Abstract
Background and objectives
B-type natriuretic peptide (BNP) concentration predicts outcome in patients undergoing dialysis. Because survival and cardiovascular risk change across the CKD continuum, serial changes in BNP were compared in patients at different CKD stages and after kidney transplantation.
Design, setting, participants, & measurements
Patients with CKD stages 3 and 4 (CKD 3–4), dialysis patients, and kidney transplant recipients (KTRs) from one center had two measurements of BNP taken a median of 161 days apart in 2003–2004 and were followed until July 2012. Both BNP-32 (Triage BNP; Biosite Diagnostics) and NT-BNP-76 (proBNP; Roche Diagnostics) were assayed. The interaction between change in log-transformed BNP concentration over time and patient group was tested by fitting regression models on panel data with random effects. Survival after the second measurement was compared by tertile of change in BNP.
Results
Patients with CKD 3–4 (n=48), dialysis patients (n=102), and KTRs (n=73) were followed for a median of 5.7, 4.8, and 5.9 years, respectively. The interaction between patient group and BNP measurements over time was significant for NT-BNP-76 (P<0.001) and BNP-32 (P<0.01). Median NT-BNP-76 increased in dialysis patients and those with CKD 3–4 from 3850 pg/ml (interquartile range [IQR], 1776–12,323 pg/ml) to 18,830 pg/ml (IQR, 6114–61,009 pg/ml; P<0.001) and from 698 pg/ml (IQR, 283–2922 pg/ml) to 2529 pg/ml (IQR, 347–9277 pg/ml; P=0.002), respectively. Change was not significant for KTRs or comparisons made with BNP-32. Survival rate was significantly lower for patients with the highest tertile of change in NT-BNP-76 among patients with CKD 3–4 (P=0.02), but not in the dialysis or KTR groups. In 11 patients who received a kidney transplant during the study, median NT-BNP-76 decreased from 9607 pg/ml (IQR, 2292–31,282 pg/ml) to 457 pg/ml (IQR, 203–863 pg/ml) after transplant (P<0.01).
Conclusions
The temporal trajectory of BNP differs between dialysis patients and those with CKD 3–4 and KTRs. This has important implications for the development of BNP-guided management strategies in CKD.
Keywords: B-type natriuretic peptide, cardiovascular disease, chronic kidney disease, dialysis, kidney transplant
Introduction
Patients with CKD have a high prevalence of left ventricular (LV) abnormalities and high cardiovascular mortality (1). Because B-type natriuretic peptide (BNP) is released by the left ventricle in response LV end-diastolic wall stress (2), this biomarker has been studied as a predictor of patient outcome. Our group and others have demonstrated that a single elevated BNP concentration strongly predicts cardiovascular and all-cause mortality in patients undergoing dialysis (3,4). Concentrations of BNP are frequently elevated in patients with CKD, particularly those receiving dialysis, and more so for the inactive N-terminal end NT-BNP-76 than the active 32–amino acid peptide BNP-32 (3). These high BNP concentrations reflect intermittent increases in LV wall stress (2), which is highly deleterious and an important target of successful therapies in chronic heart failure, such as mineralocorticoid receptor antagonists (5). In patients receiving hemodialysis, an increase over time of both NT-BNP-76 (6,7) and BNP-32 (8) was associated with an increased risk of all-cause mortality. Therefore, assessment of long-term exposure to high LV wall stress by repeated measurement of BNP in patients with CKD is the focus of this study.
We hypothesized that changes in BNP over time would be greater in dialysis patients than patients with CKD stages 3 and 4 (CKD 3–4) or kidney transplant recipients (KTRs). The primary aim of this study was to directly compare the change in BNP-32 and NT-BNP-76 over time in these CKD groups and to determine which factors are associated with this change. Secondary aims were to confirm the association of change in BNP with mortality and the fall in BNP following successful kidney transplantation demonstrated in previous studies.
Materials and Methods
The baseline BNP concentrations of this prospective cohort study of patients attending one center (Austin Health, Victoria, Australia) have been previously reported (3). The Austin Health Human Research Ethics Committee approved the study. The clinical and research activities being reported adhered to the Declaration of Helsinki and are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Study Population
Patients receiving treatment for CKD 3–4, prevalent dialysis patients, and prevalent KTRs were recruited between August 2003 and August 2004. We defined CKD 3–4 according to an eGFR<60 ml/min per 1.73 m2 on two occasions using the Modification of Diet in Renal Disease (MDRD) equation 7 (9). Although the CKD-Epidemiology Collaboration equation has replaced the MDRD equation at our institution, the MDRD equation was used at the time patients were recruited to this study; the patients with CKD 3–4 had GFR in the lower range, where the MDRD equation performs best (10). Baseline data were recorded, and blood samples were collected at baseline and on one occasion a median of 161 (interquartile range [IQR], 139–189) days later.
