Abstract
Excess weight gain is the most significant, preventable cause of increased blood pressure (BP) in patients with primary (essential) hypertension and increases the risk for cardiovascular and renal diseases. In this review, we discuss the role of the brain melanocortin system in causing increased sympathetic activity in obesity and other forms of hypertension. In addition, we highlight potential mechanisms by which the brain melanocortin system modulates metabolic and cardiovascular functions.
Obesity is a major health problem worldwide, and it is estimated that ∼36% of the U.S. adult population is obese, with body mass index (BMI) of >30, while more than two-thirds of the population is overweight, accounting for more than $150 billion/year in medical costs (11). Being overweight or obese markedly increases the risk for developing metabolic, cardiovascular, and renal diseases, including diabetes, dyslipidemia, atherosclerosis, coronary artery disease, hypertension, and chronic kidney disease (20, 53). Risk estimates from population studies suggest that excess weight gain contributes to as much as 65–75% of the risk for primary (essential) hypertension (53). Although not all obese individuals are hypertensive when assessed by standard clinical criteria, obesity is associated with a shift in blood pressure (BP) distribution toward higher BP values. Obese individuals not classified as hypertensive exhibit lower BP when they lose weight, and there is an almost linear relationship between BMI and BP (9, 20). Weight loss also is effective in primary prevention of hypertension (43), and multiple studies have shown that weight loss is effective in reducing BP in most hypertensive patients whether the weight loss is achieved by restriction of energy intake and increased physical activity or by bariatric surgery (43). Although an early analysis of the Swedish Obesity Study reported that patients who received bariatric surgery had a lower incidence of diabetes and dyslipidemia but similar incidence of hypertension compared with control subjects (40), a recent follow-up of these patients showed reduced systolic and diastolic pressures in the patients who underwent gastric bypass compared with obese controls and patients who underwent gastric banding and that the reduction in BP was proportional to the amount of weight lost during the 10-year follow-up (22). Thus most of the available evidence suggests that excess weight gain is the single most important risk factor for developing hypertension and that weight loss, if it can be achieved and sustained, is an important means of reducing BP in most hypertensive patients.
Sympathetic Nervous System Activation Contributes to Elevated BP in Obesity
Previous studies have implicated several mechanisms linking weight gain with elevations in BP. For instance, in all forms of hypertension studied thus far, including obesity hypertension, there is impaired renal-pressure natriuresis that is characterized by a rightward shift in the pressure-natriuresis curve toward hypertensive levels (18, 19). Some of the most powerful mechanisms contributing to impaired renal-pressure natriuresis in obesity hypertension are activation of the renin-angiotensin-aldosterone system (RAAS), physical compression of the kidneys by surrounding visceral fat and increased renal sinus fat, and sympathetic nervous system (SNS) overactivity (9, 19, 20). In this review, we focus on the role of increased SNS activity in contributing to elevated BP in obesity.
Obesity, especially when increased visceral adiposity is present, is accompanied by increased SNS activation to several organs and tissues, and it can occur as early as 1 wk after exposure to high-fat diets in experimental animal models and may also be observed after modest weight gain in nonobese subjects (1, 10, 21). There is strong evidence that excess weight gain is associated with selective increases in SNS to certain organs and tissues, including the kidneys and skeletal muscle, instead of widespread whole-body SNS activation (10, 44). In addition, the rise in SNS activity to the kidneys is modest, albeit enough to promote sodium retention and to activate the RAAS, and is considered the main mechanism by which SNS activation contributes to increased BP with weight gain rather than peripheral vasoconstriction that would occur during massive widespread SNS activation (9, 20). Evidence for an important role of increased SNS activity and renal sympathetic activation in obesity hypertension derives from studies showing that pharmacological blockade of adrenergic receptors elicits more pronounced BP reductions in obese vs. lean hypertensive subjects (54), and bilateral renal denervation markedly attenuates sodium retention and elevated BP in obese dogs fed a high-fat diet (25).
Several factors have been suggested to contribute to increased SNS activity in obesity, including increased angiotensin II levels, hyperleptinemia, hyperinsulinemia, sleep apnea, hypoghrelinemia, hypoadiponectemia, and impaired baroreflex sensitivity, to name a few (6). However, one key factor that appears to play a critical role linking increased SNS activity and elevated BP in obesity is activation of the brain pro-opiomelanocortin (POMC) neurons and consequent stimulation of melanocortin-4 receptors (MC4R) in brain regions involved in cardiovascular regulation.
