Abstract
Attention-deficit/hyperactivity disorder (ADHD) is a childhood disorder that is associated with many behavioral and social problems. These problems may continue when an individual continues to meet criteria for ADHD as an adult. In this study, we describe the outcome patterns for three different groups: individuals who had ADHD as children, but no longer meet criteria as adults (Childhood-Limited ADHD, n = 71); individuals who met ADHD criteria as children and continue to meet criteria as young adults (Persistent ADHD n = 79); and a control group of individuals who did not meet ADHD diagnostic criteria in childhood or adulthood (n = 69). Groups were compared to examine differences in change in rates of alcohol, marijuana, and nicotine dependence over three time points in young adulthood (mean ages 18, 20 and 22 years). The method used is notable as this longitudinal study followed participants from childhood into young adulthood instead of relying on retrospective self-reports from adult participants. Results indicated that there were no significant group differences in change in rates of substance dependence over time. However, individuals whose ADHD persisted into adulthood were significantly more likely to meet DSM-IV criteria for alcohol, marijuana, and nicotine dependence across the three time points after controlling for age, sex, childhood stimulant medication use, and childhood conduct problems. Implications of these findings, as well as recommendations for future research, are discussed.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a childhood disorder with prevalence rates estimated at 6–9% worldwide (Faraone, Sergeant, Gillberg, & Biederman, 2003). It is associated with several psychosocial problems, including employment problems, criminal behavior, and lower academic achievement (Realmuto, Winters, August, Lee, Fahnhorst, & Botzet, 2009; Wilens, Biederman, & Spencer, 2002). Numerous prospective studies have also found that adolescents with ADHD, or those with a proxy measure of ADHD based on symptoms of Inattention or Hyperactivity, are more likely to use alcohol and other drugs during adolescence than their non-ADHD peers (Lee, Humphreys, Flory, Liu, & Glass, 2011). In general, they are more prone to begin using drugs at an early age, their use tends to be more chronic, and they have a much shorter gap between onset of use and the development of a substance use disorder (Wilens, Biederman, & Mick, 1998). This relationship exists for several different drug categories, including illicit (e.g. marijuana and cocaine) and licit (alcohol and tobacco) drugs (Lee et al., 2011; Molina et al., 2013). Given the focus of our data on alcohol, marijuana, and tobacco, we will discuss the background literature pertaining to these three drugs.
Adolescent ADHD and Substance Use Disorders
Adolescents with ADHD have been found to be more likely to drink alcohol, begin drinking at an early age, and meet criteria for a dependence disorder than their non-ADHD peers (Ohlmeier et al., 2007). The transition from alcohol abuse to dependence appears to be accelerated during this age when ADHD is present (Wilens & Biederman, 2006). Recent research on the association between ADHD and marijuana, including the Cannabis Youth Treatment Study, suggests that individuals with ADHD also have greater rates of marijuana use, abuse, and dependence (Dennis, Godley, Diamond, Tims, Babor, et al., 2004; Molina et al., 2013). With respect to tobacco use, adolescents with ADHD have greater use of tobacco products than their non-ADHD peers (Molina et al., 2013; Wilens & Biederman, 2006). In addition, they report an earlier age at first use, greater amount of use, greater persistence into adulthood, and higher dependence rates (Chang, Lichtenstein, & Larsson, 2012; Lambert & Hartsough, 1998). Some authors have suggested that both tobacco and marijuana may serve a self-medicating role in ADHD (Szobot & Bukstein, 2008; Whalen, Jamner, Henker, Gehricke, & King, 2003). Regardless of ADHD status, adolescent rates of substance use disorders (SUDs) tend to persist during the adolescent and early adulthood years and then tend to subsequently decrease during adulthood (Brown, McGue, Maggs, Schulenberg, Sher, Winters, & Lowman, 2008; Jackson & Schulenberg, 2013). However, it remains unclear if this pattern is similar for individuals with ADHD that persists into adulthood.
Adult ADHD and Substance Use Disorders
Much less is known about the link between adult ADHD and substance use. It is estimated that approximately 4% of all adults meet diagnostic criteria for ADHD (Faraone, Sergeant, Gillberg, & Biederman, 2003). For those individuals who had ADHD as children, between 35 and 60% still meet full criteria for a diagnosis as an adult with up to 78% experiencing at least some persistent symptoms (Biederman, Petty, Evans, Small, & Faraone, 2010). These adults with ADHD have more employment, interpersonal, legal, and mental health problems, as well as higher health care costs when compared to non-persistent ADHD adults, suggesting that persistent adult ADHD is an important problem to address (Barkley, Murphy, & Fischer, 2008; Biederman et al., 2006; Kessler et al., 2006). However, although several studies exist which examine the relationship between adult ADHD and substance use, there are several methodological challenges. One problem with the non-longitudinal studies is the reliance on retrospective reports of childhood symptoms when establishing that the adult met ADHD criteria prior to the age of seven. Research indicates that individuals are frequently inaccurate in these retrospective reports (Miller, Newcorn, & Halperin, 2010). In addition, the existence of ADHD as a genuine adult medical disorder is controversial, as well as how it should be properly assessed (Barkley, Murphy, & Fischer, 2008; Kessler et al., 2006).
Despite these challenges, there have been important findings in the relationship between adult ADHD and substance use disorders. Alcohol use data generally indicate that adults with ADHD are no more likely to drink alcohol compared to adults without a history of ADHD (e.g. Weiss & Hechtman, 1993). However, the prevalence rate of alcohol use disorders are higher among adults with ADHD compared to their non-ADHD counterparts (Charach, Yeung, Climans, & Lillie, 2011; Wilens, 2004). For example, in the Wilens publication (2004), it is reported that 17–45% of adults with ADHD meet criteria for a co-existing alcohol use disorder. Adults with ADHD are also more likely to report a higher lifetime prevalence rate of marijuana use and more chronic use than their non-ADHD peers (Fergusson & Boden, 2008; Flory, Milich, Lynam, Leukefeld, & Clayton, 2003). Further, they are more likely to be regular smokers, meet criteria for tobacco dependence, begin smoking earlier, and are less likely to successfully quit smoking (Lambert & Hartsough, 1998; Rodriguez, Tercyak, & Audrain-McGovern, 2008).