Laboratory Procedures
Blood was collected in serum (for NT-BNP-76) and EDTA (for BNP-32) tubes and transported on ice to the laboratory, where it was centrifuged at 3200 g for 10 minutes. Serum and plasma was divided into aliquots and frozen at −80°C until assayed. For patients undergoing hemodialysis, blood was drawn from the arterial needle before the hemodialysis procedure. Otherwise, blood was processed, stored, and assayed under the same conditions for all patients in the study.
BNP-32 was measured by a paramagnetic particle chemiluminescent immunoenzymatic assay (Triage BNP; Biosite Diagnostics) on a Beckman Coulter Access analyzer (Beckman Instruments Inc., Chaska, MN), and NT-BNP-76 was measured by an electrochemiluminescence assay (proBNP, Roche Diagnostics, Indianapolis, IN) on an E170 analyzer (Roche Diagnostics). The total coefficient of variation (CV) at different concentrations of BNP-32 between 40 and 4000 pg/ml is reported to be <7% (11). The CV at different concentrations of NT-BNP-76 (494 pg/ml, 7828 pg/ml, and 13,143 pg/ml) is <6% (12). Cardiac troponin T (cTnT) was measured by electrochemiluminescence immunoassay (Troponin T; Roche Diagnostics) using the Elecsys E170 analyzer. A concentration ≥0.04 ng/ml was considered detectable.
Statistical Analyses
For comparison of baseline factors between groups, chi-squared tests, ANOVA, and Kruskal–Wallis tests were performed according to distribution. The change in BNP was categorized into tertiles. Pairwise comparisons of serial BNP were made using the nonparametric Wilcoxon matched-pairs signed-rank test.
Concentrations of BNP at baseline and follow-up were compared between patients with CKD 3–4, dialysis patients, and KTRs by fitting generalized least-squares regression models on panel data with random effects. The significance of the difference between the changes over time between treatment groups was tested as the interaction term between patient group and time of measurement. Factors that differed between the groups were entered into the model individually to determine their effect on the interaction. In addition, variables that increased the overall R2 of the model by the largest amount were sequentially added to create a multivariate model of this interaction. Concentrations of BNP were log-transformed for this analysis to give a normal distribution. Intraindividual variability of BNP was calculated from the SD of the natural logarithm–transformed BNP values and expressed as the CV according to the method of Bland, where CV=antilog(intraindividual SD of log-transformed BNP)−1 (13). The association of change in BNP over time with change in GFR over time was assessed by inspection of scatter plots of change in BNP versus change in GFR and by regression on panel data. A Hausman test was used to determine whether to use random effects or fixed effects for this analysis.
Patients were followed for all-cause mortality until July 31, 2012. Our earlier study reported shorter-term follow-up (3). Survival analysis was performed from the date of the second measurement with censoring at transplantation, death, or end of study. Separate analyses were performed for BNP-32 and NT-BNP-76, and statistical analysis was done using Stata software, version 11.2 (College Station, TX).
Results
Background Data
Of 252 patients who provided blood at baseline, 5 patients did not meet the definition of CKD 3–4 and were excluded from further analysis. Two patients received a kidney transplant before the second sample was due and were included in the analysis of change in BNP following transplant; however, they were excluded from other analyses. There were 59 patients with CKD 3–4, 106 receiving dialysis (7 on peritoneal dialysis), and 80 KTRs; of these, 10 (17%), 4 (4%), and 9 (11%), respectively, did not give a second sample (P=0.02). These patients did not differ from those who provided two samples with regard to age, sex, history of diabetes, or cardiovascular disease. Overall, 9 (39%) patients who did not provide a second sample died (3 in each patient group) compared with 25 (11%) patients who did provide that sample (P<0.001). Five patients died before the second sample was due. We thus included 49 patients with CKD 3–4, 102 dialysis patients, and 71 KTRs with serum available to measure NT-BNP-76 and 48, 102, and 73 patients, respectively, with plasma to measure BNP-32.
Dialysis patients had required RRT for a median of 2.6 (IQR, 1.1–9.0) years, and KTRs had undergone transplantation a median of 4.9 (IQR, 2.2–13.6) years previously. GN was the primary cause of kidney disease in 6% of patients with CKD 3–4, 40% of dialysis patients, and 48% of KTR. Diabetes was the primary cause of kidney disease in 20%, 13%, and 1% of participants, respectively; hypertension and vascular disease were the cause in 18%, 9%, and 3%, respectively. At baseline, dialysis patients had higher systolic BP, higher C-reactive protein, and lower serum albumin levels (Table 1). However, patients with CKD 3–4 were older and more likely to have diabetes and cardiovascular disease and had lower GFR than did KTRs.
Table 1.
