Abstract
BACKGROUND
Despite dramatic declines in children’s blood lead concentrations and a lowering of the Centers for Disease Control and Prevention’s level of concern to 10 µg per deciliter (0.483 µmol per liter), little is known about children’s neurobehavioral functioning at lead concentrations below this level.
METHODS
We measured blood lead concentrations in 172 children at 6, 12, 18, 24, 36, 48, and 60 months of age and administered the Stanford–Binet Intelligence Scale at the ages of 3 and 5 years. The relation between IQ and blood lead concentration was estimated with the use of linear and nonlinear mixed models, with adjustment for maternal IQ, quality of the home environment, and other potential confounders.
RESULTS
The blood lead concentration was inversely and significantly associated with IQ. In the linear model, each increase of 10 µg per deciliter in the lifetime average blood lead concentration was associated with a 4.6-point decrease in IQ (P=0.004), whereas for the subsample of 101 children whose maximal lead concentrations remained below 10 µg per deciliter, the change in IQ associated with a given change in lead concentration was greater. When estimated in a nonlinear model with the full sample, IQ declined by 7.4 points as lifetime average blood lead concentrations increased from 1 to 10 µg per deciliter.
CONCLUSIONS
Blood lead concentrations, even those below 10 µg per deciliter, are inversely associated with children’s IQ scores at three and five years of age, and associated declines in IQ are greater at these concentrations than at higher concentrations. These findings suggest that more U.S. children may be adversely affected by environmental lead than previously estimated.
Lead is neurotoxic, and young children are at particular risk for exposure.1 Numerous studies indicate that blood lead concentrations above 10 µg per deciliter (0.483 µmol per liter) are associated with adverse outcomes on measures of intellectual functioning and social–behavioral conduct.2–9 Such studies led to the identification of a blood lead concentration of 10 µg per deciliter or higher as a “level of concern” by the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).1,10
It remains unclear whether lead-associated cognitive deficits occur at concentrations below 10 µg per deciliter. The CDC and WHO recognized that no evidence of a threshold existed for lead-associated deficits but noted an absence of research on the possible effects of blood lead concentrations below 10 µg per deciliter. Although some studies in which the average blood lead concentration was below 10 µg per deciliter have reported associations between the blood lead concentration and cognitive deficits, the analyses did not focus specifically on children whose concentrations remained below 10 µg per deciliter throughout life.6,11 Other evidence suggesting lead-related deficits at concentrations below 10 µg per deciliter relied on linear extrapolation or on data unadjusted for important potential confounders such as maternal intelligence and the quality of caregiving.12–15 We examined associations between low-level exposure to lead and children’s performance on intelligence tests at the ages of three and five years in a population that included many children whose blood lead concentrations remained below 10 µg per deciliter.
METHODS
STUDY COHORT
Participants had been enrolled at five to seven months of age for a prior study of dust-control efficacy.16 The children had been born between July 1994 and January 1995. Families were invited to participate in the current study when the children were 24 to 30 months of age. Thirty-six of the 276 children in the original study were excluded from the current study because of premature birth (less than 37 weeks’ gestation), low birth weight (less than 2500 g), Down’s syndrome, speech and hearing abnormalities, or death or because their parents were short-term custodians or lacked English proficiency. Of the 240 eligible participants, 54 were not assessed at the age of three years and 65 were not assessed at the age of five years because they missed appointments, relocated, declined to participate, or died. Children were tested at three and five years of age. The institutional review board of the University of Rochester Medical Center (Rochester, N.Y.) approved the study protocol, and parents or guardians of all children provided written informed consent.
ANALYSIS AND QUALITY CONTROL OF BLOOD SAMPLES
Blood lead concentrations were determined by electrothermal atomic absorption spectrometry (Wadsworth Laboratories). Lead values were calculated as the means of six analyses of each sample (SD, 0.03 µg per deciliter [0.001 µmol per liter]). The results of repeated analyses, separated by five days, were highly consistent (SD, 0.40 µg per deciliter [0.019 µmol per liter]) for blood lead concentrations below 20 µg per deciliter (0.966 µmol per liter). The limit of detection was 1.0 µg per deciliter (0.048 µmol per liter), and values below this limit were set to 1.0 µg per deciliter.17
ASSESSMENT OF INTELLIGENCE
Children were assessed with the Stanford–Binet Intelligence Scale, fourth edition, which tests vocabulary, spatial pattern analysis, quantitative ability, and memory. We used the composite score (mean [±SD], 100±16) to represent IQ, because it is similar to the IQ score of other intelligence tests.18,19 A different examiner administered an abbreviated Stanford–Binet Scale at each age. Examiners were unaware of children’s lead status. Scores from the abbreviated batteries are highly correlated with the Stanford–Binet full composite score (0.94 at the age of three years and 0.99 at the age of five years).20 Because of the limited diagnostic value of Stanford– Binet subscales at these ages, the composite score was the dependent variable.19
LEAD EXPOSURE VARIABLES
Venous blood samples were obtained at 6, 12, 18, 24, 36, 48, and 60 months of age. Four exposure indexes were analyzed: lifetime average, peak, concurrent, and average blood lead concentration in infancy. The lifetime average blood lead concentration was estimated at 3 and 5 years of age by computing the area under the blood lead curve (AUC) from 6 through 36 months of age and from 6 through 60 months of age, respectively. Dividing the AUC by the corresponding age span yields an average concentration expressed in micrograms per deciliter. The peak blood lead concentration is the child’s highest measured lead concentration through the age of three or five years. The concurrent blood lead concentration is that measured on the day of cognitive testing. The average blood lead concentration in infancy is the AUC for values measured between 6 and 24 months of age.
The lifetime average blood lead concentration best reflects chronic exposure and was used as the primary exposure variable. The blood lead concentration was specified as an untransformed continuous variable. To compute the AUC, conditional means regression21 was used to impute values for 72 of the 1168 age-specific lead values (6.2 percent).
