Abstract
Babesia microti-like parasites were detected for the first time in Ixodes ovatus in Hyogo Prefecture, Japan, where two reported types of B. microti-like parasites were recognized in many rodents. Of 80 adult I. ovatus ticks collected, 5 possessed the reported type and 1 possessed a new type of B. microti-like parasite.
Human babesiosis is most frequently caused by Babesia microti, a rodent babesia species. Human babesiosis caused by B. microti has occurred almost exclusively in the northeastern and upper midwestern United States of America (6, 7, 19). The first human babesiosis case in Japan was identified in Kobe, Hyogo Prefecture, in 1999 (11, 12). It was shown that the patient was infected through blood transfusion from an asymptomatic donor infected with a geographical variant of B. microti (the Kobe isolate or strain). A small subunit of the rRNA gene (SSU rDNA) sequence of the etiologic parasite (Kobe type of SSU rDNA) was the most homologous but not identical to that of B. microti from the United States (U.S. type of SSU rDNA). The antigenicity of the Kobe strain was substantially different from that of B. microti from the United States (12). The Otsu strain was established by Shiota et al. from Apodemus speciosus captured in 1999 at the same place where the B. microti-like parasite infection was first demonstrated in Japanese field rodents, A. speciosus and Apodemus argenteus, in Otsu, Shiga Prefecture, in the early 1980s (13). The SSU rDNA of the Otsu strain (Otsu type of SSU rDNA) differed from the Kobe and U.S. types. The Kobe type of B. microti was later discovered in A. speciosus on Awaji Island, Hyogo Prefecture, the residential area of the donor (20, 23). Tsuji et al. reported that the Kobe type of B. microti was only identified in rodents on Awaji Island, while the Hobetsu type (corresponding to the Otsu type) was widely distributed in Japan but not in Kobe (20). Thus, it has been shown that at least two SSU rDNA types of B. microti are parasitizing rodents in Japan. However, the tick vector, as well as the detailed epizootiologic status of B. microti, remains unknown. Accordingly, we have surveyed B. microti infection in wild rodents and ticks in Hyogo Prefecture, the human babesiosis emergence area of Japan.
From June 2000 to May 2002, field rodent and unfed tick collections were carried out with Sherman live traps and by flagging vegetation both on Awaji Island, the residential island of the asymptomatic blood donor, and in the Rokko Mountains, mainly located in Kobe, in Hyogo Prefecture (see the map at http://www.kobe-u.ac.jp/hyogo/stage.html). The species of rodents and ticks were identified by the key characteristics described by Abe et al. (1) and Takada (14), respectively. For rodents captured alive, Giemsa-stained blood smears were examined for intraerythrocytic parasites. B. microti-specific fragments of SSU rDNA were amplified from DNA extracted from rodent blood, rodent spleens, and tick salivary glands by nested PCR with two sets of B. microti-specific primers, Bab1-Bab4 and Bab2-Bab3 (B. microti detection PCR), in accordance with the method established by Persing et al. (10). The first PCR product (one of the most divergent parts of the SSU rDNA sequences of the Kobe, Otsu, and U.S. types) was directly sequenced and defined as the Kobe, Otsu, or U.S. SSU rDNA type. For B. microti detection PCR-positive samples, a different nested PCR (B. microti confirmation PCR) was performed with two sets of Babesia-specific primers: CF1 (5′-GACGGTAGGGTATTGGCCT-3′)-BabD (5′-TCAAGGTGCTGAAGGAGTCG-3′) and BabA (5′-GCTCGTAGTTGAATTTCTGCCT-3′)-BabB (5′-AGTAGTTCGTCTTTAACAAATCT-3′). By sequencing the part from BabA to BabB (the other one of the most divergent parts), the respective types were confirmed. To avoid errors caused by contamination of PCR products, DNA was extracted and divided into several aliquots in a safety cabinet placed in a room in which PCR products had never been treated. Detection and confirmation PCR assays were separately carried out with the different aliquots. For the sample showing the new type of SSU rDNA, the nearly full length of the SSU rDNA sequence was determined as described before (3).
B. microti infection was highly enzootic both on Awaji Island and in the Rokko Mountains (Table 1). The Kobe type of B. microti was frequently and exclusively detected in rodents in the middle area of Awaji Island, while the Otsu type of B. microti was frequently found in the Rokko Mountains and also occasionally on Awaji Island, including the middle area.
TABLE 1.