Baseline characteristics of the patients who had two measurements of NT-BNP-76 by treatment group
| Characteristic | CKD 3–4 (n=49) | Dialysis (n=102) | Transplant (n=71) | P Value |
|---|---|---|---|---|
| Age (yr) | 67.1±11.2 | 62.6±14.3 | 52.8±10.7 | <0.001 |
| Men | 37 (76) | 60 (59) | 46 (63) | 0.13 |
| Diabetes | 21 (43) | 20 (20) | 8 (11) | <0.001 |
| Cardiovascular disease | 19 (39) | 32 (31) | 9 (13) | 0.003 |
| Coronary artery disease | 15 (31) | 24 (24) | 4 (6) | 0.001 |
| Heart failure | 8 (16) | 19 (19) | 1 (1) | 0.002 |
| Cerebrovascular disease | 1 (2) | 7 (7) | 2 (3) | 0.29 |
| Peripheral vascular disease | 8 (16) | 16 (16) | 3 (4) | 0.05 |
| Smoking | 30 (61) | 55(54) | 36 (51) | 0.52 |
| Systolic BP (mmHg) | 132±21 | 145±24 | 129±14 | <0.001 |
| Diastolic BP (mmHg) | 76±9 | 78±13 | 77±6 | 0.46 |
| BMI (kg/m2) | 27.3 (24.2–31.8) | 24.2 (21.8–27.4) | 26.5 (23.3–29.8) | <0.001 |
| Antiplatelet agent | 24 (49) | 39 (38) | 15 (21) | <0.01 |
| RAS blockade | 33 (67) | 39 (38) | 41 (58) | 0.001 |
| Statin | 26 (53) | 46 (45) | 44 (62) | 0.09 |
| β-Blocker | 17 (35) | 35 (34) | 35 (49) | 0.11 |
| eGFR (ml/min per 1.73 m2) | 24±11 | — | 35±16 | <0.001 |
| Hemoglobin (g/dl) | 12.3±1.6 | 12.1±1.2 | 12.9±1.9 | 0.003 |
| C-reactive protein (mg/L) | 3.8 (1.8–7.6) | 5.0 (3.0–11.8) | 1.6 (0.6–4.6) | <0.001 |
| Albumin (g/dl) | 4.01±0.39 | 3.76±0.41 | 4.12±0.32 | <0.001 |
| cTnT-positive (sample 1) | 9 (19) | 48 (47) | 6 (8) | <0.001 |
| cTnT-positive (sample 2) | 10 (21) | 59 (58) | 8 (11) | <0.001 |
Results are similar for patients with two measurements of 32–amino acid BNP (data not shown). Values expressed with a plus/minus sign are the mean±SD; all other values are the number (percentage) of patients. NT-BNP-76, N-terminal end B-type natriuretic peptide; BMI, body mass index; RAS, renin-angiotensin system; cTnT-positive, cardiac troponin T≥0.04 ng/ml.
Change in BNP Concentrations
The intraindividual CVs of NT-BNP-76, based on two measurements obtained a median of 161 days apart, were 131% for patients with CKD 3–4, 151% for dialysis patients, and 35% for KTRs. The corresponding values for BNP-32 were 45%, 58%, and 29%, respectively.
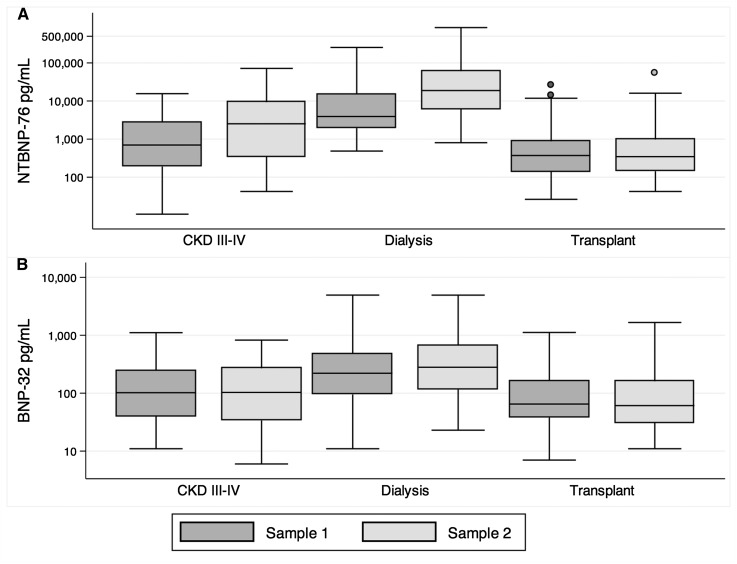
The change in concentrations of BNP over time was significantly different among the three groups for both NT-BNP-76 (P for interaction between patient group and time of measurement <0.001) and BNP-32 (P<0.01). The largest rise occurred for NT-BNP-76 in patients undergoing dialysis (Figure 1, Table 2). Concentrations of BNP did not change over time for KTRs. In pairwise comparisons of nontransformed BNP concentrations, the concentrations of NT-BNP-76 were significantly different between baseline and the second sample in patients with CKD 3–4 (P=0.004) and dialysis patients (P<0.001). No pairwise differences were significant for BNP-32.
Figure 1.
Concentrations of NT-BNP-76 (A) measured at baseline (sample 1) and 5–6 months later (sample 2) increased in patients with CKD and dialysis patients, but not kidney transplant recipients. For BNP-32 (B), no significant change was detected. The median and interquartile ranges are based on natural logarithm–transformed values of B-type natriuretic peptide (BNP) and actual values are given on the y axis. The whiskers represent the data point closest to 1.5 times the interquartile range away from the nearer quartile. BNP-32, 32–amino acid BNP; NT-BNP-76, N-terminal end BNP.