Although the above studies support the relationship between current ADHD symptomatology and substance use disorders in adulthood, there is minimal longitudinal research that examines the relationship between persistence of childhood ADHD and substance use disorders in young adulthood. Among the longitudinal studies we located in the literature, participants are typically followed only through adolescence (Chang, Lichtenstein, & Larsson, 2012). Furthermore, little is known about what happens to individuals who had ADHD in childhood but no longer meet ADHD diagnostic criteria as a young adult (i.e., those with ADHD limited to childhood) (Young & Gudjonsson, 2008). Finally, another area of interest is the effect of co-existing conduct disorder on substance abuse outcomes. Some investigations support the notion that conduct disorder, rather than ADHD, explains the negative substance-related outcomes in adulthood (Fergusson, Horwood, & Ridder, 2007), whereas others suggest that both ADHD and conduct disorder are contributory as an additive effect (e.g., August et al., 2006; Barkley, Murphy, & Fischer, 2008; Biederman, Wilens, Mick, & Faraone, 1997; Molina & Pelham, 2003). Still other research indicates that conduct disorder may play an important role in certain outcome measures, such as alcohol use, but play less of a role in others (e.g. tobacco use) (Glass & Flory, 2012).
Present Study
In prior analyses of our longitudinal data we have reported that childhood ADHD status confers a risk toward late adolescent/young adulthood substance use disorders (18–22-years-old) (August et al., 2006; Lee, Winters, & Wall, 2010). The present study examines whether the course of ADHD status during this age period (i.e., persistent ADHD or Childhood-limited ADHD) is associated with higher rates of substance dependence disorders assessed at 18, 20, and 22 years of age. Specifically, this paper compares three groups of young adults: 1) Those with no history of childhood ADHD (controls), 2) Individuals with childhood ADHD that persisted into adulthood (Persistent ADHD), and 3) Those with childhood ADHD who no longer meet diagnostic criteria as adults (Childhood-Limited ADHD). Age, gender, childhood stimulant medication use, and baseline assessment for conduct disorder are entered as covariates to control for their effects on substance dependence outcomes. Given the extant literature that ADHD is a risk for drug involvement, we hypothesize that the Persistent ADHD group will show higher rates of substance dependence across the three assessment waves compared to the other two groups. The longitudinal analytic method will be applied to the substance dependence outcomes because it provides a more parsimonious approach than multiple, separate comparisons at each time point. It also allows inclusion of all participants with missing data on the outcome variable, whereas multiple, separate comparisons utilize pairwise deletion of participants with missing data.
Method
Participants
Participants for this analysis were derived from a longitudinal study initiated in 1990 entitled The Minnesota Competence Enhancement Program (MNCEP); for a detailed description, see August et al., 1995. Briefly, a screening procedure was conducted among 7,231 children aged 7 to 11 who attended 22 suburban elementary schools that utilized the teacher version of the Conners’ Hyperactivity Index (HI-T; Goyette, Conners, & Ulrich, 1978). Students whose HI-T score was 1.75 SD above the mean were then screened by their parent using the Conners’ Hyperactivity Index (HI-P; Goyette et al., 1978). Those students with a HI-P score of greater than 1.75 were placed in the “disruptive” group (n = 318). A comparison group was derived from the entire sample whose HI-T score was less than 1.0 SD. HI-P scores were not obtained on the comparison group (n = 144). All students had an IQ score of 80 or higher and were predominantly middle class (levels II and III of the Hollingshead socioeconomic index; Hollingshead, 1975), Caucasian (95%), and resided in suburban neighborhoods of a major metropolitan area (August, Realmuto, Crosby, & MacDonald, 1995).
Initial childhood participation (T1–T3)
Detailed baseline (T1) and follow-up (T2 and T3) assessments (all conducted roughly within a 5-year period, approximately 2 years apart) determined that among the disruptive children, 205 met DSM-III-R criteria for ADHD (based on the Diagnostic Interview for Children and Adolescents-Revised Parent Version (DICA-R-P; Reich, Shayla, & Taibleson, 1992) at one or more of these assessment points (T1–T3). Late adolescent and young adult assessments (FU1–FU3), as dictated by the grant that funded these evaluations, required that the case have a full set of T1–T3 data. Thus, the small number of families assessed at baseline (T1) who then dropped out of the study and did not receive a T2 and T3 assessment (37 ADHD subjects and 28 controls) were not eligible for FU1–FU3 evaluations and were not approached. Also not included in the present sample were 17 participants in the comparison group who were diagnosed with either childhood ADHD or an externalizing disorder at the T2 or T3 assessments.
Current study participation (FU1–FU3)
Sample inclusion and exclusion considerations led to the following eligible sample sizes for the present analysis: ADHD-childhood (n = 168), and controls (n = 98). Among them a total of 150 (ADHD-childhood) and 93 (controls) participated in the follow-up assessments. Thus, follow-up rates were 89.2% (ADHD-childhood) and 94.9% (controls). FU1 assessments were timed to coincide with subjects’ status as either a senior in high school or one-year post-graduate, thus the age range at FU1 is narrower than the age range at baseline (T1). Finally, participants in the comparison group who met criteria for adult ADHD at any time point during the FU1–FU3 assessments were excluded from the present analysis (n = 24, see below for more details). The final sample for the present study included ADHD-childhood (n = 150) and controls (n = 69).
Measures
Background variables
Several assessments were administered at baseline (T1) or early in individuals’ study participation (e.g. T2 or T3). The Four Factor Index of Social Status (Hollingshead, 1975) was administered to parents at baseline (T1). A score of 8 (low SES) to 66 (high SES) is derived based on parent occupation and education that correlates with five levels of SES (unskilled to major business/professionals).
The Behavioral Assessment Scale for Children – Parent Report Form and -Teacher Report Form (BASC-PRF; BASC-TRF; Reynolds & Kamphaus, 1992) is a multidimensional system used to assess broad domains of behavioral and emotional problems as well as adaptive skills. The measure was administered to parents and teachers at T1. Items were rated on a 4-point Likert scale ranging from 0 = never to 3 = almost always. T-scores were derived with a mean of 50 and a standard deviation of 10. These measures have been normed and validated on both clinical and normative populations and have favorable psychometric properties.
The Kaufman Brief Intelligence Test (K-BIT; Kaufman & Kaufman, 1990) was individually administered to child participants at T1 to assess expressive and receptive vocabulary and nonverbal problem solving (matrices). Age-based standard scores with a mean of 100 and a standard deviation of 15 were derived.
