of the specific PCR products. A size-dependent discrimination (gel electrophoresis) of target and IAC DNA will then not be possible. However, homologous target and IAC sequences can form heteroduplex PCR products, which often leads to a reduced detection limit for the desired target pathogen. Addition of the recognition primer sites could also be constructed by, e.g., overlap-extension PCR (18). The addition method is easier than the modification method, as IACs are synthesized in one step PCR. The primers used in this reaction possess 5′ overhanging ends, which are identical to the primers used in the diagnostic reaction, whereas their 3′ ends are complementary to a predetermined DNA sequence (pUC19 for example) of defined length and sequence. The advantage of the methodology is that the possibility of heteroduplex formation during PCR due to sequence similarity of the target DNA is avoided (14, 22).
Another methodology allows the construction of an IAC with completely designed nucleotide sequence (16, 20, 21). Several pairs of partially overlapping oligonucleotides that contain the entire IAC sequence are annealed and extended with E. coli DNA polymerase I Klenow fragment. An alternative method is the artificial construction of the complete IAC in one run, which could be easily obtained commercially. The latter method is currently limited to construction of IACs up to 100 bp. However, restriction of the length of the PCR target to less than 150 bp is an aspect, which should be considered to achieve optimal PCR efficiency (per the ABIPRISM [model 7900 HT; Applied Biosystems, Foster City, Calif.] sequence detection system user guide).
CLONING VERSUS NONCLONING
One of the ways of constructing a competitive IAC is to insert the (modified) target sequence in a plasmid. This has several benefits, such as better control of stability, size and copy number. In addition the plasmid can be safely stored in convenient quantities for long periods in minimum-degradation tubes (see data of D. Gaillard and F. Strauss [http://research.bmn.com/tto]). However, the modified target sequence could be obtained completely artificial from a commercial source as well.
Usually, IAC DNA is embedded in a vector system. Here, linearized (loop-free), recombinant plasmid DNA, including the IAC sequence, serves as template in the PCR. The advantage of cloned IAC DNA is that it allows simple storage of a recombinant plasmid DNA within bacterial cells, which guarantees the continuous availability and quality of the IAC. Care must however be taken to minimize laboratory contamination with plasmid DNA, as it can be quite persistent. It is also possible to use purified PCR products of the IAC with flanking sequences of plasmid DNA (16). The direct use of a single-stranded oligonucleotide as IAC template might be a new strategy. The advantage of using artificial oligonucleotides as IACs is easy access of synthesis, as well as guaranteed consistent quality assurance worldwide, which would be an added advantage in regard to standardization of diagnostic PCR.
STORAGE LOSS OF IAC
The safest IAC storage method seems to be as part of a recombinant plasmid in an E. coli strain, which can be kept as a glycerine culture, lyophilized, or frozen as a “microbank.” However, free DNA should be stored undiluted in an alkaline buffer such as 0.1 M TE (pH 8.0), which stabilizes it. We have experienced substantial unintentional loss of IAC signal during storage at low concentrations or in distilled water (15). Therefore, addition of EDTA is important to chelate ions that can function as cofactors of DNA degrading enzymes. Carrier nucleic acid can be also used to aid stabilization. A usually overlooked factor is the storage of DNA in appropriate plastic tubes. DNA can bind to polypropylene and interact with the tube walls inducing conformational changes, which influence the amplification efficiency and accuracy, and non-the-least detection limit (4). Incidentally, many PCRs assay claim detection limits of one DNA target copy number, although some DNA could have been, unintentionally, absorbed to the tube. It has been shown that polyallomer tubes are more suitable for storage as they do not show adsorption and denaturation of DNA (http://research.bmn.com/tto). Alternatively, DNA can be freeze-dried by a novel production method, with minimal loss or degradation (S. Trapmann, P. Catalani, J. Hoorfar, J. Prokisch, P. van Iwaarden, and H. Schimmel, submitted for publication).