Table 2.
Median (interquartile range) concentrations of BNP-32 and NT-BNP-76 at baseline (first sample) and 5–6 months later (second sample) and median difference between the two by treatment group
| Variable | CKD 3–4 | Dialysis | Transplant |
|---|---|---|---|
| BNP-32 (pg/ml) | n=48 | n=102 | n=73 |
| First sample | 102 (48–252) | 218 (93–445) | 66 (41–148) |
| Second sample | 103 (34–269) | 280 (116–668) | 61 (31–161) |
| ΔBNP-32 | −9 (−47 to 39) | 13 (−66 to 171) | −4 (−20 to 19) |
| NT-BNP-76 (pg/ml) | n=49 | n=102 | n=71 |
| First sample | 698 (283 to 2,922) | 3850 (1776–12,323) | 379 (141–875) |
| Second sample | 2529 (347–9277)a | 18,830 (6114– 61,009)b | 347 (144–981) |
| ΔNT-BNP-76 | 778 (−57 to 7468) | 13,772 (2058–41,244) | 2 (−74 to 105) |
BNP-32, 32–amino acid BNP; BNP, B-type natriuretic peptide; ΔBNP, change between first and second samples.
P=0.004 compared with the first sample.
P<0.001 compared with the first sample.
In the cohort as a whole, NT-BNP-76 was significantly associated with several variables that differed among groups (Table 1). All of these variables demonstrated significant associations with logarithm-transformed NT-BNP-76, except for diabetes (Supplemental Table 1). Addition of each of these variables to the model individually did not change the significance of the interaction between patient group and time of measurement. In the full model, the interaction remained significant (P<0.001) when adjusted for history of cardiovascular disease, systolic BP, body mass index, use of renin-angiotensin system inhibitor, serum albumin, hemoglobin, and serum troponin T. For BNP-32, addition of variables in Supplemental Table 2 individually also did not change the significance of the interaction, and when adjusted for age, history of cardiovascular disease, systolic BP, body mass index, use of a β-blocker agent, serum albumin, hemoglobin, and serum troponin T, the P for interaction was <0.01.
Associations with Change in BNP
Associations with change in BNP were examined for the three groups of patients (Table 3). Because the change in BNP could be positive or negative, the ratio of the second to the first measure was used; this was natural logarithm–transformed to correct a skewed distribution. The variable most consistently associated with a rise in both forms of BNP in all groups was cTnT≥0.04 ng/ml in sample 2. In dialysis patients, the degree of increase in NT-BNP-76 lessened as systolic BP increased, and the lowest tertile of change in BNP-32 had the greatest proportion of patients with a history of heart failure. In CKD 3–4, higher C-reactive protein was associated with a lower ratio of second to first BNP-32. In KTRs, higher hemoglobin or albumin was associated with a lower ratio of second to first BNP-32 measurements. However, none of these continuous variable associations was consistent across the patient groups or for forms of BNP.
Table 3.
Summary of variables measured at baseline or sample 2 that were associated with change in BNP at the P≤0.05 level
| Variable and Predictor | Continuous Variables | Categorical Variables | ||||
|---|---|---|---|---|---|---|
| β-Coefficient (95% CI) | P Value | Tertile 1, n (%) | Tertile 2, n (%) | Tertile 3, n (%) | P Value | |
| NT-BNP-76 | ||||||
| CKD 3–4 | ||||||
| Male sex | 10 (60) | 11 (69) | 16 (100) | 0.02 | ||
| cTnT-positive (sample 1) | 0 | 2 (13) | 7 (44) | 0.004 | ||
| cTnT-positive (sample 2) | 0 | 2 (13) | 8 (50) | 0.001 | ||
| Dialysis | ||||||
| SBP (mmHg) | −0.01 (−0.02 to 0.0) | 0.05 | ||||
| cTnT-positive (sample 1) | 15 (44) | 11 (32) | 22 (65) | 0.03 | ||
| cTnT-positive (sample 2) | 17 (50) | 15 (44) | 27 (79) | <0.01 | ||
| KTR | ||||||
| cTnT-positive (sample 2) | 1 (4) | 0 | 7 (30) | 0.002 | ||
| BNP-32 | ||||||
| CKD 3–4 | ||||||
| logCRP (mg/L) | −0.21 (−0.38 to −0.03) | 0.02 | ||||
| Statin use | 10 (59) | 4 (27) | 11 (69) | 0.05 | ||
| cTnT-positive (sample 2) | 4 (24) | 0 | 6 (18) | 0.04 | ||
| Dialysis | ||||||
| Heart failure | 11 (32) | 2 (6) | 6 (18) | 0.02 | ||
| KTR | ||||||
| Hemoglobin (per 10 g/dl) | −0.01 (−0.01 to 0.0) | 0.02 | ||||
| Albumin (per 1 g/dl) | −0.04 (0.07 to 0.0) | 0.05 | ||||
| cTnT-positive (sample 1) | 1 (4) | 0 | 5 (21) | 0.02 | ||
| cTnT-positive (sample 2) | 1 (4) | 0 | 7 (30) | 0.002 | ||
The β-coefficient is the change in the natural logarithm–transformed ratio of the second to the first BNP sample for each unit change of the variable presented. Categorical variables are presented as the number (percentage) in each progressive tertile of change in BNP. Actual BNP concentrations that define tertiles are available in Supplemental Table 3. SBP, systolic BP; KTR, kidney transplant recipient; CRP, C-reactive protein; 95% CI, 95% confidence interval.