The Diagnostic Interview for Children and Adolescents – Revised (DICA; Reich et al., 1992) was used to assess childhood psychiatric disorders and was administered to one parent (usually the mother) over-the-phone at three assessment points (T1 [excluding controls], T2, and T3). Structured diagnostic interviews administered to parents over the telephone have been shown to be valid (Holmes et al., 2004; Todd, Joyner, Heath, Neuman, & Reich, 2003). The DICA-R generates standardized diagnoses as reflected by the DSM-III-R. Each item was scored yes if the behavior was definitely endorsed or no if the respondent indicated “sometimes”, “rarely”, or “never”. A data sheet was prepared for each child and the number of symptoms endorsed was tallied and entered. Specific diagnostic parameters, including age of onset, duration of symptoms, and frequency were recorded. Diagnostic scoring algorithms were adopted from the DICA-R by which categorical diagnoses were made. Twenty percent (every fifth interview completed) of the interviews were independently rated by an assessment technician via an extension phone, in order to obtain interrater reliability on item scoring and to prevent interviewer drift. Kappas for symptoms were derived for each disorder; average symptom kappas ranged from 0.88 for conduct disorder at T1 to 1.00 for ADHD at T2 (see August, Braswell, & Thuras, 1998).
Psychostimulant medication history
A continuing debate in the child psychopathology literature is the extent to which psychostimulant medication for children with ADHD confers a risk of subsequent drug abuse. Although some exceptions exist (Lambert & Hartsough, 1998), the weight of empirical evidence is that childhood stimulant treatment does not increase the risk for later development of drug abuse (e.g., Barkley, Fischer, Smallish, & Fletcher, 2004; Mannuzza, Klein, & Moulton, 2003; Paternite, Loney, Salisbury, & Whaley, 1999). Some studies have shown that stimulant treatment may contribute to protective effects against drug abuse risk (e.g., Molina, Pelham, & Roth, 1999), and it may moderate the persistence of ADHD into young adulthood (Barkley, 1998). The present authors have reported elsewhere, based on the same sample analyzed in the present paper, that history of childhood psychostimulant medication was not related to drug use, including nicotine use, at any of the three young adult outcomes (mean ages: 18, 20 and 22; Winters et al., 2011). Also, as shown in Table 1, a history of stimulant medication use was not significantly different between the Childhood-Limited ADHD and Persistent ADHD groups. Nonetheless, we took a conservative approach and included history of psychostimulant medication in the present study to control for its possible effects on the drug involvement outcomes. Using a semistructured format, the parent provided at each childhood time point (T1 to T3) a record of their child’s psychostimulant medication history. Based on these data, youths were categorized into three psychostimulant medication groups: (1) never used; (2) medication prescribed and used up to age 12 but not later; and (3) medication prescribed and used after age 12 (includes both childhood and later prescription).
Table 1.
Demographic Characteristics of the Normal Control and Young Adult ADHD Groups
| Variable | Normal Control (n=69) |
Childhood- Limited ADHD (n=71) |
Persistent ADHD (n=79) |
χ2 | F | p |
|---|---|---|---|---|---|---|
| Male % | 62.3 | 88.7 | 74.7 | 13.17 | <.01 | |
| White % | 100.0 | 93.0 | 93.7 | 4.86 | .09 | |
| Mean Age (SD) at T1 | 8.81 (1.14) | 9.00 (1.06) | 8.97 (1.19) | 0.60 | .55 | |
| FU1 | 18.13 (1.12) | 18.42 (1.08) | 18.30 (1.11) | 1.23 | .29 | |
| FU2 | 19.88 (1.48)a | 20.28 (1.42) | 20.54 (1.58)a | 3.48 | .03 | |
| FU3 | 21.77 (1.32) | 21.96 (1.25) | 21.97 (1.29) | 0.45 | .64 | |
| High school graduate % | 100.0 | 92.6 | 88.3 | 8.03 | .02 | |
| Mean IQ (SD) at T1 | 109.55 (12.81)a | 104.93 (12.47) | 104.18 (10.56)a | 4.25 | .02 | |
| Mean SES (SD) at T1 | 49.68 (10.78) | 45.50 (10.32) | 46.58 (11.78) | 2.65 | .07 | |
| Single parent family at T1 % | 7.2 | 23.5 | 15.2 | 7.02 | .03 | |
| Childhood Stimulant Medication % | 1.5 | 39.4 | 48.7 | 40.52 | <.001 | |
| CD/ODD at T1, T2 or T3 % | 0.0 | 60.6 | 59.5 | 70.30 | <.001 | |
| BASC-TRS at T1 | ||||||
| Aggression | 46.02 (6.57)ab | 57.72 (9.78)a | 60.99 (14.30)b | 37.12 | <.001 | |
| Attention Problems | 46.02 (8.81)ab | 61.76 (8.92)a | 65.02 (8.56)b | 94.52 | <.001 | |
| Conduct Problems | 46.14 (6.67)ab | 54.38 (9.53)a | 58.06 (16.78)b | 18.39 | <.001 | |
| Hyperactivity | 45.13 (9.22)ab | 58.54 (10.64)ac | 63.01 (10.84)bc | 57.75 | <.001 | |
| BASC-PRS at T1 | ||||||
| Aggression | 46.52 (6.63)ab | 64.40 (11.42)a | 64.75 (12.50)b | 55.54 | <.001 | |
| Attention Problems | 44.75 (7.56)ab | 65.63 (7.13)a | 67.67 (8.12)b | 158.86 | <.001 | |
| Conduct Problems | 44.33 (6.82)ab | 61.99 (12.36)a | 63.82 (16.79)b | 40.34 | <.001 | |
| Hyperactivity | 42.43 (6.61)ab | 67.09 (14.25)a | 69.69 (14.63)b | 83.46 | <.001 |
Note. Within each row, cells with the same letter subscripts are significantly (p < .05) different from one another (Tukey HSD test). BASC-TRS = The Behavioral Assessment Scale for Children – Teacher Report Form (Reynolds & Kamphaus, 1992); BASC-PRS = The Behavioral Assessment Scale for Children – Parent Report Form (Reynolds & Kamphaus, 1992); CD = conduct disorder; ODD = oppositional defiant disorder; SES = social economic status.
Substance dependence and ADHD diagnosis
Prior year DSM-IV-based diagnostic mental health information, including abuse and dependence disorders for nicotine, alcohol, marijuana and other drugs, as well as ADHD diagnosis, was obtained from the youth using the Adolescent Diagnostic Interview (DSM-IV; American Psychiatric Association, 2000; ADI; Winters & Henly, 1993). The ADI has established reliability and validity in adolescent clinical and non-clinical populations (Winters & Henly, 1993). Barkley’s recommended cutoff scores for adult ADHD (five inattentive symptoms or four hyperactivity/impulsivity symptoms) were used to make a current adult diagnosis of ADHD (Barkley, 1998). It is worth noting that the ADHD diagnostic criteria based on DSM-III-R (which was applied at T1–T3) and DSM-IV (which was applied at FU1–FU3) are not identical. However, if DSM-IV criteria were applied at T1–T3, only one DSM-III-R ADHD case would have lost a diagnosis of ADHD (nearly reached DSM-IV criteria), and all non-ADHD individuals would have retained their non-ADHD status. For the data analysis, SUD refers to the presence of a prior year DSM-IV substance dependence diagnosis. Base rate considerations in terms of a substance dependence diagnosis limited our analysis to alcohol, marijuana, and nicotine dependence.
