Abstract
Pseudomonas aeruginosa is the major opportunistic bacterial pathogen in persons with cystic fibrosis (CF); pulmonary infection occurs in approximately 80% of adult CF patients. Much of CF patient management depends on accurate identification of P. aeruginosa from sputum culture. However, identification of this species may be problematic due to the marked phenotypic variability demonstrated by CF sputum isolates and the presence of other closely related species. To facilitate species identification, we used 16S ribosomal DNA (rDNA) sequence data to design PCR assays intended to provide genus- or species-level identification. Both assays yielded DNA fragments of the predicted size. We tested 42 culture collection strains (including 14 P. aeruginosa strains and 28 strains representing 16 other closely related Pseudomonas species) and 43 strains that had been previously identified as belonging to 28 nonpseudomonal species also recovered from CF patient sputum. Based on these 85 strains, the specificity and sensitivity of both assays were 100%. To further assess the utility of the PCR assays, we tested 66 recent CF sputum isolates. The results indicated that preliminary phenotypic testing had misidentified several isolates. The 16S rDNA sequence was determined for 38 isolates, and in all cases it confirmed the results of the PCR assays. Thus, we have designed two PCR assays: one is specific for the genus Pseudomonas, while the other is specific for P. aeruginosa. Both assays show 100% sensitivity and specificity.
Pseudomonas aeruginosa is the most common bacterial pathogen causing respiratory tract infection in persons with cystic fibrosis (CF). Infection occurs throughout childhood, ultimately affecting some 80% of adult CF patients, and is associated with increased rates of morbidity and mortality (9, 13). P. aeruginosa is also a well-known opportunistic and nosocomial pathogen, the identification of which typically presents little challenge. Accurate identification of CF isolates, however, can be difficult. CF-derived isolates often demonstrate phenotypic diversity due to loss of pigment production, exopolysaccharide production (mucoidy), and synthesis of rough lipopolysaccharide (23). Commercial test systems and other phenotype-based identification methods may therefore misidentify P. aeruginosa (15, 18, 28, 29, 31). Identification is often further hampered by the presence of other closely related nonfermenting gram-negative bacilli, including other Pseudomonas species (2, 6). The potential for misidentification of this species from sputum culture presents an obstacle to CF patient management, particularly with respect to antimicrobial therapy, patient prognosis, and infection control.
Genotype-based identification methods circumvent the problem of variable phenotype to provide more accurate species identification. However, the taxonomic complexity, uncertain phylogeny, and paucity of genomic sequence data of the dozens of species within the broad genus Pseudomonas present an obstacle to genotypic identification assays. We took advantage of a recent comprehensive reassessment of the phylogenetic affiliation of the pseudomonads based on 16S ribosomal DNA (rDNA) sequence data (1) to identify genus- and species-specific 16S rDNA signature sequences. Based on these sequences we designed simple, rapid, and accurate PCR assays that allow the differentiation of P. aeruginosa from other Pseudomonas species that may also be recovered from CF sputum cultures.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
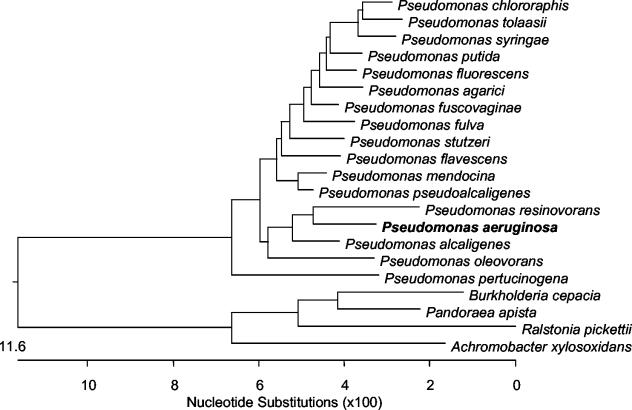
Forty-two Pseudomonas strains were obtained from the BCCM/LMG Bacteria Collection (Laboratorium voor Microbiologie, Universiteit Gent, Ghent, Belgium) or the American Type Culture Collection (Manassas, Va.). This included 14 P. aeruginosa strains and at least one strain each of 16 other Pseudomonas species (Table 1; Fig. 1). Another 43 strains had been identified in previous studies as belonging to 28 nonpseudomonal species (4-8, 21, 22, 24, 33). This group included 15 Burkholderia cepacia complex, 5 Pandoraea spp., 5 Ralstonia spp., 5 Achromobacter xylosoxidans, 4 Stenotrophomonas maltophilia, 2 Acinetobacter spp., and 2 Serratia marcescens strains and 1 strain each of Herbaspirillum frisingense, Klebsiella pneumoniae, Morganella morganii, Moraxella osloensis, and Escherichia coli. An additional 66 isolates (recovered from 66 CF patients) were selected from isolates referred to the Burkholderia cepacia Research Laboratory and Repository (BcRLR, University of Michigan) for analysis. This group consisted of isolates that had been referred to the BcRLR identified as either P. aeruginosa (n = 14 isolates) or another Pseudomonas species (n = 20). It also included isolates that had been unidentified (n = 12) or identified by the referring laboratory as a nonpseudomonal species (n = 20) but which were identified by the BcRLR as a Pseudomonas species by using the RapID NF Plus system (Remel, Lenexa, Kans.). All bacteria were stored at −80°C. Bacteria from frozen stocks were grown aerobically at 34°C for as long as 48 h on Mueller-Hinton medium supplemented with 1.6% (wt/vol) agarose.
