Abstract
Background and objectives
There is conflicting evidence comparing peritonitis rates among patients treated with continuous ambulatory peritoneal dialysis (CAPD) or automated peritoneal dialysis (APD). This study aims to clarify the relationship between peritoneal dialysis (PD) modality (APD versus CAPD) and the risk of developing PD-associated peritonitis.
Design, setting, participants, & measurements
This study examined the association between PD modality (APD versus CAPD) and the risks, microbiology, and clinical outcomes of PD-associated peritonitis in 6959 incident Australian PD patients between October 1, 2003, and December 31, 2011, using data from the Australia and New Zealand Dialysis and Transplant Registry. Median follow-up time was 1.9 years.
Results
Patients receiving APD were younger (60 versus 64 years) and had fewer comorbidities. There was no association between PD modality and time to first peritonitis episode (adjusted hazard ratio [HR] for APD versus CAPD, 0.98; 95% confidence interval [95% CI], 0.91 to 1.07; P=0.71). However, there was a lower hazard of developing Gram-positive peritonitis with APD than CAPD, which reached borderline significance (HR, 0.90; 95% CI, 0.80 to 1.00; P=0.05). No statistically significant difference was found in the risk of hospitalizations (odds ratio, 1.12; 95% CI, 0.93 to 1.35; P=0.22), but there was a nonsignificant higher likelihood of 30-day mortality (odds ratio, 1.33; 95% CI, 0.93 to 1.88; P=0.11) at the time of the first episode of peritonitis for patients receiving APD. For all peritonitis episodes (including subsequent episodes of peritonitis), APD was associated with lower rates of culture-negative peritonitis (incidence rate ratio [IRR], 0.81; 95% CI, 0.69 to 0.94; P=0.002) and higher rates of gram-negative peritonitis (IRR, 1.28; 95% CI, 1.13 to 1.46; P=0.01).
Conclusions
PD modality was not associated with a higher likelihood of developing peritonitis. However, APD was associated with a borderline reduction in the likelihood of a first episode of Gram-positive peritonitis compared with CAPD, and with lower rates of culture-negative peritonitis and higher rates of Gram-negative peritonitis. Peritonitis outcomes were comparable between both modalities.
Keywords: peritoneal dialysis, peritonitis, registry study
Introduction
Peritonitis is a serious complication of peritoneal dialysis (PD), and is responsible for significant morbidity and mortality. It accounted for 22% of all technique failures, excluding death and transplantation, in Australian and New Zealand PD patients in 2010 (1), and has been found to be associated with a higher likelihood of mortality (2). Multiple risk factors for developing peritonitis have been identified, but there remains conflicting evidence on peritonitis risk between continuous ambulatory peritoneal dialysis (CAPD) and automated peritoneal dialysis (APD).
There have been reports of lower (3–6), similar (7–12), or higher (13,14) peritonitis rates in patients treated with APD compared with CAPD. The available evidence is predominantly based on observational studies, with only two small randomized controlled trials (3,8) and a meta-analysis of these trials (15). These studies are limited by the small sample size and/or retrospective nature of study design. Some of these studies used a cycler machine that is no longer available (3). Moreover, the observed peritonitis rates may have also been variably affected by several center-specific and patient-specific factors (including medical comorbidities, prior RRT, and unit practices). Furthermore, these studies were conducted at different time periods, raising the possibility of vintage bias. The last 2 decades have witnessed a substantial change in PD practice in terms of connectology, cycler machines, PD solutions, adequacy targets, and guidelines for prevention and treatment of infections. Therefore, the results of some of these studies cannot be generalized to contemporary PD practice.
This study aimed to examine the association between PD modality (APD versus CAPD) and the risks, microbiology, and clinical outcomes of PD-associated peritonitis in all Australian PD patients.
Materials and Methods
Study Population
This study included all incident Australian adult PD patients who commenced PD between October 1, 2003 (when collection of detailed peritonitis data started), and December 31, 2011. Follow-up was until the end of 2011.
Data Collection
Deidentified data were obtained from the Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry. This registry collects data on all patients receiving RRT in Australia and New Zealand, and details regarding its method of collection were previously described (16). The data collected included demographic data, cause of primary renal disease, comorbidities at the time of commencement of RRT, body mass index (BMI), and, for Australian patients, microbiology of peritonitis episodes (up to three organisms for polymicrobial episodes).
