Abstract
Background and objectives
Previous studies suggested that intravenous methylprednisolone possibly accelerates remission of proteinuria in adult-onset minimal change disease; its impact on relapse of proteinuria is unknown.
Design, setting, participants, & measurements
This multicenter retrospective cohort study included 125 adult-onset minimal change disease patients diagnosed by kidney biopsy between 2000 and 2009 and treated initially with corticosteroid in five nephrology centers in Japan participating in the Study of Outcomes and Practice Patterns of Minimal Change Disease. Times to first remission and first relapse of proteinuria after initiating the first immunosuppressive therapy were compared between 65 patients with initial use of intravenous methylprednisolone followed by prednisolone and 60 patients with initial use of prednisolone alone using multivariate Cox proportional hazards models. After calculating the probability of receiving methylprednisolone and prednisolone using a logistic regression model (propensity score), the results were ascertained using propensity score-matched and -stratified models.
Results
During the median 3.6 years of observation (interquartile range=2.0–6.9), all 65 patients in the methylprednisolone and prednisolone group achieved remission within 11 (8–20) days of the corticosteroid initiation, whereas in the prednisolone group, 58 of 60 patients (96.7%) achieved remission within 19 (12–37) days (P<0.001). After achieving first remission, 32 (49.2%) patients in the methylprednisolone and prednisolone group and 43 (74.1%) patients in the prednisolone group developed at least one relapse. Multivariate Cox proportional hazards models revealed that methylprednisolone and prednisolone use was significantly associated with early remission (multivariate-adjusted hazard ratio, 1.56; 95% confidence interval, 1.06 to 2.30) and lower incidence of relapse (0.50; 95% confidence interval, 0.29 to 0.85) compared with prednisolone use alone. These results were ascertained in propensity score-based models. No significant difference was observed in incidence of adverse events, including infection, aseptic osteonecrosis, cataract, diabetes, and gastrointestinal bleeding.
Conclusions
Initial use of methylprednisolone was associated with earlier remission and lower incidence of relapse in adult-onset minimal change disease patients. Efficacy of methylprednisolone should be evaluated in randomized controlled trials.
Keywords: nephrotic syndrome, GN, drug interactions
Introduction
Along with membranous nephropathy and FSGS, minimal change disease (MCD) is one of the major glomerulonephritides in adult patients with primary nephrotic syndrome (1–6) and especially common in Asian countries (4–6). Although few patients with MCD progress to ESRD, a series of studies reported that approximately 30%–70%, most commonly 60%, of adult MCD patients experienced at least one relapse of proteinuria (7–18) and that 50%–60% of patients with a first relapse subsequently experienced a second relapse (7–12). Thus, clinical goals in MCD patients are early induction of remission of proteinuria and more importantly, prevention of relapse of proteinuria.
A few small randomized controlled studies showed that intravenous methylprednisolone (mPSL) accelerated remission in MCD patients. An Italian randomized controlled trial showed that pediatric (not adult) MCD patients treated with mPSL and prednisone achieved remission earlier than those patients with prednisone alone (19). In another Japanese nonrandomized controlled trial of adult MCD patients, initial mPSL use followed by cyclosporine (not a corticosteroid) resulted in shorter time to remission compared with cyclosporine monotherapy and prednisolone (PSL) monotherapy (20). Although these studies showed that mPSL accelerated remission, no obvious impact on relapse was observed. By contrast, an interesting Japanese retrospective cohort study recently reported that adult MCD patients treated with mPSL followed by PSL (mPSL+PSL) experienced significantly earlier remissions and earlier relapses (13), suggesting that mPSL did not benefit the long-term prognosis of adult MCD patients.
The purpose of this study was to assess the effectiveness of mPSL+PSL for first remission and first relapse of proteinuria in the largest retrospective adult MCD cohort worldwide, the Study of Outcomes and Practice Patterns of Minimal Change Disease (STOP-MCD), including 125 adult MCD patients (21).
Materials and Methods
Patients
A multicenter retrospective cohort study, the STOP-MCD, included 147 adult patients ages 15 years or older who developed a first episode of nephrotic syndrome and were diagnosed with primary MCD by kidney biopsy between 2000 and 2009 in five nephrology centers in Japan (21). We excluded 22 patients (15.0%) because of spontaneous remission of proteinuria (n=6), loss to follow-up without immunosuppressive therapy (n=3), initial use of nonsteroidal immunosuppressives (n=9), and missing data (n=4). Finally, this study included 125 MCD patients (85.0%) who initiated a corticosteroid as the first immunosuppressive therapy. According to the intention-to-treat principle, 125 patients were categorized into two groups: one group comprised 65 patients treated initially with intravenous mPSL followed by PSL (mPSL+PSL group), and the other group comprised 60 patients receiving initial therapy with PSL only (PSL group). The study protocol was approved by the ethical committees in each facility.
Measurements
Baseline characteristics preceding corticosteroid initiation were collected retrospectively from patients’ medical records and included age, sex, body mass index, systolic and diastolic BPs, serum concentration of creatinine, total cholesterol, albumin, urinary protein (UP), intravenous administration of 25% human albumin, and initial doses of mPSL (0.5 or 1.0 g/d for 3 consecutive days) and PSL. Data on the use of mPSL, PSL, and other immunosuppressants during the follow-up period were also collected.