Positivity rate of B. microti-like parasites in field rodents in Hyogo Prefecture from June 2000 to May 2002
| Survey area | Rodent species | No. of animals captured | No. positive/no. examined
|
|||||
|---|---|---|---|---|---|---|---|---|
| Smear | Blood PCR | Spleen PCR | Total | SSU rDNA type
|
||||
| Kobe | Otsu | |||||||
| Awaji Island | ||||||||
| Northern | Apodemus speciosus | 10 | 0/5 | 0/6 | 0/10 | 0/10 | ||
| Middle | Apodemus speciosus | 41 | 13/27 | 13/26 | 18/41 | 18/41 | 16/17 | 1/17 |
| Apodemus argenteus | 1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| Southern | Apodemus speciosus | 4 | 1/4 | 1/4 | 1/4 | 1/4 | 0/1 | 1/1 |
| Total | 56 | 14/37 | 14/37 | 19/56 | 19/56 | 16/18 | 2/18 | |
| Rokko Mountains | ||||||||
| Eastern | Apodemus speciosus | 10 | 4/7 | 3/5 | 6/10 | 6/10 | 0/6 | 6/6 |
| Apodemus argenteus | 6 | 1/5 | 1/3 | 1/6 | 1/6 | 0/1 | 1/1 | |
| Crocidura dsinezumi | 1 | 1/1 | 1/1 | 0/1 | 1/1 | 0/1 | 1/1 | |
| Middle | Apodemus speciosus | 7 | 3/7 | 3/7 | 3/7 | 3/7 | 0/3 | 3/3 |
| Western | Apodemus speciosus | 4 | 1/2 | 1/2 | 2/4 | 2/4 | 0/2 | 2/2 |
| Total | 28 | 10/22 | 9/18 | 12/28 | 13/28 | 0/13 | 13/13 | |
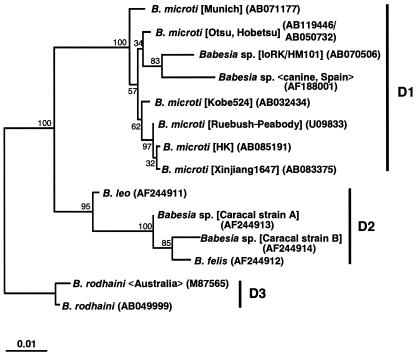
Because only tick species belonging to the genus Ixodes are known to transmit B. microti (18), we first attempted to demonstrate B. microti infection in Ixodes ovatus, which is the most prevalent species in both areas. Of a total of 80 adult ticks of I. ovatus (30 on Awaji Island and 50 in the Rokko Mountains), two from the middle area of Awaji Island and four from the eastern area of the Rokko Mountains showed positive PCR results with DNA extracted from salivary glands. Sequencing analysis showed that, except for one tick from the Rokko Mountains, all of the infected ticks on Awaji Island and in the Rokko Mountains were infected with the Otsu type of B. microti. The type of SSU rDNA in the tick from the Rokko Mountains was neither the Otsu, the Kobe, nor the U.S. type. The new SSU rDNA sequence (1,730 bp) of the presumed B. microti-like parasite (IoRK/HM101) in the tick was determined, and a primary phylogenetic analysis was performed by the neighbor-joining and maximum-likelihood methods as previously reported (3), with 1,300 unambiguously aligned positions selected from the alignment of 29 piroplasm and 6 outgroup SSU rDNA sequences. The phylogenetic analysis supported the previous finding that four monophyletic groups (A, Babesia sensu stricto; B, Theileria spp.; C, piroplasms isolated in the western United States; D, B. microti and related Babesia spp.) are present in piroplasms (3) and showed that the IoRK/HM101 parasite was clearly positioned within group D together with all six B. microti isolates examined (100% bootstrap support) (data not shown). A further phylogenetic analysis with 1,572 unambiguously aligned positions for 14 sequences in group D demonstrated that subgroups D1, D2, and D3 are present in group D and that the IoRK/HM101 parasite was identified as being closest to a Spanish canine isolate (83%) in subgroup D1 (Fig. 1).
FIG. 1.
Phylogenetic tree based on the SSU rDNA sequences of 14 Babesia spp. closely related to B. microti. Maximum-likelihood analysis (HKY85 + Γ model with eight categories for site rates) reconstructed three monophyletic subgroups, D1, D2, and D3. The sequence of IoRK/HM101 was the closest relative of a Spanish canine isolate in subgroup D1. GenBank accession numbers are given in parentheses; the name of the isolate, strain, clone, or genotype is shown in brackets; and the host origin or the isolation site is shown in chevrons. The SSU rDNA sequences used for the primary analysis are as follows; they were selected on the basis of the results of a FASTA similarity search (http://www.ddbj.nig.ac.jp/E-mail/homology-j.html) (12, 15) by using the SSU rDNA sequence of IoRK/HM101 as a query: B. microti (AB032434, AB119446, AB050732, AB071177, AB083375, AB085191, and U09833), Babesia sp. (AF188001), B. felis (AF244912), B. leo (AF244911), Cytauxzoon felis (L19080), Babesia sp. (AF244913 and AF244914), B. rodhaini (M87565 and AB049999), B. bigemina (X59604), B. bovis (L19077), B. caballi (Z15104), B. canis (L19079), B. divergens (U07885), B. equi (Z15105), B. gibsoni (AF175300 and AF158702), B. odocoilei (U16369), Babesia sp. (AB053216), Piroplasmida gen. sp. CA1 (AF158703), Babesia sp. WA1 (AY027815), Theileria parva (AF013418), Theileria mutans (AF078815), Plasmodium falciparum (M19172), Plasmodium berghei (M14599), Toxoplasma gondii (U03070), Hepatozoon sp. Boiga (AF297085), Sarcocystis tenella (L24383), and Eimeria tenella Houghton (AF026388).
