Abstract
Background and objectives
AKI is a risk factor for development or worsening of CKD. However, diagnosis of renal dysfunction by serum creatinine could be confounded by loss of muscle mass and creatinine generation after critical illness.
Design, setting, participants, & measurements
A retrospective, single center analysis of serum in patients surviving to hospital discharge with an intensive care unit admission of 5 or more days between 2009 and 2011 was performed.
Results
In total, 700 cases were identified, with a 66% incidence of AKI. In 241 patients without AKI, creatinine was significantly lower (P<0.001) at hospital discharge than admission (median, 0.61 versus 0.88 mg/dl; median decrease, 33%). In 160 patients with known baseline, discharge creatinine was significantly lower than baseline in all patients except those patients with severe AKI (Kidney Disease Improving Global Outcomes category 3), who had no significant difference. In a multivariable regression model, median duration of hospitalization was associated with a predicted 30% decrease (95% confidence interval, 8% to 45%) in creatinine from baseline in the absence of AKI; after allowing for this effect, AKI was associated with a 29% (95% confidence interval, 10% to 51%) increase in predicted hospital discharge creatinine. Using a similar model to exclude the confounding effect of prolonged major illness on creatinine, 148 of 700 patients (95% confidence interval, 143 to 161) would have eGFR<60 ml/min per 1.73 m2 at hospital discharge compared with only 63 of 700 patients using eGFR based on unadjusted hospital creatinine (a 135% increase in potential CKD diagnoses; P<0.001).
Conclusion
Critical illness is associated with significant falls in serum creatinine that persist to hospital discharge, potentially causing inaccurate assessment of renal function at discharge, particularly in survivors of AKI. Prospective measurements of GFR and creatinine generation are required to confirm the significance of these findings.
Keywords: acute renal failure, creatinine, glomerular filtration rate, progression of chronic renal failure
Introduction
AKI complicates over one half of intensive care unit (ICU) admissions (1) and has been associated with the development or progression of CKD and long-term mortality (2–5). Despite these concerns and the recommendations of international guidelines (6), survivors of critical illness complicated by AKI rarely receive specific renal follow-up (7), despite potential benefit (8), and the true prevalence of CKD in this group is not well understood. Importantly, calculation of eGFR (9) has not been calibrated in survivors of critical illness, which is associated with substantial and persistent decrease of muscle mass (the site of creatinine generation) (10). Accordingly, we performed a single center retrospective analysis of renal function in patients admitted to The Royal London Hospital ICU in 2009–2011. We hypothesized that, in critically ill patients who did not experience AKI, serum creatinine might fall over the course of hospitalization and might be related to surrogates of decreased muscle creatinine generation, including length or severity of illness. Furthermore, we hypothesized that the same processes causing decrease in creatinine might also occur in survivors of critical illness with AKI, confounding our ability to accurately assess risk for CKD in these patients at hospital discharge.
Materials and Methods
We performed a retrospective observational study examining renal function in all patients admitted to The Royal London Hospital Adult Critical Care Unit between January 1, 2009, and January 31, 2011. Approval was obtained from Barts Health/Queen Mary University of London Joint Research Office as a retrospective review of data collected as part of the usual care, and waiver of full research ethics committee review was obtained. We screened our admissions database for ICU admissions with survival to hospital discharge. To ensure comparable exposure with critical illness, we considered only patients with an ICU admission of 5 or more days. Because we aimed to understand the determinants of serum creatinine after recovery from acute critical illness, this study was limited to patients surviving to hospital discharge. Patients with new or preexisting ESRD and renal transplant recipients were excluded. We examined hospital pathology records for serum creatinine results at baseline, during hospitalization, and postdischarge. Baseline creatinine was defined as the last available measurement from 365 to 7 days before hospital admission (11). Where baseline creatinine was not available, hospital admission creatinine was used as baseline. AKI diagnosis was defined by the serum creatinine criteria of the 2012 Kidney Disease Improving Global Outcomes (KDIGO) criteria (6), and urine output values were not available for AKI diagnosis. Follow-up creatinine was defined as the last measurement obtained 90–365 days after index hospital discharge.
Statistical analysis was performed using R: A Language and Environment for Statistical Computing (12). Continuous variables are reported as medians and interquartile ranges (IQRs). Paired continuous data, including repeated creatinine samples in matched individuals, were compared using the Wilcoxon signed rank test. Paired categorical data were compared with McNemar’s test. Between-group comparisons were made using the Kruskal–Wallis test or the chi-squared test. We performed log-transformed multivariable linear regression analyses examining influences of AKI and critical illness on hospital discharge and follow-up creatinine using stepwise forward and backward selection based on minimization of the Akaike Information Criterion in the optimal model.