COVARIATES
All analyses used the same set of prespecified covariates, which were based on established predictors of children’s intellectual outcomes and those widely used in studies of pediatric lead exposure.2–4,8,22,23 The following variables were used: the child’s sex, birth weight, and iron status (defined by the serum transferrin saturation at three and five years of age) and the mother’s IQ (determined with use of the abbreviated Stanford–Binet Intelligence Scale), years of education, race (self-assigned as white or nonwhite), tobacco use during pregnancy (user or nonuser), yearly household income, and the total score for the Home Observation for Measurement of the Environment Inventory.24
STATISTICAL ANALYSIS
Mixed-model methods25,26 were used to estimate and test parameters in linear, polynomial, and semi-parametric models that always included the child’s sex and the mother’s race and prenatal smoking status as fixed classification effects, and a lead measure, the child’s iron status, and the mother’s income, level of education, IQ, and Home Observation for Measurement of the Environment score as covariates. The child’s IQ (the composite score on the Stanford–Binet Intelligence Scale) was the dependent variable. The longitudinal study design provides repeated measures of the IQ variable at the ages of three and five years, and the models also include a fixed classification factor for age and a random factor for individual children. The mother’s income and level of education, the child’s iron status, and all lead measures (except the infancy average) were measured at both time points and are time-varying covariates. The error structure for each child assumes different variances at each age and a covariance between ages; these were assumed to be the same for all children, and covariances between children were assumed to be negligible. All significance tests were two-tailed.
For a given lead variable, regressions were specified separately according to age, and the homogeneity of these estimates was tested (i.e., the interaction of age with lead concentration). In the absence of a difference between the age-specific estimates, their unweighted average (based on all available data) is the best estimate of the association between the blood lead concentration and IQ and is referred to as the overall estimate.
Regression diagnostics were carried out for the mixed models.27 Only one value had a standardized residual of more than 3.0 (a child who had a low IQ and a low lead concentration). It did not pass a discordancy test27 and was retained in all analyses.
The linear relations of IQ scores to lifetime average, concurrent, peak, and infancy average blood lead concentrations were estimated in the full sample. A second, parallel set of analyses estimated the relation between IQ and the lead concentration for children whose peak lead concentration was below 10 µg per deciliter. Observations for children who were three years of age were included in these calculations only when their maximal blood lead concentration through that age was below 10 µg per deciliter and were included at the age of five years only when their maximal concentration was below 10 µg per deciliter during the entire five-year span.
Nonlinearity in the relation between IQ and the blood lead concentration across the full range of lead values was examined with the use of the mixed models described above in two types of analyses: quadratic, cubic, and higher-degree polynomials were estimated for each lead variable; and semiparametric models were estimated with the use of parametric adjustment for covariates and penalized spline smoothing for the nonparametric relation between IQ and the blood concentration.28 The semiparametric models estimate the regression locally and, unlike the polynomial models, do not require the restrictive assumption that the true relation between IQ and the blood lead concentration conforms to a particular parametric function. Inference is less well developed in the mixed semiparametric model, and confidence intervals are not reported.
RESULTS
A total of 198 children completed at least one assessment. Of these, 172 (86.9 percent) had complete data for all variables included in the model (305 observations; 151 at the age of three years and 154 at the age of five years). There were no significant differences in the background characteristics among children with complete data, those with incomplete data, and those who did not participate (Table 1).
Table 1.
Characteristics of the Children at the Age of Five Years and of Their Mothers.*
| Characteristic | Children with Complete Data (N=154) |
Children with Incomplete Data (N=21) |
Children Who Did Not Participate (N=65) |
|---|---|---|---|
| Children | |||
| Age at testing (mo) | 60.6±1.0 | 60.6±0.9 | — |
| Female sex (%) | 52.6 | 45.5 | 53.9 |
| Weeks of gestation | 39.5±1.2 | 39.8±1.0 | 39.4±1.2 |
| Birth weight (g) | 3295±405 | 3400±496 | 3304±473 |
| Transferrin saturation (%) | 22.5±9.4 | 23.5±6.6 | — |
| Blood lead concentration (µg/dl)† |
|||
| Lifetime average | 7.4±4.3 | 7.3±3.6 | — |
| Peak | 11.1±7.1 | 12.6±8.2 | — |
| Concurrent | 5.8±4.1 | 6.4±7.5 | — |
| Average in infancy | 7.0±3.8 | 7.4±3.4 | 7.2±4.1 |
| IQ‡ | 89.8±11.4 | 85.6±12.2 | — |
| Mothers | |||
| No. of prenatal visits | 11.1±4.1 | 10.2±5.0 | 10.4±3.7 |
| HOME total score§ | 27.3±7.1 | 28.7±6.1 | 27.8±6.2 |
| Yearly income >$15,000 (%) | 35.7 | 45.5 | — |
| Smoked during pregnancy (% | )20.1 | 38.1 | 27.7 |
| Age at delivery (yr) | 25.0±6.7 | 25.8±4.6 | 23.8±5.6 |
| Parity | 1.4±1.4 | 1.6±1.3 | 1.3±1.4 |
| Nonwhite race (%) | 73.4 | 68.2 | 66.2 |
| Education >12 yr (%) | 31.2 | 22.7 | — |
| IQ‡ | 81.9±12.7 | 80.5±13.6 | 83.8±10.2 |
Data obtained at the age of three years were similar to the data obtained at five years of age and are not shown. Differences among the groups were not significant (P<0.05) for any variable at the age of either three or five years. Plus– minus values are means ±SD. To convert values for lead to micromoles per liter, multiply by 0.0483.
The lifetime average blood lead concentration was estimated at the ages of 3 and 5 years by computing the area under the blood lead curve (AUC) from 6 through 36 months and from 6 through 60 months, respectively, and then dividing the AUC by its corresponding age span to yield an average on the mi-crogram-per-deciliter scale. The peak blood lead concentration was the child’s highest measured blood lead concentration through the age of three or five years. The concurrent blood lead concentration was the concentration measured on the day of cognitive testing, and the average blood lead concentration in infancy was the AUC from 6 through 24 months.
The Stanford–Binet Intelligence Scale, fourth edition (abbreviated), was used to assess IQ.
The Home Observation for Measurement of Environment Inventory (HOME) is an index that reflects the quality and quantity of emotional and cognitive stimulation in the home environment. The total score is the sum of 39 items, each scored as present (1) or absent (0), in six categories (maternal responsivi-ty, acceptance of child, organization of the home environment, provision of play materials, maternal involvement with the child, and the variety of stimulation).