Activation of Central Nervous System MC4Rs is Required for Excess Weight Gain to Activate the SNS and Increase BP
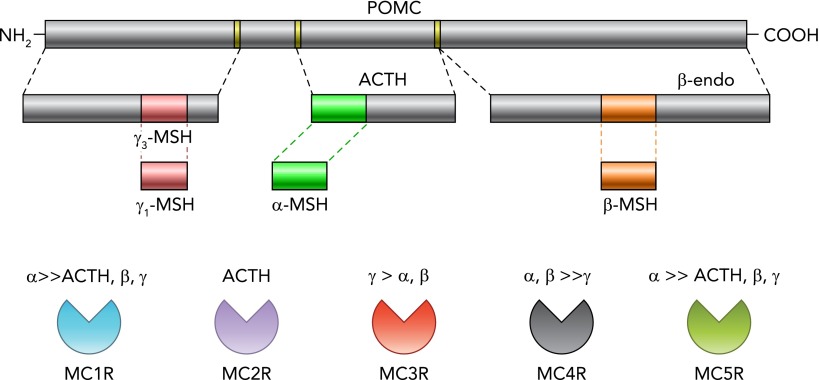
As shown in FIGURE 1, there are five melanocortin receptor subtypes (namely melanocortin receptors 1–5, MC1R–MC5R) that are activated by prohormone POMC cleavage peptides, including adrenocorticotropin hormone (ACTH), α-, β-, and γ-melanocyte stimulating hormone (α-MSH, β-MSH, γ-MSH) (5, 49). However, some byproducts of POMC cleavage, such as β-endorphins, for example, do not act primarily on melanocortin receptors (49). POMC-containing neurons are mainly located in the arcuate nucleus of the hypothalamus and a few nuclei in the brain stem [e.g., nucleus of the tractus solitarius (NTS)] (49) and project to several other brain regions involved not only in cardiovascular regulation but also appetite, energy balance, glucose homeostasis, pituitary function, etc. (5, 49). Among the five melanocortin receptor subtypes, only MC3R and MC4R are abundant in the central nervous system (CNS) (49). Although MC3Rs have been shown to contribute to body weight regulation and to help prevent salt sensitivity of BP (23), the MC4R, a G-protein-coupled 7 transmembrane receptor that is activated mainly by α-MSH, is thought to be the dominant efferent arm of the brain melanocortin system's actions on body weight homeostasis, SNS activation, and BP regulation. Impaired MC4R activation caused by mutations of the MC4R or the POMC gene, for example, is estimated to account for as much as 5–6% of early onset morbid obesity in humans (50), and, as discussed below, MC4R activation appears to be an important link between obesity, SNS activation, and hypertension.
FIGURE 1.
Schematic representation of POMC cleavage and proposed affinities of its byproducts to melanocortin receptors (MC1R–MC5R)
ACTH, adrenocorticotropin hormone; β-endo, β-endorphin; MSH, melanocyte-stimulating hormone; POMC, pro-opiomelanocortin.
Pharmacological blockers of MC4R have been shown to cause hyperphagia, rapid weight gain, and pronounced obesity in rodents (8, 26), whereas activation of MC4R using synthetic agonists promotes weight loss by reducing appetite and increasing energy expenditure (26, 49). A remarkable difference in the obesity observed in MC4R-deficient mice and rats with that observed in rodents fed high-calorie diets is that BP is not elevated in MC4R deficiency. In fact, SNS activity and BP are often reduced in MC4R-deficient rodents, even though they exhibit many characteristics of the metabolic syndrome, such as visceral adiposity, hyperleptinemia, hyperinsulinemia, and insulin resistance (42, 48). Conversely, chronic MC4R activation causes sustained increases in BP despite reducing food intake and promoting weight loss, and the rise in BP can be prevented by adrenergic receptor blockade (27). These observations highlight the importance of MC4R in modulating SNS activity and contributing to elevated BP in obesity. The importance of MC4R to obesity hypertension is also supported by observations in humans with MC4R deficiency who, despite being markedly obese, exhibit lower BP, reduced 24-h norepinephrine excretion, and reduced prevalence of hypertension compared with obese subjects with normal MC4R function (17, 31). Individuals with MC4R mutations also exhibit reduced muscle SNS activity and impaired SNS responses to a hypoxia stress test (17). These observations indicate that a functional MC4R is necessary for excess weight gain to increase SNS activity and elevate BP, and that MC4R also modulates sympathetic responses to stress.
Although the rise in BP during MC4R activation with pharmacological agonists is modest (usually between 8 and 10 mmHg), the real impact of MC4R activation on BP regulation may be clouded by factors that offset part of MC4R hypertensive effects, such as the weight loss that often occurs when MC4R agonists are administered. Also, in obese subjects, there is often endothelial dysfunction, which may amplify the BP effects of MC4R activation. We have shown, for example, that chronic administration of the nitric oxide synthase inhibitor l-NAME amplified the hypertensive effects of MC4R activation in Sprague-Dawley rats (12).
We and others have also demonstrated a critical role for the POMC neuron-MC4R axis in mediating the BP effects of other factors implicated in obesity hypertension. Rahmouni et al. showed that the acute effects of insulin to increase renal SNS activity were attenuated and abolished in heterozygous and homozygous MC4R knockout mice, respectively (37). A similar observation was found for the acute effects of leptin on renal SNS activity (37).