Separate analyses of the average effect of changing GFR over time on the change in BNP in patients with CKD 3–4 and KTRs demonstrated no significant effect for NT-BNP-76 (P=0.48) or BNP-32 (P=0.35) in CKD 3–4, or for NT-BNP-76 in KTRs (P=0.27). The average effect of 1 ml/min per 1.73 m2 increase in GFR over the time period lowered the natural logarithm of BNP-32 by 0.015 (95% confidence interval, −0.063 to −0.007; P<0.001) in KTRs.
Associations of Change in BNP with Mortality
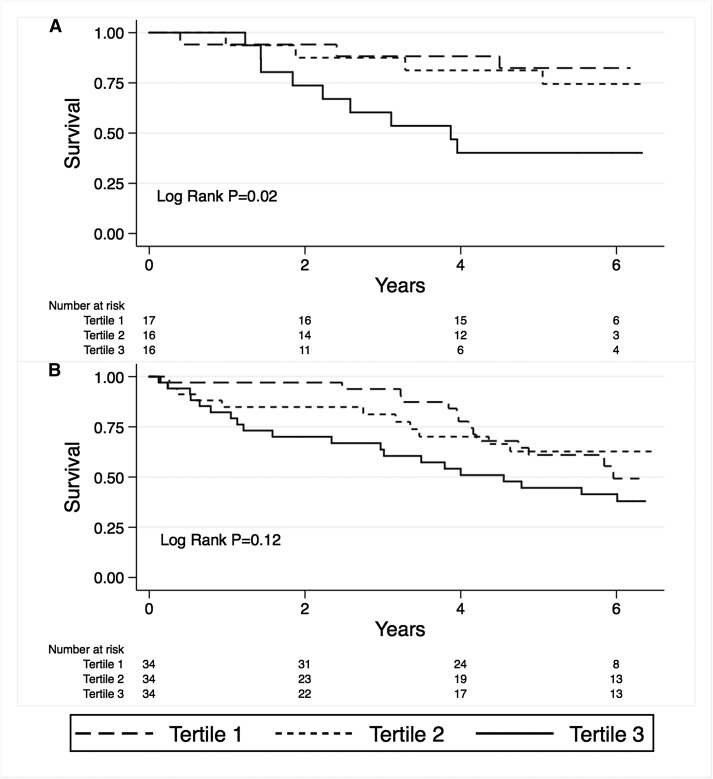
Over a median of 5.7, 4.8, and 5.9 years of follow-up, 16 patients with CKD 3–4 died, as did 45 dialysis patients and 8 KTRs. Survival analysis demonstrated significantly poorer survival with the highest tertile of change in NT-BNP-76 in patients with CKD 3–4 (Figure 2A) but no significant difference in survival by tertile of NT-BNP-76 in dialysis patients (Figure 2B). Survival did not differ by BNP-32 tertile, and no difference in survival was seen in the KTRs.
Figure 2.
Survival was significantly reduced in patients with the greatest tertile of change of NT-BNP-76 for CKD stages 3–4 (A), but not dialysis patients (B).
Change in BNP after Transplant
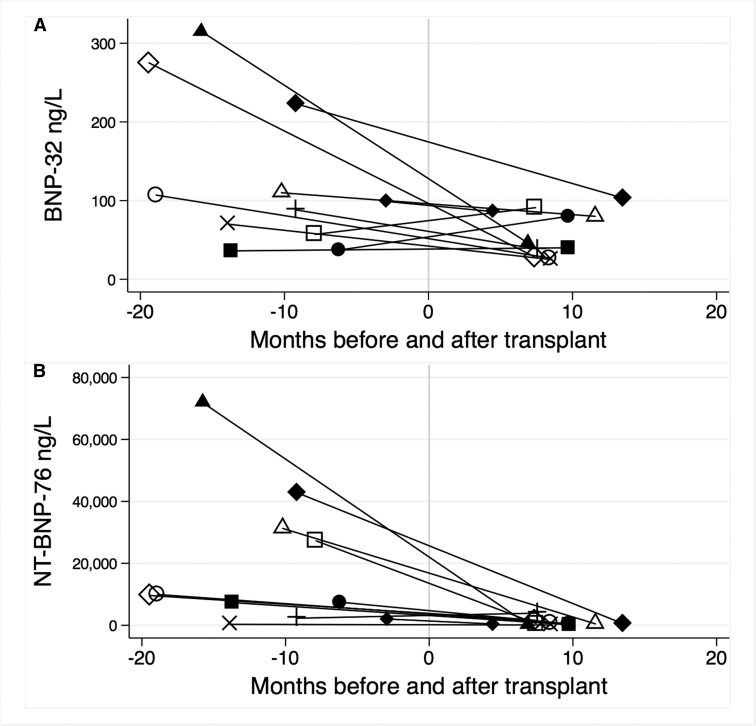
When this study commenced, 11 dialysis patients received a kidney transplant and had serum and plasma available before and after the transplant (Figure 3). The GFR increased in all patients from a mean of 7.2±0.9 ml/min per 1.73 m2 to 44.7±4.9 ml/min per 1.73 m2 (P<0.001). The median BNP-32 was 99 pg/ml (IQR, 57–223) pg/ml before and 46 pg/ml (IQR, 29–86 pg/ml) after receiving a kidney transplant (P=0.041). The median NT-BNP-76 was 9607 pg/ml (IQR, 2292–31,282 pg/ml) before and 457 pg/ml (IQR, 203–863 pg/ml) after receiving a kidney transplant (P<0.01).