Procedure
At each wave of follow-up assessment (FU1, FU2, and FU3), participants were contacted by telephone and offered participation in the study. Informed consent was obtained and structured interviews were conducted either in-person or over the telephone. Youth participants were paid $50 for their participation at each assessment wave. Interviews took approximately one hour and were administered by trained interviewers with Bachelor’s or Master’s degrees in Psychology.
Statistical Analyses
One-way ANOVAs (for continuous variables) and chi-square tests (for categorical variables) were used to test for differences in subgroup characteristics. Significant group effects from ANOVAs were followed up with post hoc group comparisons using Tukey HSD (Honestly Significant Difference) tests. Group differences in substance dependence outcomes (dichotomous yes/no) were examined by using the generalized estimating equations (GEE; Liang & Zeger, 1986). The GEE method appropriately models correlations in repeatedly measured categorical data and allows inclusion of participants with missing data on the outcome variable (Lee et al. 2007, MacKinnon & Lockwood, 2003). This method has been applied to longitudinal substance use data (e.g., Chou et al. 1998; Molina et al. 2013; Patrick et al. 2012). The GEE model in this study included main effects of group and time, as well as the interaction effect of Group × Time. In the analyses, age, gender, childhood stimulant medication use, and baseline teacher-rated conduct problems (BASC-TRF) were entered as covariates to control for their effects on substance dependence outcomes. The GEE methods were applied using the PROC GENMOD procedure in SAS 9.2.
Results
Attrition Analysis
Two sets of attrition analyses were conducted. The first set of analyses compared those who participated in the FU1 assessment versus those who did not from the pool of “eligible” participants. A one-way ANOVA was used for continuous variables and chi-square tests were used for categorical data within the ADHD-childhood and control groups across several (1) demographic (age, gender, IQ, SES, single parent status) and (2) T1 clinical measures (HI-T, HI-P, number of ADHD symptoms, teacher- and parent-rated externalizing problems, internalizing problems, school problems and behavioral symptom index on the BASC). Analysis revealed only one significant difference: within the ADHD-childhood group, participants had significantly more single parent households than non-single parent households compared to nonparticipants (11% vs. 1%, p < .05).
The second set of analyses compared those who dropped out of the study at FU2 or FU3 versus those who were retained. Of those who participated in the FU1 assessment, one participant dropped out of the study at FU2 and 37 at FU3. The main reason for attrition was lost to follow-up. Attrition analysis comparing those who dropped out (n = 38) versus those who were retained (n = 205) on the demographic and T1 clinical measures revealed that there was no significant group difference in all variables except for SES (attrited M = 43.9, SD = 10.9; retained M = 48.4, SD = 11.1, p < .05).
Adult ADHD Diagnosis
Among the 150 participants with childhood ADHD, 35%, 32% and 36% met criteria for current adult ADHD at FU1, FU2 and FU3, respectively. Across the three time points, 27% of this group met criteria for ADHD once, and 26% met criteria twice or three times. Forty-seven percent did not meet criteria for ADHD at any of the three time points. Study participants who met criteria for childhood ADHD were re-categorized into Persistent ADHD or Childhood-Limited ADHD groups using their young adult diagnosis of ADHD. Those who met criteria for adult ADHD one or more times during the FU1–FU3 assessment were categorized into the Persistent ADHD group (n = 79) and those who did not meet criteria for adult ADHD at any of the time points were categorized into the Childhood-Limited ADHD group (n = 71).
Although the control group (n = 93) consisted of individuals who did not meet diagnostic criteria for ADHD during childhood, some of them met adult ADHD diagnostic criteria in young adulthood. Across the three assessment waves (FU1, FU2, FU3), 18% (n = 17) of the control participants met criteria for adult ADHD once, and 8% (n = 7) met the criteria twice. Seventy-four percent of the control group (n = 69) did not meet criteria for adult ADHD at any of the three time points. Those who met criteria for adult ADHD in the control group were excluded from the analyses.
Demographic information for the two ADHD groups and the normal controls are provided in Table 1. Chi-square tests on categorical variables showed that there were significant group differences in proportions of males, high school graduate status, single parent households at baseline, childhood stimulant medication use, and childhood conduct disorder (CD)/oppositional defiant disorder (ODD). Inspection of the data (Table 1) showed that compared to the normal controls, the two ADHD groups had higher proportions of males, single parent families, childhood stimulant medication use, and childhood CD/ODD. Also, there were slightly fewer high school graduates in the Persistent ADHD group. ANOVAs conducted on continuous variables showed significant group differences in mean age at FU2, IQ (T1), and teacher- and parent-rated externalizing scale scores (T1). Compared to the normal controls, individuals in the Persistent ADHD group were, on average, slightly older at FU2 and had lower IQ at baseline. Normal controls had lower mean scores of teacher- and parent-rated externalizing problems. It is noteworthy that there was no significant difference between the Persistent ADHD and Childhood-Limited ADHD groups in the demographic variables except the baseline teacher-rated Hyperactivity scale score.