Leptin, a peptide hormone produced by adipocytes in proportion to the amount of body fat, is also thought to be a key link between obesity, SNS overactivity, and hypertension. Rodents with leptin gene mutations (ob/ob mice) are morbidly obese and have multiple metabolic abnormalities, including severe insulin resistance, hyperinsulinemia, and dyslipidemia, but have normal or reduced BP (30). Similar results have also been reported in a small number of humans, with leptin gene mutations that generally are not hypertensive when BP is measured at a young age, before development of target organ injury (30, 35). Ozata and colleagues studied four patients with leptin gene mutations and found that three of them had BPs in the normal range despite morbid obesity and many features of the metabolic syndrome (35). Only one patient had elevated BP, and this individual (age 30 yr) also had very high levels of adrenocorticotrophic hormone (ACTH), which could have contributed to the hypertension. Despite severe obesity, all of these patients exhibited features suggesting SNS hypofunction, including orthostatic hypotension and attenuated RAAS response to upright posture as well as attenuated BP responses to a cold pressor test (30, 35).
Chronic leptin infusion in lean rodents to mimic leptin levels observed in severe obesity causes sustained elevations in BP, which is dependent on adrenergic receptor activation (4). These chronic effects of hyperleptinemia on SNS activity and BP regulation also require an intact POMC neuron-MC4R axis since genetic disruption or pharmacological blockade of MC4R or deletion of leptin receptors specifically in POMC neurons completely abolished the chronic effects of leptin to raise BP (8, 13, 46). Of note, MC4R-deficient mice are also unresponsive to the appetite-suppressing effects of leptin (46), indicating that MC4R not only plays a major role as a downstream pathway by which many factors raise SNS activity but is also crucial in mediating the metabolic effects of other important factors that regulate body weight.
MC4R Plays a Role in Regulation of SNS Activity Beyond Its Role in Obesity Hypertension
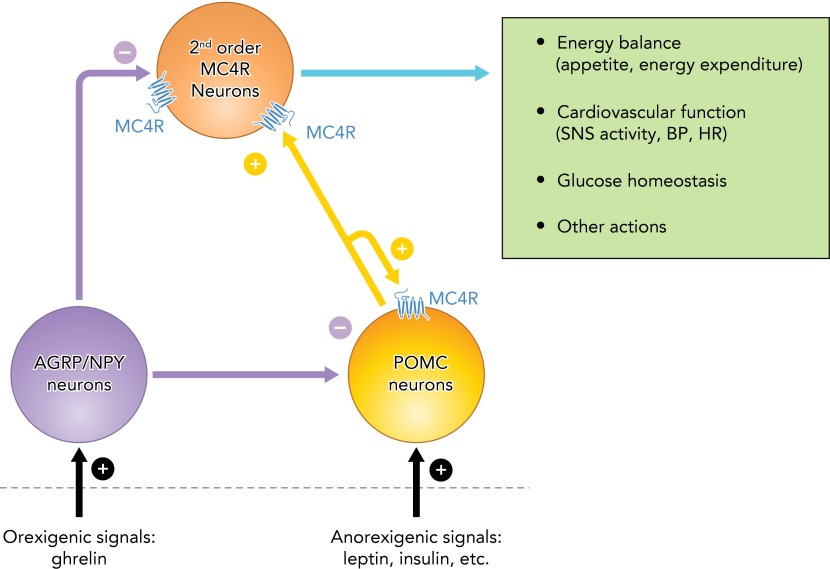
The MC4R also appears to exert a role in modulating SNS activity in response to stimuli other than excess weight gain or hyperleptinemia. We showed, for example, that MC4R blockade markedly reduced BP in spontaneous hypertensive rats (SHR), a widely used model of hypertension that is associated with increased SNS activity in the absence of obesity (7). Moreover, the BP reduction achieved by MC4R antagonism in this model was comparable to that observed during adrenergic receptor blockade (7). MC4R antagonism also caused greater BP reduction in obese vs. lean Zucker rats, supporting a role for MC4R in regulating SNS activity and BP even in models with impaired leptin actions (14). MC4R antagonism also attenuates or abolishes the acute pressor responses to some peptides such as nesfatin-1 and neuronostatin that raise BP by SNS stimulation (55, 57). Moreover, other peptides known to reduce SNS activity are thought to exert their actions, at least in part, by stimulating the release of endogenous antagonists of MC4R [e.g., agouti-related peptide (AGRP)] or by antagonizing the actions of the POMC neuron-MC4R axis (e.g., neuropeptide Y) (33) (FIGURE 2). Collectively, these observations suggest that the MC4R may play a key role in contributing to elevated BP in several forms of hypertension that accompany SNS overactivity.