The outcomes of interest in this study were time from corticosteroid initiation to first remission of proteinuria and time from corticosteroid initiation to subsequent first relapses of proteinuria. Remission was defined as UP<0.3 g/d, UP/creatinine ratio<0.3, and/or negative to trace dipstick UP values. Relapse was defined as UP≥1.0 g/d, UP/creatinine ratio≥1.0, and/or dipstick UP value ≥1+ followed by an increase in corticosteroid dosing and/or add-on use of immunosuppressive agents after developing remission. Patients were followed up until December 2010 and censored at death (n=1) or on the last day of urinary examination before December 2010. For the duration of the observational period, we also collected information on hospitalization because of infection, surgical therapy for aseptic osteonecrosis or cataract, initiation of antidiabetic drugs, and diagnosis of gastroduodenal bleeding.
Because of the retrospective nature of this study, the immunosuppressive therapy protocol was not predetermined and depended exclusively on individual nephrologists. Details of use of immunosuppressants were obtained from patient medical records for the assessment of potential confounding by corticosteroid dose and concurrent use of other immunosuppressants during the follow-up period. The cumulative PSL dose was calculated by conversion of the mPSL dose (1 mg mPSL was considered equivalent to 1.25 mg PSL) (22,23).
Statistical Analyses
Between-group comparisons of clinical characteristics were performed using the unpaired t test or Wilcoxon’s rank-sum test for continuous variables and the chi-squared test or Fisher’s exact test for categorical variables as appropriate. Because of the highly skewed distributions of serum creatinine and UP, their natural logarithms were used in subsequent analyses.
Cumulative probabilities of first remission and first relapse were calculated using Kaplan–Meier methods. The effectiveness of mPSL+PSL and PSL on these outcomes was compared using the log-rank test and univariate and multivariate Cox proportional hazards (CPH) models. The proportional hazards assumption for covariates was tested by using scaled Schoenfeld residuals. We performed a propensity score (PS) analysis to estimate the probability of initial mPSL+PSL therapy to control for the imbalance of the baseline characteristics between the mPSL+PSL and PSL groups (24). The PS was calculated using a multivariate logistic regression model including baseline age, sex, body mass index, systolic BP, antihypertensive use, serum albumin and creatinine concentration with logarithmic transformation, urinary protein with logarithmic transformation, and use of intravenous 25% albumin as independent variables. Calibration was assessed using the Hosmer–Lemeshow goodness-of-fit test. The area under the receiver operating characteristics curve was calculated to assess the predictive ability of the PS. After PS calculations, associations between the initial use of mPSL+PSL and the study outcomes were examined in CPH models stratified by PS tertiles and matched by PS. In the PS-matching analysis, each patient in the mPSL+PSL group was matched to a patient in the PSL group with the nearest PS at a ratio of 1:1 without replacement using a greedy matching algorithm with a caliper width equal to 0.2 of the SD of the logit of the PS (25). Differences in clinical characteristics between the matched pairs were assessed using paired t test or Wilcoxon’s signed-rank test for continuous variables and McNemar’s test for categorical variables as appropriate.
To assess the potential influence of corticosteroid dose and concurrent use of other immunosuppressants during follow-up between immunosuppressive therapy initiation and the first relapse (or censoring in patients without relapse), point use of immunosuppressants and point and cumulative PSL doses at 0, 1, 3, 6, 12, 18, and 24 months of immunosuppressive therapy were compared between the mPSL+PSL group and the PSL group. The point PSL dose was divided by body weight on the day of first remission or hospital discharge, whichever came first. To assess the effectiveness of initial mPSL use, we subtracted the initial mPSL dose (1 g [n=51] or 0.5 g [n=14] mPSL for 3 days, which is equivalent to 1.25 or 0.625 g PSL for 3 days, respectively) from the cumulative PSL dose in the mPSL+PSL group.
Continuous variables were expressed as mean±SD or median (interquartile range), and categorical variables were expressed as number and proportion as appropriate. The statistical significance level was set at P<0.05. All statistical analyses were conducted using STATA version 11 (STATA Corp.).
Results
The baseline characteristics of the mPSL+PSL (n=65) and PSL (n=60) groups are described in Table 1. The mPSL+PSL group had significantly lower systolic BP (mean±SD=118±12 versus 125±19 mmHg, P=0.01), lower serum creatinine concentration (median=0.8 [interquartile range=0.7–1.0] versus median=0.9 [interquartile range=0.7–1.5] mg/dl, P=0.05), and lower proportion of intravenous administration of 25% albumin (46.2% versus 66.7%, P=0.02) compared with those characteristics for the PSL group.
Table 1.
Clinical characteristics of 65 patients treated with initial methylprednisolone use followed by prednisolone (methylprednisolone+prednisolone group) and 60 patients treated with initial prednisolone use (prednisolone group)
| Patient Characteristics | Initial Use of Corticosteroid | P Value | |
|---|---|---|---|
| mPSL+PSLa | PSL | ||
| Number | 65 | 60 | |
| Baseline characteristics at initiating corticosteroid | |||
| Age, yr | 40 [26−59] | 41 [23−64] | 0.67 |
| Men, n (%) | 45 (69.2) | 35 (58.3) | 0.20 |
| Body mass index, kg/m2 | 22.5±2.7 | 23.1±2.8 | 0.24 |
| Systolic BP, mmHg | 118±12 | 125±19 | 0.01 |
| Diastolic BP, mmHg | 71±11 | 74±12 | 0.22 |
| Use of antihypertensives, n (%) | 8 (12.3) | 11 (18.3) | 0.35 |
| Serum creatinine, mg/dl | 0.8 [0.7−1.0] | 0.9 [0.7−1.5] | 0.05 |
| Serum total cholesterol, mg/dl | 374±105 | 389±108 | 0.45 |
| Serum albumin, g/dl | 1.7±0.5 | 1.9±0.6 | 0.07 |
| Urinary protein, g/d | 7.5 [4.1−13.2] | 9.5 [3.9−15.1] | 0.50 |
| Use of intravenous 25% albumin, n (%) | 30 (46.2) | 40 (66.7) | 0.02 |
| Initial PSL dose, mg/kg | 0.75±0.13 | 0.71±0.16 | 0.06 |
| Remission and relapse during observational period | |||
| First remission, n (%) | 65 (100.0) | 58 (96.7) | |
| Time to first remission, db | 11 [8−20] | 19 [12−37] | <0.001 |
| First relapse, n (%)b | 32 (49.2) | 43 (74.1) | 0.01 |
| Time to first relapse, yrc | 1.0 [0.6−1.5] | 0.8 [0.4−1.6] | 0.17 |
| Entire observational period, yr | 3.5 [1.8−6.4] | 4.0 [2.1−7.9] | 0.17 |
| Total number of relapses, n per persond | 0 (0−8) | 1 (0−9) | |
| Incidence of relapse, per person-yrd | 0.0 [0.0−0.5] | 0.5 [0.0−0.7] | 0.007 |
Values are given as mean±SD, median [interquartile range], or median (range). Conversion factors for units: serum creatinine in milligrams per deciliter to moles per liter, ×88.4; serum total cholesterol in milligrams per deciliter to moles per liter, ×0.02586. mPSL, methylprednisolone; PSL, prednisolone.