To assess effect of critical illness on the assessment of renal dysfunction at hospital discharge, we developed a regression model from patients with known baseline creatinine, predicting baseline to discharge creatinine ratio and allowing estimation of the illness-related change in creatinine in all patients. In developing our model, we examined AKI status as a factor variable (AKI category) or a continuous variable (peak to baseline creatinine ratio). Model-predicted baseline to discharge creatinine ratios allowed estimation of baseline creatinine by multiplying the predicted ratio by the hospital discharge creatinine, effectively removing the predicted change in creatinine related to nature and duration of illness. To validate this model, we compared the distribution of predicted baseline creatinine values with the distribution of observed admission creatinine values in patients who had no known baseline creatinine, no AKI, and admission creatinine of <1.4 mg/dl, defining a test set of new patients where admission creatinine would be expected to be similar to baseline creatinine. After validation, we used our model to derive adjusted creatinine values from hospital discharge values in all 700 admissions. For this analysis, we assigned a peak to baseline creatinine ratio of 1.0 (implying no AKI) to all patients, thereby correcting for only the decrease in discharge creatinine related to major illness. The final predictions of this model, thus, provide an estimate of the true baseline creatinine (extent of premorbid CKD) plus any deterioration in renal function that may have occurred as a consequence of AKI (unrecovered AKI or de novo CKD). From these adjusted creatinine values, we then calculated hospital discharge eGFRs using the Chronic Kidney Disease Epidemiology Collaboration creatinine formula (9) and compared these values against eGFRs calculated using unadjusted discharge creatinine, categorizing eGFR according to the GFR categories of the KDIGO CKD criteria (13).
Results
Univariate Analyses
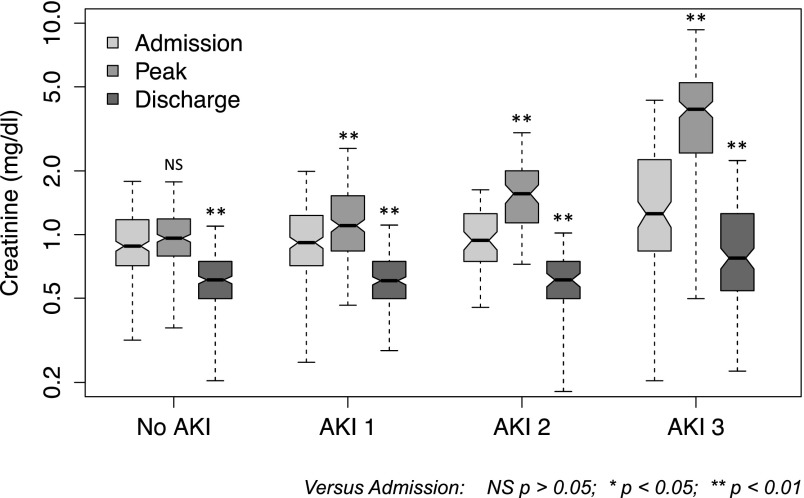
From 3101 ICU admissions to the Royal London Hospital ICU from 2009 to 2011, we identified 700 eligible hospitalizations (in 698 patients) with an ICU admission of 5 or more days and survival to discharge from the acute hospital (Supplemental Figure 1). AKI occurred in 459 cases (66%), AKI-1 occurred in 218 cases (31% of total), AKI-2 occurred in 75 cases (11%), and AKI-3 occurred in 166 cases (24%); 121 patients received RRT in the ICU (AKI-3 by definition). Increasing AKI category was associated with older age, increasing ICU admission illness severity scores, medical reason for hospital admission, and longer hospital length of stay (Table 1). Hospital discharge creatinine was significantly lower than at hospital admission (P<0.001; median, 0.63 versus 0.95 mg/dl; median difference, 0.31 mg/dl; median decrease from admission, 33%). Discharge creatinine values were significantly lower than admission across all AKI categories (Figure 1, Table 2) and admission creatinine values (Supplemental Figure 2). Of patients with AKI, 80% had their peak serum creatinine or required RRT in the ICU. Median time from ICU to hospital discharge was 22 days (IQR=10–39). In the 20% of patients with peak creatinine outside of the ICU, median hospital discharge creatinine was 0.61 (IQR=0.48–0.75), lower than the peak in patients who had peak creatinine or RRT in ICU, which was 0.66 (IQR=0.52–0.88; P=0.03).
Table 1.
Clinical variables by AKI category
| Patient Characteristics | All Patients | No AKI | AKI-1 | AKI-2 | AKI-3 | P Value |
|---|---|---|---|---|---|---|
| Number | 700 | 241 | 218 | 75 | 166 | |
| Age, yr | 49 (33–63) | 46 (32–60) | 48 (30–62) | 54 (38–65) | 55 (40–65) | 0.002 |
| Men, % | 68 | 70 | 70 | 68 | 62 | 0.32 |
| Racial background, % | 0.1 | |||||
| White/other | 74 | 69 | 78 | 81 | 73 | |
| Black | 12 | 18 | 8 | 7 | 12 | |
| Asian | 14 | 13 | 14 | 12 | 14 | |
| Diagnostic category, % | <0.001 | |||||
| Medical | 31 | 27 | 27 | 31 | 43 | |
| Surgical | 25 | 20 | 22 | 28 | 37 | |
| Trauma | 43 | 53 | 51 | 41 | 20 | |
| APACHE II | 15 (11–20) | 12 (9–16) | 13.5 (10–17) | 16 (13–20) | 20 (16–24) | <0.001 |
| SAPS II | 38 (31–47) | 35 (27–41) | 36 (30–44) | 43 (32–51) | 46 (38–52) | <0.001 |
| ICNARC | 19 (15–24) | 17 (14–20) | 18 (14–22) | 20 (16–24) | 25 (20–30) | <0.001 |
| Length of stay, d | ||||||
| Intensive care unit | 10 (7–16) | 8 (6–12) | 11.5 (7–16) | 11 (7–19) | 12 (8–19) | <0.001 |
| Hospital | 36 (23–59) | 28 (17–42) | 39 (26–62) | 47 (29–66) | 47 (29–90) | <0.001 |
Continuous variables are presented as medians and interquartile ranges; categorical variables are presented as percentages. P values represent between-column comparisons for no AKI, AKI-1, AKI-2, and AKI-3 using the Kruskal–Wallis test for continuous variables or the chi-squared test for categorical variables across categories. APACHE II, Acute Physiology, Age, Chronic Health Evaluation II; SAPS II, Simplified Acute Physiology Score II; ICNARC, Intensive Care National Audit & Research Centre Model Physiology Score.