FIGURE 2.
Schematic representation of the modulation of POMC neurons and MC4R containing neurons by peripheral signals involved in the regulation of energy balance, glucose levels, and cardiovascular function
The figure also highlights the opposing actions of POMC neurons vs. AGRP/NPY neurons on MC4R activation, and an auto-potentiation/auto-stimulation loop on POMC neurons mediated by the MC4R. AGRP, agouti-related peptide; MC4R, melanocortin 4 receptors; NPY, neuropeptide Y.
The MC4R is Located in Several Brain Nuclei Involved in Cardiovascular Regulation
Although MC4R mRNA expression has been observed in widespread areas of the CNS, the regions with the greatest abundance of MC4R are the paraventricular nucleus of the hypothalamus (PVN), lateral hypothalamus (LH), the amygdala, the dorsal motor complex, which encompasses the NTS and the dorsal motor nucleus of the vagus (DMV) (2, 38, 49), and preganglionic sympathetic neurons of the intermediolateral medulla (IML) (38), which are all important sites for regulation of autonomic function. Although the specific contribution of MC4R in distinct CNS nuclei in mediating the actions of the brain melanocortin system on energy balance, appetite, and glucose homeostasis have been the subject of intense investigation, the particular regions of the brain where MC4R are most important in regulation of SNS activity and BP are still unclear. Most of what we know in this area comes from acute studies where MC4R agonists and antagonists are microinjected into discrete CNS nuclei of anesthetized animals. For instance, Li et al. showed that microinjection of an MC4R agonist into the PVN increased renal SNS activity and BP (28). Ward et al. also found that the effect of hyperinsulinemia to acutely raise lumbar SNS activity was completely prevented by pharmacological blockade of MC4R in the PVN (52). Iwasa and colleagues observed increased HR after α-MSH was microinjected into the right IML (24). Previous studies also indicate that the effects of acute manipulations of MC4R activity on SNS activity and BP are not uniform for all CNS areas tested. Tai et al. (45) and Pavia et al. (36) observed reduced BP and bradycardia following microinjection of α-MSH or a synthetic agonist, MTII, into the NTS of anesthetized SHRs and normotensive Sprague-Dawley rats.
The few studies that have examined the chronic cardiovascular actions of MC4R in specific neuronal populations in conscious animals suggest an important role for MC4R located on cholinergic preganglionic parasympathetic and sympathetic neurons in contributing, at least in part, to obesity hypertension (41), and that MC4R on POMC neurons may play a role in modulating POMC activity and autonomic function (15). We demonstrated, for example, that rescue of MC4R function specifically in POMC neurons of mice with whole-body MC4R deficiency restored the BP response to acute stress, suggesting that MC4R may act as an auto-potentiation and/or auto-excitation mechanism on POMC neuronal activity (15). The specific groups of neurons and particular nuclei that mediate the effects of MC4R to evoke sustained increases in SNS activity to cardiovascular-relevant tissues and to promote chronic increases in BP are still largely unknown and remain an important area for future investigation.
Intrinsic MC4R Activity Plays a Major Role in Tonic Regulation of Appetite, Energy Balance, and Cardiovascular Function
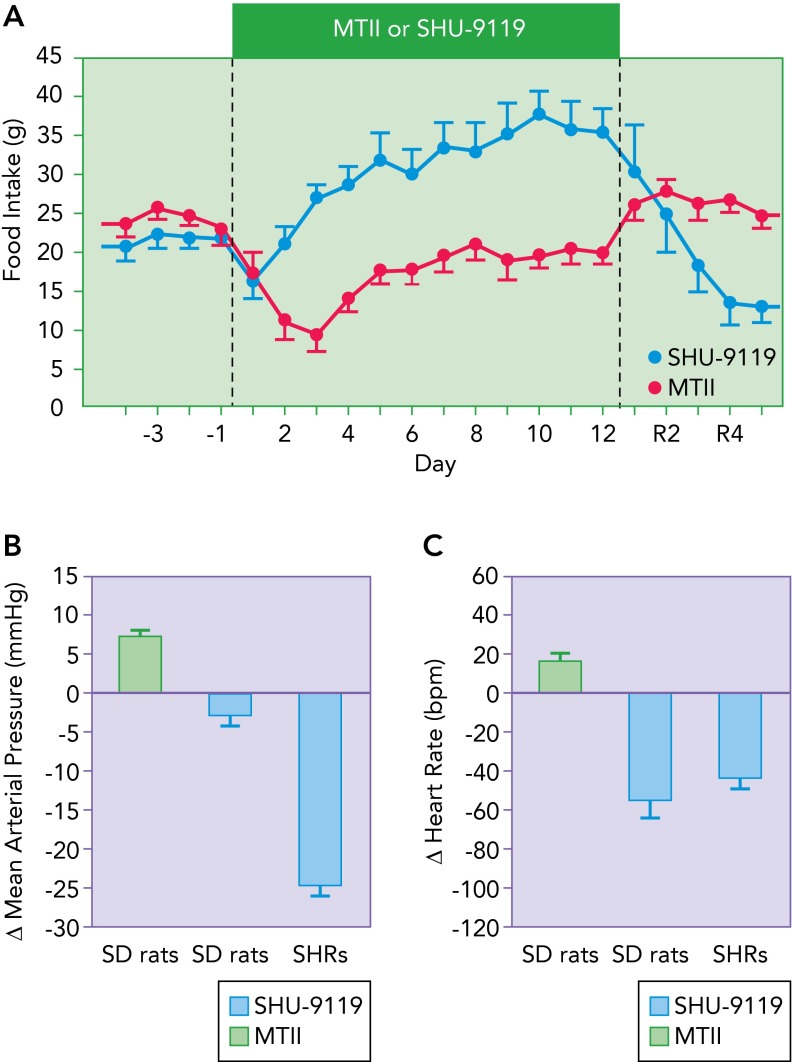