Including 51 (78.5%) patients with initial use of 1 g/d mPSL for 3 days and 14 (21.5%) patients with initial use of 0.5 g/d PSL for 3 days.
Excluding two patients (PSL group) who were censored without remission after receiving immunosuppressive therapy for 29 and 58 days.
Excluding 50 patients who were censored without relapse after receiving immunosuppressive therapy.
During the entire observational period until censoring.
Initial mPSL Use and First Remission of Proteinuria
During 3.6 years (interquartile range=2.0–6.9 years) of the entire observational period of the whole cohort (n=125), 65 (100.0%) and 58 (96.7%) patients in the mPSL+PSL and PSL groups, respectively, achieved first remission (Table 1). Two patients in the PSL group were lost to follow-up before achieving remission at days 29 and 58. The mPSL+PSL group achieved first remission significantly earlier (cumulative probabilities of remission of the mPSL+PSL group versus the PSL group: 0.66 versus 0.37 at day 15, 0.88 versus 0.65 at day 30, and 0.94 versus 0.92 at day 90; log-rank test: P=0.006) (Figure 1A). Because add-on use of mPSL before the first remission was significantly higher in the PSL group than the mPSL+PSL group (6 [9.2%] patients in the mPSL+PSL group versus 13 [21.7%] in the PSL group, P=0.04) and the proportions of add-on use of other immunosuppressants before remission were comparable between the two groups (4 [6.2%] versus 5 [8.3%], P=0.66), earlier remission in the mPSL+PSL group can hardly be ascribed to subsequent immunosuppressive therapy given before first remission. Median times to first remission were 11 days (interquartile range=8–20 days) in 65 (100.0%) mPSL+PSL patients who achieved remission and 19 days (interquartile range=12–37 days) in 58 (96.7%) PSL patients who achieved remission (P<0.001) (Table 1). In univariate CPH models, younger age, lower systolic BP, lower levels of serum creatinine and UP, no intravenous administration of 25% albumin, higher initial PSL dose, and initial mPSL use were significantly associated with earlier remission (Table 2). Even after adjusting for clinically relevant factors, initial use of mPSL was identified as a significant predictor of earlier remission (mPSL+PSL versus PSL: hazard ratio [HR], 1.56; 95% confidence interval [95% CI], 1.06 to 2.30; P=0.03) along with younger age (per 10 years: HR, 0.87; 95% CI, 0.78 to 0.97; P=0.01), lower serum creatinine concentration (per 1.0 ln mg/dl: HR, 0.41; 95% CI, 0.25 to 0.69; P=0.001), and lower UP level (per 1.0 ln g/d: HR, 0.73; 95% CI, 0.56 to 0.96; P=0.02).
Figure 1.
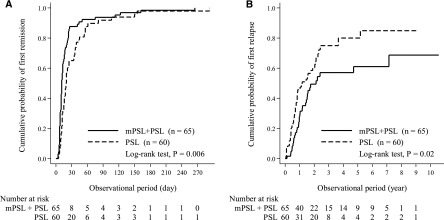
Earlier remission and lower incidence of relapse in patients with initial methylprednisolone use followed by prednisolone (mPSL+PSL group), compared with those with initial prednisolone use (PSL group). Cumulative probabilities of (A) first remission and (B) first relapse of mPSL+PSL group (n=65) and PSL group (n=60) were calculated using Kaplan–Meier method and compared using log-rank test.
Table 2.