Figure 1.
Hospital admission, peak, and hospital discharge creatinine (log scale) in 700 hospitalizations involving an intensive care unit stay of 5 or more days with survival to hospital discharge. Boxes indicate 25th to 75th percentiles, with a solid line at the median; the whiskers are 1.5× interquartile range from the box. Nonoverlap of notches suggests significant difference in medians at the P<0.05 level. Asterisks indicate statistical difference between paired creatinine values with the Wilcoxon signed rank test. NS, P>0.05. *P<0.05; **P<0.01.
Table 2.
Creatinine changes in subgroups of patients with baseline and follow-up creatinine
| Patient Groups | All Patients | No AKI | AKI-1 | AKI-2 | AKI-3 |
|---|---|---|---|---|---|
| Creatinine changes in all 700 patients | |||||
| Number of patients | 700 | 241 | 218 | 75 | 166 |
| HA | 0.95 (0.74–1.33) | 0.88 (0.71–1.18) | 0.92 (0.71–1.23) | 0.94 (0.75–1.26) | 1.26 (0.84–2.26) |
| ICU admission | 0.88 (0.64–1.29) | 0.80 (0.63–0.98) | 0.81 (0.60–1.07) | 0.86 (0.60–1.30) | 1.44 (1.03–2.82) |
| Peak | 1.28 (0.89–2.20) | 0.96 (0.79–1.20) | 1.10 (0.84–1.50) | 1.56 (1.14–2.00) | 3.92 (2.44–5.20) |
| ICU discharge | 0.63 (0.50–0.85) | 0.59 (0.50–0.71) | 0.61 (0.48–0.76) | 0.66 (0.50–0.87) | 1.05 (0.53–2.06) |
| HD | 0.63 (0.51–0.81) | 0.61 (0.50–0.75) | 0.61 (0.50–0.74) | 0.61 (0.50–0.75) | 0.77 (0.54–1.25) |
| P value (HA versus HD) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Creatinine changes in 160 patients with premorbid BL creatinine | |||||
| Number of patients | 160 | 38 | 28 | 24 | 70 |
| BL | 0.88 (0.73–1.11) | 0.81 (0.71–0.89) | 0.92 (0.69–1.06) | 0.95 (0.80–1.16) | 0.94 (0.76–1.21) |
| HA | 1.05 (0.78–1.48) | 0.86 (0.74–1.18) | 0.92 (0.60–1.17) | 1.03 (0.83–1.45) | 1.24 (0.84–2.51) |
| ICU admission | 1.11 (0.72–1.97) | 0.80 (0.68–0.98) | 0.88 (0.51–1.14) | 1.18 (0.63–1.50) | 2.07 (1.11–2.99) |
| Peak | 1.77 (1.10–3.46) | 1.04 (0.83–1.26) | 1.11 (0.75–1.57) | 1.73 (1.28–2.46) | 3.69 (2.71–5.11) |
| ICU discharge | 0.70 (0.49–1.37) | 0.61 (0.50–0.77) | 0.57 (0.39–0.76) | 0.63 (0.52–0.89) | 1.33 (0.58–2.24) |
| HD | 0.72 (0.57–1.01) | 0.66 (0.53–0.84) | 0.65 (0.51–0.82) | 0.71 (0.63–0.89) | 0.86 (0.66–1.31) |
| P value (BL versus HD) | <0.001 | <0.001 | <0.001 | 0.004 | 0.14 |
| Creatinine changes in 221 patients with FU creatinine | |||||
| Number of patients | 221 | 58 | 62 | 23 | 78 |
| HA | 1.02 (0.76–1.47) | 1.01 (0.79–1.33) | 0.84 (0.58–1.13) | 0.87 (0.73–1.15) | 1.35 (0.99–2.44) |
| ICU admission | 1.01 (0.63–1.56) | 0.88 (0.67–1.07) | 0.75 (0.52–1.05) | 0.74 (0.57–1.24) | 1.78 (1.11–2.92) |
| Peak | 1.50 (0.96–2.98) | 1.03 (0.81–1.45) | 1.00 (0.74–1.47) | 1.48 (1.08–1.87) | 4.13 (2.62–5.32) |
| ICU discharge | 0.67 (0.50–1.22) | 0.60 (0.52–0.74) | 0.56 (0.41–0.74) | 0.69 (0.55–0.87) | 1.50 (0.70–2.67) |
| HD | 0.67 (0.53–0.90) | 0.64 (0.56–0.76) | 0.56 (0.48–0.72) | 0.64 (0.50–0.84) | 0.92 (0.67–1.47) |
| FU | 0.85 (0.67–1.07) | 0.77 (0.67–0.89) | 0.72 (0.60–0.90) | 0.87 (0.64–1.10) | 1.04 (0.82–1.49) |
| P value (HD versus FU) | <0.001 | <0.001 | <0.001 | 0.01 | 0.02 |
| Creatinine changes in 96 patients with both BL and FU creatinine | |||||
| Number of patients | 96 | 17 | 16 | 16 | 47 |