Substance Dependence Outcomes
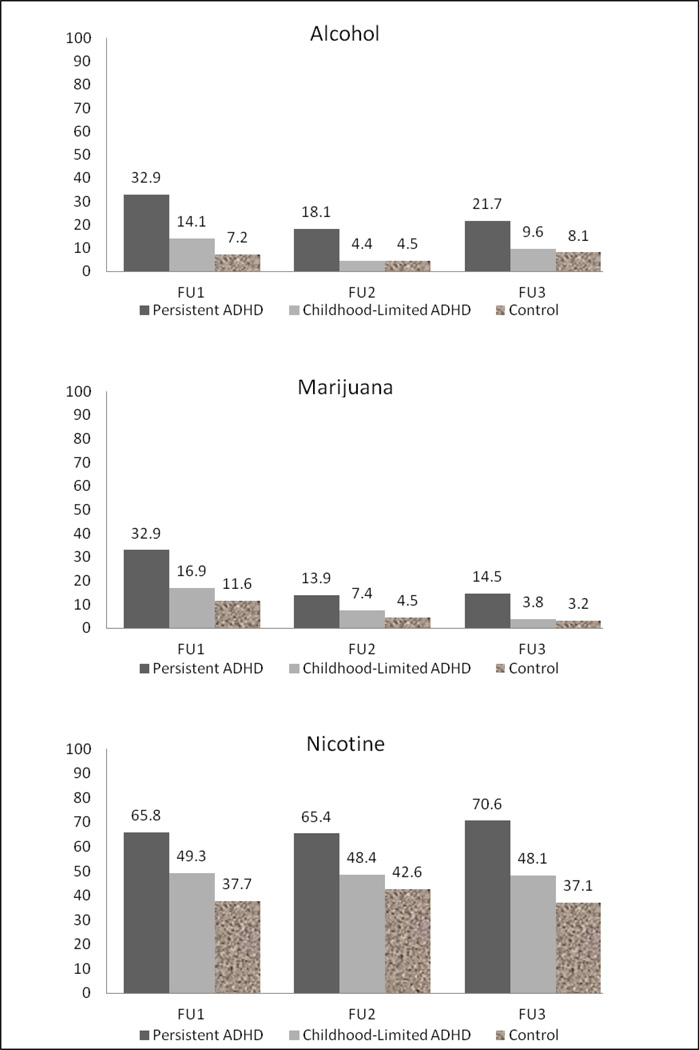
Proportions of participants with substance dependence at each follow-up for the three groups (Persistent ADHD, Childhood-Limited ADHD, and controls) are depicted in Figure 1. Across the three points, higher percentages of substance dependence were found for the Persistent ADHD group, while lower rates of substance dependence were found for the Childhood-Limited ADHD and control groups. For example, at FU1, 33% of individuals in the Persistent ADHD group met criteria for alcohol dependence, compared to 14% of the Childhood-Limited ADHD group and 7% of controls.
Figure 1.
Proportion of participants with substance dependence across follow-ups. Sample size differs slightly by types of substance due to missing data. Overall sample sizes were Persistent ADHD, FU1 n = 79, FU2 n = 79, FU3 n = 69; Childhood-Limited ADHD, FU1 n = 71, FU2 n = 70, FU3 n = 52; and Normal Control, FU1 n = 69, FU2 n = 69, FU3 n = 62.
The GEE model fit to the data on alcohol dependence outcomes suggested that there was no significant time or Group × Time effect after controlling for covariates (Table 2). All covariates had no significant impact on the alcohol dependence outcome. However, there was a significant group effect of Persistent ADHD: the Persistent ADHD group had a significantly higher proportion of alcohol dependence compared to the normal control group across the three assessment waves. The Childhood-Limited ADHD group, however, was not statistically different from the normal control group and had comparable rates of alcohol dependence. We then ran the same GEE model with Childhood-Limited ADHD as a comparison group in order to see if the Persistent ADHD group and Childhood-Limited ADHD group were significantly different from each other.1 The Persistent ADHD group had a significantly higher rate of alcohol dependence compared to the Childhood-Limited ADHD group across the three time points (estimate = 1.16, SE = 0.48, p = .016), controlling for all other covariates.
Table 2.
Summary of GEE Analyses Predicting Longitudinal Drug Use Outcomes
| Variables | Alcohol Dependence | Marijuana Dependence | Nicotine Dependence | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Est | SE | p | Est | SE | p | Est | SE | p | |
| Age | 0.13 | 0.12 | .26 | 0.11 | 0.11 | .36 | −0.07 | 0.12 | .55 |
| Gender (male=1) | 0.67 | 0.46 | .15 | 1.08 | 0.46 | .02 | 0.46 | 0.32 | .15 |
| Childhood stimulant medication (yes=1) | −0.02 | 0.34 | .95 | −0.11 | 0.37 | .76 | 0.22 | 0.31 | .48 |
| Childhood conduct problems | 0.01 | 0.01 | .69 | 0.02 | 0.01 | .11 | 0.01 | 0.01 | .41 |
| Time | 0.04 | 0.35 | .90 | −0.66 | 0.40 | .10 | 0.11 | 0.13 | .38 |
| Childhood-limited ADHD a | 0.46 | 0.67 | .49 | 0.16 | 0.58 | .78 | 0.34 | 0.40 | .41 |
| Persistent ADHD a | 1.62 | 0.64 | .01 | 1.18 | 0.54 | .03 | 0.99 | 0.41 | .01 |
| Time × Childhood-limited ADHD | −0.40 | 0.45 | .38 | −0.16 | 0.52 | .76 | −0.13 | 0.18 | .48 |
| Time × Persistent ADHD | −0.33 | 0.40 | .41 | −0.02 | 0.46 | .97 | 0.02 | 0.16 | .89 |
Comparison group is normal control.
The GEE model fit to the marijuana dependence outcome outcomes suggested that there was a significant Persistent ADHD group effect, but no main effect of time or Group × Time interaction after controlling for covariates (Table 2). None of the covariates had a significant impact on the marijuana dependence outcome except for gender. The results indicated that male participants compared to female participants, as well as the Persistent ADHD group compared to normal controls, had higher rates of marijuana dependence across the three assessment waves. The Childhood-Limited ADHD group was not statistically different from the normal control group and had comparable rates of marijuana dependence. We then ran the same GEE model with Childhood-Limited ADHD as a comparison group in order to compare between Persistent ADHD and Childhood-Limited ADHD groups on the marijuana dependence outcome. There was a significant main effect of Persistent versus Childhood-Limited ADHD, where the Persistent ADHD group had significantly higher rates of marijuana dependence across the three time points (estimate = 1.02, SE = 0.43, p = .019).
Table 2 also shows the GEE summary for the nicotine dependence variable. Similar to the findings for alcohol and marijuana dependence, the results suggested that there was no significant time effect or Group × Time interaction after controlling for covariates. All covariates had no significant impact on the nicotine dependence outcome. However, there was a significant group effect of Persistent ADHD versus normal control group. The results indicated that the Persistent ADHD group compared to the normal control group had a significantly higher proportion of nicotine dependence across the three time points. The Childhood-Limited ADHD group was not statistically different from the normal control group and had comparable rates of nicotine dependence. When we ran the same GEE model comparing Persistent ADHD and Childhood-Limited ADHD, there was a marginally significant group effect of Persistent ADHD versus Childhood-Limited ADHD (estimate = 0.66, SE = 0.34, p = .055), where the Persistent ADHD group showed higher rates of nicotine dependence across the three assessment waves.