Predictors of first remission and first relapse
| Predictors | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| Hazard Ratio [95% Confidence Interval] | P Value | Hazard Ratio [95% Confidence Interval] | P Value | |
| Predictors of first remission | ||||
| Age, per 10 yr | 0.85 [0.78 to 0.94] | 0.001 | 0.87 [0.78 to 0.97] | 0.01 |
| Men (versus women) | 1.07 [0.73 to 1.55] | 0.74 | 1.14 [0.77 to 1.70] | 0.50 |
| Body mass index, per 1.0 kg/m2 | 0.93 [0.87 to 1.00] | 0.06 | 1.06 [0.97 to 1.16] | 0.17 |
| Systolic BP, per 10 mmHg | 0.89 [0.81 to 0.98] | 0.02 | 0.99 [0.88 to 1.11] | 0.83 |
| Creatinine, per 1.0 ln mg/dl | 0.38 [0.25 to 0.58] | <0.001 | 0.41 [0.25 to 0.69] | 0.001 |
| Urinary protein, per 1.0 ln g/d | 0.74 [0.60 to 0.91] | 0.005 | 0.73 [0.56 to 0.96] | 0.02 |
| Use of intravenous 25% albumin | 0.69 [0.48 to 0.99] | 0.04 | 1.07 [0.67 to 1.70] | 0.78 |
| Initial dose of PSL, per 0.1 mg/kg | 1.16 [1.02 to 1.32] | 0.02 | 1.14 [0.97 to 1.35] | 0.11 |
| Initial use of mPSL (versus PSL) | 1.63 [1.14 to 2.34] | 0.008 | 1.56 [1.06 to 2.30] | 0.03 |
| Predictors of first relapse | ||||
| Age, per 10 yr | 0.82 [0.72 to 0.92] | 0.001 | 0.81 [0.71 to 0.93] | 0.003 |
| Men (versus women) | 1.03 [0.64 to 1.64] | 0.91 | 1.08 [0.64 to 1.83] | 0.77 |
| Body mass index, per 1.0 kg/m2 | 0.97 [0.90 to 1.05] | 0.49 | 0.94 [0.85 to 1.04] | 0.23 |
| Systolic BP, per 10 mmHg | 0.95 [0.82 to 1.09] | 0.44 | 0.97 [0.82 to 1.14] | 0.72 |
| Creatinine, per 1.0 ln mg/dl | 0.96 [0.58 to 1.59] | 0.88 | 0.93 [0.49 to 1.76] | 0.82 |
| Urinary protein, per 1.0 ln g/d | 1.30 [1.02 to 1.67] | 0.04 | 1.36 [1.01 to 1.84] | 0.04 |
| Use of intravenous 25% albumin | 1.22 [0.77 to 1.94] | 0.39 | 0.97 [0.54 to 1.73] | 0.91 |
| Initial dose of PSL, per 0.1 mg/kg | 0.90 [0.77 to 1.05] | 0.18 | 0.85 [0.70 to 1.04] | 0.12 |
| Initial use of mPSL (versus PSL) | 0.59 [0.37 to 0.94] | 0.02 | 0.50 [0.29 to 0.85] | 0.01 |
To control for significant differences in baseline characteristics between the mPSL+PSL and the PSL groups, we computed the PS of initial use of mPSL using a multivariate logistic regression model. The model was well calibrated (Hosmer–Lemeshow goodness-of-fit test: P=0.27) and had moderate discrimination in determining initial use of corticosteroid (area under the receiver operating characteristics curve=0.71). In each PS tertile, no significant differences in baseline characteristics were observed between the mPSL+PSL and the PSL groups, indicating that they were well balanced (data not shown). Multivariate CPH models stratified by PS tertiles verified the significant association between initial use of mPSL and earlier remission (mPSL+PSL versus PSL: HR, 1.47; 95% CI, 1.01 to 2.14; P=0.04) (Table 3). After each patient in the mPSL+PSL group was matched to a patient in the PSL group with the closest PS, leading to 42 matched pairs with well balanced baseline characteristics (Table 4), initial use of mPSL+PSL was also identified as a significant predictor of earlier remission in the model matched by PS (HR, 1.93; 95% CI, 1.04 to 3.61; P=0.04) (Table 3).
Table 3.
Initial use mPSL+PSL as a predictor of first remission and first relapse in propensity score-based models
| Outcome/Model | HR [95% CI] of mPSL+PSL (Versus PSL) | P Value |
|---|---|---|
| First remission | ||
| Univariate model | 1.63 [1.14 to 2.34] | 0.008 |
| Multivariate modela | 1.56 [1.06 to 2.30] | 0.03 |
| Stratification by PS tertiles | 1.47 [1.01 to 2.14] | 0.04 |
| Matching by PSb | 1.93 [1.04 to 3.61] | 0.04 |
| First relapse | ||
| Univariate model | 0.59 [0.37 to 0.94] | 0.02 |
| Multivariate modela | 0.50 [0.29 to 0.85] | 0.01 |
| Stratification by PS tertiles | 0.55 [0.33 to 0.89] | 0.02 |
| Matching by PSb | 0.48 [0.23 to 0.98] | 0.04 |
HR, hazard ratio; 95% CI, 95% confidence interval; PS, propensity score.
Adjusting for age, sex, body mass index, systolic BP, use of antihypertensives, serum concentration of creatinine, urinary protein, and initial dose of PSL at initiation of mPSL+PSL and PSL.
Cox proportional hazards models stratified by 42 matched pairs.
Table 4.
Clinical characteristics of 42 PS-matched pairs of patients in the mPSL+PSL and PSL groups
| Patient Characteristics | Initial Use of Corticosteroid | P Value | |
|---|---|---|---|
| mPSL+PSL | PSL | ||
| Number | 42 | 42 | |
| Baseline characteristics at initiation of corticosteroid | |||
| Age, yr | 45 [28−62] | 41 [21−67] | 0.94 |
| Men, n (%) | 27 (64.3) | 28 (66.7) | 0.83 |
| Body mass index, kg/m2 | 22.9±2.8 | 23.0±3.1 | 0.91 |
| Systolic BP, mmHg | 119±13 | 121±16 | 0.69 |
| Diastolic BP, mmHg | 72±11 | 71±11 | 0.70 |
| Use of antihypertensives, n (%) | 8 (19.0) | 8 (19.0) | 1.00 |
| Creatinine, mg/dl | 0.9 [0.8−1.2] | 0.9 [0.7−1.3] | 0.96 |
| Total cholesterol, mg/dl | 370±103 | 391±111 | 0.38 |
| Albumin, g/dl | 1.9±0.5 | 1.9±0.6 | 0.91 |
| Urinary protein, g/d | 7.4 [4.2−15.3] | 10.3 [4.0−15.6] | 0.86 |
| Use of intravenous 25% albumin, n (%) | 22 (52.4) | 24 (57.1) | 0.56 |
| Initial PSL dose, mg/kg | 0.75±0.13 | 0.69±0.15 | 0.08 |
| First remission and relapse | |||
| First remission, n (%) | 42 (100.0) | 41 (97.6) | |
| Time to first remission, da | 12 [7−24] | 18 [12−25] | |
| First relapse, n (%)a | 22 (52.4) | 31 (75.6) | |
| Time to first relapse, yrb | 1.3 [0.8−1.7] | 0.8 [0.4−1.2] | |
Values are given as mean±SD, median [interquartile range], or median (range). Conversion factors for units: serum creatinine in milligrams per deciliter to moles per liter, ×88.4; serum total cholesterol in milligrams per deciliter to moles per liter, ×0.02586.