APD use was reported by the individual centers and could be performed either at home, within a satellite dialysis unit temporarily for either training or an acute medical reason, or in the hospital during an admission. When reporting baseline characteristics, we classified patients according to whether they ever received APD during the study period. For all other analyses, PD modality was treated as a time-varying covariate—patients could change between groups and contribute exposure time with or without peritonitis episodes to each group.
Peritonitis was reported by the treating centers at the time of diagnosis. The primary outcome examined was peritonitis-free survival, based on the time to the first peritonitis event. Additional analyses were performed according to whether the organisms recovered from dialysate during peritonitis episodes were Gram-positive, Gram-negative, or culture-negative. Secondary outcomes included peritonitis-associated hospitalization, catheter removal, temporary hemodialysis transfer (in which patients subsequently resumed PD), permanent hemodialysis transfer, and mortality. We defined peritonitis-associated mortality as death within 30 days of an episode of peritonitis (2). Overall peritonitis rates, including subsequent peritonitis episodes, for both APD and CAPD were also determined.
Statistical Analyses
Results were expressed as frequencies and percentages, means±SD, or medians (25th to 75th percentiles) as appropriate. Differences between groups were analyzed by the chi-squared test for categorical data, the unpaired t test for continuous normally distributed data, and the Mann–Whitney test for continuous non-normally distributed data.
Peritonitis-free survival was determined by the Kaplan–Meier method and by multivariable Cox proportional hazards models, with shared frailty to account for patient clustering within centers. Each model was adjusted for age at PD commencement, racial origin, BMI, comorbidities (diabetes mellitus, coronary artery disease, cerebrovascular disease), smoking history, and whether the patient received any form of RRT before PD. Proportional hazards assumptions were checked by scaled Schoenfeld residuals, which were examined graphically as well as by formal hypothesis testing. For the Gram-positive, Gram-negative, and culture-negative subanalyses, a peritonitis event was only recorded if the patient experienced a Gram-positive, Gram-negative, or culture-negative peritonitis episode, respectively. Infections due to different organisms were censored. Follow-up was censored at renal transplantation, recovery of renal function, hemodialysis transfer, or death. Hemodialysis transfer was defined as a transfer from PD to hemodialysis without return to PD within 30 days. First-order interaction terms between the significant covariates were examined where appropriate.
The independent predictors of the clinical outcomes of peritonitis were determined by multilevel multivariable logistic regression, according to whether patients were receiving APD or CAPD at the time of peritonitis. In addition to the variables utilized in the previous model, these analyses were further adjusted for the peritonitis organism (classified as culture-negative, Gram-positive, Gram-negative nonpseudomonal, Gram-negative pseudomonal, fungal, other, or polymicrobial). A random intercept was included for each treating center to allow each center to have a different baseline infection rate.
Finally, a multivariable, three-level mixed-effects Poisson model was utilized to determine the association between APD and overall peritonitis rate, with random intercepts for both treating center and individual patients. Each model was adjusted for the above-described confounders, and an additional term was included to indicate whether a patient had already had at least one episode of peritonitis. Data were analyzed using Stata/IC 12.1 software (StataCorp LP, College Station, TX). P values <0.05 were considered statistically significant for both main effects and interactions.
Results
Population Characteristics
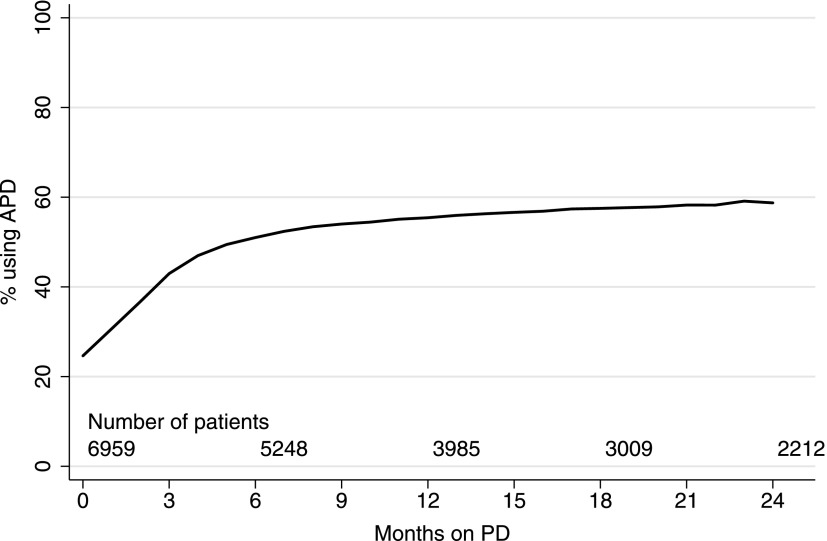
A total of 6959 patients commenced PD in Australia during the study period (October 1, 2003, to December 31, 2011). The baseline characteristics of the patients are shown in Table 1. Compared with patients who never received APD, those receiving APD were generally younger with fewer comorbidities. The use of APD increased with the increasing duration of PD (Figure 1). Patients who ever received APD spent a mean of 22% of their PD time receiving CAPD. The median technique survival was 1.9 years (95% confidence interval [95% CI], 1.9 to 2.0 years).