QUANTIFICATION OF IAC
There is a requirement to titrate the internal control DNA template prior to use in the PCR assay. For further use it is possible to prepare ready-to-go mixtures containing every reagent, including final concentration of IAC template and primers. They can be aliquoted and stored frozen for several months until use (11). The titration and calculation of the correct IAC copies used in the PCR assay is important for the exact function of the IAC. With low concentrations (approximately 20 to 40 copies per reaction), IAC molecules are distributed among replicate amplifications according to Poisson's law. Rosenstrauss et al. (21) describe a method for the titration of IAC molecules based on this type of distribution, calculating the average number of molecules in a given volume of solution and the probability that no molecule exists in a particular sample of given volume. The advantage of the method is the independence of fluorometric or spectrophotometric measurement, which each have a different reproducibility (13). However, it must be noticed that residual reagents from the purification of plasmids or PCR products might interfere with both fluorometric and spectrophotometric measurements. Also, the method of Rosenstrauss et al. (21) can be laborious and time-consuming.
It has been shown that the concentration of PCR products (after purification by gel filtration to separate nucleotides, salt and primers) can be correctly measured by absorbance at 260 nm according to standard procedures using spectrophotometric measurements that correspond well to the empirical detection limit of the IAC (Malorny et al., unpublished data). A more accurate approach is the use of quantitative real-time PCR, although this requires costly instruments.
DETECTION OF IAC AMPLICON
Amplicons are usually detected and identified according to their size. Restriction fragment analysis allows unambiguous confirmation of specificity of the amplification. However, this conventional method uses ethidium bromide that requires strict and constraining regulations in many countries. The use of this reagent is facing increased precaution measures and restrictions in most laboratories. Moreover, gel electrophoresis requires additional time- and labor-intensive processing of amplicons, and increases the risk of contaminating the laboratory environment with the carry-over products. Development of the so-called ELISA-PCR assays has helped to avoid the use of gel electrophoresis (9, 10).
Closed-tube fluorogenic PCR methods based on addition of SybrGreen and analysis of melting curves can in some situations be more helpful than gel electrophoresis. This system can be based on the measurement of the increasing fluorescence due to the incorporation of the SybrGreen I dye during the synthesis of amplicons. By using an IAC that melts at a different temperature than the target amplicons, it is possible to separate the target signal from IAC signal. Specific identification of melting peaks permits the IAC and target DNA to be coamplified in the same tube and still be distinguished. However, this would only be the case when the melting curve of the IAC amplicon is quite different from the target sequence.
There are a number of more specific real-time PCR techniques, which use fluorescence-labeled hybridization probes. These are designed to bind adjacent to one another on the amplicon (6). Hybridization probes, such as fluorescence resonance energy transfer (FRET), labeled by fluorescent dyes can be used to design real-time PCR detection (Fig. 3). In the FRET system, the IAC has the same sequence as the target PCR product, except that the LC-Red 640 probe region has been replaced with a sequence complementary to the IAC probe LC-Red 705. FRET detection of the target DNA is with the probe labeled with the LC-Red 640 dye in channel 2 of the Light Cycler instrument, while the IAC is detected with a probe labeled with the LC-Red 705 dye in channel 3.
FIG. 3.
Hybridization probes, such as FRET, labeled by fluorescent dyes can be used to design real-time PCR detection. In the FRET system, the IAC has the same sequence as the target PCR product, except that the LC-Red 640 probe region has been replaced with a sequence complementary to the IAC probe LC-Red 705. FRET detection of the target DNA is with the probe labeled with the LC-Red 640 dye in channel 2 of the Light Cycler instrument, while the IAC is detected with a probe labeled with the LC-Red 705 dye in channel 3.
Dual fluorogenic probes (e.g., TaqMan probes and molecular beacons, etc.) used in 5′-nuclease PCR assays hybridize within the target sequence amplified by the PCR primers. Different dye-labeled probes can bind specifically to the target and IAC sequence making it possible to perform duplex detection of the target and IAC.
The 5′-nuclease PCR based on the use of a fluorogenic probe (TaqMan probe) that hybridizes within the target sequence bound by PCR primers can differentiate between the wavelengths emitted by different dyes, making it possible to perform duplex detection of the target and IAC (Fig. 2). Usually, the probes specific of the target are 5′-labeled with the fluorescent reporter dye 6-carboxyfluorescein (FAM) or fluorescein (Fluo), those targeting the IAC with a different fluorescent reporter dye like VIC, JOE or TET. Thus, using two probes labeled with distinct reporter dyes, allows simultaneous detection of the target gene and the IAC in a duplex reaction. The specificity of the probes ensures that no signal is generated by nontarget amplicons.