Excluding one patient who was censored without remission after receiving immunosuppressive therapy.
Excluding 31 patients who were censored without relapse after receiving immunosuppressive therapy.
Initial mPSL Use and First Relapse of Proteinuria
After achieving remission, 32 (49.2%) patients in the mPSL+PSL group and 43 (74.1%) patients in the PSL group developed at least one relapse (up to eight and nine relapses, respectively) during the entire observational period (Table 1). In the mPSL+PSL group, 13 (40.6%), 4 (12.5%), 5 (15.6%), and 10 (31.3%) patients developed one, two, three, and four or more times of relapses during the entire observational period, respectively, whereas 16 (37.2%), 7 (16.3%), 3 (7.0%), and 17 (39.5%) patients did so in the PSL group, respectively. The incidence of relapse was significantly lower in the mPSL+PSL group than the PSL group (HR, 0.0; 95% CI, 0.0 to 0.5 versus HR, 0.5; 95% CI, 0.0 to 0.7 relapses per person-year; P=0.007). Cumulative probabilities of first relapse in the mPSL+PSL group versus the PSL group were 0.28 versus 0.46 at year 1, 0.49 versus 0.60 at year 2, and 0.61 versus 0.80 at year 5, indicating that the incidence of first relapse was significantly lower in the mPSL+PSL group than the PSL group (log-rank test: P=0.02) (Figure 1B). In univariate models, younger age, higher UP, and initial mPSL use were significantly associated with relapse (Table 2). Multivariate adjustment for clinically relevant factors did not attenuate the association between initial use of mPSL and first relapse (mPSL+PSL versus PSL: HR, 0.50; 95% CI, 0.29 to 0.85; P=0.01) along with older age (per 10 year: HR, 0.81; 95% CI, 0.71 to 0.93; P=0.003) and higher UP level (per 1.0 ln g/d: HR, 1.36; 95% CI, 1.01 to 1.84; P=0.04). PS-based models also ascertained that initial use of mPSL was significantly associated with a lower incidence of relapse (mPSL+PSL versus PSL: HR, 0.55; 95% CI, 0.33 to 0.89; P=0.02 with stratification by PS tertiles; HR, 0.48; 95% CI, 0.23 to 0.98; P=0.04 with matching by PS) (Table 3). After excluding two patients censored before first remission, similar results were obtained (data not shown).
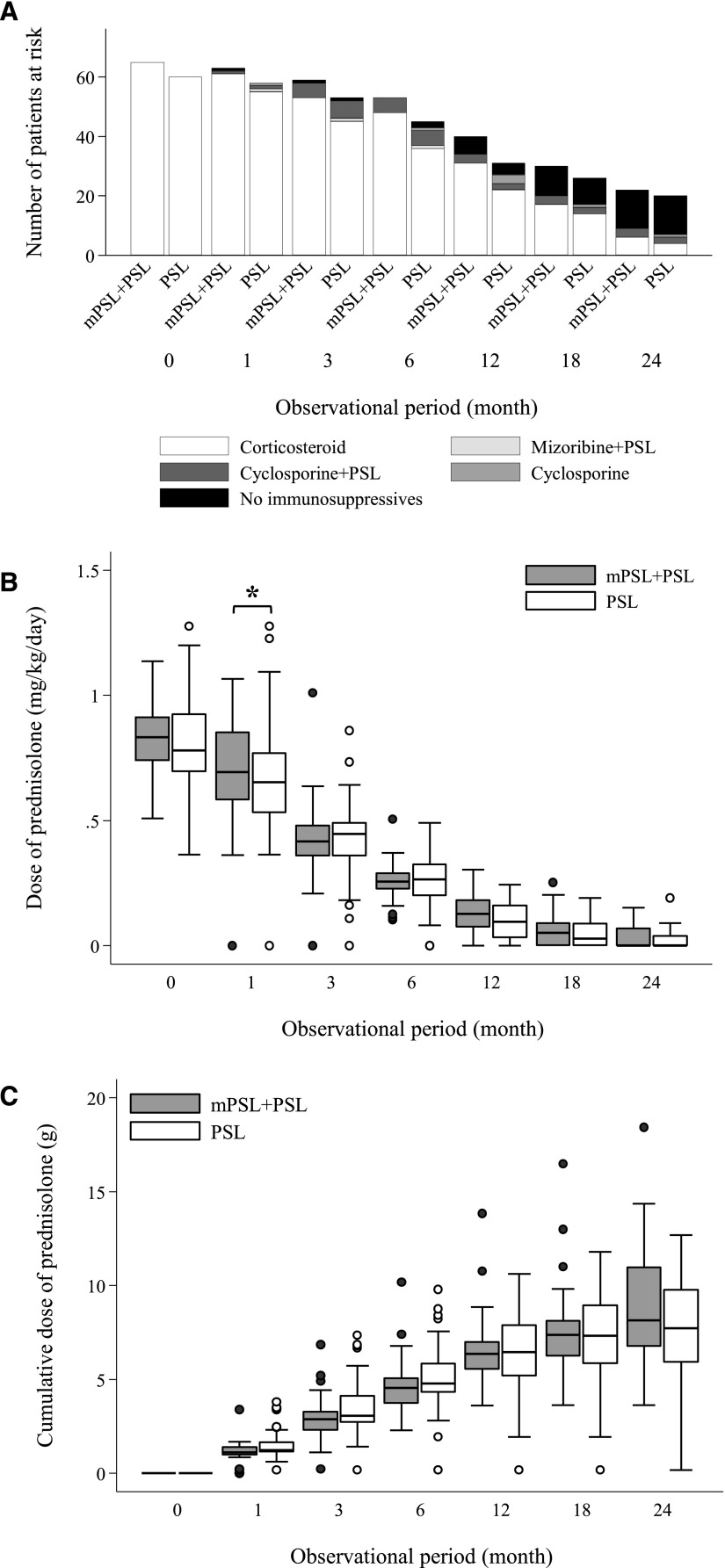
During the observational period before the first relapse (or censoring without relapse), no significant difference was observed between the mPSL+PSL and the PSL groups in the proportion of use of immunosuppressants and the point and cumulative PSL doses at months 1, 2, 3, 6, 12, 18, and 24 after initiating immunosuppressive therapy, with the exception of a significant but clinically minor difference in the point dose of PSL at month 1 (mPSL group [n=63]: HR, 0.69; 95% CI, 0.58 to 0.85 mg/kg per day; PSL group [n=54]: HR, 0.65; 95% CI, 0.53 to 0.77 mg/kg per day; P=0.04) (Figure 2). These data strongly suggest that immunosuppressive therapy was clinically comparable between the mPSL+PSL and the PSL groups.
Figure 2.