BLOOD LEAD CONCENTRATIONS
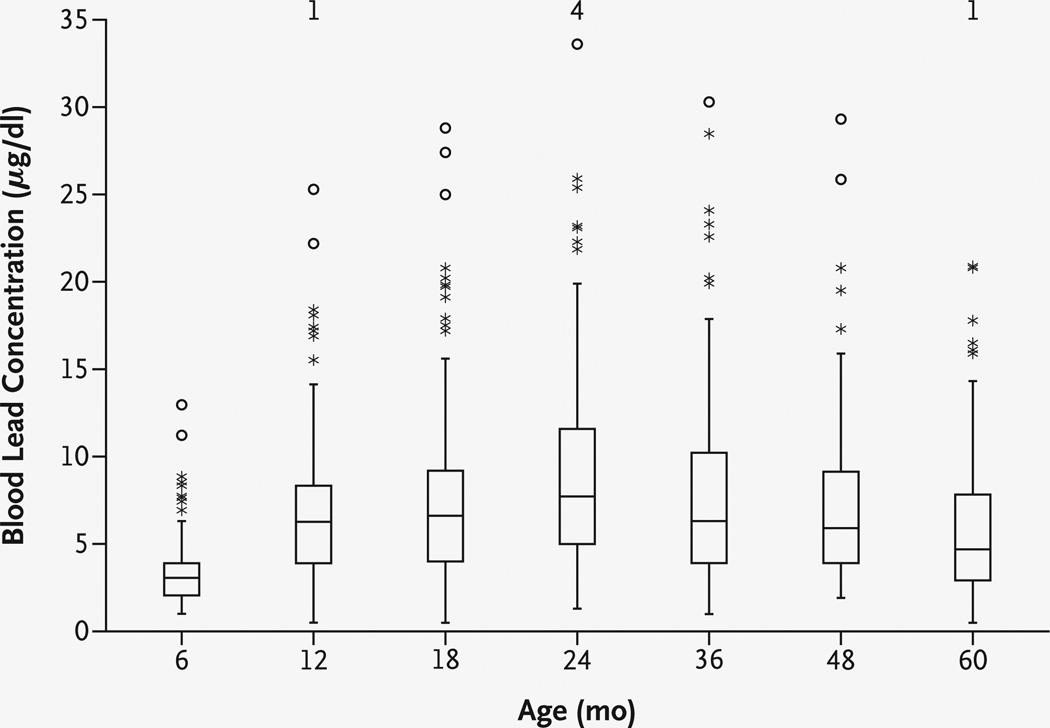
The mean blood lead concentration was lowest at the age of six months (3.4 µg per deciliter [0.164 µmol per liter]), was maximal at two years (9.7 µg per deciliter [0.483 µmol per liter]), and then decreased to 6.0 µg per deciliter (0.290 µmol per liter) at five years (Fig. 1). The lifetime average blood lead concentration was 7.7 µg per deciliter (0.372 µmol per liter) at the age of three years and 7.4 µg per deciliter (0.368 µmol per liter) at the age of five years. At three years of age, 86 children (57.0 percent) had a peak blood lead concentration below 10 µg per deciliter, as did 86 (55.8 percent) at the age of five years (71 of these children had such a concentration at both ages, and the remaining 30 had data at either three or five years).
Figure 1. Distributions of Blood Lead Concentrations at Each Assessment.
In each box plot, the median value is indicated by the center horizontal line and the 25th and 75th percentiles are indicated by the lower and upper horizontal lines, respectively. The vertical lines represent 1.5 times the interquartile range, the asterisks represent values that are between 1.5 and 3 times the interquartile range, and circles represent values that are more than 3 times the interquartile range. The numbers at the top of the graph are the numbers of children with concurrent blood lead concentrations of more than 35 µg per deciliter. To convert values for lead to micromoles per liter, multiply by 0.0483.
INTELLIGENCE TEST RESULTS
The mean IQ was approximately 90 at both three and five years of age (Table 1), a value consistent with the sample demographics.20,29 Children’s IQ scores at three and five years of age were strongly correlated (r=0.67, P<0.001), and these scores were correlated with maternal IQ (r=0.43, P<0.001, and r=0.52, P<0.001, respectively), consistent with prior reports.22,30 In other bivariate analyses, the associations among the children’s IQ, the children’s blood lead concentrations, and the other covariates were in the expected direction (Table 2).
Table 2.
Relation of Covariates to Lifetime Average Blood Lead Concentration and Mean IQ Score at Five Years of Age.*
| Covariate† | No. of Children | Lifetime Average Blood Lead |
IQ |
|---|---|---|---|
| µg/dl | |||
| Mothers | |||
| Education level | |||
| <12 yr | 56 | 8.9±4.6 | 85.4±9.4 |
| 12 yr | 50 | 6.4±3.5 | 91.2±12.4 |
| >12 yr | 48 | 6.6±4.1 | 93.4±10.8 |
| Race‡ | |||
| Nonwhite | 113 | 8.2±4.4 | 87.5±9.5 |
| White | 41 | 4.9±2.6 | 96.1±13.6 |
| Income level | |||
| $6,000 | 37 | 8.8±3.8 | 83.8±9.3 |
| $6,001-$20,000 | 80 | 7.4±4.2 | 89.2±9.8 |
| >$20,000 | 37 | 5.8±4.4 | 97.0±12.7 |
| HOME total score§ | |||
| Low (<20) | 24 | 10.1±3.2 | 85.8±8.1 |
| Middle (20–30) | 76 | 7.6±4.8 | 87.9±9.7 |
| High (>30) | 54 | 5.8±2.9 | 94.2±13.3 |
| Prenatal smoking | |||
| No | 122 | 7.3±4.4 | 90.2±12.0 |
| Yes | 32 | 7.6±3.9 | 88.3±8.5 |
| IQ¶ | |||
| Low (<75) | 52 | 8.6±4.1 | 85.7±8.8 |
| Middle (75–85) | 45 | 7.7±5.0 | 86.9±8.5 |
| High (>85) | 57 | 5.9±3.3 | 95.9±12.8 |
| Children | |||
| Birth weight | |||
| <3500 g | 106 | 7.6±4.3 | 88.9±10.8 |
| ≥3500 g | 48 | 6.9±4.1 | 91.8±12.3 |
| Sex | |||
| Male | 73 | 7.6±3.9 | 88.3±12.5 |
| Female | 81 | 7.2±4.5 | 91.2±10.1 |
| Transferrin saturation | |||
| <20% | 60 | 7.0±4.2 | 89.5±8.5 |
| ≥20% | 94 | 7.6±4.3 | 90.0±12.9 |
The lifetime average blood lead concentration was estimated at the ages of 3 and 5 years by computing the area under the blood lead curve (AUC) from 6 through 36 months and from 6 through 60 months, respectively, and then dividing the AUC by its corresponding age span to yield an average on the micro-gram-per-deciliter scale. Data obtained at the age of three years were similar to the data obtained at five years of age and are not shown. Plus–minus values are means ±SD. To convert values for lead to micromoles per liter, multiply by 0.0483.
Some continuous variables were categorized for this analysis.
Race was self-assigned as white or nonwhite.
The Home Observation for Measurement of Environment Inventory (HOME) is an index that reflects the quality and quantity of emotional and cognitive stimulation in the home environment. The total score is the sum of 39 items, each scored as present (1) or absent (0), in six categories (maternal responsivi-ty, acceptance of child, organization of the home environment, provision of play materials, maternal involvement with the child, and the variety of stimulation).