Figure 3.
Concentrations of both BNP-32 (A) and NT-BNP-76 (B) were reduced after kidney transplantation. Each line represents an individual patient. Time zero is the date of transplant.
Discussion
For BNP to become a useful biomarker in the management of patients with all forms of CKD, clinicians must better understand (1) appropriate clinical decision limits, (2) what degree of change over time is clinically significant, and (3) what management to initiate in response to the BNP measurement. This study addresses the second issue in relation to the spectrum of patients with CKD patients. The amount of change in BNP over time was greatest for NT-BNP-76 in dialysis patients. Only cTnT at the time of the second sample was consistently associated with a greater increase in NT-BNP-76 in the three groups. We did not confirm the mortality associations of change in BNP demonstrated previously in dialysis patients (6–8), but we did demonstrate that patients with CKD 3–4 who were in the highest tertile of change in NT-BNP-76 had significantly poorer survival compared with those in the other tertiles.
An increase in BNP concentration over time in dialysis patients has been previously demonstrated for both NT-BNP-76 (7,14,15) and BNP-32 (16), although two other studies demonstrated no significant change in these markers (17,18). The concurrent measurement of both forms of BNP in three groups of patients allowed us to directly compare patients with CKD 3–4 and KTRs to dialysis patients. Thus, the patients with CKD 3–4 and KTR were “control” groups, so we can be confident that the change in BNP over time in dialysis patients is real.
Dialysis patients have unique factors, such as short-term effects of the hemodialysis procedure, arteriovenous vascular access, fluctuations in volume status, and highly prevalent LV abnormalities, that might influence degree of change in BNP. All samples in this study were collected just before the hemodialysis procedure to eliminate effects of this procedure (19,20). Studies of the effect of vascular access creation on BNP concentrations are inconclusive (21,22), and studies of BNP as a biomarker of volume overload in patients receiving dialysis also vary in their conclusions (15,16,18).
Interpreting BNP as a marker of volume state requires knowledge of the underlying LV structure and function. The change in NT-BNP-76 on serial measurement was independently associated with the serial change in the peak early transmitral flow velocity to peak early diastolic mitral annular velocity ratio, which estimates LV filling pressure (14). In hemodialysis patients, the percentage change in LV mass index over time was significantly associated with the percentage change in log-transformed NT-BNP-76, but not BNP-32 (23).
Despite a large increase in NT-BNP-76 over time, this study did not demonstrate a mortality association with change in BNP in dialysis patients. This may be due to lack of statistical power. Examination of the survival curves demonstrates separation of the curves for the first 2 years but convergence of particularly the first and second tertiles thereafter. Thus, change in BNP may reflect an acute change in volume state or LV function that influences subsequent risk of death, whereas other noncardiac causes of death might cause more mortality events over the longer term. In studies with larger sample sizes than ours, the association of change in NT-BNP-76 or BNP-32 and mortality was reported at 1 year (6) and at 18 months (8), respectively. However, the 4D Study reported a significant association of doubling of NT-BNP-76 with death after a longer median follow-up of 4 years (7). We also demonstrated that patients with CKD 3–4 with greatest tertile of change in NT-BNP-76 had the poorest survival. There was no evidence that this was associated with declining GFR.
In some patients in this cohort, a change in GFR did result in a change in BNP. Eleven patients received a kidney transplant during the study and demonstrated a significant reduction in BNP concentration in concert with the rise in GFR. Both BNP-32 (24) and NT-BNP-76 (25) were reduced after transplant and were inversely associated with measures of kidney function. NT-BNP-76 was higher after transplant if graft function was delayed (25). A fall in BNP following restoration of GFR by renal transplantation may result from increased elimination of BNP or reduction in LV stretch. The relative contribution to the fall in BNP of these factors requires serial LV imaging to be determined. A limitation of our study is that prospective serial cardiac imaging, assessment of volume state, and long-term assessment of cardiovascular events were not performed.