Ixodes scapularis, a member of the Ixodes ricinus complex, and Peromyscus leucopus are the common vector and reservoir, respectively, not only for B. microti but also for Borrelia burgdorferi, the etiologic agent of Lyme disease, and the etiologic agent of human granulocytic ehrlichiosis in the United States (6, 7). The nymphs of I. scapularis feed indiscriminately on large mammals, including humans, as well as small rodents (6, 7, 19). In Europe, human babesiosis caused by B. microti is rarely reported, although B. microti has been highly enzootic in rodents. Ixodes trianguliceps is believed to be a main vector of B. microti in Europe (7, 19). The ultimately low incidence of human cases caused by B. microti is attributed to the exceeding host specificity of I. trianguliceps (19). Instead, a few cases of human babesiosis caused by Babesia divergens, a bovine babesia species, have been reported, and I. ricinus is believed to transmit B. divergens (7, 19). I. ricinus is also well known to be the main vector of Lyme disease, tick-borne encephalitis, and human granulocytic ehrlichiosis in Europe (2, 5, 9). I. ricinus has been recently revealed to possess and transmit B. microti in Europe (4, 22). In Japan, Lyme disease was first reported in 1987 (8). Ixodes persulcatus, an Asian member of the I. ricinus complex, was shown to have replaced I. scapularis and I. ricinus as the vector of Lyme disease in Japan (2). Therefore, I. persulcatus seems to be a possible candidate for the vector of B. microti. In this survey, no I. persulcatus was identified on Awaji Island or in the Rokko Mountains (data not shown), where rodent babesiosis has been confirmed to be highly enzootic. Instead, I. ovatus was the most prevalent and common tick species in these areas—the species in which B. microti was first detected. I. ovatus has recently been shown to possess R. helvetica, Borrelia japonica, a new Ehrlichia sp., and a tick-borne encephalitis virus (15, 16). I. ovatus thus seems to be related to multiple important tick-borne diseases, including babesiosis, in Japan. The rate of B. microti infection in adult I. ovatus ticks was comparable to that in adult I. scapularis ticks in some areas of the United States where it is endemic (17, 21) and to that in I. ricinus ticks in Europe (4). Interestingly, only the Otsu type of B. microti was detected in I. ovatus ticks, not only in the Rokko Mountains, where only the Otsu type of B. microti has been demonstrated in rodents, but also in the middle area of Awaji Island, where the Kobe type is much more predominant in rodents. The number of ticks examined should be increased and other kinds of tick species should be included to determine in greater detail the status of B. microti vectors.
The detection of a new type of SSU rDNA of a B. microti-like parasite in I. ovatus suggested that a third type of B. microti may exist in Japanese field rodents, although we have not identified B. microti with the new type of SSU rDNA in any rodents. Alternatively, the Babesia parasite may have originated from other species of animals. The parasite was, indeed, placed closest to a Babesia isolate from a Spanish dog (24). Irrespective of this question, the present result suggests for the first time that I. ovatus is deeply related to the transmission of B. microti or B. microti-like parasites in Japan.
Nucleotide sequence accession numbers.
The SSU rDNA sequence of IoRK/HM101 has been submitted to the DDBJ database and assigned accession no. AB070506.
Acknowledgments
We thank A. Kawai for excellent technical assistance.
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (13670244, 15590367, and 13576014) and a grant from Chyama Health Foundation Inc.
REFERENCES
- 1.Abe, H., N. Ishii, Y. Kaneko, K. Maeda, S. Miura, and M. Yoneda. 1994. A pictorial guide to the mammals of Japan. Tokai University Press, Tokyo, Japan. (In Japanese.)