Discussion
This study examined the impact of persistence of ADHD into young adulthood on the course of alcohol, marijuana, and nicotine dependence. We found that individuals with childhood ADHD that persisted into early young adulthood had a greater likelihood of alcohol and marijuana dependence throughout the young adulthood years (18–22-years-old) compared to the normal controls and those with childhood-limited ADHD. These individuals with persistent ADHD were also more likely than controls to meet criteria for nicotine dependence, although these results were marginally significant when compared to those with childhood-limited ADHD. There were no significant differences between the latter two groups (i.e., Childhood-Limited ADHD and the normal controls) on any of the substance dependence outcomes.
The non-significant Group × Time and time effects in the analyses indicated that rates of change for all three drugs were generally consistent across all follow ups for all three groups. However, despite the non-significant group × time and time effects, there appeared to be a decline in the proportion with alcohol and marijuana dependence from FU1 to FU3, which did not reach statistical significance (Persistent ADHD, alcohol dependence 33% to 22%, marijuana dependence 33% to 14%; Childhood-Limited ADHD, alcohol dependence 14% to 10%, marijuana dependence 17% to 4%). These trend data should be considered in the context that our statistical power to detect significant yet small effects is compromised because of small sample sizes.
Nonetheless, the overall results provide a meaningful contribution to the current literature by illustrating that the relationship between persistent ADHD and substance dependence, particularly alcohol and marijuana dependence, is significant throughout young adulthood. These data are consistent with the view that for many youth as they age into late adolescence and early adulthood, there continues a risk for a substance dependence disorder (Brown et al., 2008).
The findings associated with nicotine are noteworthy. We observed significantly higher rates of nicotine dependence among those in the Persistent-ADHD group across all three time points (FU1–FU3) compared to the normal controls. Nicotine dependence rates for the Persistent-ADHD group ranged from 66% – 71%, whereas the control group was lower at 37% – 43%. Although the Childhood-Limited group was not significantly different from the controls, their nicotine dependence rates were elevated as well (range, 48% – 49%). These data are consistent with the large body of literature showing that individuals with ADHD (either solely a childhood history or a current adult diagnosis) are at elevated risk for nicotine dependence (e.g., Barkley, Anastopoulod, Guevremont, & Fletcher, 1990; Lambert & Hartsough, 1998; McMahon, 1999; Milberger et al., 1997; Symmes, Winters, Fahnhorst, Botzet, Lee, et al., in press) and earlier age of onset of tobacco use (Lambert & Harsough, 1998; Milberger et al., 1997). To put our data in another perspective, we computed the prevalence of regular smoking among the childhood ADHD group (n = 150) by counting any presence of regular smoking at any of our FU1, FU2 and FU3 time points. This produced a rate of young adult regular smoking at 63%, which is comparable to the research cited above.
The study is strengthened by the longitudinal nature of the data and some unique features of our sample. Our youth are well-defined and drawn from a community sample in an affluent suburban area (e.g., only 13% of students at the participating schools were receiving free or reduced priced lunches). The great majority of ADHD studies that have prospectively followed their samples into young adulthood were originally drawn from clinics residing in urban, low SES settings (Biederman, Monuteaux, Mick, Spencer, Wilens et al., 2006). Thus, our findings add to the generalizability of the collective research on the ADHD liability for substance abuse. Also, the study’s longitudinal data avoid the problem of some ADHD studies that have used potentially unreliable retrospective data from adult participants to answer questions about their behavior as children (e.g. Ohlmeier, Peters, Wildt, Zedler, Ziegenbein et al., 2008).
It is important to consider our findings in the context of several study limitations. Our sample is predominantly Caucasian and male. Therefore, the results may not generalize to more diverse ethnic populations and to females. Also, the sample size and its composition limited our opportunity to examine the possible impact of sex and ethnicity. The change in informant from parent to youth for the assessment of ADHD symptoms may have affected symptom disclosure and categorization of youth, although it is standard in ADHD research to move from parent to youth report for ADHD (Barkley et al. 2004). There were relatively few cases with respect to some of the alcohol and marijuana-related variables. Last, our study suffered from some sample attrition, although the attrition analysis indicated that subjects lost to attrition were similar on most baseline variables compared to the non-attrition cases.
The results of this study have several implications for education. Approximately half of the participants with childhood ADHD continued to meet ADHD criteria in young adulthood during at least one young adult time point, and many of them were either currently or previously enrolled in college (nearly half of the two ADHD groups attended some college during FU1–FU3). This presence of adult ADHD, along with a co-occurring substance use disorder, can have a significant impact on college performance and completion rates. This suggests that students with ADHD may struggle with their education not only due to their ADHD symptoms, but due to the effects of substance disorders as well. This may be particularly true in early adulthood, around the age that these individuals are entering college. This suggests that early intervention in the college setting may be particularly important.
The results also have implications for treatment. For those young adults already in treatment for substance dependence, they may be further helped by assessment and treatment of possible co-occurring ADHD. In terms of tobacco, the results suggest that young adults with ADHD are important to target for smoking cessation (Fuemmeler, Kollins, & McClernon, 2007). It is likely that these young adults with ADHD will continue to use tobacco throughout later adulthood, which presents a significant and costly public health problem (U.S. Department of Health and Human Services, 2000).
Future research would benefit from large and relatively diverse samples to allow for more detailed analyses as described above. In addition, future longitudinal studies of ADHD and substance use would benefit from the inclusion of additional variables in order to further examine underlying influences of the risk toward and protection against substance abuse among individuals with ADHD. These variables may include specific externalizing traits (e.g., persistence of aggression), ADHD symptoms of Inattention and Hyperactivity, educational status, and other possible co-occurring problems (e.g., internalizing disorders).
As previously discussed, young adults with ADHD that persists into adulthood are an important group to study. They have much higher rates of alcohol and drug dependence in early young adulthood, and are significantly more likely to be dependent on tobacco throughout their young adult years. Although much remains to be known, this longitudinal study provides a meaningful contribution to the literature on ADHD and substance dependence by examining this relationship with an epidemiological sample across several time points in early adulthood. Future studies will need to continue to investigate the further clarify this complicated relationship.
Acknowledgments
This study was supported by grants DA012995 and K02-DA15347 from the National Institute on Drug Abuse
Footnotes
Summary of the results is not reported here and is available by contacting the corresponding author.