The Stanford–Binet Intelligence Scale, fourth edition (abbreviated), was used to assess IQ.
BLOOD LEAD CONCENTRATIONS AND IQ
Before adjustment for covariates, all four lead measures were inversely and significantly associated with IQ at three and five years of age (Table 3). The associations did not differ significantly according to age. From the overall estimate, an increase in the lifetime average blood lead concentration of 1 µg per deciliter was associated with a decrease of 0.87 IQ point; estimates for concurrent blood lead concentrations and average concentrations in infancy were similar, whereas that for the peak lead concentration was somewhat smaller.
Table 3.
Unadjusted and Adjusted Changes in IQ for Each Increase in the Blood Lead Concentration of 1 µg per Deciliter for All Children in the Study.*
| Type of Blood Lead Measurement |
No. of Children |
At 3 Years of Age | At 5 Years of Age | Overall | |||
|---|---|---|---|---|---|---|---|
| β±SE (95% CI) | P Value | β±SE (95% CI) | P Value | β±SE (95% CI) | P Value | ||
| Unadjusted estimate† | |||||||
| Lifetime average | 172 | −0.74±0.18 (−1.09 to −0.39) |
<0.001 | −1.00±0.19 (−1.38 to −0.63) |
<0.001 | −0.87±0.16 (−1.19 to −0.55) |
<0.001 |
| Peak | 172 | −0.40±0.11 (−0.62 to −0.18) |
<0.001 | −0.47±0.11 (−0.70 to −0.25) |
<0.001 | −0.44±0.10 (−0.63 to −0.24) |
<0.001 |
| Concurrent‡ | 171 | −0.60±0.15 (−0.89 to −0.31) |
<0.001 | −1.02±0.19 (−1.38 to −0.65) |
<0.001 | −0.81±0.14 (−1.09 to −0.53) |
<0.001 |
| Average in infancy (6–24 mo) |
172 | −0.73±0.21 (−1.15 to −0.31) |
<0.001 | −0.97±0.22 (−1.40 to −0.54) |
<0.001 | −0.85±0.19 (−1.23 to −0.47) |
<0.001 |
| Adjusted estimate§ | |||||||
| Lifetime average | 172 | −0.35±0.17 (−0.69 to 0.00) |
0.05 | −0.57±0.18 (−0.93 to −0.20) |
0.003 | −0.46±0.15 (−0.76 to −0.15) |
0.004 |
| Peak | 172 | −0.19±0.10 (−0.39 to 0.01) |
0.06 | −0.26±0.11 (−0.47 to −0.05) |
0.02 | −0.23±0.09 (−0.40 to −0.05) |
0.01 |
| Concurrent‡ | 171 | −0.31±0.15 (−0.60 to −0.01) |
0.04 | −0.61±0.19 (−0.99 to −0.24) |
<0.001 | −0.46±0.14 (−0.74 to −0.18) |
0.002 |
| Average in infancy (6–24 mo) |
172 | −0.32±0.20 (−0.71 to 0.07) |
0.10 | −0.53±0.20 (−0.93 to −0.13) |
0.01 | −0.43±0.17 (−0.77 to −0.09) |
0.02 |
The lifetime average blood lead concentration was estimated at the ages of 3 and 5 years by computing the area under the blood lead curve (AUC) from 6 through 36 months and from 6 through 60 months, respectively, and then dividing the AUC by its corresponding age span to yield an average on the microgram-per-deciliter scale. The peak blood lead concentration was the child’s highest measured blood lead concentration through the age of three or five years. The concurrent blood lead concentration was the concentration measured on the day of cognitive testing, and the average blood lead concentration in infancy was the AUC from 6 through 24 months. CI denotes confidence interval. /3 values are the estimated unstandardized regression coefficients.
The unadjusted model includes only classification factors for age and for individual children.
One child was lacking a concurrent blood lead measurement at the age of three years.
Estimates were adjusted for maternal IQ, race, level of education, use of tobacco during pregnancy, household income, and Home Observation for Measurement of Environment Inventory score, and the child’s sex, birth weight, and iron status.
After adjustment for the nine additional covariates, there were significant inverse associations with IQ for all blood lead variables, with no significant differences according to age (Table 3). The overall estimate indicated that an increase in the lifetime average blood lead concentration of 1 µg per deciliter was associated with a change of –0.46 IQ point (95 percent confidence interval, –0.76 to −0.15). Estimated effects were similar for the concurrent blood lead concentration and the average blood lead concentration in infancy and smaller, but still significant, for peak lead concentrations (Table 3). Other significant predictors of the child’s IQ were the same in all models: maternal IQ and income and the child’s birth weight.
IQ AT BLOOD LEAD CONCENTRATIONS BELOW 10 µG PER DECILITER
To examine the relation between IQ and blood lead concentrations consistently below 10 µg per deciliter, linear models for each lead variable were estimated for the subgroup of children whose peak lead concentration was below 10 µg per deciliter. Without exception, the estimates were larger in this subgroup. Lifetime average, peak, and concurrent blood lead concentrations, but not the average in infancy, were inversely and significantly associated with IQ, both before and after adjustment for covariates (Table 4) and at both three and five years of age. The estimated overall difference in IQ for each increase in the lifetime average lead concentration of 1 µg per deciliter was –1.37 points (95 percent confidence interval, –2.56 to −0.17).
Table 4.