Table 1.
Characteristics of patients commencing PD, according to APD exposure
| Factor | CAPD Group | APD Group | P Value |
|---|---|---|---|
| Patients (n) | 2761 | 4198 | |
| RRT before PD | 992 (35.9) | 1603 (38.2) | 0.06 |
| Age at PD start (yr) | 64.0 (52.0, 73.0) | 60.0 (48.0, 70.0) | <0.001 |
| Men | 1508 (54.6) | 2506 (59.7) | <0.001 |
| Race | 0.01 | ||
| Caucasian | 2066 (74.8) | 3219 (76.7) | |
| Indigenous | 257 (9.3) | 294 (7.0) | |
| Asian | 329 (11.9) | 512 (12.2) | |
| Other | 109 (3.9) | 173 (4.1) | |
| Primary renal disease | <0.001 | ||
| GN | 646 (23.4) | 1196 (28.5) | |
| Analgesic nephropathy | 82 (3.0) | 82 (2.0) | |
| Polycystic kidney disease | 164 (5.9) | 267 (6.4) | |
| Reflux nephropathy | 75 (2.7) | 166 (4.0) | |
| Hypertension | 428 (15.5) | 569 (13.6) | |
| Diabetic nephropathy | 963 (34.9) | 1306 (31.1) | |
| Other | 235 (8.5) | 364 (8.7) | |
| Uncertain | 168 (6.1) | 248 (5.9) | |
| BMI (kg/m2) | 26.1 (22.8, 30.1) | 26.2 (22.8, 30.0) | 0.95 |
| Smoking at RRT entry | 0.99 | ||
| Never | 1322 (48.0) | 2008 (47.9) | |
| Former | 1080 (39.2) | 1641 (39.2) | |
| Current | 352 (12.8) | 539 (12.9) | |
| Diabetes | 1234 (44.7) | 1716 (40.9) | 0.002 |
| Coronary artery disease | 1114 (40.3) | 1531 (36.5) | 0.001 |
| Peripheral vascular disease | 751 (27.2) | 942 (22.4) | <0.001 |
| Chronic lung disease | 455 (16.5) | 607 (14.5) | 0.02 |
| Cerebrovascular disease | 444 (16.1) | 561 (13.4) | 0.002 |
| PET at baseline (4-h dialysate/plasma creatinine)a | 0.69 (0.14) | 0.69 (0.14) | 0.29 |
Data are presented as n (%) or median (interquartile range). The CAPD group never received APD, whereas the APD group received APD at least once. APD, automated peritoneal dialysis; BMI, body mass index; CAPD, continuous ambulatory peritoneal dialysis; PD, peritoneal dialysis; PET, peritoneal equilibrium test.
Missing in 29% of cohort. All other values missing in <1%.
Figure 1.
APD use over time: the proportion of incident Australian PD patients using APD according to time spent on PD during the period from 2003 to 2011. APD, automated peritoneal dialysis; CAPD, continuous ambulatory peritoneal dialysis; PD, peritoneal dialysis.