TABLE 1.
Culture collection Pseudomonas strains used in this study
| Species | Straina |
|---|---|
| P. aeruginosa | ATCC 10145, ATCC 14207, ATCC 19154, ATCC 19429, ATCC 23993, ATCC 25006, ATCC 25010, ATCC 25619, ATCC 27315, ATCC 27316, ATCC 35032, ATCC 35422, ATCC 35554, ATCC 9721 |
| P. agarici | LMG 2112T |
| P. alcaligenes | LMG 1224T, LMG 6353 |
| P. chlororaphis | LMG 5004T |
| P. flavescens | LMG 18387T |
| P. fluorescens | LMG 14564, LMG 1794T, LMG 5940 |
| P. fulva | LMG 11722T |
| P. fuscovaginae | LMG 2158T |
| P. mendocina | LMG 1223T, LMG 5941, LMG 6396 |
| P. oleovorans | LMG 2229T |
| P. pertucinogena | LMG 1874T |
| P. pseudoalcaligenes | LMG 1225, LMG 2854, LMG 5516 |
| P. putida | LMG 2257T, LMG 2259 |
| P. resinovorans | LMG 2274T |
| P. stutzeri | ATCC 17588, LMG 11199T, LMG 1228, LMG 2232 |
| P. syringae | LMG 1247T, LMG 12648 |
| P. tolaasii | LMG 2342 |
ATCC, American Type Culture Collection, Manassas, Va.; LMG, BCCM/LMG Bacteria Collection, Laboratorium voor Microbiologie, Universiteit Gent, Ghent, Belgium.
FIG. 1.
Phylogenetic tree generated by using ClustalV-based alignment for 16S rDNA sequences of select Pseudomonas and other CF-related species. P. resinovorans, P. aeruginosa, P. alcaligenes, and P. oleovorans are species within the P. aeruginosa group (1).
DNA preparation.
DNA was prepared from bacteria as described previously (22). In brief, a single CFU was suspended in 20 μl of lysis buffer containing 0.25% (vol/vol) sodium dodecyl sulfate and 0.05 N NaOH. After heating for 15 min at 95°C, 180 μl of high-performance liquid chromatography-grade H2O (Fisher) was added, and the lysis suspension was stored at −20°C.
Primer design.
Relevant 16S rDNA sequences available in the GenBank database were aligned by using the MegAlign software package (DNASTAR Inc., Madison, Wis.). These included 136 sequences from 42 validly described Pseudomonas species (1), as well as several other phylogenetically related γ-Proteobacteria and CF-relevant species. Based on this alignment, putative genus- and species-specific primers were designed.
PCR.
Amplification of targeted DNA was carried out in 25-μl reaction volumes, each containing 2 mM MgCl2, 50 mM Trizma (pH 8.3; Sigma, St. Louis, Mo.), 250 μM (each) deoxynucleoside triphosphates (Promega, Madison, Wis.), 0.4 μM (each) primer, 1 U of Taq polymerase (Invitrogen, Carlsbad, Calif.), and 2 μl of whole-cell bacterial lysate, and adjusted to 25 μl by the addition of high-performance liquid chromatography-grade H2O. Amplification was carried out in a RapidCycler (Idaho Technology Inc., Salt Lake City, Utah) thermocontroller. After an initial denaturization for 2 min at 95°C, 25 cycles were completed, each consisting of 20 s at 94°C, 20 s at the appropriate annealing temperature (Table 2), and 40 s at 72°C. A final extension of 1 min at 72°C was applied. With this program, the total time for amplification of target DNA was approximately 45 min.