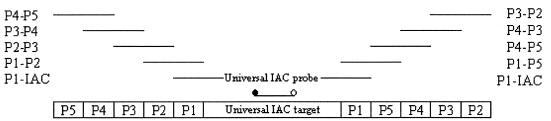
An interesting development in real-time PCR is generation of multiple IACs for a panel of PCR assays with a single DNA fragment (Fig. 4) (24). In routine laboratories with many real-time PCRs, a single IAC and IAC probe is generated rapidly by a multiple primer composite technique and is used for many assays.
FIG. 4.
Production of a single “universal” IAC and a single hybridization probe (TaqMan) for addition to different diagnostic real-time, fluorescence PCR assays for detection of, e.g., five pathogens (P1 to P5). The composite primers for the preparative PCRs are presented as horizontal lines from bottom up in order of their use (24).
CONCLUDING REMARKS
Construction of IACs can be performed in several ways, at the choice and discretion of the user. However, we recommend the competitive method to avoid the risk of undesired interactions of multiple primers and to have both PCRs (the target-specific and the IAC-specific) working with the same primer set and under identical PCR conditions. Regardless of the strategy, an optimal IAC should fulfill the criteria summarized in Table 1.
TABLE 1.
Recommendations for optimal IAC for use in diagnostic PCR
| Recommendation no. | Recommendation |
|---|---|
| 1 | Target DNA and IAC should share the same primer-binding sites. This avoids interference of two primer pairs in a multiplex reaction. |
| 2 | IAC amplicons should be easily distinguishable from the target DNA amplicons. |
| 3 | It is not strictly necessary that the amplification efficiencies of target and IAC DNA be identical. An experimental approach is needed to show the amplification efficiencies of both DNAs during PCR. |
| 4 | The source of IAC should be plasmid DNA carrying the cloned IAC sequence or purified PCR products. |
| 5 | Depending on the detection method of the assay, the IAC should be detected by sequence-dependent hybridization probes or, after gel electrophoresis, by size. |
| 6 | Only highly purified templates should be used. |
| 7 | The concentration of IAC should be determined by titration according the method described by Rosenstrauss et al. (22). |
| 8 | IAC should be added to PCR mix to ensure equal distribution in all PCR tubes. |
| 9 | Polyallomer tubes should be used for diluting IAC DNA, using aerosol-resistant and sterile pipette tips. IAC DNA can be stabilized with carrier DNA, and storage should be at high concentrations of stabilized IAC DNA (>103 copies in aliquots at −20°C). |
| 10 | The amount of IAC in the assay should be as low as possible, while still eliciting a signal through amplification. This will maximize the potential to identify false negatives by detecting PCR inhibition and will ensure the reliable detection of low target concentrations. |
On the other hand, the competitive method can require more optimization work in order to achieve a sensitive detection limit. The overlap-extension technique is simple and effective and creates IAC DNA with the same primer-binding sequence as the target DNA. The cloning of IAC into a plasmid provides an unlimited amount of IAC. Using the same primers for IAC and target is an advantage, because multiple sets of primers might interfere with the amplification of one or both of the target genes, due to the differences in primer sequence. Differences in size, internal sequences of the amplified products, and the relative amounts of the two targets might also interfere with the amplification.
