Abstract
Mesenchymal stem cells are sensitive to changes in feature height, order and spacing. We had previously noted that there was an inverse relationship between osteoinductive potential and feature height on 15-, 55- and 90 nm-high titania nanopillars, with 15 nm-high pillars being the most effective substrate at inducing osteogenesis of human mesenchymal stem cells. The osteoinductive effect was somewhat diminished by decreasing the feature height to 8 nm, however, which suggested that there was a cut-off point, potentially associated with a change in cell–nanofeature interactions. To investigate this further, in this study, a scanning electron microscopy/three-dimensional scanning electron microscopy approach was used to examine the interactions between mesenchymal stem cells and the 8 and 15 nm nanopillared surfaces. As expected, the cells adopted a predominantly filopodial mode of interaction with the 15 nm-high pillars. Interestingly, fine nanoscale membrane projections, which we have termed ‘nanopodia,’ were also employed by the cells on the 8 nm pillars, and it seems that this is analogous to the cells ‘clinging on with their fingertips’ to this scale of features.
Keywords: Nanotopography, mesenchymal stem cell, nanopodia, filopodia, titanium, orthopaedics
Introduction
Mesenchymal stem cells (MSCs) are highly sensitive to environmental cues, including nanoscale topographical features, and changes in the feature height1 and extent of disorder of the features2,3 have been shown to modulate stem cell fate. Osteogenic nanotopographies would have great potential for use in patterning implants for orthopaedic applications, if reproducible features could be generated in clinically relevant load-bearing materials such as titanium (Ti). Thus, we have previously investigated the potential of nanoscale osteogenic features fabricated in Ti using through-mask anodisation to generate 15-, 55- and 90 nm-high pillar-like features1 and block copolymer masking to produce 8- and 15 nm-high pillars.4 In the former study, the osteogenic potential was inversely proportional to feature height, with 15 nm-high features being most osteogenic, and these data correlate well with other reports with nanoscale features on polymers.5,6 In a follow-up study, the feature height was reduced to 8 nm to investigate whether this would enhance the osteogenic effect even further.4 Interestingly, however, the osteogenic effect was diminished at 8 nm, but was still enhanced compared to planar control materials. Relative to cells on the 15 nm pillars, cells on the 8 nm pillars had a reduced abundance of the bone marker osteocalcin, and fewer of the largest sized super-mature cell adhesions. Super-mature adhesions are generally defined as adhesions in excess of 5 or 8 µm (the definition varies in the literature) and have been implicated in osteogenesis,7,8 as such adhesions are likely to serve as anchors to support the increased levels of intracellular tension noted during the differentiation of MSCs to osteoblasts.9,10 This increased tension promotes direct mechanotransduction, the process by which mechanical stimuli are transferred from focal adhesions, via the cytoskeleton, into the nucleus, where gene and protein level changes can be induced that modulate the cellular physiology and stem cell fate, in this case promoting an osteogenic phenotype (discussed further in McNamara et al.11 and Wang et al.12).
Filopodia have previously been noted to be important for the cellular response to nanotopography13,14 and have been implicated in modulating the development of adhesions in response to nanotopographies. In this study, we sought to determine whether the cells ‘sensed’ and interacted with the 8- and 15 nm-high features in a distinct manner using a field emission scanning electron microscopy (SEM) and stereo-SEM approach, in order to investigate the basis for the diminished osteogenic effect below the previously reported optimal 15 nm feature height. We noted that on the 15 nm pillars, the MSCs predominantly adopted a filopodial sensory mechanism, whereas on the smaller pillars, the cells largely employed nanoscale membrane projections that we have termed ‘nanopodia’ to detect the surface features. In some cases, nanopodium-like structures were also observed extending from filopodia on the 15 nm-high pillars.
Materials and methods
Fabrication
The 8- and 15 nm-high pillars were fabricated on commercially pure Grade 1 Ti discs (Titanium Metals UK) using block copolymer masking, as described in Sjöström et al.4 Briefly, poly(styrene-b-4-vinylpyridine; PS-b-P4VP) solution was spin-coated onto the Ti discs (2000 r/min, 60 s), and the surfaces were exposed to tetrahydrofuran vapour (3 h at room temperature in a sealed glass vessel), prior to rapid air-drying of the polymer. Ti samples were anodised at room temperature in 0.01 M oxalic acid, and the cathode was a platinum strip. The anodisation potential was increased (1 V/s) until the final voltage had been achieved; to anodise the 8 nm samples, a potential of 2 V was used, and 10 V was used to anodise the 15 nm samples. Samples were anodised for 60 s, then the power supply was switched off and samples were rinsed in distilled water and air-dried under an air flow. Oxygen plasma treatment (100 W for 60 min in a Femto plasma system (Diener Electronics), gas flow 10 sccm) was utilised to remove the polymer films.