Similar immunosuppressive therapy in the mPSL+PSL group and the PSL group. Immunosuppressive therapy until the incidence of first relapse (or censoring without relapse) in the mPSL+PSL group and the PSL group: (A) the proportion of immunosuppressants, (B) the point dose of PSL (milligrams per kilogram per day), and (C) the cumulative dose of PSL (grams). The cumulative dose of PSL excludes the initial use of mPSL (1 g/d for 3 days [n=51] and 0.5 g/d for 3 days [n=14]) but includes the add-on use of mPSL (6 [9.2%] patients in the mPSL+PSL group versus 13 [21.7%] patients in the PSL group). The whiskers indicate the lowest and highest values within the range of the lower adjacent value (the lower quartile − 1.5× interquartile range) and the upper adjacent value (the upper quartile+1.5× interquartile range). *P=0.04.
Initial mPSL Use and Adverse Events
During a median 3.6 years (interquartile range=2.0–6.9 years) of the entire observational period, no patients progressed to ESRD requiring dialysis, and one patient in the mPSL group died of pancreatic cancer. No significant difference was observed between the mPSL+PSL and the PSL groups in hospitalization caused by infection (n=7 [10.8%] versus n=10 [16.7%]; P=0.34), surgical therapy for aseptic osteonecrosis (n=1 [1.5%] versus n=1 [1.7%]; P=0.95) or cataract (n=4 [6.2%] versus n=5 [8.3%]; P=0.64), and initiation of antidiabetic drugs (n=12 [18.5%] versus n=14 [23.3%]; P=0.50). No patients in either group were diagnosed with gastroduodenal bleeding.
Discussion
This retrospective cohort study of 125 adult-onset MCD patients revealed that mPSL was associated with earlier remission of proteinuria and interestingly, lower incidence of relapses of proteinuria. In addition, baseline age and UP levels were identified as the predictors of remission and relapse, whereas baseline serum creatinine concentration was associated with remission only. There were several advantages of this study. First, it was the largest cohort of adult MCD patients in the world with a long observational period (median=3.6 years [interquartile range=2.0–6.9 years]). Second, it used PS-based models to deliberately control for the imbalance of baseline characteristics between the mPSL+PSL and the PSL groups. Third, immunosuppressive therapy during the observational period was carefully assessed.
Only a few past studies assessed the clinical impact of mPSL in adult MCD patients. A small Italian randomized controlled trial of 67 pediatric and 22 adult MCD patients reported no efficacy of mPSL in accelerating remission and reducing relapse in 22 adult MCD patients (19). A Japanese retrospective cohort study reported a significantly higher incidence rate of relapse in patients treated with mPSL followed by PSL than in those patients who received PSL only (13). Unfortunately, we cannot draw any conclusion on the antiproteinuric effect of mPSL from these studies, because prednisone and PSL dosing between mPSL-treated and untreated patients was quite different. By contrast, the present study showed that the mPSL+PSL and PSL groups received clinically comparative PSL dosing (Figure 2), strongly suggesting that the initial mPSL use was associated with earlier remission and lower incidence of relapse.