| BL | 0.96 (0.77–1.16) | 0.86 (0.74–1.02) | 0.92 (0.62–1.05) | 0.98 (0.76–1.13) | 1.02 (0.82–1.33) |
| HA | 1.11 (0.80–1.49) | 0.84 (0.77–1.29) | 0.92 (0.59–1.17) | 0.97 (0.75–1.41) | 1.32 (0.94–2.51) |
| ICU admission | 1.15 (0.68–2.20) | 0.80 (0.64–0.98) | 0.99 (0.49–1.16) | 0.88 (0.59–1.38) | 2.12 (1.14–3.13) |
| Peak | 2.00 (1.20–3.83) | 1.09 (0.81–1.29) | 1.21 (0.72–1.69) | 1.52 (1.20–1.88) | 3.81 (2.72–4.93) |
| ICU discharge | 0.78 (0.51–1.65) | 0.63 (0.51–0.85) | 0.53 (0.39–0.89) | 0.62 (0.52–0.77) | 1.43 (0.70–2.60) |
| HD | 0.76 (0.55–1.09) | 0.67 (0.54–0.85) | 0.57 (0.50–0.82) | 0.68 (0.63–0.89) | 1.01 (0.70–1.67) |
| FU | 0.96 (0.72–1.28) | 0.84 (0.78–0.97) | 0.72 (0.60–0.85) | 1.00 (0.81–1.31) | 1.12 (0.88–1.96) |
| P value (BL versus HD) | <0.001 | 0.003 | 0.01 | 0.1 | 0.4 |
| P value (HD versus FU) | <0.001 | 0.03 | 0.11 | 0.01 | 0.02 |
| P value (BL versus FU) | 0.1 | 0.7 | 0.2 | 0.30 | 0.01 |
Paired comparisons were by the Wilcoxon signed rank test. HA, hospital admission; ICU, intensive care unit; HD, hospital discharge; BL, baseline; FU, follow-up.
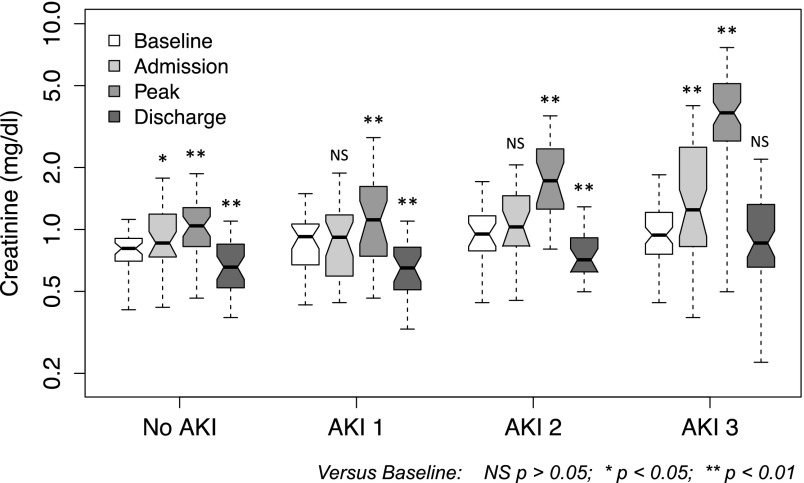
In patients without AKI, creatinine fell significantly (P<0.001; median, 0.88–0.61 mg/dl; median decrease, 33%) (Figure 1, Table 2). In 160 patients with premorbid baseline values, there was a significant decrease in creatinine from baseline to hospital discharge in patients with no AKI, AKI-1, or AKI-2 (P<0.001; median, 0.84–0.66 ml/dl; median decrease, 19%); however, after AKI-3, discharge values were similar to baseline (P=0.14; median, 0.94–0.86) (Figure 2, Table 2).
Figure 2.
Premorbid baseline, hospital admission, peak, and hospital discharge creatinine (log scale) in 160 hospitalizations with baseline values. Boxes indicate 25th to 75th percentiles, with a solid line at the median; the whiskers are 1.5× interquartile range from the box. Nonoverlap of notches suggests significant difference in medians at the P<0.05 level. Asterisks indicate statistical difference between paired creatinine values with the Wilcoxon signed rank test. NS, P>0.05. *P<0.05; **P<0.01.
Multivariable Regression Analyses
We developed a regression model using data from patients with premorbid baseline creatinine to examine factors independently influencing discharge creatinine. Only baseline creatinine, hospital length of stay, diagnostic category, and AKI category were retained in the final model (Table 3, column 1). Increasing hospital stay was significantly associated with lower hospital discharge creatinine; for example, in a typical patient (a trauma patient) with the median baseline creatinine of 0.88 mg/dl and the population median hospital length of stay of 39 days, our model predicts a 29% (95% confidence interval, 8% to 45%) fall in hospital discharge creatinine from baseline in the absence of AKI. In this model, AKI-3 would then confer a 29% increase (95% confidence interval, 10% to 51%) from that prediction, resulting in a predicted discharge creatinine not significantly different from baseline. Similar associations were seen when we considered all 700 patients in a model using hospital admission creatinine as a surrogate for baseline creatinine (Supplemental Table 1).
Table 3.