A PCR-based method cannot be given diagnostic status, no matter how limited the application, before it includes, as a minimum, an IAC, a processing-positive control, a processing-negative control, and a reagent control (blank), as suggested by the MicroVal protocol (Table 2) (3, 12) and draft standard document (2). However, an IAC is only an indicator of PCR failure, and does not in itself have any counter-effect against inhibitory factors. The detection limit and thereby the diagnostic sensitivity of a PCR assay, particularly on subclinical samples with low target pathogens, depends also on an effective sample treatment procedure (15). Even then, an IAC will not show whether the purified DNA can be readily amplified. When the DNA to be amplified is derived from very complex matrices using harsh extraction protocols, the amplification of a sequence of a housekeeping gene or 16S ribosomal gene, i.e., a sequence that is definitely present in the DNA, should be performed as one of the positive controls in every setup to check the integrity of the purified DNA. Here it must be emphasized that inclusion of an IAC should not be a replacement for good laboratory practice, such as proper treatment and storage of samples on arrival. Finally, the support of editorial boards and reviewers is important if we are going to maintain the credibility of PCR as a useful tool for laboratory diagnostics. We have proposed that publication guidelines should be extended to require inclusion of IAC in any PCR intended for diagnostic use, either as a detection or subtyping tool (J. Hoorfar, N. Cook, B. Malorny, M. Wagner, D. De Medici, and P. Fach, Letter, J. Clin. Microbiol. 41:5835, 2003). This should certainly bring home to developmental scientists the importance of an IAC.
TABLE 2.
Test controls necessary for performance of diagnostic PCRa
| Type of control | Description |
|---|---|
| IAC | Containing chimeric nonrelevant DNA added to master mixture and to be amplified by the same primer set as the target DNA but with an amplicon size visually distinguishable from the target amplicon |
| Processing positive control | Negative sample spiked with sufficient amount of pathogen and processed throughout the entire protocol |
| Processing negative control | Negative sample spiked with sufficient amount of closely related, but nontarget strain processed throughout the entire protocol |
| Nontemplate control (blank) | Containing all reagents, but no target of IAC nucleic acids |
| Premise control | Tube containing master mixture left open in the PCR setup room to detect possible contaminating DNA in the environment (to be done at certain intervals as part of the quality assurance program) |
| Standard concentrations | 3-4-samples containing 10-fold dilution concentrations of known number of target DNA copies in a range above the detection limit (necessary cutoff determination and for quantitative PCR) |
See reference 3.
Acknowledgments
The work was supported in part by the EU research project Food-PCR (QLK1-CT-1999-00227), under the 5th RTD Framework, and DFFE (Denmark). N.C. acknowledges the support of the United Kingdom Food Standards Agency.
We thank Stefan Jensen for editorial assistance with the manuscript and preparation of the figures. We thank P. Rådström, M. H. Josefsen, P. Ahrens, Ø. Angen, L. O. Andresen, and M. Lund for critical reading of the manuscript.
REFERENCES
- 1.Abdulmawjood, A., S. Roth, and M. Bülte. 2002. Two methods for construction of internal amplification controls for the detection of Escherichia coli O157 by polymerase chain reaction. Mol. Cell. Probes 16:335-339. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 2002. Microbiology of food and animal feeding stuffs. Polymerase chain reaction (PCR) for the detection of food-borne pathogens. General method specific requirements (EN ISO 22174). International Organization for Standardization, Geneva, Switzerland.
- 3.Anonymous. 2002. Microbiology of food and animal feeding stuffs. Protocol for the validation of alternative methods (EN ISO 16140). European Committee for Standardization, AFNOR, Paris, France.