Unadjusted and Adjusted Changes in IQ for Each Increase in the Blood Lead Concentration of 1 µg per Deciliter for Children with Peak Blood Lead Concentrations below 10 µg per Deciliter.*
| Type of Blood Lead Measurement |
No. of Children |
At 3 Years of Age | At 5 Years of Age | Overall | |||
|---|---|---|---|---|---|---|---|
| β±SE (95% CI) | P Value | β±SE (95% CI) | P Value | β±SE (95% CI) | P Value | ||
| Unadjusted estimate† | |||||||
| Lifetime average | 101 | −2.30±0.67 (−3.64 to −0.96) |
<0.001 | −2.54±0.74 (−4.01 to −1.07) |
<0.001 | −2.42±0.63 (−3.67 to −1.17) |
<0.001 |
| Peak | 101 | −2.09±0.58 (−3.25 to −0.93) |
<0.001 | −2.12±0.60 (−3.32 to −0.91) |
<0.001 | −2.10±0.53 (−3.16 to −1.04) |
<0.001 |
| Concurrent | 101 | −2.19±0.49 (−3.18 to −1.21) |
<0.001 | −2.56±0.58 (−3.71 to −1.40) |
<0.001 | −2.38±0.45 (−3.26 to 1.49) |
<0.001 |
| Average in infancy (6–24 mo) |
105 | −1.29±0.67 (−2.61 to 0.04) |
0.06 | −1.58±0.67 (−2.92 to −0.24) |
0.02 | −1.43±0.61 (−2.65 to −0.21) |
0.02 |
| Adjusted estimate‡ | |||||||
| Lifetime average | 101 | −1.22±0.66 (−2.53 to 0.09) |
0.07 | −1.52±0.71 (−2.94 to −0.09) |
0.04 | −1.37±0.60 (−2.56 to −0.17) |
0.03 |
| Peak | 101 | −1.36±0.55 (−2.46 to −0.27) |
0.02 | −1.44±0.56 (−2.55 to −0.33) |
0.01 | −1.40±0.48 (−2.37 to −0.44) |
0.005 |
| Concurrent | 101 | −1.36±0.51 (−2.37 to −0.35) |
0.009 | −1.79±0.60 (−3.00 to −0.60) |
0.004 | −1.58±0.46 (−2.50 to −0.65) |
0.001 |
| Average in infancy (6–24 mo) |
105 | −0.58±0.58 (−1.75 to 0.59) |
0.32 | −0.92±0.59 (−2.09 to 0.25) |
0.12 | −0.75±0.51 (−1.78 to 0.28) |
0.15 |
The lifetime average blood lead concentration was estimated at the ages of 3 and 5 years by computing the area under the blood lead curve (AUC) from 6 through 36 months and from 6 through 60 months, respectively, and then dividing the AUC by its corresponding age span to yield an average on the microgram-per-deciliter scale. The peak blood lead concentration was the child’s highest measured blood lead concentration through the age of three or five years. The concurrent blood lead concentration was the concentration measured on the day of cognitive testing, and the average blood lead concentration in infancy was the AUC from 6 through 24 months. A total of 71 children were found to have a peak blood lead concentration below 10 µg per deciliter at both ages; an additional 15 children had a peak concentration below 10 µg per deciliter at three years of age but at five years of age had a higher concentration or were not tested, and another 15 children had a peak concentration below 10 µg per deciliter at five years but were not tested at three years. The total number of children in the analysis of the average concentration in infancy is 105 because in 4 children the peak blood lead concentration occurred after the age of 24 months. CI denotes confidence interval. b values are the estimated unstand-ardized regression coefficients.
The unadjusted model includes only classification factors for age and for individual children.
Estimates were adjusted for maternal IQ, race, level of education, use of tobacco during pregnancy, household income, and Home Observation for Measurement of Environment Inventory score, and the child’s sex, birth weight, and iron status.
NONLINEAR ANALYSES
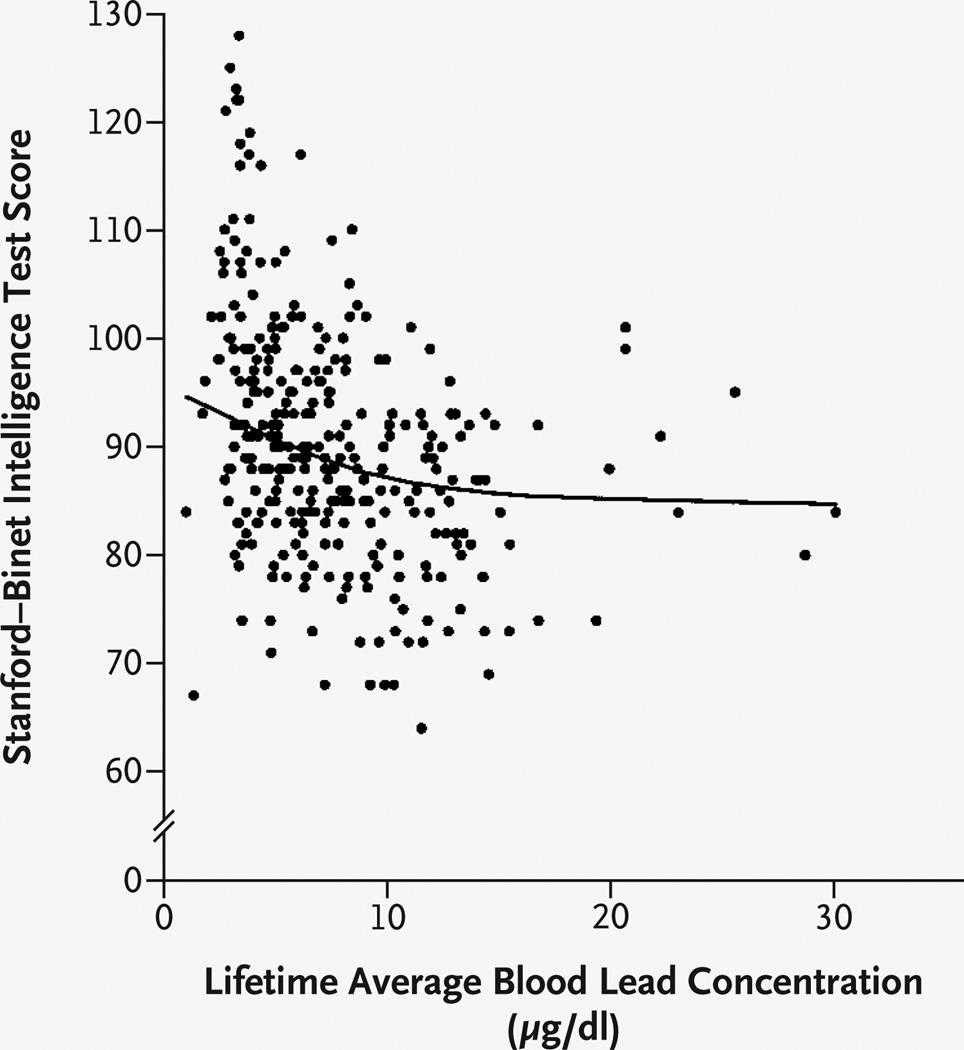
Nonlinear mixed models were analyzed with the use of the full range of blood lead values. Semiparametric analysis indicated a decline in IQ of 7.4 points for a lifetime average blood lead concentration of up to 10 µg per deciliter (Fig. 2). For lifetime average blood lead concentrations ranging from more than 10 µg per deciliter to 30 µg per deciliter, a more gradual decrease in IQ was estimated (approximately 2.5 points). An analysis using polynomial models confirmed this departure from linearity. The quadratic term was significant in the model for lifetime average blood lead concentration (P=0.05), and as the blood lead concentration increased from 1 to 10 µg per deciliter, the total change in IQ was −8.0 points (95 percent confidence interval, −12.9 to −3.2). Significant nonlin-earity was also found for the relations between IQ and the peak lead concentration (P=0.003 for the quadratic term) and between IQ and the concurrent lead concentration (P=0.007 for the cubic term). The spline estimates for these variables had shapes similar to that for the lifetime average. The same covariates that were significant in the linear models were also significant in the nonlinear models.