Peritonitis-Free Survival
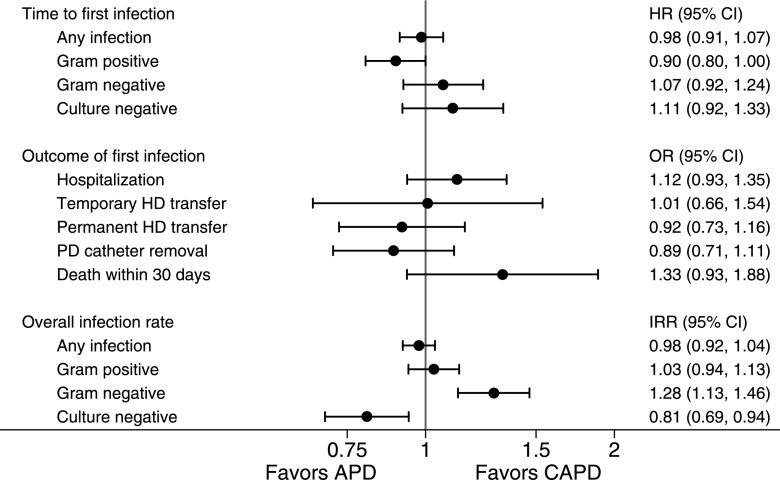
The analysis of time to first peritonitis included 3175 episodes of peritonitis over 8100 patient-years. The unadjusted incidence rates of first peritonitis were 0.43 infections per patient-year (95% CI, 0.41 to 0.45) in the CAPD group and 0.36 infections per patient-year (95% CI, 0.34 to 0.37) in the APD group. The overall median time to peritonitis was 1.8 years (95% CI, 1.7 to 1.9 years). After adjusting for confounders, there was no association between PD modality and time to first peritonitis episode (hazard ratio for APD versus CAPD, 0.98; 95% CI, 0.91 to 1.07; P=0.71) (Figure 2, Table 2). The other independent predictors of increased peritonitis were any RRT modality before the commencement of PD, race, higher BMI, diabetes mellitus, cerebrovascular disease, and current or former cigarette smoking.
Figure 2.
Association between APD (versus CAPD) and peritonitis-associated outcomes in incident Australian PD patients during the period from 2003 to 2011. All estimates are adjusted for previous RRT before PD, age, race, body mass index, diabetes mellitus, coronary artery disease, cigarette smoking, and cerebrovascular disease. Outcome of first infection also adjusted for organism. 95% CI, 95% confidence interval; HD, hemodialysis; HR, hazard ratio; IRR, incidence rate ratio; OR, odds ratio.
Table 2.
Results of multivariable Cox proportional hazards model analyses for time to first episode of peritonitis in all patients commencing PD in Australia between 2003 and 2011
| Factor | HR | 95% CI | P Value |
|---|---|---|---|
| APD | 0.98 | 0.91 to 1.07 | 0.71 |
| RRT before PD | 1.16 | 1.07 to 1.25 | <0.001 |
| Age at PD start | 1.00 | 1.00 to 1.00 | 0.24 |
| Race | |||
| Caucasian | 1a | ||
| Indigenous | 1.67 | 1.45 to 1.92 | <0.001 |
| Asian | 0.88 | 0.78 to 0.99 | 0.04 |
| Other | 1.08 | 0.91 to 1.29 | 0.38 |
| BMI (kg/m2) | 1.01 | 1.01 to 1.02 | <0.001 |
| Diabetes | 1.09 | 1.07 to 1.18 | 0.03 |
| Coronary artery disease | 1.07 | 0.99 to 1.16 | 0.11 |
| Smoking at RRT entry | |||
| Never | 1a | ||
| Former | 1.12 | 1.04 to 1.21 | 0.004 |
| Current | 1.12 | 1.01 to 1.26 | 0.04 |
| Cerebrovascular disease | 1.14 | 1.03 to 1.27 | 0.01 |
95% CI, 95% confidence interval; HR, hazard ratio.
Reference group.
Microbiology of First Peritonitis Episodes
The risks of developing a first peritonitis episode categorized by the microorganism isolated from dialysate cultures are summarized in Figure 2. There was a lower hazard of developing Gram-positive peritonitis with APD than CAPD that reached borderline significance (hazard ratio, 0.90; 95% CI, 0.80 to 1.00; P=0.05).
Outcomes of Peritonitis Episodes
Clinical outcomes were comparable between patients receiving CAPD or APD at the time of peritonitis (Figure 2). However, there was a nonsignificant higher likelihood of 30-day mortality in patients on APD who developed peritonitis (odds ratio, 1.33; 95% CI, 0.93 to 1.88; P=0.11). There was also a nonsignificant higher likelihood of hospitalization in patients on APD (odds ratio, 1.12; 95% CI, 0.93 to 1.35; P=0.22).