Log-log regression models
| Covariates or Factors Included in Model | Dependent Variable | ||
|---|---|---|---|
| Log(Hospital Discharge Creatinine) | Log(Baseline to Hospital Discharge Creatinine) | ||
| Model 1 | Model 2 | Model 3 | |
| Log(baseline creatinine) | 0.746a (0.073) | ||
| Log(hospital length of stay) | −0.107a (0.037) | 0.077b (0.038) | 0.075b (0.033) |
| Sex | 0.088 (0.061) | 0.068 (0.055) | |
| Log(age) | 0.135 (0.091) | 0.112 (0.081) | |
| Trauma | Reference | Reference | Reference |
| Surgical | −0.005 (0.140) | 0.021 (0.144) | 0.039 (0.129) |
| Medical | 0.166 (0.135) | −0.154 (0.140) | −0.133 (0.126) |
| No AKI | Reference | Reference | |
| AKI-1 | −0.040 (0.092) | 0.071 (0.094) | |
| AKI-2 | 0.086 (0.099) | −0.055 (0.101) | |
| AKI-3 | 0.254a (0.079) | −0.191b (0.079) | |
| Log(peak to baseline creatinine) | −0.290a (0.042) | ||
| Constant | 0.019 (0.185) | −0.566 (0.384) | −0.335 (0.344) |
| Observations | 160 | 160 | 160 |
| R2 | 0.53 | 0.16 | 0.31 |
| Adjusted R2 | 0.51 | 0.12 | 0.28 |
Regression coefficients for covariates and factors (SEM in parentheses).
P<0.01.
P<0.05.
Modeling the Effect of AKI on CKD Diagnosis
To predict the effect of prolonged major illness on diagnosis of renal dysfunction after recovery from critical illness, we constructed regression models predicting baseline to hospital discharge creatinine ratio in patients with known baseline creatinine (Table 3, columns 2 and 3). Hospital length of stay, age, sex, and diagnostic category were included in the final model. The model (Table 3, column 3) incorporating a continuous measure of AKI severity (peak to baseline creatinine ratio) had the best fit and was chosen. In our test cohort, model predictions very closely matched the actual distribution of admission creatinine (Supplemental Figure 3, Supplemental Table 2), Kolmogorov–Smirnov P value was 0.98, which is strong evidence that the actual data and the model estimates could be drawn from the same continuous distribution.
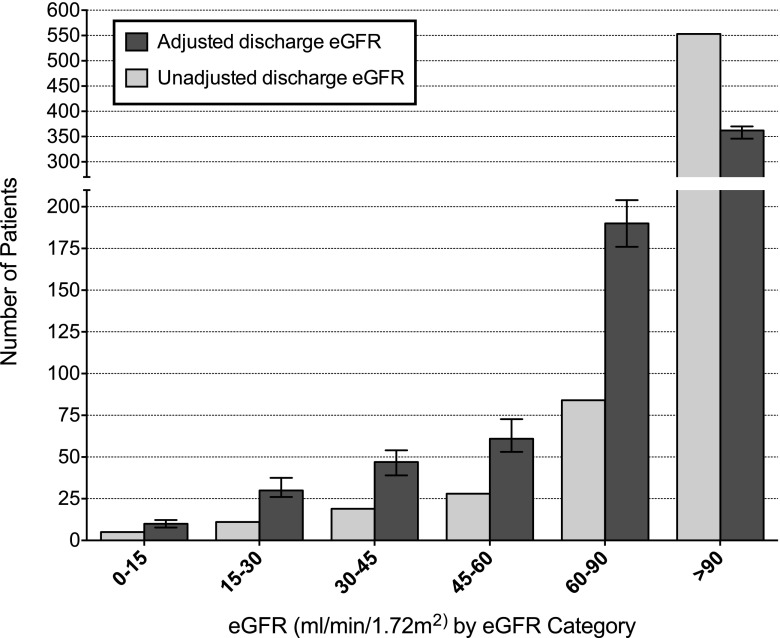
Using this regression model, we generated adjusted hospital discharge creatinine in all 700 patients, correcting for the effect of creatinine decrease related to major illness. Median adjusted creatinine was 0.93 mg/dl (IQR=0.75–1.20; eGFR=91 ml/min per 1.73 m2 [62–111]) compared with a median measured creatinine of 0.63 mg/dl (0.51–0.81; eGFR=115 ml/min per 1.73 m2 [96–131]); 63 patients had eGFR<60 based on measured creatinine, and this number rose to 148 patients when using eGFR based on adjusted creatinine (a 135% increase; P<0.001) (Figure 3, Table 4). Of 85 patients who newly had an eGFR<60 ml/min per 1.73 m2 after adjustment, 79% of patients had AKI. In the adjusted analysis, the proportion of AKI-3 patients with eGFR<60 at hospital discharge rose from 27% to 46% (P<0.001), and the proportion of AKI-3 patients with eGFR<30 at hospital discharge doubled from 9% to 18% (P<0.001) (Table 4).
Figure 3.
CKD category at hospital discharge in 700 survivors of critical illness based on actual discharge creatinine and discharge creatinine adjusted for decreases in serum creatinine associated with duration and severity of illness. Error bars represent 95% confidence intervals for the mean model prediction of eGFR distribution.
Table 4.