Cell culture
Human MSCs (passage 2–3; PromoCell, Germany) were seeded at 1 × 104 cells per Ti disc (planar controls, 8 and 15 nm Ti; three discs per surface type) and cultured for 3 days in α-modified Eagle’s medium (α-MEM; PAA Laboratories) supplemented with 10% foetal bovine serum, 0.2 µg/mL Fungizone, 67 U/mL Penicillin, 66 µg/mL streptomycin and 1% (v/v) 200 mM l-glutamine in a humidified atmosphere of 5% CO2.
SEM/stereo-SEM
The culture medium was removed and cells were rinsed once in warm phosphate buffered saline (PBS) prior to fixation. Cells were fixed in 1.5% glutaraldehyde in 0.1 M cacodylate buffer for 1 h at 4°C. The samples were dehydrated using an alcohol series, dried with hexamethyldisilazane (HMDS) as previously described5 and sputter-coated with gold/palladium. For SEM, samples were imaged using a Hitachi S4700 field emission SEM with an accelerating voltage of 10 keV. Stereo-SEM images were obtained in pairs using a Carl Zeiss (Jena, Germany) Sigma high definition (HD) field emission SEM with a 6° tilt between pairs of images, to enable projection into pseudo three-dimensional (3D) images and reconstruction into stereo images that could be viewed using red-green filter “3D” stereo glasses.
Results
To investigate whether there was a distinction in the mechanism by which cells sensed the different heights or arrangement of features, a SEM and stereo-SEM approach was used to examine the fine-scale interactions between the cells and surface features. Figure 1 illustrates that the cells on the 15 nm-high features predominantly interacted with the surface using filopodial projections, but as will be discussed in greater detail in relation to Figure 2, some smaller scale interactions were also noted on the surfaces with 15 nm nanopillars.
Figure 1.
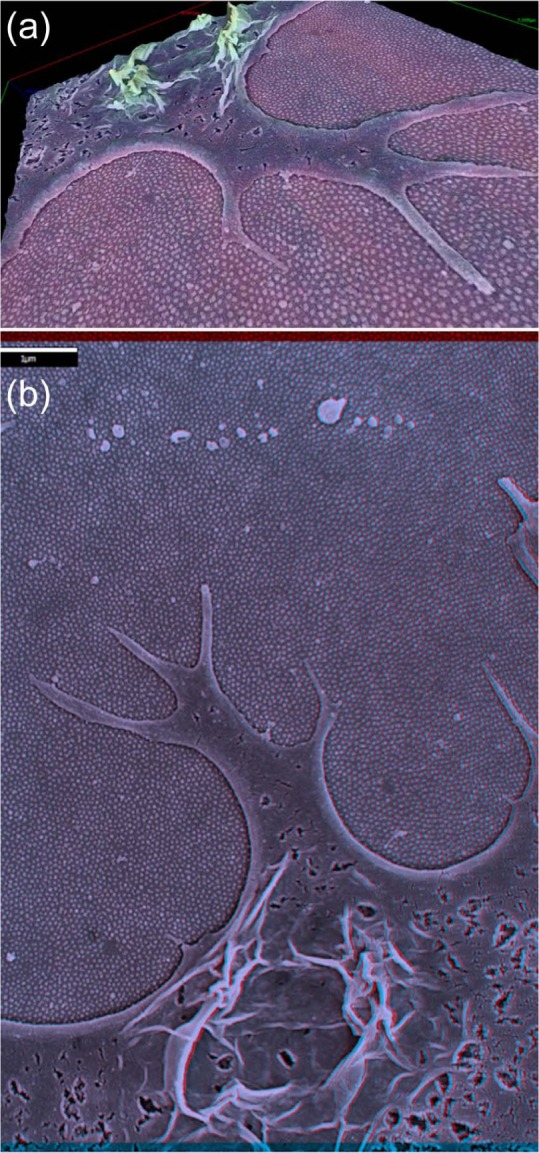
MSC investigating the 15 nm pillared substrate: (a) 3D projection (depth-coded by height) and (b) stereo-SEM image of the same MSC exploring the 15 nm nanopillared substrate using filopodia (can be viewed in 3D with red-green stereo glasses) (scale bar in (b): 1 µm).