TABLE 2.
16S rDNA-based primer sets
| Primer | Sequence (5′-3′) | Target | Annealing temp (°C) | Locationa | Product size (bp) |
|---|---|---|---|---|---|
| PA-GS-F | GACGGGTGAGTAATGCCTA | Pseudomonas species | 54 | 95-113 | 618 |
| PA-GS-R | CACTGGTGTTCCTTCCTATA | 693-712 | |||
| PA-SS-F | GGGGGATCTTCGGACCTCA | P. aeruginosa | 58 | 189-206 | 956 |
| PA-SS-R | TCCTTAGAGTGCCCACCCG | 1124-1144 |
Position and size relative to 16S rDNA sequence of P. aeruginosa AT2 (AB091760).
Amplification and sequence determination of 16S rDNA.
In order to confirm PCR-based identification results, we performed comparative 16S rDNA sequence analysis. Nearly complete 16S rRNA genes (corresponding to positions 9 to 1500 in the E. coli numbering system) were amplified by PCR using Pfu DNA polymerase (Stratagene, La Jolla, Calif.) with conserved primers UFPL and URPL as previously described (22). Amplified DNA was purified with the QIAquick PCR purification kit (Qiagen Inc., Valencia, Calif.) according to the manufacturer's instructions. DNA sequencing was carried out with an Applied Biosystems ABI model 3700 sequencer and the protocols of the manufacturer (PE Applied Biosystems, Foster City, Calif.) by using the BigDye Terminator cycle sequencing ready reaction kit. Resultant sequences were visualized as chromatograms and manually edited using Chromas version 2.22 (Technelysium Pty. Ltd., Helensvale, Australia). Edited sequences were assembled using EditSeq (DNASTAR Inc.) and identified by using BLASTN and comparison to sequences currently available in the NCBI database (www.ncbi.nlm.nih.gov/BLAST). Isolates were considered P. aeruginosa if their 16S rDNA sequences showed the highest degree of similarity to 16S rDNA sequences of P. aeruginosa culture collection reference strains.
Nucleotide sequence accession number.
The GenBank accession numbers for the 16S rDNA sequences generated in this study are AY486350 through AY486387.
RESULTS
Primer design.
Based on alignment of 16S rDNA sequences available in GenBank, two primer pairs were designed. Primer pair PA-GS-F and PA-GS-R was intended to amplify all Pseudomonas species, while the pair PA-SS-F and PA-SS-R was designed to amplify only P. aeruginosa (Table 2). The latter primers targeted species-specific signature sequences in 16S rDNA variable regions 2 and 8 (V2 and V8), respectively.
Sensitivity and specificity of PCR assays.
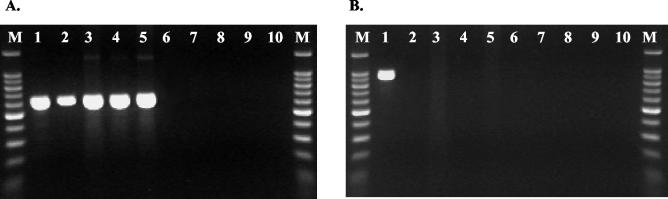
PCR assays employing each primer pair produced DNA products of the predicted sizes (Fig. 2). The sensitivity and specificity of the putative genus-specific and species-specific PCR assays were determined by testing the 42 culture collection Pseudomonas strains (Table 1; Fig. 1) and the 43 previously identified non-Pseudomonas strains. The results are summarized in Table 3; the sensitivity and specificity of each PCR assay were 100%.
FIG. 2.
PCR analysis of P. aeruginosa, Pseudomonas species, and CF-relevant bacteria. (A) PCR using Pseudomonas genus-specific primers PA GS-F and PA GS-R. (B) PCR using P. aeruginosa-specific primers PA SS-F and PA SS-R. Lanes M, reference markers; lane 1, P. aeruginosa; lanes 2 to 5, P. pertucinogena, P. stutzeri, P. putida, and P. syringae, respectively; lanes 6 to 9, CF-relevant genera (Burkholderia, Pandoraea, Ralstonia, and Achromobacter, respectively); lane 10, negative control (water).