Although it is clear that direct activation of MC4R using pharmacological agents or indirectly via stimulation of POMC neurons leading to release of the endogenous MC4R agonist α-MSH reduces appetite, promotes weight loss, and raises SNS activity and BP in humans and rodents, the magnitude of some of these effects is less pronounced than what is observed when endogenous MC4R is blocked. For example, although exogenous long-term stimulation of MC4R causes transient reductions in food intake lasting for a few days and weight loss of ∼10–15% (26, 27), chronic MC4R antagonism using synthetic agents or by infusing the endogenous MC4R inverse agonist, AGRP, in lean animals leads to profound hyperphagia (sometimes more than doubling food consumption) accompanied by rapid weight gain at a rate of up to 25% in 7–10 days in adult rats (7, 26, 47) (FIGURE 3). In addition, contrary to the effects of long-term exogenous stimulation of MC4R where appetite is only transiently reduced and returns to normal levels within 5–7 days, the hyperphagia observed with MC4R blockade lasts as long as the antagonists are infused (7, 47). One teleological explanation for these observations is that there has not been evolutionary pressure for animals to develop safeguards against overeating and weight gain; thus impairment of a powerful inhibitor of food intake (such as the brain melanocortin system) would not suffer opposing actions of various compensatory mechanisms to prevent the overfeeding and excess weight gain. However, for most of human history, there has been pressure to avoid starvation; compensatory systems are therefore likely activated during chronic exogenous MC4R stimulation to increase food intake back to normal and avoid continuous weight loss.
FIGURE 3.
Compilation of data demonstrating the impact of MC4R activation with the MC3/4 agonist MTII or the MC3/4 antagonist SHU-9119 on food intake
A: compilation of data demonstrating the impact of MC4R activation with the MC3/4 agonist MTII or the MC3/4 antagonist SHU-9119 on food intake in Sprague-Dawley (SD) rats. The average changes (Δ) in mean arterial pressure (B) and heart rate (C) in normotensive and hypertensive rats (SHR) during days 7 to 12 of treatment with MTII or SHU-9119. Image modified from Refs. 7, 26, 27 and used with permission.
A powerful tonic action of MC4R is also found on SNS activity in some instances. As mentioned previously, blockade of endogenous MC4R activity markedly reduced the hypertension in SHRs by ∼25–30 mmHg and caused greater BP reduction in obese Zucker rats or obese rats fed high-fat diets compared with lean controls (7, 14, 16). It is also important to highlight that, even in lean normotensive animals, chronic MC4R antagonism causes sustained bradycardia and reduces BP (albeit only a few mmHg), and these effects occur despite hyperphagia and rapid weight gain, which normally would evoke tachycardia and elevated BP (7, 14, 16, 26) (FIGURE 3). Taken together, these findings demonstrate a major role of MC4R in controlling energy balance and SNS activity and as a potential target for drugs aimed at modulating appetite, energy expenditure, SNS activation, and BP.
Because of its potent effects on body weight regulation, the MC4R has been a target for development of anti-obesity drugs. One apparent challenge, however, is that anti-obesity drugs that target activation of MC4R to reduce appetite and promote weight loss often evoke undesirable increases in SNS activity and BP. A novel approach to increase MC4R activity that has been recently developed is to prevent degradation of α-MSH using prolylcarboxypeptidade inhibitors. Although the few studies using this approach have reported reduced appetite and weight loss (39, 51), the cardiovascular impact of these compounds and whether their effects are mediated by activation of MC4R or by some off-target effects are still unknown.
What are the Downstream Mediators of MC4R Action on Energy Balance and Cardiovascular Function?