MSC: mesenchymal stem cell; 3D: three-dimensional; SEM: scanning electron microscopy.
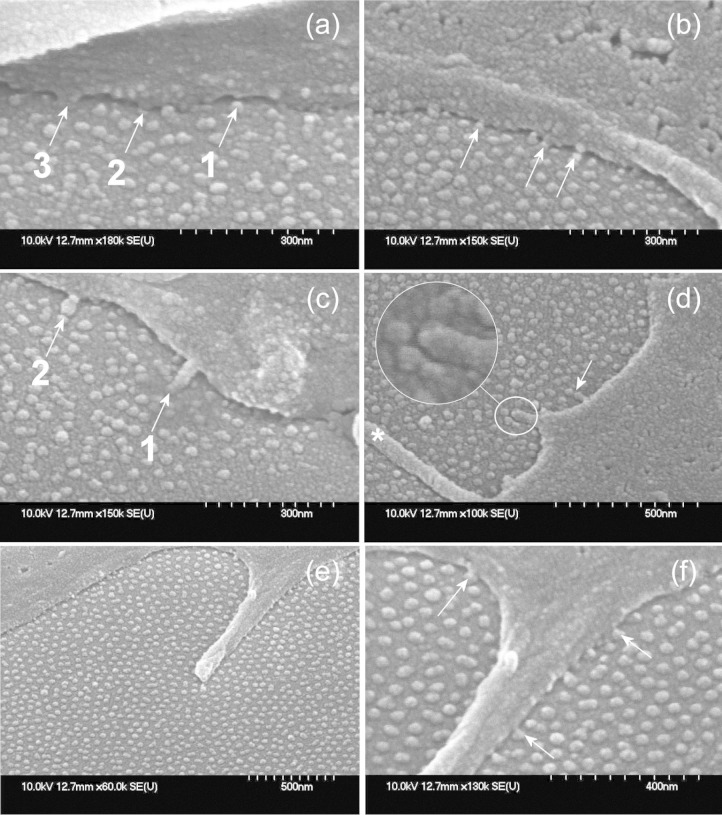
Figure 2.
MSCs interact directly with the nanofeatures. SEM images of MSCs cultured on (a–d) 8 nm and (e, f) 15 nm nanopillared Ti surfaces. (a) Cell membrane showing (1) the initiation of a nanopodial projection, (2) a longer projection and (3) a nanopodial structure contacting the 8 nm nanopillars directly. (b) Arrows indicate further examples of nanopodia on another cell. (c, d) Slightly larger nanoscale membrane projections were also responsive to the arrangement of the 8 nm nanofeatures, arrows indicate the termini of projections directly contacting pillars (c1, arrow in (d)) and appearing ‘blunted’ in shape on contacting the pillar-like features (c2), inset image in (d) shows a nanopodium-like projection being guided or confined by a group of nanofeatures, and the asterisk (*) in (d) shows a filopodium interacting with the substrate. (e, f) Filopodium exploring the surface of the 15 nm pillars ((f) is a higher magnification image of the cell shown in (e)): note the presence of nanopodia-like protrusions extending from the filopodium (scale bars: (a–c) 300 nm; (d, e) 500 nm; (f) 400 nm).
MSC: mesenchymal stem cell; SEM: scanning electron microscopy.
On the 8 nm-high pillars, the cells appeared to employ nanoscale membrane projections to detect the surface features, which we have termed ‘nanopodia’ (Figure 2). Interestingly, similar membrane structures were sometimes detected as finer protrusions emanating from larger filopodia in MSCs cultured on the 15 nm surface (Figure 2(e) and (f)). These nanopodial projections seemed to extend from the cell body and project down towards the topographical features, eventually contacting the features: Figure 2(a) shows examples of these membrane extensions apparently (1) forming/beginning to initiate contact with the 8 nm surface features, (2) extending to increase contact and (3) making extensive contact with the pillars. Interestingly, some slightly larger nanoscale projections, nanopodium-like structures, could also contact the features directly (Figure 2(c) and (d)) and could appear ‘blunted’ by contact with the features, where the nanopodium-like structures conformed to the shape of the pillars (Figure 2(c)). Other such structures appeared to have been guided or confined by contact with the 8 nm nanopillars (Figure 2(d) shows such a projection seemingly confined by a group of nanofeatures). These projections looked slightly ‘thicker’ than the majority of the nanopodia, however, and perhaps also had the potential to become filopodia, particularly since filopodia were also noted on the 8 nm surface (Figure 2(d)).