TABLE 3.
Sensitivity and specificity of PCR assays
| PCR using primer pair | Target | No. of PCR positive/no. tested
|
Sensitivity (%) | Specificity (%) | ||
|---|---|---|---|---|---|---|
| Pseudomonas sp.a | P. aeruginosab | Otherc | ||||
| PA GS-F and PA GS-R | Pseudomonas species | 28/28 | 14/14 | 0/43 | 100 | 100 |
| PA SS-F and PA SS-R | P. aeruginosa | 0/28 | 14/14 | 0/43 | 100 | 100 |
Culture collection Pseudomonas species other than P. aeruginosa (detailed in Table 1).
Culture collection P. aeruginosa only (detailed in Table 1).
Previously identified non-Pseudomonas species recovered from CF sputum (includes 15 B. cepacia complex, 5 Pandoraea sp., 5 Ralstonia sp., 5 A. xylosoxidans, 4 S. maltophilia, 2 Acinetobacter sp., and 2 S. marcescens strains and 1 strain each of H. frisingense, K. pneumoniae, M. morganii, M. osloensis, and E. coli).
Further assessment of PCR assays.
As another test of the utility of the two new PCR assays, we investigated 66 sputum culture isolates (recovered from 66 CF patients) that had been referred to the BcRLR for analysis. The results are summarized in Table 4. Fourteen isolates in this group were identified by the referring laboratories as P. aeruginosa. Thirteen of these were also identified as P. aeruginosa by PCR assay; 16S rDNA sequence analysis was performed on six of these, which confirmed all as P. aeruginosa. The single isolate that was not a Pseudomonas species by PCR assay was identified as a Burkholderia species by 16S rDNA sequence analysis.
TABLE 4.
PCR analysis of CF sputum isolates referred to BcRLR
| Referring lab identificationa | PCR analysisb
|
||
|---|---|---|---|
| P. aeruginosa | Pseudomonas sp. (non-aeruginosa) | Not Pseudomonas sp. | |
| P. aeruginosa | 13 (6c) | 1 (1d) | |
| Pseudomonas sp. (non-aeruginosa) | 17 (6c) | 1 (1e) | 2 (2f) |
| Not Pseudomonas sp. | 12 (6c) | 5 (5g) | 3 (3h) |
| No identification | 8 (4c) | 2 (2i) | 2 (2j) |
Identification provided by referring laboratory.
Results obtained with PCR assays using species-specific primers PA SS-F and PA SS-R and genus-specific primers PA GS-F and PA GS-R. Numbers in parentheses indicate number of isolates for which 16S rDNA sequence analysis was performed. Superscript, italic letters c through j indicate species tentatively identified by 16S rDNA sequence analysis as follows:
P. aeruginosa
Burkholderia sp.
P. synxantha
Burkholderia sp. and S. maltophilia
P. fluorescens, P. stutzeri, P. lundensis/fragi, and two Pseudomonas sp.
H. seropedicae and two Herbaspirillum sp.
P. lundensis/fragi and P. pseudoalcaligenes
j, Staphylococcus sp. and Acinetobacter sp.
Twenty isolates had been identified by the referring laboratory as either “Pseudomonas sp.” (n = 10), P. alcaligenes (n = 1), P. mendocina (n = 2), P. oleovorans (n = 1), P. fluorescens (n = 3), P. putida (n = 2), or P. stutzeri (n = 1). Seventeen of these were identified as P. aeruginosa by PCR assay; 16S rDNA sequence analysis of six of these confirmed that they were P. aeruginosa. One isolate was identified as a non-aeruginosa pseudomonal species by PCR and confirmed as P. synxantha by 16S rDNA analysis. The remaining two isolates were not Pseudomonas sp. by PCR and were identified as Burkholderia sp. and S. maltophilia by 16S rDNA sequence analysis.