The MC4R is a G-protein-coupled 7 transmembrane receptor that increases cAMP phosphorylation and activates protein kinase A (PKA), and blockade of each of these intracellular pathways attenuates or abolishes MC4R actions (28, 45, 49). Although alternative cAMP-independent second-messenger mediators of MC4R have been proposed (34), their physiological importance is unknown. Also unclear are potential downstream mechanisms by which MC4R regulates metabolic and cardiovascular function. Most previous studies examining potential downstream mediators of MC4R function are acute (effects examined for only minutes to a few hours). For example, Bariohay et al. showed that pharmacological activation or inhibition of MC4R, respectively, increased and reduced brain-derived neurotrophic factor (BDNF) protein content in the dorsal motor complex of adult rats and that the acute orexigenic effect of a selective MC4R antagonist injected into the fourth ventricle was blocked by co-administration of BDNF (3). A similar finding was reported by Nicholson and colleagues, who found that the reduction in 24-h food intake caused by a MC4R agonist, MK1, was attenuated by prior central injection of an anti-BDNF antibody (32). They also found that the acute hypertensive effect of MK1 was markedly attenuated by the anti-BDNF antibody. Other candidates such as oxytocin, corticotrophin-releasing hormone (CRH), and melanin-concentrating hormone (MCH), which are modulated by MC4R activity, have also been proposed to contribute to MC4R's actions on appetite (49, 56). However, it is still unclear whether oxytocin also participates in the cardiovascular effects of MC4R activation.
Thus, despite the major role of the CNS MC4R in regulating several physiological functions including appetite, energy expenditure, autonomic nervous system activity, and cardiovascular responses to stress, among many other important functions, the brain regions and neuronal connections as well as the peripheral and central stimuli that activate/inhibit the POMC-MC4R axis and the downstream pathways that mediate MC4R actions are only beginning to be elucidated.
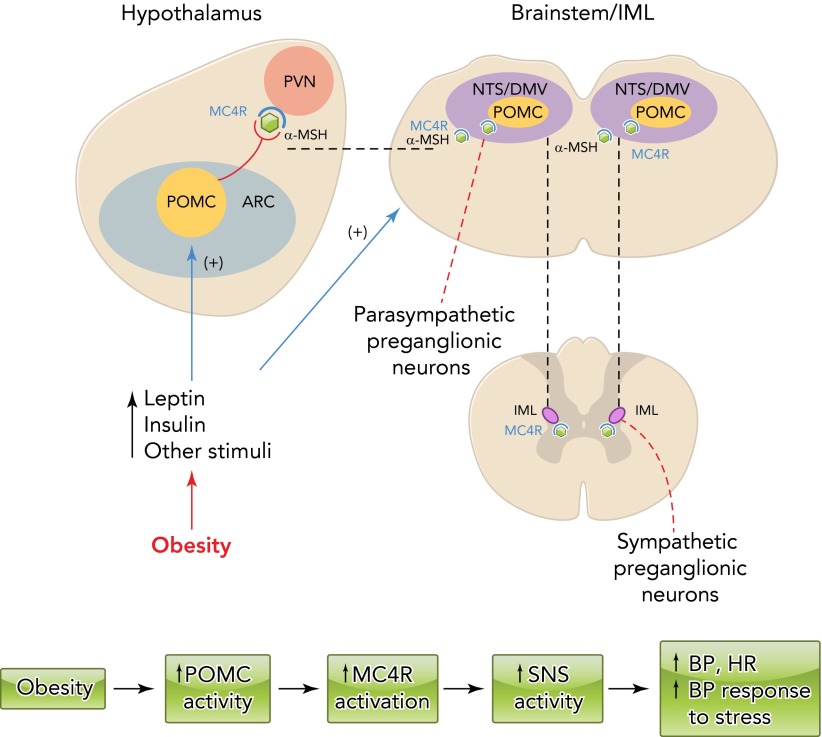
In conclusion, obesity is a major cause of hypertension as well as cardiovascular and renal diseases worldwide. As summarized in FIGURE 4 the brain melanocortin system, mainly via activation of the POMC neuron-MC4R axis, is a key link between excess weight gain and SNS activation leading to impaired renal-pressure natriuresis and elevated BP. Considerable evidence suggests that the importance of the brain MC4R in controlling SNS activity goes beyond its role in obesity hypertension. MC4R also appears to play a role in modulating SNS activity in response to various other stimuli and other forms of hypertension that are accompanied with sympathetic overactivity. Although the MC4R has long been recognized as an important target for anti-obesity drugs given its powerful actions on appetite and energy balance, the effects of most MC4R agonists to increase SNS activity and BP currently preclude their use in treating obesity. A better understanding of the mechanisms by which MC4R exerts its actions on energy balance and cardiovascular function may offer new approaches to treatment of obesity and cardiovascular disease.
FIGURE 4.