Discussion
MSCs cultured on the 15 nm-high pillars had previously been shown to exhibit a more osteogenic phenotype than those cultured on the 8 nm-high features. In this study, it was noted that the sensory mechanism by which the cells detect the features was distinct between cells on the 8- and 15 nm-high surfaces. Cells on the 15 nm-high pillars adopted a more filopodial adhesion mechanism, while cells on the smaller features made use of smaller nanoscale membrane projections that we have termed ‘nanopodia’. A previous SEM-based study highlighted that fibroblasts were able to sense 10-nm-high polymeric nanoislands using filopodia.13 To date, the 8 nm-high features in this study are the smallest topographical features that stem cells have been shown to be able to directly detect and interact with.
Integrins, a crucial component of focal adhesions, are heterodimers incorporating an α and a β subunit and bind to recognition sequences such as RGD (arginine, glycine, aspartic acid) in extracellular matrix proteins adsorbed onto biomaterial surfaces. As discussed by Chen et al.,15 in inactive integrins, the N-terminal ligand binding region is held in the ‘bent’ conformation <5 nm from the membrane binding region; however, this can shift to 15–20 nm upon activation. The parallels between the length scales of these two conformational states and the 8 and 15 nm pillars are striking: perhaps the ~15 nm integrin displacement affords an osteogenic advantage for MSCs cultured on the pillars of the same height, if the 15 nm pillars can potentially facilitate integrin activation and thus promote adhesion formation. Interestingly, the 8 nm features are also ~25 nm in diameter, while the diameter of an integrin molecule is approximately 10 nm,16 and clustering of multiple integrin molecules is needed to initiate the signalling required to promote focal adhesion development.17 This could potentially promote the formation of fine nanopodial projections, if the clustering of integrins is more restricted on top of the surface features. In addition, the nearest neighbours of the 8 nm features are typically spaced 20–50 nm away, which is within the integrin clustering distance established by the Spatz group.18,19 Thus, it is tempting to postulate that osteogenesis is permitted on the 8 nm features via bridging between adjacent nanofeatures or by permitting integrin clustering in the inter-feature regions, but that the osteogenic effect is reduced compared to the 15 nm features, where multiple integrin units should be able to cluster on a single feature, as well as in inter-feature regions or between bridged features. This also ties in with work from the Sutherland group that shows that focal adhesion bridging is enhanced if multiple integrins can gather closely, and their observations that although adhesions were able to bridge between adjacent engineered adhesive protein patches, the integrins did not bridge between them.20 If the situation is similar with the 8 nm topography, it seems likely that the inter-feature regions would be more effective at initiating adhesion formation due to the restrictive diameter of the topographical features, which could account for the reduced osteogenic capacity of this substrate compared to the 15 nm features with larger diameter.4 Furthermore, in view of the theory that the convex membrane curvature (such as that induced by nanopits) can be sensed by depolarisation of ion channels, and that the concave membrane curvature (such as that induced by nanopillars) can potentially be detected by BAR (Bin–amphiphysin-Rvs) domain proteins,21 the shape of the cell membrane over these features could also be a crucial determinant of the downstream cellular response. If the smaller feature height is also less efficient at inducing a mechanoresponse via such mechanisms, this could be having a dual effect on reducing the osteogenic effect of the substrate. In future studies, it would be interesting to investigate the role of these aspects in inducing osteogenesis on the topographies. It would also be intriguing to study even smaller nanofeatures, to examine whether nanopodia could detect these features, and study the functional and mechanotransductive consequences of nanopodial interactions in greater detail. In addition, use of techniques such as atomic force microscopy (AFM) and environmental SEM (ESEM) would be valuable to investigate the cell–material interactions, in order to study the forces exerted by the nanopodia, to visualise the interactions in samples in a ‘wet’ state and facilitate the production of computational models of the interactions on both the 8 and 15 nm pillars.
Together, these results indicate that human MSCs can directly sense features 8 nm high using nanoscale membrane projections we have termed ‘nanopodia’. To our knowledge, the 8 nm pillars are currently the smallest height of topographical features that MSCs have been shown to sense and interact with using membrane projections. It appears that the scale of the 8 nm features, similar to that of individual integrin receptors, has the cells ‘hanging on with their fingertips’ for the last traces of topographical information. Given the distinctions between the osteogenic potential of the 8- and 15 nm-high pillars, a greater understanding of the variety of cellular sensory mechanisms on different topographies would be valuable for the design of future biomaterial substrates.