All Peritonitis Episodes
When all peritonitis episodes were included, the unadjusted peritonitis rates were 0.58 infections per patient-year (95% CI, 0.56 to 0.60) in the CAPD group and 0.52 infections per patient-year (95% CI, 0.50 to 0.54) in the APD group. After adjusting for confounders, the risk of developing peritonitis was not different between the two PD modalities (Figure 2). However, APD was associated with lower rates of culture-negative peritonitis (incidence rate ratio, 0.81; 95% CI, 0.69 to 0.94; P=0.002) and higher rates of Gram-negative peritonitis (incidence rate ratio, 1.28; 95% CI, 1.13 to 1.46; P=0.01).
Discussion
This large multicenter registry study demonstrated that PD modality was not associated with a patient’s overall risk of developing peritonitis. However, we found that APD was associated with a lower risk of culture-negative peritonitis, which was balanced by an increased risk of Gram-negative peritonitis compared with CAPD. When only the first peritonitis episodes were analyzed, APD was not associated with overall peritonitis risk but was associated with a lower risk of Gram-positive peritonitis. At the same time, the clinical outcomes of peritonitis were comparable between both patient groups.
The overall finding of our analysis is in agreement with a number of other observational studies (7,9–12) and one randomized control trial (8), which reported comparable peritonitis rates between CAPD and APD. Two of the observational studies were also registry-based analyses from Canada (11) and Scotland (12). Each consisted of a smaller cohort of patients compared with our analysis, and each was performed almost a decade ago. The randomized trial reporting no association between PD modality and peritonitis was performed in three Danish dialysis units >10 years ago, and was limited by its small sample size (8). The study involved only 13 CAPD and 12 APD patients, and was limited by a low event rate (only three peritonitis events in total) (8).
By contrast, two other observational studies showed higher rates of peritonitis in APD (13,14). Golper et al. compared 866 CAPD patients with 213 APD patients, and found that the peritonitis-free interval was inferior in the APD patients (14.9 versus 20.2 months), such that the relative risk for peritonitis was 28% higher in APD patients (13). Similarly, in a retrospective study of the US Renal Data System, Oo et al. found that CAPD patients had a lower risk of peritonitis (relative risk, 0.94; 95% CI, 0.88 to 0.99) compared with APD patients (14). Unfortunately, this study only examined the time to first peritonitis after 9 months of PD had been completed. This is a clear limitation of the analysis and would have resulted in significant survivor bias. This study was also performed over a decade ago, limiting its applicability to current practice.
Several other studies have demonstrated lower peritonitis rates in association with APD therapy. A randomized controlled trial by de Fijter et al. found that APD was associated with lower rates of peritonitis than CAPD (0.51 versus 0.94 episodes per patient-year; P=0.03) (3). However, this trial was limited by its small sample size and short duration. A meta-analysis performed by Rabindranath et al. concluded that APD reduced peritonitis rates compared with CAPD (rate ratio, 0.54; 95% CI, 0.35 to 0.83) (15). However, this analysis was limited by both the number of available trials and patients (139 patients from three randomized controlled trials). Several other single-center, observational cohort studies have demonstrated reduced peritonitis rates with APD (4–6).
The apparent disparity in peritonitis rates according to PD modality between the previously mentioned studies and our study may be explained by the fact that many of the previously mentioned studies involved small patient numbers, had low peritonitis event rates, were conducted at different time periods, and utilized different cycler connection methodologies from study to study. As a result, the majority of these studies would have been confounded by both center-specific factors (e.g., patient training, center size, and regular auditing of infection rates) and patient-specific factors (e.g., compliance, age, diabetic status, connection systems used, and previous peritonitis episodes). Our study overcomes these limitations by including all PD patients within Australia, resulting in both a large patient population and a large number of events. However, we did find that in our patient cohort, patients receiving APD were younger (60 versus 64 years) and had slightly fewer comorbidities (Table 1). It is also important to note that the majority of the above-discussed studies were performed over a decade ago. By contrast, our study was restricted to 2003–2011, meaning that our results remain relevant to current clinical practice.