Twenty isolates were identified by referring laboratories as species other than Pseudomonas sp. Twelve of these were identified as P. aeruginosa by PCR; six were tested by 16S rDNA sequence analysis and confirmed as P. aeruginosa. Five of the 20 isolates were identified by both PCR assay and 16S rDNA analysis as non-aeruginosa pseudomonal species. Three isolates were not Pseudomonas sp. by PCR and were identified as Herbaspirillum sp. by 16S rDNA analysis. The remaining 12 isolates were unidentified by the referring laboratories. Eight of these were identified as P. aeruginosa by PCR assay; four were examined by 16S rDNA and confirmed as P. aeruginosa. PCR assays identified two isolates as non-aeruginosa pseudomonal species and two as not Pseudomonas species; 16S rDNA sequence analysis of these four isolates confirmed the PCR results.
In summary, the novel species- and genus-specific PCR assays indicated that several of the 66 clinical isolates had been misidentified by the referring laboratories. Thirty-eight (58%) of these were further examined by 16S rDNA sequence analysis, and in each case the results were consistent with the results of the PCR assays. Thus, when assessed against 16S rDNA sequence analysis of this set of isolates, the sensitivity and specificity of both PCR assays were again 100%.
DISCUSSION
Persons with CF are susceptible to chronic respiratory tract infection by a number of opportunistic bacterial pathogens, including P. aeruginosa, S. maltophilia, A. xylosoxidans, and several Ralstonia, Pandoraea, and B. cepacia complex species (2, 20). Recent work has shown that CF patients also can become infected with other unusual or novel taxa, such as Acinetobacter sp., Bordetella sp., Moraxella sp., Comomonas sp., Chryseobacterium sp., Rhizobium sp., Herbaspirillum sp., and Inquilinus limosus (6). Accurate identification of these phenotypically similar species from respiratory samples is critical to patient management. This is particularly true with respect to P. aeruginosa. Sustained infection by this species is typically regarded as a poor prognostic indicator in young CF patients (13, 19). In addition, recent evidence of interpatient spread of P. aeruginosa among CF patients (14) has prompted increasingly stringent infection control measures in CF care centers; the effectiveness of such measures relies in the first instance on accurate species identification. The differentiation of P. aeruginosa from other Pseudomonas species is also fundamental to efforts to better understand the natural history of P. aeruginosa infection in CF and to develop and assess new therapeutic strategies (e.g., aggressive antimicrobial therapy aimed at eradication of initial pulmonary infection) (19). In fact, entry into clinical trials evaluating novel therapies relies on the accurate and rapid identification of P. aeruginosa and other infecting species.
The CF lung, however, constitutes a microenvironment that promotes phenotypic alteration of chronically infecting bacteria (26). Consequently, the atypical phenotypes often exhibited by Pseudomonas CF isolates present a challenge to commercial test systems commonly used in clinical microbiology laboratories (15, 18, 28, 29, 31). Genotypic identification methods would be expected to circumvent this problem, and molecular assays based on specific genes have been described. These include PCR assays targeting the P. aeruginosa oprL (11), algD (10, 25), and exotoxin A genes (17, 32). The performance of these assays in differentiating P. aeruginosa from other closely related Pseudomonas species has not been examined, however, and the paucity of sequence data for these loci from non-P. aeruginosa strains limits predictions based on in silico analyses. More recently, Clarke and colleagues (3) described a PCR assay that amplifies a fragment of the groE heat shock protein gene from several Pseudomonas species. Restriction fragment length polymorphism analysis of the amplified DNA was reported to differentiate P. aeruginosa from P. stutzeri, P. fluorescens, and P. putida. Qin and colleagues (28) used real-time PCR amplification of multiple targets, including exotoxin A, algD, oprL, and gyrB, together with biochemical tests and 16S rDNA sequencing to identify phenotypically atypical P. aeruginosa isolates recovered from CF specimens.
16S rDNA sequence has long been used as a taxonomic “gold standard” in determining the phylogenies of bacterial species (35). Selective amplification of Pseudomonas 16S rDNA by PCR followed by restriction fragment length polymorphism analysis or denaturing gradient gel electrophoresis has been used to detect and differentiate Pseudomonas species from clinical and environmental samples (12, 27, 30, 34). Karpati and Jonasson (16) used a conserved 16S rDNA primer with a Pseudomonas genus-specific primer in a PCR assay to detect Pseudomonas DNA in CF sputum; this assay was not designed to differentiate P. aeruginosa from other Pseudomonas species.