Figure 2. IQ as a Function of Lifetime Average Blood Lead Concentration.
IQ was assessed with use of the Stanford–Binet Intelligence Scale, fourth edition. The line represents the relation between IQ and lifetime average blood lead concentration estimated by the covariate-adjusted penalized-spline mixed model. Individual points are the unadjusted lifetime average blood lead and IQ values. To convert values for lead to micromoles per liter, multiply by 0.0483.
DISCUSSION
Two findings from this investigation raise questions about the consequences of blood lead concentrations commonly found among U.S. children today. Of primary importance is that children’s intellectual functioning at three and five years of age is inversely associated with blood lead concentrations, even when their peak concentrations remain below the CDC and WHO level of concern.1,10 This finding was consistent for lifetime average, concurrent, and peak lead concentrations and in adjusted as well as unadjusted models. In the linear model involving the full range of lead values in this sample, the estimated IQ loss was 4.6 points for each increase in the blood lead concentration of 10 µg per deciliter, a result consistent with prior research in other cohorts.2,11,31 In contrast, for children whose lead concentrations remained below 10 µg per deciliter, the estimated loss in IQ was considerably greater.
The second, related finding is that the relation between children’s IQ score and their blood lead concentration is nonlinear. The best estimate, from the semiparametric analysis, indicates a loss of 7.4 IQ points for a lifetime average blood lead concentration of up to 10 µg per deciliter. These findings suggest that the total lead-related impairment in this cohort is due largely to the initial IQ loss at blood lead concentrations of 10 µg per deciliter or less and that the linear model for children with peak concentrations of less than 10 µg per deciliter overestimates the lead-associated impairment.
Previous research is consistent with the interpretation that the effects of lead on IQ are proportionally greater at lower lead concentrations. A cross-sectional study of children with lead concentrations ranging from 3 to 34 µg per deciliter (0.145 to 1.643 µmol per liter) suggested a larger decrement in scores on ability tests over the range of 5 to 10 µg per deciliter (0.242 to 0.483 µmol per liter) than over the range from more than 10 through 20 µg per deciliter.6 A second cross-sectional study that used data from the third National Health and Nutrition Examination Survey indicated greater possible effects on reading and math scores among children with blood lead concentrations below 5 µg per deciliter than among those with higher concentrations.12 In addition, a prospective study32 suggested that the effects of prenatal exposure to lead were proportionally greater at lower levels of exposure, and a meta-analysis33 reported that studies in which average blood lead concentrations were below 15 µg per deciliter (0.725 µmol per liter) had larger slope estimates than studies in which concentrations were higher. However, we have documented this finding in children whose blood lead concentrations remained below 10 µg per deciliter, using a prospective design and adjusting for maternal intelligence and the quality of the home environment. Moreover, our findings were similar when the children were tested at three years and at five years of age.
Our results are also consistent with findings from meta-analyses that an increase in the blood lead concentration from 10 to 30 µg per deciliter is associated with a decline in IQ of 2 to 6 points.7,33,34 Although the estimation was less precise for lead concentrations above 10 µg per deciliter in our study, the curve estimated by the semiparametric analysis suggests a loss of 2.5 IQ points as blood lead concentrations increase from more than 10 through 30 µg per deciliter. The estimates from meta-analy-ses reflect primarily findings from studies involving a low proportion of children with lead concentrations of 0 to 10 µg per deciliter. Our findings suggest that when linear estimation from such samples is extrapolated to lower blood lead concentrations, the results do not accurately reflect the greater magnitude of the lead-associated impairment at these lower concentrations.
The larger associations with IQ at lower lead concentrations may appear counterintuitive. Although we did not explore possible biologic mechanisms that could explain this finding, there is evidence that high concentrations of heavy metals may enhance cellular defense mechanisms and thereby lessen the rate at which additional damage occurs.35
As with any observational study, it is not possible to draw causal inferences from these findings. Instead, the plausibility of a causal interpretation must be judged by the consistency of findings from numerous epidemiologic studies and the relevant experimental studies in animals.7,36,37 An inevitable limitation of the observational design is that it is not possible to control for all potentially confounding variables. However, the available evidence suggests that, in this area of research, a relatively small number of variables (e.g., the Home Observation for Measurement of the Environment score, socioeconomic status, and maternal IQ) are the primary confounders and that including other variables does not appreciably change the estimated effect of lead.11,38 For example, Tong and Lu compared the results of two empirical model-selection procedures using the Port Pirie cohort study.38 One procedure resulted in a model with 4 covariates, and the other in a model with 14. The estimated effect of lead on IQ was nearly identical in the two models and was consistent with the linear estimates we report.
Our findings (both linear and nonlinear) for the four lead-exposure variables suggest a high degree of consistency for lifetime average, concurrent, and peak exposure. In their pattern of association with children’s IQ scores, concurrent blood lead concentration was nearly identical to the lifetime average and the peak exposure. By contrast, the average blood lead concentration in infancy was less predictive of IQ, particularly for children whose lead concentrations remained below 10 µg per deciliter. We note, however, that these variables are by definition highly intercorrelated, and our results for them are not fully independent.
The results of any individual study depend, of course, on the study population. Our study group included a cluster of children with high IQ scores and low lead concentrations, but these subjects were not unduly influential in the statistical models. Regardless, our findings should be replicated in other cohorts and with the use of other cognitive assessments.
The definition of an elevated blood lead concentration has been incrementally but consistently lowered over the past two decades. Our findings suggest that children with blood lead concentrations below 10 µg per deciliter merit more intensive investigation. These and other data suggest that there may be no threshold for the adverse consequences of lead exposure6,7,33 and that lead-associated impairments may be both persistent and irreversible.39–42 Furthermore, although typically investigated because of its neurotoxic properties, an elevated lead concentration is also a risk factor for other public health problems, including delinquency, cardiovascular disease, renal disease, and dental caries.43–47
Our findings suggest that considerably more U.S. children are adversely affected by environmental exposure to lead than previously estimated. Because there is no effective treatment for children with moderately elevated blood lead concentrations,40 the collective evidence argues for a shift toward primary prevention of lead exposure in contrast to the current, almost exclusive emphasis on the treatment of children with elevated blood lead concentrations.48–50
Acknowledgments
Supported by a grant (R01 ES08388) from the National Institute of Environmental Health Sciences (NIEHS) and in part by grants from the NIEHS Environmental Health Sciences Center at the University of Rochester (ES01247), the Cornell University Bronfenbren-ner Life Course Institute in the College of Human Ecology, the Strong Memorial Hospital Children’s Research Center, and a joint research and extension program funded by the Cornell University Agricultural Experiment Station and Cornell Cooperative Extension.