Contributor Information
Jessie L. Breyer, Century College
Susanne Lee, University of Minnesota Medical School
Ken Winters, University of Minnesota Medical School
Gerald August, University of Minnesota Medical School
George Realmuto, University of Minnesota Medical School
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (text revision) Washington, DC: Author; 2000. [Google Scholar]
- August GJ, Braswell L, Thuras P. Diagnostic stability of ADHD in a community sample of school-age children screened for disruptive behavior. Journal of Abnormal Child Psychology. 1998;26:345–356. doi: 10.1023/a:1021999722211. [DOI] [PubMed] [Google Scholar]
- August GJ, Realmuto GM, Crosby RD, MacDonald AW. Community-based multiple gate screening of children at risk for conduct disorder. Journal of Abnormal Child Psychology. 1995;23:521–544. doi: 10.1007/BF01447212. [DOI] [PubMed] [Google Scholar]
- August GJ, Winters KC, Realmuto G, Fahnhorst T, Botzet A, Lee S. Prospective study of adolescent drug abuse among community samples of ADHD and non-ADHD participants. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:824–832. doi: 10.1097/01.chi.0000219831.16226.f8. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit hyperactivity disorder: A handbook for diagnosis and treatment. 2nd ed. New York: Guilford; 1998. [Google Scholar]
- Barkley RA, Anastopoulod AD, Guevremont DG, Fletcher KE. Adolescents with attention deficit hyperactivity disorder: Patterns of behavioral adjustment, academic functioning, and treatment utilization. Journal of American Academic Child Adolescent Psychiatry. 1990;35:343–351. doi: 10.1016/s0890-8567(10)80010-3. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. The Journal of Child Psychology and Psychiatry. 2004;45:195–211. doi: 10.1111/j.1469-7610.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in adults: What the science says. New York: Guilford; 2008. [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, Faraone SV. Young adult outcome of attention deficit hyperactivity disorder: A controlled 10-year follow up study. Psychological Medicine. 2006;36:167–179. doi: 10.1017/S0033291705006410. [DOI] [PubMed] [Google Scholar]
- Biederman J, Petty CR, Evans M, Small J, Faraone SV. How persistent is ADHD? A controlled 10-year follow-up study of boys with ADHD. Psychiatry Research. 2010;177:299–304. doi: 10.1016/j.psychres.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, Wilens T, Mick B, Faraone S. VFindings from a four-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry. 36:21–29. doi: 10.1097/00004583-199701000-00013. [DOI] [PubMed] [Google Scholar]
- Brown SA, McGue M, Maggs J, Schulenberg J, Sher K, Winters KC, Lowman C. A developmental perspective on alcohol and youth ages 16 – 20. Pediatrics. 2008;121:S290–S310. doi: 10.1542/peds.2007-2243D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Larsson H. The effects of childhood ADHD symptoms on early-onset substance use: a Swedish twin study. Journal of Abnormal Child Psychology. 2012;40:425–435. doi: 10.1007/s10802-011-9575-6. [DOI] [PubMed] [Google Scholar]
- Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: Comparative meta-analyses. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(1):9–21. doi: 10.1016/j.jaac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- Chou C-P, Montgomery S, Pentz MA, Rohrbach LA, Johnson A, Flay BR, MacKinnon DP. Effects of a community-based prevention program on decreasing drug use in high-risk adolescents. American Journal of Public Health. 1998;88:944–948. doi: 10.2105/ajph.88.6.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis ML, Godley SH, Diamond G, Tims F, Babor T, Donaldson J, Funk R, et al. The Cannabis Youth Treatment (CYT) study: Main findings from two randomized trials. Journal of Substance Abuse Treatment. 2004;27:197–213. doi: 10.1016/j.jsat.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Sergeant J, Gillberg C, Biederman J. The worldwide prevalence of ADHD: Is it an American condition? World Psychiatry. 2003;2:104–113. [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM. Cannabis use and adult ADHD symptoms. Drug and Alcohol Dependence. 2008;95:90–96. doi: 10.1016/j.drugalcdep.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM. Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence: Results of a 25 year longitudinal study. Drug and Alcohol Dependence. 2007;885:S14–S26. doi: 10.1016/j.drugalcdep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Flory K, Milich R, Lynam DR, Leukefeld C, Clayton R. Relation between childhood disruptive behavior disorders and substance use and dependence symptoms in young adulthood: Individuals with symptoms of attention-deficit/hyperactivity disorder and conduct disorder are uniquely at risk. Psychology of Addictive Behaviors. 2003;17(2):151–158. doi: 10.1037/0893-164x.17.2.151. [DOI] [PubMed] [Google Scholar]
- Glass K, Flory K. Are symptoms of ADHD related to substance use among college students? Psychology of Addictive Behaviors. 2012;26:124–132. doi: 10.1037/a0024215. [DOI] [PubMed] [Google Scholar]
- Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners parent and teacher rating scales. Journal of Abnormal Child Psychology. 1978;6:221–236. doi: 10.1007/BF00919127. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Department of Sociology; 1975. [Google Scholar]
- Holmes J, Lawson D, Langley K, Fitzpatrick H, Trumper A, Pay H, Thapar A. The Child Attention-Deficit Hyperactivity Disorder Teacher Telephone Interview (CHATTI): Reliability and validity. British Journal of Psychiatry. 2004;184:74–78. doi: 10.1192/bjp.184.1.74. [DOI] [PubMed] [Google Scholar]
- Jackson KM, Schulenberg JE. Alcohol use during the transition from middle school to high school: National panel data on prevalence and moderators. Developmental Psychology, online first posting. 2013 doi: 10.1037/a0031843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman brief intelligence test manual. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O. The prevalence and correlates of adult ADHD in the United States: Results from the national comorbidity survey replication. American Journal of Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NM, Hartsough CS. Prospective study of tobacco smoking and substance dependencies among samples of ADHD and non-ADHD participants. Journal of Learning Disabilities. 1998;31(6):533–544. doi: 10.1177/002221949803100603. [DOI] [PubMed] [Google Scholar]