In this study, we took advantage of a recent reassessment of the phylogenetic affiliation of the pseudomonads (1) to reexamine the rapidly expanding 16S rDNA sequence data available in public databases. Based on an alignment of 136 16S rDNA sequences from 42 validly described Pseudomonas species as well as several other γ-Proteobacteria, we identified Pseudomonas genus-specific and P. aeruginosa-specific signature sequences. PCR assays using primers targeting these sequences were designed and tested against a panel of 85 previously identified strains. We included in this test panel 43 strains representing 28 nonpseudomonal species that are also found in CF sputum, as well as 42 culture collection Pseudomonas strains. In the latter group we were careful to include strains from Pseudomonas species phylogenetically most closely related to P. aeruginosa. This group included P. resinovorans, P. alcaligenes, P. oleovorans, P. pseudoalcaligenes, P. mendocina, and P. flavescens, all of which cluster within the P. aeruginosa group described by Anzai and colleagues (1). We also tested our PCR assays against P. putida, P. fluorescens, P. stutzeri, and P. syringae, common environmental pseudomonads that may also be recovered occasionally from CF sputum. Both the genus-specific and P. aeruginosa-specific assays demonstrated 100% sensitivity and specificity.
Our examination of recent CF sputum isolates further showed the utility of the new PCR assays. Sixty-six isolates, recovered from 66 patients and referred from several clinical microbiology laboratories, were examined. All had been assessed phenotypically with a commercial test system by the referring laboratory. Among these, 34 had been identified by the referring laboratory as either P. aeruginosa, another Pseudomonas species, or as Pseudomonas (i.e., not identified to the species level). The remaining 32 had been referred either unidentified or identified as a nonpseudomonal species but were preliminarily identified by us as Pseudomonas using our routine phenotypic test kit (RapID NF Plus system). Analysis of these 66 isolates with the two novel PCR assays indicated that phenotypic testing had misidentified several isolates. We performed 16S rDNA sequence analysis on more than half of these isolates, and in every case the sequence data confirmed the PCR results.
Although our PCR and DNA sequence analyses revealed isolates that had been misidentified by phenotypic testing, we must be clear in pointing out that our study was not designed to ascertain the frequency of misidentification of CF sputum isolates nor to compare the relative accuracy of different phenotypic identification systems. Isolates referred to us for testing most likely represent a biased set of atypical and difficult-to-identify isolates, and so extrapolation of misidentification rates from this study is not appropriate. Nevertheless, such isolates were well suited to provide a rigorous test of our new PCR assays and represented strains for which molecular analysis would be expected to be most useful. Our study also reiterates that various non-aeruginosa pseudomonal species can occasionally be recovered from CF sputum culture. Among isolates testing positive with the genus-level PCR, but negative with the P. aeruginosa-specific PCR, we identified P. fluorescens, P. lundensis/fragi P. pseudoalcaligenes, P. stutzeri, and P. synxantha, based on 16S rDNA sequence analysis.
In summary, we have designed 16S rDNA-based PCR assays that provide rapid, simple, and reliable identification of P. aeruginosa and its differentiation from other phylogenetically closely related Pseudomonas species. Both assays have 100% sensitivity and specificity for their intended targets. We have also demonstrated the utility of these PCR assays in accurately identifying P. aeruginosa among isolates not correctly identified by phenotypic analyses. These assays should serve as a useful adjunct in the evaluation of gram-negative nonfermenting bacteria recovered from CF sputum culture.
Acknowledgments
This work was supported by a grant (to J.J.L.) from the Cystic Fibrosis Foundation.
We acknowledge the generosity and cooperation of participating CF centers and microbiology laboratories for submission of clinical isolates. T.C. and P.V. are indebted to the Fund for Scientific Research—Flanders (Belgium) for a position as postdoctoral fellow and research grants, respectively. T.C. also acknowledges the support from the Belgian Federal Government (Federal Office for Scientific, Technical and Cultural Affairs).