Distinguishing clinically important change from biologic variation is important if serial measurement of BNP is to be clinically useful. In our study, the intraindividual variability of BNP concentrations was high. The reference change value is the degree of change that is clinically important and not just attributable to biologic variability. This was demonstrated in different studies to be 102% for NT-BNP-76 in hemodialysis patients (26). In patients with stable heart failure, this value ranges from 23% (27) to 98% (28).
Studies to define a role for routine measurement of BNP in CKD should be performed in the specific populations, and data derived from the NT-BNP-76 assay cannot be applied to the use of BNP-32 assays. From our data, one might favor NT-BNP-76 over BNP-32 because the differences between groups were more pronounced and only NT-BNP-76 demonstrated some mortality associations. In addition, NT-BNP-76 is more stable in vitro, and the higher concentration makes it easier to measure than BNP-32 (29). A different effect of renal elimination between BNP-32 and NT-BNP-76 has been described (30) but not confirmed by a study of the dependence of NT-BNP-76 and BNP-32 on renal clearance (31). Further studies of both markers simultaneously are required to better elucidate the factors causing the differential increase in their concentrations.
Randomized controlled trials in patients with heart failure have demonstrated a reduced mortality when interventions are applied in a BNP-driven management strategy (32); in patients undergoing dialysis, appropriate interventions remain to be identified. A BNP-guided management strategy might include improving volume control with dialysis or titrating medications with prognostic benefit in cardiovascular studies, such as β-blocker therapy, which has been demonstrated to decrease BNP in dialysis patients (33,34).
In conclusion, change in BNP over time differs by stage of CKD, with the greatest change evident in dialysis patients. Higher all-cause mortality is associated with rising BNP in CKD 3–4. Further studies in larger cohorts are necessary to enable development of clinical decision limits specific to the stage of CKD so that BNP-guided management strategies can be developed for clinical use in these patients.
Disclosures
None.
Supplementary Material
Acknowledgments
M.A.R. is supported by a National Health and Medical Research Council of Australia Health Professional Training Fellowship (628902). This study was supported by unrestricted grants from Amgen, Bristol-Myers Squibb, Janssen-Cilag, and Servier. Abbott Diagnostics, Roche Diagnostics Australia, and Medtec Products Australia provided assay kits at various levels of discount for the biochemical assays.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08640813/-/DCSupplemental.
References
- 1.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Niizuma S, Iwanaga Y, Yahata T, Tamaki Y, Goto Y, Nakahama H, Miyazaki S: Impact of left ventricular end-diastolic wall stress on plasma B-type natriuretic peptide in heart failure with chronic kidney disease and end-stage renal disease. Clin Chem 55: 1347–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Roberts MA, Srivastava PM, Macmillan N, Hare DL, Ratnaike S, Sikaris K, Ierino FL: B-type natriuretic peptides strongly predict mortality in patients who are treated with long-term dialysis. Clin J Am Soc Nephrol 3: 1057–1065, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D: N-terminal pro brain natriuretic peptide predicts mortality in patients with end-stage renal disease in hemodialysis. Kidney Int 71: 548–554, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS-HF Study Group : Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 364: 11–21, 2011. 21073363 [Google Scholar]
- 6.Gutiérrez OM, Tamez H, Bhan I, Zazra J, Tonelli M, Wolf M, Januzzi JL, Chang Y, Thadhani R: N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations in hemodialysis patients: Prognostic value of baseline and follow-up measurements. Clin Chem 54: 1339–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Winkler K, Wanner C, Drechsler C, Lilienthal J, März W, Krane V, German Diabetes and Dialysis Study Investigators : Change in N-terminal-pro-B-type-natriuretic-peptide and the risk of sudden death, stroke, myocardial infarction, and all-cause mortality in diabetic dialysis patients. Eur Heart J 29: 2092–2099, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breidthardt T, Kalbermatter S, Socrates T, Noveanu M, Klima T, Mebazaa A, Mueller C, Kiss D: Increasing B-type natriuretic peptide levels predict mortality in unselected haemodialysis patients. Eur J Heart Fail 13: 860–867, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Pöge U, Gerhardt T, Palmedo H, Klehr H-U, Sauerbruch T, Woitas RP: MDRD equations for estimation of GFR in renal transplant recipients. Am J Transplant 5: 1306–1311, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS: Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56: 486–495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Product Information. Triage BNP, San Diego, CA, Biosite Inc., 2003 [Google Scholar]
- 12.Product Information proBNP, Pro Brain Natriuretic Peptide, Elecsys 1010/2010/Modular Analytics E170, Indianapolis, IN, Roche Diagnostics, 2004 [Google Scholar]