The decision for choosing a PD modality for individual patients in Australia is currently based on the Caring for Australasians with Renal Insufficiency guidelines and is dominated by lifestyle considerations. Although there may have initially been a preference for commencing patients more prone to infections on APD, the International Society of Peritoneal Dialysis (ISPD) Peritonitis Guidelines have acknowledged that there is conflicting evidence as to the rates of peritonitis in APD and CAPD (17). The available evidence, updated with our findings, indicates that the PD modality does not affect the overall risk of peritonitis. However, the risk of developing peritonitis due to a Gram-positive organism appears to be higher with CAPD, whereas the risk of developing Gram-negative peritonitis is lower in these patients. The higher likelihood of Gram-positive peritonitis has also been reported by other investigators (4,7,10) and may be related to the greater number of daily connections required in CAPD versus APD, thereby increasing the likelihood of contamination with Gram-positive skin micro-organisms. Because ANZDATA does not collect any data on PD exit-site care, exit-site management may have differed between treating units in our study, potentially altering the risk of infections secondary to skin-based bacteria. However, exit-site care of patients on APD and CAPD within the same unit are not likely to have been different. The explanation for the observed lower risk of Gram-negative peritonitis in patients receiving CAPD compared with those receiving APD is uncertain. Although indigenous racial origin was more common in patients receiving APD and was previously identified by our group as a key independent risk factor for Pseudomonas peritonitis (18), race was adjusted for in the multivariable analysis.
With regard to other risk factors identified for the development of peritonitis, our study findings were consistent with a number of previous studies. For instance, our study confirmed the previous finding that the Australian indigenous population was at a higher risk of developing peritonitis (19). This study also confirmed the findings that an increased BMI at the time of PD commencement (20,21), the requirement for hemodialysis before commencing PD (11), diabetes mellitus (14,22,23), and cigarette smoking (24) all increase the likelihood of developing peritonitis. The fact that patients who transferred to PD from hemodialysis do poorly was also evident in a study performed by McDonald et al., who found that patients who changed from PD to hemodialysis had a higher mortality rate compared with those who commenced PD from the start (25). Additional risk factors that were identified but were not able to be measured in our model included socioeconomic status (26) and level of education (27).
The other important finding of this study is that there was a nonsignificant higher likelihood of both hospitalization and mortality in patients undergoing APD, a finding that may be of clinical significance. There was no statistical difference found in regard to hospitalization, and all other outcomes, including catheter removal and temporary or permanent transfer to hemodialysis, were comparable between the two modalities. These results can be compared with a single-center study performed by Rüger et al., which found no difference between CAPD and APD patients during treatment of 508 episodes of PD-related peritonitis in 205 patients with respect to the outcomes of relapse, mortality, or the combined end point of mortality plus catheter removal (28). There are a number of possible explanations for our findings. First, APD patients are less likely to observe the initial changes in their PD fluid at the time of peritonitis, because the majority of these patients are exchanging at night. Second, we found that APD patients were more likely to develop a Gram-negative infection as opposed to a Gram-positive infection. Previous studies demonstrated that Gram-negative peritonitis is associated with worse outcomes compared with Gram-positive infections, including higher rates of mortality (29) and technique failure (29,30). Another explanation may be related to a concern raised in the ISPD guidelines that the rapid exchanges in APD may lead to inadequate time to achieve satisfactory intraperitoneal levels of antibiotics, resulting in suboptimal peritonitis outcomes (31). Anecdotally, it appears to be common practice for patients to change modality to CAPD while being treated for peritonitis, although there is no means of assessing how commonly this occurs with our data.
The strengths of this study include its large sample size and inclusiveness. We included all incident PD patients during the study period, such that all centers providing PD service in Australia were included. Because there are substantial variations in the prevention, diagnosis, treatment, and auditing of peritonitis in Australian PD centers (32), the multicenter design of this study greatly enhanced the external validity of our results. In addition to center-specific factors, we corrected for several patient-specific risk factors in the multivariable analysis. These strengths should be balanced against the study’s limitations, which included limited depth of data collection. ANZDATA does not collect information such as the presence of concomitant exit-site and tunnel infections, patient compliance, socioeconomic status, severity of comorbidities, dialysate collection techniques or culture methods, and antibiotic dosages or routes of administration. Although we adjusted for a large number of patient characteristics, the possibility of residual confounding could not be excluded. Similar to other registries, ANZDATA is a voluntary registry and there is no external audit of data accuracy, including the diagnosis of peritonitis. Consequently, the possibility of coding/classification bias cannot be excluded. Finally, because this is an observational study, we are only able to demonstrate association and not causation.