REFERENCES
- 1.Anzai, Y., H. Kim, J.-Y. Park, H. Wakabayashi, and H. Oyaizu. 2000. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. E vol. Microbiol. 50:1563-1589. [DOI] [PubMed] [Google Scholar]
- 2.Burns, J. L., J. Emerson, J. R. Stapp, D. L. Yim, J. Krzewinski, L. Louden, B. W. Ramsey, and C. R. Clausen. 1998. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27:158-163. [DOI] [PubMed] [Google Scholar]
- 3.Clarke, L., J. E. Moore, B. C. Millar, L. Garske, J. Xu, M. W. Heuzenroeder, M. Crowe, and J. S. Elborn. 2003. Development of a diagnostic PCR assay that targets a heat-shock protein gene (groES) for detection of Pseudomonas spp. in cystic fibrosis patients. J. Med. Microbiol. 52:759-763. [DOI] [PubMed] [Google Scholar]
- 4.Coenye, T., E. Mahenthiralingam, D. Henry, J. J. LiPuma, S. Laevens, M. Gillis, D. P. Speert, and P. Vandamme. 2001. Burkholderia ambifaria sp. nov., a novel member of the Burkholderia cepacia complex comprising biocontrol and cystic fibrosis-related isolates. Int. J. Syst. E vol. Microbiol. 51:1481-1490. [DOI] [PubMed] [Google Scholar]
- 5.Coenye, T., J. Goris, P. De Vos, P. Vandamme, and J. J. LiPuma. 2003. Classification of Ralstonia pickettii-like isolates from the environment and clinical samples as Ralstonia insidiosa sp. nov. Int. J. Syst. Evol. Microbiol. 53:1075-1080. [DOI] [PubMed] [Google Scholar]
- 6.Coenye, T., J. Goris, T. Spilker, P. Vandamme, and J. J. LiPuma. 2002. Characterization of unusual bacteria isolated from respiratory secretions of cystic fibrosis patients and description of Inquilinus limosus gen. nov., sp. nov. J. Clin. Microbiol. 40:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coenye, T., L. Liu, P. Vandamme, and J. J. LiPuma. 2001. Identification of Pandoraea species by 16S ribosomal DNA-based PCR assays. J. Clin. Microbiol. 39:4452-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coenye, T., P. Vandamme, and J. J. LiPuma. 2002. Infection by Ralstonia species in cystic fibrosis patients: identification of R. pickettii and R. mannitolilytica by polymerase chain reaction. Emerg. Infect. Dis. 8:692-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cystic Fibrosis Foundation. 2001. Patient registry 2001. Annual data report. Cystic Fibrosis Foundation, Bethesda, Md.
- 10.da Silva Filho, L. V., J. E. Levi, C. N. Oda Bento, S. R. da Silva Ramos, and T. Rozov. 1999. PCR identification of Pseudomonas aeruginosa and direct detection in clinical samples from cystic fibrosis patients. J. Med. Microbiol. 48:357-361. [DOI] [PubMed] [Google Scholar]
- 11.De Vos, D., A. Lim, Jr., J. P. Pirnay, M. Struelens, C. Vandenvelde, L. Duinslaeger, A. Vanderkelen, and P. Cornelis. 1997. Direct detection and identification of Pseudomonas aeruginosa in clinical samples such as skin biopsy specimens and expectorations by multiplex PCR based on two outer membrane lipoprotein genes, oprI and oprL. J. Clin. Microbiol. 35:1295-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duineveld, B. M., G. A. Kowalchuk, A. Keijzer, J. D. van Elsas, and J. A. van Veen. 2001. Analysis of bacterial communities in the rhizosphere of chrysanthemum via denaturing gradient gel electrophoresis of PCR-amplified 16S rRNA as well as DNA fragments coding for 16S rRNA. Appl. Environ. Microbiol. 67:172-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson, V. L., C. L. Wielinski, and W. E. Regelmann. 1993. Prognostic implications of initial oropharyngeal bacterial flora in patients with cystic fibrosis diagnosed before the age of two years. J. Pediatr. 122:854-860. [DOI] [PubMed] [Google Scholar]
- 14.Jones, A. M., M. E. Dodd, C. J. Doherty, J. R. W. Govan, and A. K. Webb. 2002. Increased treatment requirements of patients with cystic fibrosis who harbour a highly transmissible strain of Pseudomonas aeruginosa. Thorax 57:924-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joyanes, P., M. del Carmen Conejo, L. Martinez-Martinez, and E. J. Perea. 2001. Evaluation of the VITEK 2 system for the identification and susceptibility testing of three species of nonfermenting gram-negative rods frequently isolated from clinical samples. J. Clin. Microbiol. 39:3247-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpati, F., and J. Jonasson. 1996. Polymerase chain reaction for the detection of Pseudomonas aeruginosa, Stenotrophomonas maltophilia and Burkholderia cepacia in sputum of patients with cystic fibrosis. Mol. Cell. Probes 10:397-403. [DOI] [PubMed] [Google Scholar]