- 13.Bland JM, Altman DG: Measurement error proportional to the mean. BMJ 313: 106, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YK, Shin SJ, Ihm SH, Park CS, Kim HY, Hong TY, Song HC, Yang CW, Kim YS, Choi EJ: Longitudinal changes of left ventricular filling pressure and N-terminal pro-brain natriuretic peptide on chronic hemodialysis. Clin Nephrol 74: 190–197, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Davenport A: Changes in N-terminal pro-brain natriuretic peptide correlate with fluid volume changes assessed by bioimpedance in peritoneal dialysis patients. Am J Nephrol 36: 371–376, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Roueff S, Martin E, Chauffert ML, Poli I, Kihal K, Yazbeck F, Abbassi A, Saint-Georges M: Brain natriuretic peptide variations are linked to volume status in hemodialysis patients. Clin Nephrol 70: 508–513, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Jacobs LH, van de Kerkhof JJ, Mingels AM, Passos VL, Kleijnen VW, Mazairac AH, van der Sande FM, Wodzig WK, Konings CJ, Leunissen KM, van Dieijen-Visser MP, Kooman JP: Inflammation, overhydration and cardiac biomarkers in haemodialysis patients: A longitudinal study. Nephrol Dial Transplant 25: 243–248, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Ortega O, Rodriguez I, Gracia C, Sanchez M, Lentisco C, Mon C, Gallar P, Ortiz M, Herrero JC, Oliet A, Vigil A: Strict volume control and longitudinal changes in cardiac biomarker levels in hemodialysis patients. Nephron Clin Pract 113: c96–c103, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Wang AY: Clinical utility of natriuretic peptides in dialysis patients. Semin Dial 25: 326–333, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Roberts MA, Hedley AJ, Ierino FL: Understanding cardiac biomarkers in end-stage kidney disease: Frequently asked questions and the promise of clinical application. Nephrology (Carlton) 16: 251–260, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Hiremath S, Doucette SP, Richardson R, Chan K, Burns K, Zimmerman D: Left ventricular growth after 1 year of haemodialysis does not correlate with arteriovenous access flow: A prospective cohort study. Nephrol Dial Transplant 25: 2656–2661, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Iwashima Y, Horio T, Takami Y, Inenaga T, Nishikimi T, Takishita S, Kawano Y: Effects of the creation of arteriovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis 40: 974–982, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Choi SY, Lee JE, Jang EH, Kim MO, Baek H, Ki CS, Park SW, Kim DJ, Huh WS, Oh HY, Kim YG: Association between changes in N-terminal pro-brain natriuretic peptide levels and changes in left ventricular mass index in stable hemodialysis patients. Nephron Clin Pract 110: c93–c100, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Wei T-M, Jin L, Lv L-C, Zhang B-J, Wang L-X: Changes in plasma B-type natriuretic peptide after allograft renal transplantation. Nephrology (Carlton) 12: 102–106, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Bodlaj G, Hubmann R, Saleh K, Biesenbach G, Pohanka E, Stojakovic T, Berg J: Serum levels of N-terminal pro-B-type natriuretic peptide are associated with allograft function in recipients of renal transplants. Wien Klin Wochenschr 121: 631–637, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Aakre KM, Roraas T, Hyltoft Petersen P, Svarstad E, Saele K, Sandberg S: Week-to-week biological variation in the n-terminal prohormone of brain natriuretic peptide in hemodialysis patients and healthy individuals. Clin Chem 59: 1813–1814, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Schou M, Gustafsson F, Nielsen PH, Madsen LH, Kjaer A, Hildebrandt PR: Unexplained week-to-week variation in BNP and NT-proBNP is low in chronic heart failure patients during steady state. Eur J Heart Fail 9: 68–74, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Bruins S, Fokkema MR, Römer JW, Dejongste MJ, van der Dijs FP, van den Ouweland JM, Muskiet FA: High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clin Chem 50: 2052–2058, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Sikaris K: BNP—Considering a heartfelt message. Heart Lung Circ 13[Suppl 3]: S31–S37, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Vickery S, Price CP, John RI, Abbas NA, Webb MC, Kempson ME, Lamb EJ: B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with CKD: Relationship to renal function and left ventricular hypertrophy. Am J Kidney Dis 46: 610–620, 2005 [DOI] [PubMed] [Google Scholar]
- 31.van Kimmenade RR, Januzzi JL, Jr, Bakker JA, Houben AJ, Rennenberg R, Kroon AA, Crijns HJ, van Dieijen-Visser MP, de Leeuw PW, Pinto YM: Renal clearance of B-type natriuretic peptide and amino terminal pro-B-type natriuretic peptide a mechanistic study in hypertensive subjects. J Am Coll Cardiol 53: 884–890, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Felker GM, Hasselblad V, Hernandez AF, O’Connor CM: Biomarker-guided therapy in chronic heart failure: A meta-analysis of randomized controlled trials. Am Heart J 158: 422–430, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Hara Y, Hamada M, Shigematsu Y, Murakami B, Hiwada K: Beneficial effect of beta-adrenergic blockade on left ventricular function in haemodialysis patients. Clin Sci (Lond) 101: 219–225, 2001 [PubMed] [Google Scholar]
- 34.Kojima M, Sato K, Kimura G, Ueda R, Dohi Y: Carvedilol reduces elevated B-type natriuretic peptide in dialyzed patients without heart failure: Cardioprotective effect of the beta-blocker. J Cardiovasc Pharmacol 49: 191–196, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.