Potential CKD diagnoses at hospital discharge by AKI category
| AKI Category | Number | Number with eGFR<60 | Number with eGFR<30 | ||||
|---|---|---|---|---|---|---|---|
| Measured | Adjusted | P Value | Measured | Adjusted | P Value | ||
| All patients | 700 | 63 (9.0%) | 148 (21%) | <0.001 | 16 (2.3%) | 40 (5.7%) | <0.001 |
| No AKI | 241 | 2 (0.8%) | 20 (8.3%) | <0.001 | 0 (0.0%) | 0 (0.0%) | — |
| AKI-1 | 218 | 11 (5.0%) | 36 (17%) | <0.001 | 1 (0.5%) | 8 (3.7%) | 0.02 |
| AKI-2 | 75 | 6 (8.0%) | 16 (21%) | 0.004 | 0 (0.0%) | 2 (2.7%) | 0.5 |
| AKI-3 | 166 | 44 (27%) | 76 (46%) | <0.001 | 15 (9.0%) | 30 (18%) | <0.001 |
eGFR (ml/min per 1.73 m2) based on discharge creatinine versus eGFR based on creatinine adjusted for critical illness by McNemar's test.
Follow-Up Creatinine
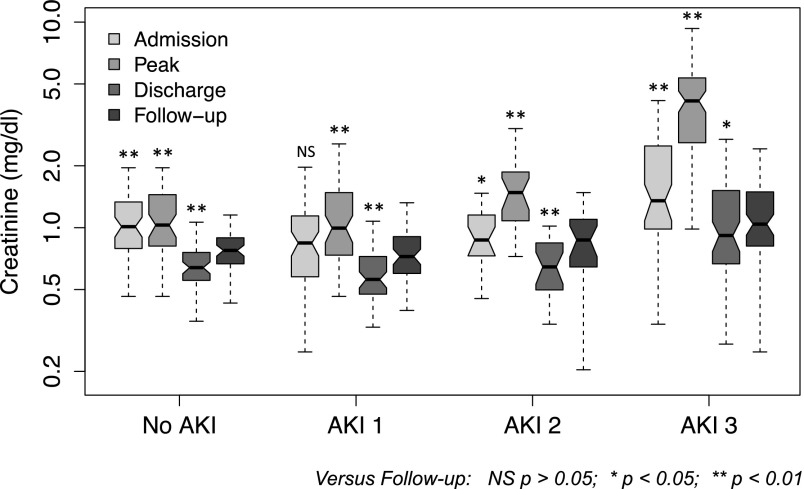
Two hundred twenty-one patients had follow-up creatinine measurements between 3 and 12 months after discharge; these values were significantly higher than the values at discharge across all AKI categories (Figure 4, Table 2). At follow-up, there were 48% more patients with eGFR<60 ml/min per 1.73 m2 than at hospital discharge (P=0.009) (Supplemental Figure 4); 24 patients had eGFR<60 at both discharge and follow-up, 22 patients had GFR<60 at follow-up despite GFR>60 at discharge, and only 7 patients had GFR<60 at discharge and GFR>60 at follow-up.
Figure 4.
Hospital admission, peak, hospital discharge, and 3- to 12-month follow-up creatinine (log scale) in 221 hospitalizations with follow-up values. Boxes indicate 25th to 75th percentiles, with a solid line at the median; the whiskers are 1.5× interquartile range from the box. Nonoverlap of notches suggests significant difference in medians at the P<0.05 level. Asterisks indicate statistical difference between paired creatinine values with the Wilcoxon signed rank test. NS, P>0.05. *P<0.05; **P<0.01.
Ninety-six patients had both baseline and follow-up creatinine values. In 47 patients who had AKI-3, follow-up creatinine values were significantly higher than baseline values (P=0.01), despite a lack of significant difference from baseline at hospital discharge (Table 2).
We examined the relationship between follow-up and hospital discharge creatinine in multivariable regression. In this model, AKI-3 was associated with a 14% (1%–28%) higher follow-up creatinine than the creatinine predicted with no AKI, whereas hospital length of stay remained associated with a lower follow-up creatinine (Supplemental Table 3, column 1). Finally, we considered variables associated with follow-up creatinine in 96 episodes where baseline and follow-up creatinine measurements were available (Supplemental Table 3, column 2). In this analysis, after allowing for differences in baseline creatinine, longer hospital length of stay was significantly associated with lower follow-up creatinine (P<0.001), whereas AKI-2 and AKI-3 were associated with 48% (23%–83%) and 53% (9%–116%) increases in predicted creatinine at follow-up compared with no AKI, respectively.
Discussion
Study Findings
We found that critical illness was associated with significant falls in serum creatinine from admission to discharge, irrespective of the occurrence or severity of AKI. When considering patients with measured baseline, significant decreases from baseline to discharge were apparent in all patients except those patients who had AKI-3, in whom discharge values were similar to baseline. In general, discharge creatinine values were abnormally low compared with expected values in the general population, and therefore, for men, median discharge creatinine (0.67 mg/dl) was below the lower limit of the reference range (0.7 mg/dl), suggesting that more than 50% of these patients had creatinine values seen in less than 2.5% of the general population of men.