- 17.Khan, A. A., and C. E. Cerniglia. 1994. Detection of Pseudomonas aeruginosa from clinical and environmental samples by amplification of the exotoxin A gene using PCR. Appl. Environ. Microbiol. 60:3739-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiska, D. L., A. Kerr, M. C. Jones, J. A. Caracciolo, B. Eskridge, M. Jordan, S. Miller, D. Hughes, N. King, and P. H. Gilligan. 1996. Accuracy of four commercial systems for identification of Burkholderia cepacia and other gram-negative nonfermenting bacilli recovered from patients with cystic fibrosis. J. Clin. Microbiol. 34:886-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch, C. 2002. Early infection and progression of cystic fibrosis lung disease. Pediatr. Pulmonol. 34:232-236. [DOI] [PubMed] [Google Scholar]
- 20.LiPuma, J. J. 2003. Burkholderia and emerging pathogens in cystic fibrosis. Semin. Respir. Crit. Care Med. 24:681-692. [DOI] [PubMed] [Google Scholar]
- 21.LiPuma, J. J., B. J. Dulaney, J. D. McMenamin, P. W. Whitby, T. L. Stull, T. Coenye, and P. Vandamme. 1999. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J. Clin. Microbiol. 37:3167-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, L., T. Coenye, J. L. Burns, P. W. Whitby, T. L. Stull, and J. J. LiPuma. 2002. Ribosomal DNA-directed PCR for identification of Achromobacter (Alcaligenes) xylosoxidans recovered from sputum samples from cystic fibrosis patients. J. Clin. Microbiol. 40:1210-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahenthiralingam, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme. 2000. DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 39:3165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIntosh, I., J. R. W. Govan, and D. J. H. Brock. 1992. Detection of Pseudomonas aeruginosa in sputum from cystic fibrosis patients by the polymerase chain reaction. Mol. Cell. Probes 6:299-304. [DOI] [PubMed] [Google Scholar]
- 26.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1253. [DOI] [PubMed] [Google Scholar]
- 27.Porteous, L. A., F. Widmer, and R. J. Seidler. 2002. Multiple enzyme restriction fragment length polymorphism analysis for high resolution distinction of Pseudomonas (sensu stricto) 16S rRNA genes. J. Microbiol. Methods 51:337-348. [DOI] [PubMed] [Google Scholar]
- 28.Qin, X., J. Emerson, J. Stapp, L. Stapp, P. Abe, and J. L. Burns. 2003. Use of real-time PCR with multiple targets to identify Pseudomonas aeruginosa and other nonfermenting gram-negative bacilli from patients with cystic fibrosis. J. Clin. Microbiol. 41:4312-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saiman, L., J. L. Burns, D. Larone, Y. Chen, E. Garber, and S. Whittier. 2003. Evaluation of MicroScan Autoscan for identification of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J. Clin. Microbiol. 41:492-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schabereiter-Gurtner, C., S. Maca, S. Rolleke, K. Nigl, J. Lukas, A. Hirschl, W. Lubitz, and T. Barisani-Asenbauer. 2001. 16S rDNA-based identification of bacteria from conjunctival swabs by PCR and DGGE fingerprinting. Invest. Ophthalmol. Vis. Sci. 42:1164-1171. [PubMed] [Google Scholar]
- 31.Shelly, D. B., T. Spilker, E. Gracely, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Utility of commercial test systems for identification of Burkholderia cepacia complex from cystic fibrosis sputum culture. J. Clin. Microbiol. 38:3112-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song, K. P., T. K. Chan, Z. L. Ji, and S. W. Wong. 2000. Rapid identification of Pseudomonas aeruginosa from ocular isolates by PCR using exotoxin A-specific primers. Mol. Cell. Probes 14:199-204. [DOI] [PubMed] [Google Scholar]
- 33.Whitby, P. W., K. B. Carter, J. L. Burns, J. A. Royall, J. J. LiPuma, and T. L. Stull. 2000. Identification and detection of Stenotrophomonas maltophilia by rRNA-directed PCR. J. Clin. Microbiol. 38:4305-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Widmer, F., R. J. Seidler, P. M. Gillevet, L. S. Watrud, and G. D. Di Giovanni. 1998. A highly selective PCR protocol for detecting 16S rRNA genes of the genus Pseudomonas (sensu stricto) in environmental samples. Appl. Environ. Microbiol. 64:2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]