In conclusion, this is the largest and most definitive study to examine the relationship between PD modality and peritonitis; in doing so, we found that PD modality was not associated with overall peritonitis risk. Future research should focus on other aspects of PD technique in order to improve peritonitis rates.
Disclosures
D.W.J. has received consultancy fees, research funds, speaker honoraria, and travel sponsorships from Baxter Healthcare Pty Ltd and Fresenius Medical Care. He was previously a consultant to Gambro and is a current recipient of a Queensland Government Health Research Fellowship. N.B. previously received research funds from Roche, travel grants from Roche, Amgen, and Jansen Cilag, and speaker honoraria from Roche. K.S. previously received speaker honoraria from Baxter Healthcare Pty Ltd and Boehringer Ingelheim, and conference sponsorships from Shire Australia Pty Ltd, Roche, Boehringer Ingelheim, and Novartis.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the Australian and New Zealand renal units, staff, and patients who contribute to the ANZDATA Registry.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09730913/-/DCSupplemental.
References
- 1.Brown F, Gulyani A, Dent H, Hurst K, McDonald SP: Peritoneal dialysis. In: 34th Annual Report 2011, edited by McDonald SP, Hurst K, Adelaide, South Australia, Australia and New Zealand Dialysis and Transplant Registry, 2011, pp 6-23–6-32 [Google Scholar]
- 2.Boudville N, Kemp A, Clayton P, Lim W, Badve SV, Hawley CM, McDonald SP, Wiggins KJ, Bannister KM, Brown FG, Johnson DW: Recent peritonitis associates with mortality among patients treated with peritoneal dialysis. J Am Soc Nephrol 23: 1398–1405, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Fijter CW, Oe LP, Nauta JJ, van der Meulen J, Verbrugh HA, Verhoef J, Donker AJ: Clinical efficacy and morbidity associated with continuous cyclic compared with continuous ambulatory peritoneal dialysis. Ann Intern Med 120: 264–271, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Sanchez AR, Madonia C, Rascon-Pacheco RA: Improved patient/technique survival and peritonitis rates in patients treated with automated peritoneal dialysis when compared to continuous ambulatory peritoneal dialysis in a Mexican PD center. Kidney Int Suppl 73: S76–S80, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Huang JW, Hung KY, Yen CJ, Wu KD, Tsai TJ: Comparison of infectious complications in peritoneal dialysis patients using either a twin-bag system or automated peritoneal dialysis. Nephrol Dial Transplant 16: 604–607, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Carmona A, Pérez Fontán M, García Falcón T, Fernández Rivera C, Valdés F: A comparative analysis on the incidence of peritonitis and exit-site infection in CAPD and automated peritoneal dialysis. Perit Dial Int 19: 253–258, 1999 [PubMed] [Google Scholar]
- 7.Akman S, Bakkaloglu SA, Ekim M, Sever L, Noyan A, Aksu N: Peritonitis rates and common microorganisms in continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Pediatr Int 51: 246–249, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Bro S, Bjorner JB, Tofte-Jensen P, Klem S, Almtoft B, Danielsen H, Meincke M, Friedberg M, Feldt-Rasmussen B: A prospective, randomized multicenter study comparing APD and CAPD treatment. Perit Dial Int 19: 526–533, 1999 [PubMed] [Google Scholar]
- 9.Troidle LK, Gorban-Brennan N, Kliger AS, Finkelstein FO: Continuous cycler therapy, manual peritoneal dialysis therapy, and peritonitis. Adv Perit Dial 14: 137–141, 1998 [PubMed] [Google Scholar]
- 10.Yishak A, Bernardini J, Fried L, Piraino B: The outcome of peritonitis in patients on automated peritoneal dialysis. Adv Perit Dial 17: 205–208, 2001 [PubMed] [Google Scholar]
- 11.Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV: Predictors of peritonitis in patients on peritoneal dialysis: Results of a large, prospective Canadian database. Clin J Am Soc Nephrol 4: 1195–1200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kavanagh D, Prescott GJ, Mactier RA: Peritoneal dialysis-associated peritonitis in Scotland (1999-2002). Nephrol Dial Transplant 19: 2584–2591, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, Strife F, Hamburger RJ: Risk factors for peritonitis in long-term peritoneal dialysis: The Network 9 peritonitis and catheter survival studies. Academic Subcommittee of the Steering Committee of the Network 9 Peritonitis and Catheter Survival