Why did initial use of mPSL contribute to earlier remission and a lower incidence of relapse in MCD? Although the precise MCD pathogenesis remains unknown, immunologic perturbations, especially in T cells (26), and subsequent injury to glomerular podocytes (27), including reduced expression of nephrin (28–30), lead to massive proteinuria in MCD. In addition to its immunomodulatory effect, corticosteroid directly affects the gene expression of podocytes (31). Dexamethasone promotes the expression of nephrin and other proteinuria-associated factors in human podocytes in a dose-dependent fashion within a range corresponding to the intravenous mPSL therapy used in this study (31). Thus, the effectiveness of mPSL+PSL in this study may be partly ascribed to the higher initial dose of corticosteroid, which normalized podocyte function more strongly. However, the lower relapse rate in the mPSL+PSL group during the several years of the observational period is hardly explained by the higher initial dose of corticosteroid in the mPSL+PSL group. One potential hypothesis for the long-term suppression of relapse by initial use of mPSL may be epigenetic modification (26). Because corticosteroid induces epigenetic changes (32,33) and because DNA methylation in PBMCs is modified in MCD patients (34), altering between remission and relapse (35), the higher initial dose of corticosteroid administered in the mPSL+PSL group may possibly induce long-term epigenetic changes, leading to a long-term suppression of relapse compared with the PSL group. Additional study is essential to clarify the various long-term impacts of different corticosteroid doses on podocytes and immune cells.
This study had several limitations. First, a significant difference in use of intravenous 25% albumin, a potentially confounding therapeutic intervention (18), suggests that therapeutic interventions between the mPSL+PSL and the PSL groups were not equal. Although the PS-based models (Tables 3 and 4) and careful assessment of immunosuppressive therapy during the observational period (Figure 2) indicated that the therapeutic interventions observed were comparable between the mPSL+PSL and the PSL groups, the differences in unobserved therapeutic interventions linked to initial use of mPSL potentially led to the biased estimate of its effectiveness. The efficacy of mPSL should be evaluated in a well designed randomized controlled trial. Second, high prevalence of the use of intravenous 25% albumin is common in adult MCD patients in Japan (13,18) but not common in other countries. Although the multivariate-adjusted models in Table 2 showed that intravenous administration of 25% albumin did not affect remission (HR, 1.07; 95% CI, 0.67 to 1.70; P=0.78) or relapse (HR, 0.97; 95% CI, 0.54 to 1.73; P=0.91) and additionally, did not modify an association between initial use of mPSL and these outcomes (P for interaction=0.23 and P for interaction=0.80 in multivariate-adjusted models regarding first remission and first relapse as the outcome, respectively), efficacy of mPSL should be ascertained in the internationally standard treatment regimen. Third, this study assessed the effectiveness of mPSL for first remission and first relapse in adult MCD patients, not for multiple remissions and relapses in MCD patients with frequent relapses. A similar efficacy of mPSL for multiple remissions and relapses in MCD patients with frequent relapses remains to be elucidated.
In conclusion, this study revealed that initial use of mPSL was associated with earlier remission and lower incidence of relapse, providing novel insight into the strategy of adult-onset MCD treatment. Its efficacy should be examined in a well designed randomized controlled trial.
Disclosures
None.
Acknowledgments
The authors thank Kakuya Niihiata for data management and Tomoko Namba for preliminary analysis of this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Korbet SM, Genchi RM, Borok RZ, Schwartz MM: The racial prevalence of glomerular lesions in nephrotic adults. Am J Kidney Dis 27: 647–651, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Kazi JI, Mubarak M, Ahmed E, Akhter F, Naqvi SA, Rizvi SA: Spectrum of glomerulonephritides in adults with nephrotic syndrome in Pakistan. Clin Exp Nephrol 13: 38–43, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Polito MG, de Moura LA, Kirsztajn GM: An overview on frequency of renal biopsy diagnosis in Brazil: Clinical and pathological patterns based on 9,617 native kidney biopsies. Nephrol Dial Transplant 25: 490–496, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Chang JH, Kim DK, Kim HW, Park SY, Yoo TH, Kim BS, Kang SW, Choi KH, Han DS, Jeong HJ, Lee HY: Changing prevalence of glomerular diseases in Korean adults: A review of 20 years of experience. Nephrol Dial Transplant 24: 2406–2410, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Zhou FD, Zhao MH, Zou WZ, Liu G, Wang H: The changing spectrum of primary glomerular diseases within 15 years: A survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant 24: 870–876, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama H, Taguchi T, Sugiyama H, Sato H, Committee for the Standardization of Renal Pathological Diagnosis and for Renal Biopsy and Disease Registry in the Japanese Society of Nephrology : Membranous nephropathy in Japan: Analysis of the Japan Renal Biopsy Registry (J-RBR). Clin Exp Nephrol 16: 557–563, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto S, Yamamoto Y, Hisanaga S, Morita S, Eto T, Tanaka K: Minimal change nephrotic syndrome in adults: Response to corticosteroid therapy and frequency of relapse. Am J Kidney Dis 17: 687–692, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Korbet SM, Schwartz MM, Lewis EJ: Minimal-change glomerulopathy of adulthood. Am J Nephrol 8: 291–297, 1988 [DOI] [PubMed] [Google Scholar]
- 9.Mak SK, Short CD, Mallick NP: Long-term outcome of adult-onset minimal-change nephropathy. Nephrol Dial Transplant 11: 2192–2201, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Nolasco F, Cameron JS, Heywood EF, Hicks J, Ogg C, Williams DG: Adult-onset minimal change nephrotic syndrome: A long-term follow-up. Kidney Int 29: 1215–1223, 1986 [DOI] [PubMed] [Google Scholar]