- 4.Belotserkovskii, B. P., and B. H. Johnston. 2003. Polypropylene tube surfaces may include denaturation and multimerisation of DNA. Science 271:222-223. [DOI] [PubMed] [Google Scholar]
- 5.Brightwell, G., M. Pearce, and D. Leslie. 1998. Development of internal controls for PCR detection of Bacillus anthracis. Mol. Cell. Probes 12:367-377. [DOI] [PubMed] [Google Scholar]
- 6.Caplin, B. E., R. P. Rasmussen, P. S. Bernard, and C. T. Wittwer. 1999. LightCycler Hybridisation probes—the most direct way to monitor PCR amplification and mutation detection. Biochemica 1:5-8. [Google Scholar]
- 7.Courtney, B. C., M. M. Smith, and E. A. Henchal. 1999. Development of internal controls for probe-based nucleic acid diagnostic assays. Anal. Biochem. 270:249-256. [DOI] [PubMed] [Google Scholar]
- 8.Cubero, J., J. van der Wolf, J. van Beckhoven, and M. M. Lopez. 2002. An internal control for the diagnosis of crown gall by PCR. J. Microbiol. Methods 51:387-392. [DOI] [PubMed] [Google Scholar]
- 9.Fach, P., F. Dilasser, J. Grout, and J. Tache. 1999. Evaluation of a polymerase chain reaction-based test for detecting Salmonella spp. in food samples: Probelia Salmonella spp. J. Food Prot. 62:1387-1393. [DOI] [PubMed] [Google Scholar]
- 10.Fach, P., S. Perelle, F. Dilasser, J. Grout, C. Dargaignaratz, L. Botella, J. M. Gourreau, F. Carlin, M. R. Popoff, and V. Broussolle. 2002. Detection by PCR-enzyme-linked immunosorbent assay of Clostridium botulinum in fish and environmental samples from a coastal area in northern France. Appl. Environ. Microbiol. 68:5870-5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoorfar, J., P. Ahrens, and P. Rådström. 2000. Automated 5′ nuclease PCR assay for identification of Salmonella enterica. J. Clin. Microbiol. 38:3429-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoorfar, J., and N. Cook. 2003. Critical aspects of standardization of PCR. Methods Mol. Biol. 216:51-64. [DOI] [PubMed] [Google Scholar]
- 13.Jensen, A. N., and J. Hoorfar. 2002. Optimal purification and sensitive quantification of DNA from fecal samples. J. Rapid Methods Automat. Microbiol. 10:231-244. [Google Scholar]
- 14.Jones, R. N., M. L. Neale, B. Beattie, D. Westmoreland, and J. D. Fox. 2000. Development and application of a PCR-based method including an internal control for diagnosis of congenital cytomegalovirus infection. J. Clin. Microbiol. 38:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lübeck, P. S., P. Wolffs, S. L. W. On, P. Rådström, and J. Hoorfar. 2003. Toward an international standard for PCR-based detection of food-borne thermotolerant campylobacters: assay development and analytical validation. Appl. Environ. Microbiol. 69:5664-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malorny, B., C. Bunge, J. Hoorfar, and R. Helmuth. 2003. Multicenter validation of the analytical accuracy of Salmonella PCR: towards an international standard. Appl. Environ. Microbiol. 69:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malorny, B., P. T. Tassios, P. Rådström, N. Cook, M. Wagner, and J. Hoorfar. 2003. Standardization of diagnostic PCR for the detection of foodborne pathogens. Int. J. Food Microbiol. 83:39-48. [DOI] [PubMed] [Google Scholar]
- 18.Müller, F.-M., N. Schnitzler, O. Cloot, P. Kockelkorn, G. Haase, and Z. Li. 1998. The rationale and method for constructing internal control DNA used in pertussis polymerase chain reaction. Diagn. Microbiol. Infect. Dis. 31:517-523. [DOI] [PubMed] [Google Scholar]
- 19.Rådström, P., C. Löfström, M. Lövenklev, R. Knutsson, and P. Wolffs. 2003. Strategies for overcoming PCR inhibition, p. 149-161. In C. W. Diefenbach and G. S. Dveksler (ed.), PCR primer: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Reddy, N. R., B. N. Wilkie, and B. A. Mallard. 1996. Construction of an internal control to quantitate multiple porcine cytokine mRNAs by RT-PCR. BioTechniques 21:868-875. [DOI] [PubMed] [Google Scholar]
- 21.Rosenstraus, M., Z. Wang, S.-Y. Chang, D. DeBonville, and J. P. Spadoro. 1998. An internal control for routine diagnostic PCR: design, properties, and effect on clinical performance. J. Clin. Microbiol. 36:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sachadyn, P., and J. Kur. 1998. The construction and use of a PCR internal control. Mol. Cell. Probes 12:259-262. [DOI] [PubMed] [Google Scholar]
- 23.Siebert, P. D., and J. W. Larrick. 1992. Competitive PCR. Nature 359:557-558. [DOI] [PubMed] [Google Scholar]
- 24.Stöcher, M., V. Leb, and J. Berg. 2003. A convenient approach to the generation of multiple internal control DNA for a panel of real-time PCR assays. J. Virol. Methods 108:1-8. [DOI] [PubMed] [Google Scholar]