Studies. Am J Kidney Dis 28: 428–436, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Oo TN, Roberts TL, Collins AJ: A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am J Kidney Dis 45: 372–380, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Rabindranath KS, Adams J, Ali TZ, Daly C, Vale L, Macleod AM: Automated vs continuous ambulatory peritoneal dialysis: A systematic review of randomized controlled trials. Nephrol Dial Transplant 22: 2991–2998, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Grace B, Hurst K, McDonald S: 34th Annual ANZDATA Report. Adelaide, South Australia: Australia and New Zealand Dialysis and Transplantation Registry. Available at: http://www.anzdata.org.au/v1/report_2011.html Accessed August 10, 2013
- 17.Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, Price V, Ramalakshmi S, Szeto CC: ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 31: 614–630, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Siva B, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, Bannister KM, Johnson DW: Pseudomonas peritonitis in Australia: Predictors, treatment, and outcomes in 191 cases. Clin J Am Soc Nephrol 4: 957–964, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim WH, Johnson DW, McDonald SP: Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: Analysis of ANZDATA. Nephrology (Carlton) 10: 192–197, 2005 [DOI] [PubMed] [Google Scholar]
- 20.McDonald SP, Collins JF, Rumpsfeld M, Johnson DW: Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 24: 340–346, 2004 [PubMed] [Google Scholar]
- 21.Lim WH, Boudville N, McDonald SP, Gorham G, Johnson DW, Jose M: Remote indigenous peritoneal dialysis patients have higher risk of peritonitis, technique failure, all-cause and peritonitis-related mortality. Nephrol Dial Transplant 26: 3366–3372, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Han SH, Lee SC, Ahn SV, Lee JE, Kim DK, Lee TH, Moon SJ, Kim BS, Kang SW, Choi KH, Lee HY, Han DS: Reduced residual renal function is a risk of peritonitis in continuous ambulatory peritoneal dialysis patients. Nephrol Dial Transplant 22: 2653–2658, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Chow KM, Szeto CC, Leung CB, Kwan BCH, Law MC, Li PKT: A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int 25: 374–379, 2005 [PubMed] [Google Scholar]
- 24.Kotsanas D, Polkinghorne KR, Korman TM, Atkins RC, Brown F: Risk factors for peritoneal dialysis-related peritonitis: Can we reduce the incidence and improve patient selection? Nephrology (Carlton) 12: 239–245, 2007 [DOI] [PubMed] [Google Scholar]
- 25.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR: Relationship between dialysis modality and mortality. J Am Soc Nephrol 20: 155–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chow KM, Szeto CC, Leung CB, Law MC, Li PK: Impact of social factors on patients on peritoneal dialysis. Nephrol Dial Transplant 20: 2504–2510, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Martin LC, Caramori JCT, Fernandes N, Divino-Filho JC, Pecoits-Filho R, Barretti P, Brazilian Peritoneal Dialysis Multicenter Study BRAZPD Group : Geographic and educational factors and risk of the first peritonitis episode in Brazilian Peritoneal Dialysis study (BRAZPD) patients. Clin J Am Soc Nephrol 6: 1944–1951, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rüger W, van Ittersum FJ, Comazzetto LF, Hoeks SE, ter Wee PM: Similar peritonitis outcome in CAPD and APD patients with dialysis modality continuation during peritonitis. Perit Dial Int 31: 39–47, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Troidle L, Gorban-Brennan N, Kliger A, Finkelstein F: Differing outcomes of gram-positive and gram-negative peritonitis. Am J Kidney Dis 32: 623–628, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Bunke CM, Brier ME, Golper TA: Outcomes of single organism peritonitis in peritoneal dialysis: Gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 52: 524–529, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, Johnson DW, Kuijper EJ, Lye WC, Salzer W, Schaefer F, Struijk DG, International Society for Peritoneal Dialysis : Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 30: 393–423, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Badve SV, Smith A, Hawley CM, Johnson DW: Adherence to guideline recommendations for infection prophylaxis in peritoneal dialysis patients. Nephrol Dial Transplant Plus 2: 508, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.