Footnotes
Editor’s note: Dr. Lanphear has served as an expert witness for the State of Rhode Island and the City of Milwaukee in lead-related cases, for which Children’s Hospital (Cincinnati) is compensated.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the Department of Agriculture.
Presented in part at the Pediatric Academic Societies Annual Meeting, Baltimore, April 28–May 1, 2001; at the 109th Convention of the American Psychological Association, San Francisco, August 24–28, 2001; and at the 21st Annual Meeting of the Behavioral Toxicology Society, Research Triangle Park, N.C., April 20–22, 2002.
We are indebted to Elliott G. Smith for assisting with the management and analysis of portions of these data and for valuable feedback on previous versions of the manuscript and to Keith Alexander, Kristine DiBitetto, and Karen Knauf for data collection and cohort management.
References
- 1.Preventing lead poisoning in young children: a statement by the Centers for Disease Control. Centers for Disease Control; Atlanta: 1991. Oct, [Google Scholar]
- 2.Baghurst PA, McMichael AJ, Wigg NR, et al. Environmental exposure to lead and children’s intelligence at the age of seven years: the Port Pirie Cohort Study. N Engl J Med. 1992;327:1279–1284. doi: 10.1056/NEJM199210293271805. [DOI] [PubMed] [Google Scholar]
- 3.Bellinger D, Sloman J, Leviton A, Rabinowitz M, Needleman HL, Waternaux C. Low-level lead exposure and children’s cognitive function in the preschool years. Pediatrics. 1991;87:219–227. [Erratum, Pediatrics 1994;93:A28.] [PubMed] [Google Scholar]
- 4.Bellinger D, Dietrich KN. Low-level lead exposure and cognitive function in children. Pediatr Ann. 1994;23:600–605. doi: 10.3928/0090-4481-19941101-08. [DOI] [PubMed] [Google Scholar]
- 5.Dietrich KN, Berger OG, Succop PA, Hammond PB, Bornschein RL. The developmental consequences of low to moderate prenatal and postnatal lead exposure: intellectual attainment in the Cincinnati Lead Study Cohort following school entry. Neu-rotoxicol Teratol. 1993;15:37–44. doi: 10.1016/0892-0362(93)90043-n. [DOI] [PubMed] [Google Scholar]
- 6.Fulton M, Raab G, Thomson G, Laxen D, Hunter R, Hepburn W. Influence of blood lead on the ability and attainment of children in Edinburgh. Lancet. 1987;1:1221–1226. doi: 10.1016/s0140-6736(87)92683-3. [DOI] [PubMed] [Google Scholar]
- 7.Pocock SJ, Smith M, Baghurst P. Environmental lead and children’s intelligence: a systematic review of the epidemiological evidence. BMJ. 1994;309:1189–1197. doi: 10.1136/bmj.309.6963.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wasserman GA, Liu X, Lolacono NJ, et al. Lead exposure and intelligence in 7-year-old children: the Yugoslavia Prospective Study. Environ Health Perspect. 1997;105:956–962. doi: 10.1289/ehp.97105956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wasserman GA, Staghezza-Jaramillo B, Shrout P, Popovac D, Graziano J. The effect of lead exposure on behavior problems in preschool children. Am J Public Health. 1998;88:481–486. doi: 10.2105/ajph.88.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Programme on Chemical Safety. Environmental health criteria 165. World Health Organization; Geneva: 1995. Inorganic lead. [Google Scholar]
- 11.Bellinger DC, Stiles KM, Needleman HL. Low-level lead exposure, intelligence and academic achievement: a long-term follow-up study. Pediatrics. 1992;90:855–861. [PubMed] [Google Scholar]
- 12.Lanphear BP, Dietrich K, Auinger P, Cox C. Cognitive deficits associated with blood lead concentrations <10 microg/dl in US children and adolescents. Public Health Rep. 2000;115:521–529. doi: 10.1093/phr/115.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz J. Beyond LOEL’s, p values, and vote counting: methods for looking at the shapes and strengths of associations. Neu-rotoxicity. 1993;14:237–246. [PubMed] [Google Scholar]
- 14.Winneke G, Altmann L, Krämer U, et al. Neurobehavioral and neurophysiologi-cal observations in six year old children with low lead levels in East and West Germany. Neurotoxicity. 1994;15:705–713. [PubMed] [Google Scholar]
- 15.Stone BM, Reynolds CR. Can the National Health and Nutrition Examination Survey III (NHANES III) data help resolve the controversy over low blood lead levels and neuropsychological development in children? Arch Clin Neuropsychol. 2003;613:1–26. doi: 10.1016/s0887-6177(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 16.Lanphear BP, Howard C, Eberly S, et al. Primary prevention of childhood lead exposure: a randomized trial of dust control. Pediatrics. 1999;103:772–777. doi: 10.1542/peds.103.4.772. [DOI] [PubMed] [Google Scholar]
- 17.Lanphear BP, Weitzman M, Winter NL, et al. Lead-contaminated house dust and urban children’s blood lead levels. Am J Public Health. 1996;86:1416–1421. doi: 10.2105/ajph.86.10.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCrowell KL, Nagle RJ. Comparability of the WPPSI-R and the S-B:IV among preschool children. J Psychoedu Assess. 1994;12:126–134. [Google Scholar]
- 19.Assessment of children. 3rd ed. J.M. Sattler; San Diego, Calif: 1992. [Google Scholar]
- 20.Thorndike RL, Hagen EP, Sattler JM. The Stanford-Binet Intelligence Scale: technical manual. 4th ed. Riverside Publishing; Chicago: 1986. [Google Scholar]
- 21.Buck SF. A method of estimation of missing values in multivariate data suitable for use with an electronic computer. J R Stat Soc [B] 1960;22:302–306. [Google Scholar]
- 22.Bouchard TJ, Segal NL. Environment and IQ. In: Wolman BB, editor. Handbook of intelligence: theories, measurements, and applications. John Wiley; New York: 1985. pp. 391–464. [Google Scholar]