Schematic representation of the impact of obesity factors
Schematic representation of the impact of obesity factors (e.g., leptin, insulin, and other stimuli) on pro-opiomelanocortin (POMC) neuron activity and melanocortin 4 receptor (MC4R) activation in forebrain and brain stem nuclei as well as in the spinal cord IML leading to elevated sympathetic nervous system (SNS) activity, blood pressure (BP), heart rate (HR), and BP response to stress. α-MSH, α-melanoctyte stimulating hormone; ARC, arcuate nucleus of the hypothalamus; DMV, dorsal motor nucleus of the vagus; IML, intermediolateral medulla; NTS, nucleus of the tractus solitarius; PVN, paraventricular nucleus of the hypothalamus.
Footnotes
The authors' research was supported by grants from the National Heart, Lung, and Blood Institute (P01 HL-51971), the National Institute of General Medical Sciences (P20 GM-104357), and American Heart Association Scientist Development Grants to Alexandre A. da Silva and Jussara M. do Carmo.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: A.A.d.S. and J.E.H. conception and design of research; A.A.d.S. prepared figures; A.A.d.S. drafted manuscript; A.A.d.S., J.M.d.C., Z.W., and J.E.H. edited and revised manuscript; A.A.d.S., J.M.d.C., Z.W., and J.E.H. approved final version of manuscript.
References
- 1.Armitage JA, Burke SL, Prior LJ, Barzel B, Eikelis N, Lim K, Head GA. Rapid onset of renal sympathetic nerve activation in rabbits fed a high-fat diet. Hypertension 60: 163–171, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123: 493–505, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Bariohay B, Roux J, Tardivel C, Trouslard J, Jean A, Lebrun B. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology 150: 2646–2653, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Carlyle M, Jones OB, Kuo JJ, Hall JE. Chronic cardiovascular and renal actions of leptin-role of adrenergic activity. Hypertension 39: 496–501, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Corander MP, Fenech M, Coll AP. How melanocortin action in the brain modulates body weight, blood pressure, and ischemic damage. Circulation 120: 2260–2268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva AA, do Carmo J, Dubinion J, Hall JE. The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep 11: 206–211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension 51: 884–890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva AA, Kuo JJ, Hall JE. Role of hypothalamic melanocortin 3/4-receptors in mediating chronic cardiovascular, renal, and metabolic actions of leptin. Hypertension 43: 1312–1317, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Davy KP, Hall JE. Obesity, and hypertension: two epidemics or one? Am J Physiol Regul Integr Comp Physiol 286: R803–R813, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Davy KP, Orr JS. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev 33: 116–124, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Health, and Human Services, Center for Disease Control and Prevention. Obesity and overweight trends in the U.S., 2011. (Online) Atlanta, GA: Center for Disease Control; http://www.cdc.gov/nccdphp/dnpa/obesity/trend/index.htm. [Google Scholar]
- 12.do Carmo JM, Bassi M, da Silva AA, Hall JE. Systemic but not central nervous system nitric oxide synthase inhibition exacerbates the hypertensive effects of chronic melanocortin-3/4 receptor activation. Hypertension 57: 428–434, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.do Carmo JM, da Silva AA, Cai Z, Lin S, Dubinion JH, Hall JE. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension 57: 918–926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.do Carmo JM, da Silva AA, Rushing JS, Hall JE. Activation of the central melanocortin system contributes to the increased arterial pressure in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 302: R561–R567, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.do Carmo JM, da Silva AA, Rushing JS, Pace B, Hall JE. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am J Physiol Regul Integr Comp Physiol 305: R359–R368, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubinion JH, da Silva AA, Hall JE. Enhanced blood pressure and appetite responses to chronic central melanocortin-3/4 receptor blockade in dietary-induced obesity. J Hypertens 28: 1466–1470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 360: 44–52, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Guyton AC. The surprising kidney-fluid mechanism for pressure control: its infinite gain! Hypertension 16: 425–430, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Hall JE. The kidney, hypertension, obesity. Hypertension 41: 625–633, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM. Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci 324: 127–137, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Hall JE, da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S, Smith G, Stec DE. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem 285: 17271–17276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallersund P, Sjöström L, Olbers T, Lönroth H, Jacobson P, Wallenius V, Näslund I, Carlsson LM, Fändriks L. Gastric bypass surgery is followed by lowered blood pressure and increased diuresis: long term results from the Swedish Obese Subjects (SOS) study. PLos One 7: e49696, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humphreys MH, Ni XP, Pearce D. Cardiovascular effects of melanocortins. Eur J Pharmacol 660: 43–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasa M, Kawabe K, Sapru HN. Activation of melanocortin receptors in the intermediolateral cell column of the upper thoracic cord elicits tachycardia in the rat. Am J Physiol Heart Circ Physiol 305: H885–H893, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kassab S, Kato T, Wilkins FC, Chen R, Hall JE, Granger JP. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 25: 893–897, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Kuo JJ, da Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 41: 768–774, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Kuo JJ, da Silva AA, Tallam LS, Hall JE. Role of adrenergic activity in pressure responses to chronic melanocortin receptor activation. Hypertension 43: 370–375, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Li P, Cui BP, Zhang LL, Sun HJ, Liu TY, Zhu GQ. Melanocortin 3/4 receptors in paraventricular nucleus modulate sympathetic outflow and blood pressure. Exp Physiol 98: 435–443, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Mark AL. Weight reduction for the treatment of obesity-associated hypertension: nuances and challenges. Curr Hypertens Rep 9: 368–372, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Mark AL. Selective leptin resistance revisited. Am J Physiol Regul Integr Comp Physiol 305: R566–R581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinelli CE, Keogh JM, Greenfield JR, Henning E, van der Klaauw AA, Blackwood A, O'Rahilly S, Roelfsema F, Camacho-Hübner C, Pijl H, Farooqi IS. Obesity due to melanocortin 4 receptor (MC4R) deficiency is associated with increased linear growth and final height, fasting hyperinsulinemia, and incompletely suppressed growth hormone secretion. J Clin Endocrinol Metab 96: E181–E188, 2011 [DOI] [PubMed] [Google Scholar]
- 32.Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol 19: 974–982, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Nogueiras R, Williams LM, Dieguez C. Ghrelin: new molecular pathways modulating appetite and adiposity. Obes Facts 3: 285–292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overton JD, Leibel RL. Mahoganoid and mahogany mutations rectify the obesity of the yellow mouse by effects on endosomal traffic of Mc4R protein. J Biol Chem 286: 18914–18929, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab 10: 3686–3695, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Pavia JM, Pavia JM, Schiöth HB, Morris MJ. Role of MC4 receptors in the depressor and bradycardic effects of alpha-MSH in the nucleus tractus solitarii of the rat. Neuroreport 14: 703–707, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating the renal sympathoactivation to leptin and insulin. J Neurosci 23: 5998–6004, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13: 195–204, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shariat-Madar B, Kolte D, Verlangieri A, Shariat-Madar Z. Prolylcarboxypeptidase (PRCP) as new target for obesity treatment. Diabetes Metab Syndr Obes 3: 67–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjöström CD, Peltonen M, Wedel H, Sjöström L. Differentiated long-term effects of intentional weight loss on diabetes and hypertension. Hypertension 36: 20–25, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Sohn JW, Harris LE, Berglund ED, Liu T, Vong L, Lowell BB, Balthasar N, Williams KW, Elmquist JK. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic preganglionic neurons. Cell 152: 612–619, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spradley Ft, Palei AC, Granger JP. Obese melanocortin-4 receptor-deficient rats exhibit augmented angiogenic balance and vasorelaxation during pregnancy. Physiol Rep 1: e00081, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med 134: 1–11, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Straznicky NE, Eikelis N, Lambert EA, Esler MD. Mediators of sympathetic activation in metabolic syndrome obesity. Curr Hypertens Rep 10: 440–447, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Tai MH, Weng WT, Lo WC, Chan JY, Lam HC, Tseng CJ. Role of nitric oxide in alpha-melanocyte-stimulating hormone-induced hypotension in the nucleus tractus solitarii of the spontaneously hypertensive rats. J Pharmacol Exp Ther 321: 455–461, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Tallam LS, da Silva AA, Hall JE. Melanocortin-4 receptor mediates chronic cardiovascular and metabolic actions of leptin. Hypertension 48: 58–64, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Tallam LS, Kuo JJ, da Silva AA, Hall JE. Cardiovascular, renal, and metabolic responses to chronic central administration of agouti-related peptide. Hypertension 44: 853–858, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Tallam LS, Stec DE, Willis MA, da Silva AA, Hall JE. Melanocortin-4 receptor-deficient mice are not hypertensive or salt-sensitive despite obesity, hyperinsulinemia, and hyperleptinemia. Hypertension 46: 326–332, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Tao YX. The melanocortin-4 receptor: physiology, pharmacology and pathophysiology. Endocr Rev 31: 506–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 106: 253–262, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating alpha-MSH in rodents. J Clin Invest 119: 2291–2303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward KR, Bardgett JF, Wolfgang L, Stocker SD. Sympathetic response to insulin is mediated by melanocortin 3/4 receptors in the hypothalamic paraventricular nucleus. Hypertension 57: 435–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 162: 1867–1872, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Wofford MR, Anderson DC, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of alpha and beta adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens 14: 694–698, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol 297: R330–R336, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol 298: R1642–R1647, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yosten GL, Pate AT, Samson WK. Neuronostatin acts in brain to biphasically increase mean arterial pressure through sympatho-activation followed by vasopressin secretion: the role of melanocortin receptors. Am J Physiol Regul Integr Comp Physiol 300: R1194–R1199, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]