- 11.Takei T, Koike M, Suzuki K, Shirota S, Itabashi M, Ohtsubo S, Sugiura H, Suzuki K, Kojima C, Takahashi M, Ino J, Ogawa T, Uchida K, Tsuchiya K, Yumura W, Nitta K: The characteristics of relapse in adult-onset minimal-change nephrotic syndrome. Clin Exp Nephrol 11: 214–217, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Waldman M, Crew RJ, Valeri A, Busch J, Stokes B, Markowitz G, D’Agati V, Appel G: Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephrol 2: 445–453, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Fukudome K, Fujimoto S, Sato Y, Kitamura K: Comparison of the effects of intravenous methylprednisolone pulse versus oral prednisolone therapies on the first attack of minimal-change nephrotic syndrome in adults. Nephrology (Carlton) 17: 263–268, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Huang JJ, Hsu SC, Chen FF, Sung JM, Tseng CC, Wang MC: Adult-onset minimal change disease among Taiwanese: Clinical features, therapeutic response, and prognosis. Am J Nephrol 21: 28–34, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Nair RB, Date A, Kirubakaran MG, Shastry JC: Minimal-change nephrotic syndrome in adults treated with alternate-day steroids. Nephron 47: 209–210, 1987 [DOI] [PubMed] [Google Scholar]
- 16.Nakayama M, Katafuchi R, Yanase T, Ikeda K, Tanaka H, Fujimi S: Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am J Kidney Dis 39: 503–512, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Tse KC, Lam MF, Yip PS, Li FK, Choy BY, Lai KN, Chan TM: Idiopathic minimal change nephrotic syndrome in older adults: Steroid responsiveness and pattern of relapses. Nephrol Dial Transplant 18: 1316–1320, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Yoshimura A, Ideura T, Iwasaki S, Taira T, Koshikawa S: Aggravation of minimal change nephrotic syndrome by administration of human albumin. Clin Nephrol 37: 109–114, 1992 [PubMed] [Google Scholar]
- 19.Imbasciati E, Gusmano R, Edefonti A, Zucchelli P, Pozzi C, Grassi C, Della Volpe M, Perfumo F, Petrone P, Picca M, Appiani AC, Pasquali S, Ponticelli C: Controlled trial of methylprednisolone pulses and low dose oral prednisone for the minimal change nephrotic syndrome. Br Med J (Clin Res Ed) 291: 1305–1308, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto H, Nakao T, Okada T, Nagaoka Y, Takeguchi F, Tomaru R, Iwasawa H: Favorable outcome of low-dose cyclosporine after pulse methylprednisolone in Japanese adult minimal-change nephrotic syndrome. Intern Med 43: 668–673, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Shinzawa M, Yamamoto R, Nagasawa Y, Oseto S, Mori D, Tomida K, Hayashi T, Izumi M, Fukunaga M, Yamauchi A, Tsubakihara Y, Rakugi H, Isaka Y: Age and prediction of remission and relapse of proteinuria and corticosteroid-related adverse events in adult-onset minimal-change disease: A retrospective cohort study. Clin Exp Nephrol 17: 839–847, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Schimmer BP, Parker KL: Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of the synthesis and actions of adrenocortical hormones. In: Goodman and Gilman's The Pharmacological Basis of Therapeutics, 9th Ed., edited by Goodman LS, Limbird LE, Milinoff PB, Ruddon RW, Gilman AG, New York, Mcgraw-Hill Companies, 1996, pp 1649–1678 [Google Scholar]
- 23.Donohoue P: The adrenal gland and its disorders. In: Principles and Practice of Pediatric Endocrinology, edited by Kappy M, Allen D, Geffner M, Springfield, IL, Charles C. Thomas Publisher, 2005, pp 357–486 [Google Scholar]
- 24.Connors AF, Jr., Speroff T, Dawson NV, Thomas C, Harrell FE, Jr., Wagner D, Desbiens N, Goldman L, Wu AW, Califf RM, Fulkerson WJ, Jr., Vidaillet H, Broste S, Bellamy P, Lynn J, Knaus WA, SUPPORT Investigators : The effectiveness of right heart catheterization in the initial care of critically ill patients. JAMA 276: 889–897, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Austin PC: The performance of different propensity-score methods for estimating relative risks. J Clin Epidemiol 61: 537–545, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Grimbert P, Audard V, Remy P, Lang P, Sahali D: Recent approaches to the pathogenesis of minimal-change nephrotic syndrome. Nephrol Dial Transplant 18: 245–248, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Hogan J, Radhakrishnan J: The treatment of minimal change disease in adults. J Am Soc Nephrol 24: 702–711, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G: Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am J Pathol 158: 1723–1731, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Furness PN, Hall LL, Shaw JA, Pringle JH: Glomerular expression of nephrin is decreased in acquired human nephrotic syndrome. Nephrol Dial Transplant 14: 1234–1237, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Wernerson A, Dunér F, Pettersson E, Widholm SM, Berg U, Ruotsalainen V, Tryggvason K, Hultenby K, Söderberg M: Altered ultrastructural distribution of nephrin in minimal change nephrotic syndrome. Nephrol Dial Transplant 18: 70–76, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW: Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ito K, Barnes PJ, Adcock IM: Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol 20: 6891–6903, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krukowski K, Eddy J, Kosik KL, Konley T, Janusek LW, Mathews HL: Glucocorticoid dysregulation of natural killer cell function through epigenetic modification. Brain Behav Immun 25: 239–249, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Dai Y, Peng W, Lu J, Zhang Y, Wang L: Genome-wide analysis of histone H3 lysine 4 trimethylation in peripheral blood mononuclear cells of minimal change nephrotic syndrome patients. Am J Nephrol 30: 505–513, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi Y, Aizawa A, Takizawa T, Yoshizawa C, Horiguchi H, Ikeuchi Y, Kakegawa S, Watanabe T, Maruyama K, Morikawa A, Hatada I, Arakawa H: DNA methylation changes between relapse and remission of minimal change nephrotic syndrome. Pediatr Nephrol 27: 2233–2241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]