- 23.McMichael AJ, Baghurst PA, Vimpani GV, Robertson EF, Wigg NR, Tong SL. Socio-demographic factors modifying the effect of environmental lead on neuropsychological development in early childhood. Neurotoxi-col Teratol. 1992;14:321–327. doi: 10.1016/0892-0362(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 24.Caldwell BM, Bradley R. Home observation for measurement of the environment. University of Arkansas at Little Rock; Little Rock: 1984. [Google Scholar]
- 25.Searle SR. Linear models. John Wiley; New York: 1971. [Google Scholar]
- 26.Henderson CR., Jr. Analysis of covariance in the mixed model: higher-level, nonhomo-geneous, and random regressions. Biometrics. 1982;38:623–640. [PubMed] [Google Scholar]
- 27.Langford IH, Lewis T. Outliers in multilevel data. J R Stat Soc [A] 1998;161:121–160. [Google Scholar]
- 28.Ruppert D, Wand MP, Carroll RJ. Semiparametric regression. Cambridge University Press; London: 2003. [Google Scholar]
- 29.Peoples CD, Fagan JF, Drotar D. The influence of race on 3-year-old children’s performance on the Stanford-Binet: fourth edition. Intelligence. 1995;21:69–82. [Google Scholar]
- 30.Bellinger DC, Needleman HL. Lead and the relationship between maternal and child intelligence. J Pediatr. 1983;102:523–527. doi: 10.1016/s0022-3476(83)80178-4. [DOI] [PubMed] [Google Scholar]
- 31.McMichael AJ, Baghurst PA, Wigg NR, Vimpani GV, Robertson EF, Roberts RJ. Port Pirie Cohort Study: environmental exposure to lead and children’s abilities at the age of four years. N Engl J Med. 1988;319:468–475. doi: 10.1056/NEJM198808253190803. [DOI] [PubMed] [Google Scholar]
- 32.Wasserman GA, Liu X, Popovac D, et al. The Yugoslavia Prospective Lead Study: contributions of prenatal and postnatal lead exposure to early intelligence. Neurotoxicol Teratol. 2000;22:811–818. doi: 10.1016/s0892-0362(00)00106-9. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz J. Low-level lead exposure and children’s IQ: a meta-analysis and search for a threshold. Environ Res. 1994;65:42–55. doi: 10.1006/enrs.1994.1020. [DOI] [PubMed] [Google Scholar]
- 34.Needleman HL, Gatsonis CA. Low-level lead exposure and the IQ of children: a meta-analysis of modern studies. JAMA. 1990;263:673–678. [PubMed] [Google Scholar]
- 35.Bae DS, Gennings C, Carter WH, Jr., Yang RS, Campain JA. Toxicological interactions among arsenic, cadmium, chromium, and lead in human keratinocytes. Toxicol Sci. 2001;63:132–142. doi: 10.1093/toxsci/63.1.132. [DOI] [PubMed] [Google Scholar]
- 36.Cory-Slechta DA. Relationships between lead-induced learning impairments and changes in dopaminergic, cholinergic, and glutamatergic neurotransmitter system functions. Annu Rev Pharmacol Toxicol. 1995;35:391–415. doi: 10.1146/annurev.pa.35.040195.002135. [DOI] [PubMed] [Google Scholar]
- 37.Rice DC. Lead-induced changes in learning: evidence for behavioral mechanisms from experimental animal studies. Neuro-toxicology. 1993;14:167–178. [PubMed] [Google Scholar]
- 38.Tong S, Lu Y. Identification of confounders in the assessment of the relationship between lead exposure and child development. Ann Epidemiol. 2001;11:38–45. doi: 10.1016/s1047-2797(00)00176-9. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich KN, Ris MD, Succop PA, Berger OG, Bornschein RL. Early exposure to lead and juvenile delinquency. Neurotoxicol Ter-atol. 2001;23:511–518. doi: 10.1016/s0892-0362(01)00184-2. [DOI] [PubMed] [Google Scholar]
- 40.Rogan WJ, Dietrich KN, Ware JH, et al. The effect of chelation therapy with succi-mer on neuropsychological development in children exposed to lead. N Engl J Med. 2001;344:1421–1426. doi: 10.1056/NEJM200105103441902. [DOI] [PubMed] [Google Scholar]
- 41.Tong SL, Baghurst P, McMichael A, Sawyer M, Mudge J. Lifetime exposure to environmental lead and children’s intelligence at 11–13 years: the Port Pirie cohort study. BMJ. 1996;312:1569–1575. doi: 10.1136/bmj.312.7046.1569. [Erratum, BMJ 1996; 313:198.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong SL, Baghurst PA, Sawyer MG, Burns J, McMichael AJ. Declining blood lead levels and changes in cognitive function during childhood: the Port Pirie Cohort Study. JAMA. 1998;280:1915–1919. doi: 10.1001/jama.280.22.1915. [DOI] [PubMed] [Google Scholar]
- 43.Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone lead and blood lead levels in relation to baseline blood pressure and the prospective development of hypertension: the Normative Aging Study. Am J Epidemiol. 2001;153:164–171. doi: 10.1093/aje/153.2.164. [DOI] [PubMed] [Google Scholar]
- 44.Lin J-L, Lin-Tan D-T, Hsu K-H, Yu C-C. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N Engl J Med. 2003;348:277–286. doi: 10.1056/NEJMoa021672. [DOI] [PubMed] [Google Scholar]
- 45.Moss ME, Lanphear BP, Auinger P. Association of dental caries and blood lead levels. JAMA. 1999;281:2294–2298. doi: 10.1001/jama.281.24.2294. [DOI] [PubMed] [Google Scholar]
- 46.Needleman HL, Riess JA, Tobin MJ, Biesecker GE, Greenhouse JB. Bone lead levels and delinquent behavior. JAMA. 1996;275:363–369. [PubMed] [Google Scholar]
- 47.Schwartz J. Lead, blood pressure, and cardiovascular disease in men. Arch Environ Health. 1995;50:31–37. doi: 10.1080/00039896.1995.9955010. [DOI] [PubMed] [Google Scholar]
- 48.Lanphear BP. The paradox of lead poisoning prevention. Science. 1998;281:1617–1618. doi: 10.1126/science.281.5383.1617. [Erratum, Science 1998;282:51.] [DOI] [PubMed] [Google Scholar]
- 49.Marsden PA. Increased body lead burden — cause or consequence of chronic renal insufficiency? N Engl J Med. 2003;348:345–347. doi: 10.1056/NEJMe020164. [DOI] [PubMed] [Google Scholar]
- 50.Rosen JF, Mushak P. Primary prevention of childhood lead poisoning — the only solution. N Engl J Med. 2001;344:1470–1471. doi: 10.1056/NEJM200105103441910. [DOI] [PubMed] [Google Scholar]