Acknowledgments
The authors thank Margaret Mullin for preparation of EM samples and Peter Chung for assistance with stereo-SEM.
Footnotes
Declaration of conflicting interests: The authors declare that there is no conflict of interest.
Funding: This study received financial support from the EPSRC. L.E.M. and M.J.D. are currently funded by the BBSRC.
References
- 1. McNamara LE, Sjöström T, Burgess KEV, et al. Skeletal stem cell physiology on functionally distinct titania nanotopographies. Biomaterials 2011; 32: 7403–7410. [DOI] [PubMed] [Google Scholar]
- 2. Dalby MJ, Gadegaard N, Tare R, et al. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat Mater 2007; 6(12): 997–1003. [DOI] [PubMed] [Google Scholar]
- 3. McMurray RJ, Gadegaard N, Tsimbouri PM, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater 2011; 10(8): 637–644. [DOI] [PubMed] [Google Scholar]
- 4. Sjöström T, McNamara LE, Meek RMD, et al. 2D and 3D nanopatterning of titanium for enhancing osteoinduction of stem cells at implant surfaces. Adv Healthc Mater 2013; 2(9): 1285–1293. [DOI] [PubMed] [Google Scholar]
- 5. Dalby MJ, Giannaras D, Riehle MO, et al. Rapid fibroblast adhesion to 27 nm high polymer demixed nano-topography. Biomaterials 2004; 25: 77–83. [DOI] [PubMed] [Google Scholar]
- 6. Dalby MJ, McCloy D, Robertson M, et al. Osteoprogenitor response to semi-ordered and random nanotopographies. Biomaterials 2006; 27: 2980–2987. [DOI] [PubMed] [Google Scholar]
- 7. Biggs MJP, Richards RG, Gadegaard N, et al. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials 2009; 30: 5094–5103. [DOI] [PubMed] [Google Scholar]
- 8. Tsimbouri PM, McMurray RJ, Burgess KV, et al. Using nanotopography and metabolomics to identify biochemical effectors of multipotency. ACS Nano 2012; 6: 10239–10249. [DOI] [PubMed] [Google Scholar]
- 9. McBeath R, Pirone D, Nelson C, et al. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 2004; 6(4): 483–495. [DOI] [PubMed] [Google Scholar]
- 10. Kilian KA, Bugarija B, Lahn BT, et al. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A 2010; 107: 4872–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McNamara LE, McMurray RJ, Biggs MJP, et al. Nanotopographical control of stem cell differentiation. J Tissue Eng 2010; 2010(1): 120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009; 10: 75–82. [DOI] [PubMed] [Google Scholar]
- 13. Dalby MJ, Riehle MO, Johnstone H, et al. Investigating the limits of filopodial sensing: a brief report using SEM to image the interaction between 10 nm high nano-topography and fibroblast filopodia. Cell Biol Int 2004; 28(3): 229–236. [DOI] [PubMed] [Google Scholar]
- 14. Albuschies J, Vogel V. The role of filopodia in the recognition of nanotopographies. Sci Rep 2013; 3: 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen W, Lou J, Evans EA, et al. Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells. J Cell Biol 2012; 199(3): 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nermut MV, Green NM, Eason P, et al. Electron microscopy and structural model of human fibronectin receptor. EMBO J 1988; 7: 4093–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiseman PW, Brown CM, Webb DJ, et al. Spatial mapping of integrin interactions and dynamics during cell migration by image correlation microscopy. J Cell Sci 2004; 117: 5521–5534. [DOI] [PubMed] [Google Scholar]
- 18. Cavalcanti-Adam EA, Volberg T, Micoulet A, et al. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys J 2007; 92: 2964–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang J, Grater SV, Corbellini F, et al. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett 2009; 9: 1111–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malmstrom J, Lovmand J, Kristensen S, et al. Focal complex maturation and bridging on 200 nm vitronectin but not fibronectin patches reveal different mechanisms of focal adhesion formation. Nano Lett 2011; 11: 2264–2271. [DOI] [PubMed] [Google Scholar]
- 21. Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol 2006; 7: 265–275. [DOI] [PubMed] [Google Scholar]