- Lee C-YS, Winters KC, Wall M. Trajectories of substance use disorders in youth: Identifying and predicting group memberships. Journal of Child & Adolescent Substance Abuse. 2010;19:135–157. doi: 10.1080/10678281003634975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Herzog TA, Meade CD, Webb MS, Brandon TH. The use of GEE for analyzing longitudinal binomial data: A primer using data from a tobacco intervention. Addictive Behaviors. 2007;32:187–193. doi: 10.1016/j.addbeh.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clinical Psychology Review. 2011;31:328–341. doi: 10.1016/j.cpr.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- MacKinnon DP, Lockwood CM. Advances in statistical methods for substance abuse prevention research. Prevention Science. 2003;4:155–171. doi: 10.1023/a:1024649822872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Moulton JI. Does stimulant treatment place children at risk for adult substance abuse?: A controlled, prospective follow-up study. Journal of Child and Adolescent Psychopharmacology. 2003;13:273–282. doi: 10.1089/104454603322572606. [DOI] [PubMed] [Google Scholar]
- McMahon RJ. Child and adolescent psychopathology as risk factors for subsequent tobacco use. Nicotine & Tobacco Research. 1999;1:S45–S50. doi: 10.1080/14622299050011801. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones K. ADHD is associated with early initiation of cigarette smoking children and adolescents. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Newcorn JH, Halperin JM. Fading memories: Retrospective recall inaccuracies in ADHD. Journal of Attention Disorders. 2010;14(1):7–14. doi: 10.1177/1087054709347189. [DOI] [PubMed] [Google Scholar]
- Molina BSG, et al. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, random assignment to childhood treatments, and subsequent medication. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:250–263. doi: 10.1016/j.jaac.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE. Childhood predictors of substance use in a longitudinal study of children with ADHD. Journal of Abnormal Psychology. 2003;112:497–507. doi: 10.1037/0021-843x.112.3.497. [DOI] [PubMed] [Google Scholar]
- Molina B, Pelham W, Roth J. Stimulant medication and substance use by adolescents with a childhood history of ADHD. Poster presented at the Biennial Meeting of the International Society for Research in Child and Adolescent Psychopathology; Barcelona, Spain. 1999. Jun, [Google Scholar]
- Ohlmeier MD, Peters K, Kordon A, Seifert A, Seifert J, Wildt B, Schneider U. Nicotine and alcohol dependence in patients with comorbid Attention-Deficit/Hyperactivity Disorder (ADHD) Alcohol and Alcoholism. 2007;42(6):539–543. doi: 10.1093/alcalc/agm069. [DOI] [PubMed] [Google Scholar]
- Ohlmeier MD, Peters K, te Wildt BT, Zelder M, Ziegenbein M, Wiese B. Comorbidity of alcohol and substance dependence with attention-deficit/hyperactivity disorder. Alcohol & Alcoholism. 2008;43(3):300–304. doi: 10.1093/alcalc/agn014. [DOI] [PubMed] [Google Scholar]
- Paternite CE, Loney J, Salisbury H, Whaley MA. Childhood inattention-overactivity, aggression, and stimulant medication history as predictors of young adult outcomes. Journal of Child and Adolescent Psychopharmacology. 1999;9:169–184. doi: 10.1089/cap.1999.9.169. [DOI] [PubMed] [Google Scholar]
- Patrick ME, O’Malley PM, Johnston LD, Terry-McElrath YM, Schulenberg JE. HIV/AIDS risk behaviors and substance use by young adults in the United States. Prevention Science. 2012;13:532–538. doi: 10.1007/s11121-012-0279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realmuto GM, Winters KC, August GJ, Lee S, Fahnhorst T, Botzet A. Drug use and psychosocial functioning of a community derived sample of adolescents with childhood ADHD. Journal of Child and Adolescent Substance Abuse. 2009;18(2):172–192. doi: 10.1080/10678280902724176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W, Shayla JJ, Taibelson C. The diagnostic interview for children and adolescents–revised (DICA-R) (structured psychiatric interview) St. Louis: Washington University; 1992. [Google Scholar]
- Reynolds C, Kamphaus RC. BASC: Behavioral assessment system for children and adolescents. Circle Pines, MN: American Guidance Services, Inc; 1992. [Google Scholar]
- Rodriguez D, Tercyak KP, Audrain-McGovern J. Effects of inattention and hyperactivity/impulsivity symptoms on development of nicotine dependence from mid adolescence to young adulthood. Journal of Pediatric Psychology. 2008;33(6):563–575. doi: 10.1093/jpepsy/jsm100. [DOI] [PubMed] [Google Scholar]
- Symmes A, Winters KC, Fahnhorst T, Botzet A, Lee S, August GJ, Realmuto G. Examining the association between attention-deficit hyperactivity disorder and nicotine use among adolescents and young adults. Journal of Child and Adolescent Substance Abuse. doi: 10.1080/1067828X.2012.756442. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szobot CM, Bukstein O. Attention deficit hyperactivity disorder and substance use disorders. Child and Adolescent Psychiatric Clinics of North America. 2008;17:309–323. doi: 10.1016/j.chc.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Todd RD, Joyner CA, Heath AC, Neuman RJ, Reich W. Reliability and stability of a semi-structured DSM-IV interview designed for family studies. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:1460–1468. doi: 10.1097/00004583-200312000-00013. [DOI] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services (USDHHS) Healthy people 2010: Understanding and improving health. 2nd ed. Washington, DC: U. S. Government Printing Office; 2000. [Google Scholar]
- Weiss G, Hechtman LT. Hyperactive children grown up. New York: Guilford; 1993. [Google Scholar]
- Whalen CK, Jamner LD, Henker B, Gehricke J, King PS. Is there a link between adolescent cigarette smoking and pharmacotherapy for ADHD? Psychology of Addictive Behaviors. 2003;17:332–335. doi: 10.1037/0893-164X.17.4.332. [DOI] [PubMed] [Google Scholar]
- Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: The nature of the relationship, subtypes at risk and treatment issues. Psychiatric Clinics of North America. 2004;27:283–301. doi: 10.1016/S0193-953X(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J. Alcohol, drugs, and attention-deficit/hyperactivity disorder: A model for the study of addictions in youth. Journal of Psychopharmacology. 2006;20(4):580–588. doi: 10.1177/0269881105058776. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Mick E. Does ADHD affect the course of substance abuse? Findings from a sample of adults with and without ADHD. The American Journal on Addiction. 1998;7(2):156–163. [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annual Revue of Medicine. 2002;53:113–131. doi: 10.1146/annurev.med.53.082901.103945. [DOI] [PubMed] [Google Scholar]
- Winters KC, Henly GA. Adolescent diagnostic interview (ADI) manual. Los Angeles, CA: Western Psychological Services; 1993. [Google Scholar]
- Winters KC, Lee S, Botzet AM, Fahnhorst T, Realmuto G, August GJ. A prospective examination of the association of stimulant medication history and drug use outcomes among community samples of ADHD youth. Journal of Child & Adolescent Substance Abuse. 2011;20:314–329. doi: 10.1080/1067828X.2011.598834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S, Gudjonsson GH. Growing out of ADHD: The relationship between functioning and symptoms. Journal of Attention Disorders. 2008;12(2):162–169. doi: 10.1177/1087054707299598. [DOI] [PubMed] [Google Scholar]