- 2.Burgdorfer, W. 1989. Vector/host relationships of the Lyme disease spirochete, Borrelia burgdorferi. Rheum. Dis. Clin. N. Am. 15:775-787. [PubMed] [Google Scholar]
- 3.Dantrakool, A., P. Somboon, T. Hashimoto, and A. Saito-Ito. 2004. Demonstration of a new type of Babesia species in wild rats (Bandicota indica) in Chiang Mai Province, Thailand. J. Clin. Microbiol. 42:850-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duh, D., M. Petrovec, and T. Avsic-Zupanc. 2001. Diversity of Babesia infecting European sheep ticks (Ixodes ricinus). J. Clin. Microbiol. 39:3395-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fingerle, V., J. L. Goodman, R. C. Johnson, T. J. Kurtti, U. G. Munderloh, and B. Wilske. 1997. Human granulocytic ehrlichiosis in southern Germany: increased seroprevalence in high-risk groups. J. Clin. Microbiol. 35:3244-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorenflot, A., K. Moubri, E. Precigout, B. Carcy, and T. P. Schetters. 1998. Human babesiosis. Ann. Trop. Med. Parasitol. 92:489-501. [DOI] [PubMed] [Google Scholar]
- 7.Homer, M. J., D. I. Aguilar, S. R. I. Telford, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawabata, M., S. Baba, K. Iguchi, N. Yamaguti, and H. Russell. 1987. Lyme disease in Japan and its possible incriminated tick vector, Ixodes persulcatus. J. Infect. Dis. 156:854. [DOI] [PubMed] [Google Scholar]
- 9.Labuda, M., and S. E. Randolph. 1999. Survival strategy of tick-borne encephalitis virus: cellular basis and environmental determinants. Zentbl. Bakteriol. 289:513-524. [DOI] [PubMed] [Google Scholar]
- 10.Persing, D. H., D. Mathiesen, W. F. Marshall, S. R. I. Telford, A. Spielman, J. W. Thomford, and P. A. Conrad. 1992. Detection of Babesia microti by polymerase chain reaction. J. Clin. Microbiol. 30:2097-2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito-Ito, A., S. K. Rai, S. He, M. Kohsaki, M. Tsuji, and C. Ishihara. 1999. First demonstration of Babesia parasitizing in human in Japan. J. Jpn. Assoc. Infect. Dis. 11:1163-1164. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 12.Saito-Ito, A., M. Tsuji, Q. Wei, S. He, T. Matsui, M. Kohsaki, S. Arai, T. Kamiyama, K. Hioki, and C. Ishihara. 2000. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J. Clin. Microbiol. 38:4511-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiota, T., H. Kurimoto, N. Haguma, and Y. Yoshida. 1984. Studies on babesia first found in murine in Japan: epidemiology, morphology and experimental infection. Zentbl. Bakteriol. Mikrobiol. Hyg. 256:347-355. [PubMed] [Google Scholar]
- 14.Takada, N. 1990. A pictorial of medical acarology in Japan. Kinpodo, Kyoto, Japan. (In Japanese.)
- 15.Takada, N. 1995. Recent findings on vector acari for rickettsia and spirochete in Japan. Jpn. J. Sanit. Zool. 46:91-108. [Google Scholar]
- 16.Takada, N. 2002. Vectorial competences and distribution patterns of parasitic acari, especially for emerging diseases in Japan and East Asia. Med. Entomol. Zool. 54:1-12. (In Japanese.) [Google Scholar]
- 17.Telford, S. R. I., J. E. Dawson, P. Katavolos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 93:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telford, S. R. I., A. Gorenflot, P. Brasseur, and A. Spielman. 1993. Babesia infection in humans and wildlife, p. 1-47. In J. P. Kreier (ed.), Parasitic protozoa, 2nd ed., vol. 5. Academic Press, San Diego, Calif. [Google Scholar]
- 19.Telford, S. R. I., and A. Spielman. 1998. Babesiosis of humans, p. 349-359. In L. Collier, A. Balows, and M. Sussman (ed.), Topley & Wilson's microbiology and microbial infection, 9th ed., vol. 5. Arnold, London, England. [Google Scholar]
- 20.Tsuji, M., Q. Wei, A. Zamoto, C. Morita, S. Arai, T. Shiota, M. Fujimagari, A. Itagaki, H. Fujita, and C. Ishihara. 2001. Human babesiosis in Japan: epizootiologic survey of rodent reservoir and isolation of new type of Babesia microti-like parasite. J. Clin. Microbiol. 39:4316-4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varde, S., J. Beckley, and I. Schwartz. 1998. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey county. Emerg. Infect. Dis. 4:97-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter, G. 1981. Isolation of Babesia microti from free-living nymphs of Ixodes ricinus. Acta Trop. 38:187-188. (Author's translation.) [PubMed] [Google Scholar]
- 23.Wei, Q., M. Tsuji, A. Zamoto, M. Kohsaki, T. Matsui, T. Shiota, S. R. I. Telford, and C. Ishihara. 2001. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J. Clin. Microbiol. 39:2178-2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zahler, M., H. Rinder, E. Schein, and R. Gothe. 2000. Detection of a new pathogenic Babesia microti-like species in dogs. Vet. Parasitol. 89:241-248. [DOI] [PubMed] [Google Scholar]