Decrease in steady state serum creatinine can be explained by increase in GFR or decrease in creatinine generation rate. However, large improvements in GFR from baseline after critical illness would seem implausible, whereas large and sustained falls in creatinine generation have been shown in animal models of sepsis (14), patients with advanced CKD (15), and critically ill humans (16–18), with greatest decrease occurring in the sickest patients (16). Skeletal muscle is the major source of creatinine production, and critical illness is associated with profound loss of skeletal muscle protein (10,19,20), with muscle thickness steadily decreasing over time after ICU admission (10,21,22). Loss of muscle mass can persist long after hospital discharge (23). Therefore, it seems plausible that the observed falls in serum creatinine across critical illness occur as the result of loss of muscle mass and reduced creatinine generation, potentially combined with reduced hepatic creatinine production and dietary changes. These changes would also compromise the use of creatinine as a measure of renal function during hospital admission (24). In addition, we would predict that use of final or lowest hospital creatinine as a surrogate for baseline creatinine in post hoc AKI diagnosis might lead to overestimation of AKI diagnosis, which was reported by other investigators (25).
In multivariable analysis, we found that decreased hospital discharge creatinine was associated longer duration of hospitalization (Table 3). These results suggest that patients who experience more lengthy and severe illness might experience the largest relative drops in creatinine generation during and after ICU admission. Because AKI was strongly associated with illness severity and longer hospital length of stay (Table 1), illness-associated falls in creatinine would be expected to be more pronounced in these patients. Such falls in creatinine can confound diagnosis of renal dysfunction after AKI. In patients with measured baseline creatinine and AKI-3, we found apparent recovery to baseline creatinine in most patients (Figure 2, Table 2). However, given the fall in creatinine seen in survivors of critical illness without AKI, an unchanged creatinine in AKI survivors might, in fact, suggest a persistent decline in renal function, which was suggested by the independent effect of AKI-3 on hospital discharge creatinine after accounting for the effect of duration of hospitalization in our multivariable model (Table 3, column 1).
When we examined the impact of the confounding effect of prolonged illness on assessment of renal function at hospital discharge by modeling creatinine values in the absence of this effect, we predicted a 135% increase in the number of patients with an eGFR<60 ml/min per 1.73 m2 at hospital discharge (Figure 3, Table 4). This result suggests that the majority of potential CKD diagnoses after critical illness may be missed or misclassified.
Additional confirmation of effect of critical illness on serum creatinine is seen in patients with follow-up data; there were significant increases in creatinine from discharge to follow-up, irrespective of AKI category (Figure 4, Table 2). Use of follow-up creatinine to classify eGFR resulted in a 48% rise in the number of patients with eGFR<60 compared with discharge (Supplemental Figure 4), a smaller increase than the increase suggested from eGFR based on adjusted discharge creatinine (Figure 3). However, because longer hospitalization remained significantly associated with lower follow-up creatinine in multivariable analysis, the influence of critical illness on serum creatinine seems to persist at follow-up. Thus, although follow-up detects many patients developing overt CKD, some patients may still be misclassified.
Strengths and Limitations
To our knowledge, this work is the first attempt to systematically examine the magnitude and implications of creatinine changes associated with critical illness in a large cohort of patients with and without AKI. Our study is observational and was restricted to a single ICU; however, we did consider a diverse population of medical, surgical, and trauma patients, representing the most common associations of AKI in critically ill patients (26).
We chose to consider patients with 5 or more ICU days, and therefore, our conclusions may not extend to patients with shorter durations of critical illness. However, the majority of critical illness complicated by AKI involves longer ICU admission, and therefore, we focused our study on a relevant cohort of patients with significant exposure to critical illness.
The majority of patients that we considered had no known baseline creatinine; however, large decreases in creatinine were seen across all levels of admission creatinine and AKI categories (Supplemental Figure 2), in patients without AKI (Figure 1), and in patients with measured baseline creatinine (Figure 2), suggesting that declines in creatinine occurring after critical illness do not just reflect recovery of AKI present at admission. In the absence of known baseline, our analysis cannot distinguish the extent to which renal dysfunction apparent after adjustment for critical illness reflects new renal dysfunction caused by AKI or the presence of preexisting CKD. However, misdiagnosis of renal dysfunction after critical illness is clinically relevant, irrespective of whether it is a preexisting or de novo condition. In addition, our analysis of patients with known baseline or follow-up creatinine supports a specific association between more severe AKI and newly sustained deterioration in renal function persisting to hospital discharge and beyond.
Finally, our conclusions based on statistical modeling require confirmation with formal measurements of GFR and creatinine generation rates in individual patients. However, the abnormally low creatinine values consistently observed after critical illness do, at least, imply the existence of an important phenomenon that requires additional characterization.
Relation to Previous Studies
Our results are in keeping with previous studies correlating AKI to the development or progression of CKD (2–5,27). Less data exists on patients who seem to recover baseline renal function after AKI. Our analysis suggests that many of these patients may have unrecognized CKD, findings that are supported by studies showing increased rates of later CKD after apparent recovery from AKI in adults (28,29) and children (30).
The use of creatinine-based eGFR in the general population has recently been the subject of a meta-analysis (31). Prevalence of CKD rose significantly when cystatin-c, a renal filtration marker less dependent on muscle mass, was used for eGFR, with better predictions of all-cause mortality and cardiovascular death. These results suggest that variations in creatinine generation might confound CKD diagnosis in the general population and that these missed diagnoses are clinically significant. Our data also suggest that these effects may be particularly marked in survivors of critical illness.
We have shown that critical illness is associated with significant and sustained decreases in serum creatinine. At the same time, AKI is associated with relatively higher creatinine at hospital discharge. In combination, these effects can offset, potentially masking significant renal dysfunction in survivors of critical illness complicated by AKI. Our findings also suggest that serum creatinine-based methods of eGFR may not be applicable to survivors of critical illness and that other methods are required to assess for potential development of CKD in this population. Prospective studies examining measured GFR and creatinine generation rate are required to confirm our findings. In the interim, given the apparent difficulty in assessing true convalescent renal function from serum creatinine at hospital discharge, we suggest that monitoring of renal function and management of CKD risk factors should be considered in survivors of critical illness complicated by significant AKI, irrespective of apparent recovery.
Disclosures
J.R.P. has received travel support and consultancy fees from Gambro AB and speakers fees from Alere, Ltd. R.M.P. has received research funding and honoraria from LiDCO, Ltd., Circassia Holdings, Covidien, Inc., Pulsion Medical Systems, and Edwards Lifesciences.
Supplementary Material
Acknowledgments
Elements of these data have been presented in abstract form at the British Transplantation Society and the Renal Association Joint Congress, March 13–15, 2013 (Bournemouth, UK), The 33rd International Symposium on Intensive Care and Emergency Medicine (Brussels, Belgium, March 19–22, 2013), and The World Congress of Nephrology (Hong Kong, China, May 31–June 4, 2013).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.11141113/-/DCSupplemental.
References
- 1.Hoste EAJ, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, Kellum JA: RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care 10: R73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta RL, Pascual MT, Soroko S, Chertow GM, PICARD Study Group : Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA 288: 2547–2553, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE: Outcomes following diagnosis of acute renal failure in U.S. veterans: Focus on acute tubular necrosis. Kidney Int 76: 1089–1097, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Hsu CY: Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidney Disease Improving Global Outcomes (KDIGO) : KDIGO clinical practice guideline for acute kidney injury. Section 2: AKI definition. Kidney Int Suppl 2: 19–36, 2012 [Google Scholar]
- 7.Siew ED, Peterson JF, Eden SK, Hung AM, Speroff T, Ikizler TA, Matheny ME: Outpatient nephrology referral rates after acute kidney injury. J Am Soc Nephrol 23: 305–312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, Ray JG, Luo J, Li P, Quinn RR, Forster A, Perl J, Bell CM: Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Padhke R, Dew T, Sidhu PS, Velloso C, Seymour J, Agley CC, Selby A, Limb M, Edwards LM, Smith K, Rowlerson A, Rennie MJ, Moxham J, Harridge SD, Hart N, Montgomery HE: Acute skeletal muscle wasting in critical illness. JAMA 310: 1591–1600, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I, Dwyer JP, Srichai M, Hung AM, Smith JP, Peterson JF: Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 7: 712–719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R Foundation for Statistical Computing : R: A Language and Environment for Statistical Computing. Version 2.15.1, Vienna, Austria, R Foundation for Statistical Computing, 2012 [Google Scholar]
- 13.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members : Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Doi K, Yuen PS, Eisner C, Hu X, Leelahavanichkul A, Schnermann J, Star RA: Reduced production of creatinine limits its use as marker of kidney injury in sepsis. J Am Soc Nephrol 20: 1217–1221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grootendorst DC, Michels WM, Richardson JD, Jager KJ, Boeschoten EW, Dekker FW, Krediet RT, NECOSAD Study Group : The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant 26: 1932–1937, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Wilson FP, Sheehan JM, Mariani LH, Berns JS: Creatinine generation is reduced in patients requiring continuous venovenous hemodialysis and independently predicts mortality. Nephrol Dial Transplant 27: 4088–4094, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark WR, Mueller BA, Kraus MA, Macias WL: Quantification of creatinine kinetic parameters in patients with acute renal failure. Kidney Int 54: 554–560, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Pickering JW, Ralib AM, Endre ZH: Combining creatinine and volume kinetics identifies missed cases of acute kidney injury following cardiac arrest. Crit Care 17: R7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monk DN, Plank LD, Franch-Arcas G, Finn PJ, Streat SJ, Hill GL: Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann Surg 223: 395–405, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill AA, Plank LD, Finn PJ, Whalley GA, Sharpe N, Clark MA, Hill GL: Massive nitrogen loss in critical surgical illness: Effect on cardiac mass and function. Ann Surg 226: 191–197, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruther W, Benesch T, Zorn C, Paternostro-Sluga T, Quittan M, Fialka-Moser V, Spiss C, Kainberger F, Crevenna R: Muscle wasting in intensive care patients: Ultrasound observation of the M. quadriceps femoris muscle layer. J Rehabil Med 40: 185–189, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Reid CL, Campbell IT, Little RA: Muscle wasting and energy balance in critical illness. Clin Nutr 23: 273–280, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Reid CL, Murgatroyd PR, Wright A, Menon DK: Quantification of lean and fat tissue repletion following critical illness: A case report. Crit Care 12: R79, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endre ZH, Pickering JW, Walker RJ: Clearance and beyond: The complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI). Am J Physiol Renal Physiol 301: F697–F707, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Siew ED, Matheny ME, Ikizler TA, Lewis JB, Miller RA, Waitman LR, Go AS, Parikh CR, Peterson JF: Commonly used surrogates for baseline renal function affect the classification and prognosis of acute kidney injury. Kidney Int 77: 536–542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M: Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis 60: 402–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, Matsell DG: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT, CKD Prognosis Consortium : Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369: 932–943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.