Abstract
Objectives
The aim of this prospective study was to evaluate the Carotid Atherosclerosis Score (CAS) for predicting the development of high-risk plaque features and plaque burden progression.
Background
Prior studies have shown that carotid intraplaque hemorrhage (IPH) and a disrupted luminal surface (DLS), as identified by magnetic resonance imaging (MRI), are associated with greater risk for cerebrovascular events. Based on data from a large cross-sectional study, a scoring system was developed to determine which plaque features are associated with the presence of IPH and DLS. However, the predictive value of CAS has not been previously tested in a prospective, longitudinal study.
Methods
120 asymptomatic subjects with 50-79% carotid stenosis underwent carotid MRI scans at baseline and three-years thereafter. Presence of IPH and DLS, wall volume, maximum wall thickness, and maximum percent lipid-rich necrotic core area were measured at both time points. Baseline CAS values were calculated based on previously published criteria.
Results
Of the 73 subjects without IPH or DLS at baseline, nine (12%) developed one or both of these features during follow-up. There was a significant increasing trend between CAS and the development of new DLS (p<0.001) and with plaque burden progression (p=0.03), but not with the development of new IPH (p=0.3). Percent carotid stenosis was not significantly associated with new DLS (p=0.2), new IPH (p=0.1) or plaque progression (p=0.6)..
Conclusion
CAS was found to have a significant increasing relationship with incident DLS and plaque progression in this prospective study. CAS can potentially provide improved risk stratification beyond luminal stenosis.
Keywords: Carotid Atherosclerosis Score, Stenosis, Plaque progression, Disrupted Luminal Surface
Introduction
Stroke is one of the leading causes of mortality and morbidity worldwide, and carotid atherosclerotic disease is a primary etiologic factor.(1,2) Currently, the risk of stroke associated with carotid atherosclerosis is stratified by the severity of luminal stenosis.(3) Several large prospective clinical trials (4-6) have supported intervention with carotid endarterectomy (CEA) or stenting for symptomatic patients with >70% stenosis to reduce stroke risk. However, for symptomatic patients with <70% stenosis and for asymptomatic patients, stenosis alone may not be a reliable measure of stroke risk. A growing body of evidence suggests that plaque composition, as detected by MRI, rather than luminal narrowing, is the critical factor governing risk of ischemic cerebrovascular events originating from the carotid artery. (7-12) Specifically, the presence of a disrupted luminal surface (e.g. fibrous cap rupture (FCR) or ulceration) and/or intraplaque hemorrhage is indicative of a high risk lesion for ischemic events. (13-16) While results from studies to better characterize carotid atherosclerosis using MRI hold great promise, progress toward its translation to clinical practice has been hindered by the complex nature of analysis of the multi-contrast weighted images. A simple-to-use method to classify plaque that has predictive value for carotid lesion progression and future ischemic events is essential to move the field forward.
In a cross-sectional study of 435 subjects collected from four imaging sites in the United States and China, Underhill et al.(17) proposed a rapid risk stratification strategy, known as CAS, based on maximum wall thickness (WT) and the maximum lipid-rich necrotic core percentage (%LRNC). Statistical analysis showed that CAS was an accurate predictor for the presence of IPH and FCR in the test subgroup of this single time-point study. Furthermore, when compared to stenosis measured by MR angiography (MRA), CAS was a stronger classifier of high-risk features.
Though CAS was successfully designed and tested in a cross-sectional study (17), it is unclear if it can predict the development of high-risk plaque features and plaque burden progression in a prospective, longitudinal study. The aims of the current work were to (i) investigate the associations between the CAS, incident IPH or DLS, and plaque burden progression during follow-up; and (ii) to compare CAS and stenosis in risk prediction amongst asymptomatic individuals with 50-79% carotid stenosis.
Methods
Study Subjects
Individuals with 50-79% carotid stenosis on at least one side consented to be recruited into this study at the University of Washington Medical Center and the Veterans Affairs Puget Sound Health Care Systems. Carotid stenosis was determined with duplex sonography using the Strandness criteria.(18) The side with greater stenosis was defined as the index artery. All subjects were asymptomatic with regard to their carotid disease for the six months prior to recruitment. One hundred twenty (120) subjects underwent a baseline carotid MRI and follow-up scan three years thereafter.
Subjects with any of following conditions were excluded: 1) carotid endarterectomy before or during the study on the index carotid artery; 2) prior radiation therapy to the neck; 3) contraindication to MRI.
MR Imaging
All subjects underwent MR imaging at baseline and at the 3-year follow-up on a 1.5T scanner (Signa, Version 5.8, GE Healthcare, Milwaukee, Wisconsin) with a bilateral 4-element, phased array surface coil (Pathway MRI, Seattle, Washington). A multi-contrast protocol for carotid MR imaging(19) was used to acquire axial images with the parameters listed in Table 1.
Table 1.
Multi-contrast MRI Imaging Parameters
| Sequence | T1w | T2w | PDw | TOF |
|---|---|---|---|---|
| Readout pulse | 2D FSE | 2D FSE | 2D FSE | 3D spoiled GRE |
| TR(ms) | 800 | 2770 | 2770 | 23 |
| TE(ms) | 10 | 40 | 20 | 3.8 |
| FOV | 16 × 16 | 16 × 16 | 16 × 16 | 16 × 16 |
| Matrix | 256 × 256 | 256 × 256 | 256 × 256 | 256 × 256 |
| Slice Thickness(mm) | 2.0 | 2.0 | 2.0 | 2.0 |
| NEX | 2 | 2 | 2 | 2 |
| No. of slices | 12 | 12 | 12 | 24 |
| Blood suppression | DIR | Mulitslice DIR | Mulitslice DIR | Venous in-flow saturation |
| Slice gap (mm) | 0 | 0 | 0 | −1.0 |
| Flip angle | 90 | 90 | 90 | 35 |
| Scan time (min) | 8 | 4 | 4 | 4 |
Image Analysis
The index arteries of all subjects were interpreted via consensus opinion by two experienced reviewers who received certified training at the Vascular Imaging Lab, University of Washington. Both reviewers were blinded to the time point and clinical information during image analysis. For each scan, multi-contrast MR images were aligned using the carotid bifurcation as a landmark. At each registered axial slice, images were graded for image quality on a four-point scale where 1=poor and 4=excellent. Slices with image quality ≥2 at both time points were interpreted using vascular image analysis software (CASCADE, Vascular Imaging Lab, University of Washington) (20). After drawing the lumen and outer wall boundaries on each slice, plaque burden measurements were derived, including wall area and volume, maximum wall thickness (WT) and mean normalized wall index (NWI). NWI was computed per slice as wall area / (lumen area + wall area). Based on validated analysis criteria, (21,22, 28) IPH, LRNC, FCR, and ulceration were identified. The percent LRNC was also recorded per slice, where %LRNC = 100% × (LRNC area / wall area). Using the carotid bifurcation as a landmark, scans from the two time points were matched and artery-based measurements were computed from the common coverage for each subject. The inter-reader and inter-scan reproducibility of these measurements has been reported in previous studies.(23,24)
The severity of carotid stenosis was quantified based on MRA images rendered from TOF data by a trained reviewer who was blinded to clinical information and cross-sectional carotid MR images. Luminal stenosis was measured on the index side of the carotid artery using the NASCET criterion: 1 – (luminal diameter at the location with maximal narrowing / luminal diameter of normal distal internal carotid artery) × 100%.(4) All measurements were acquired on Maximum Intensity Projection (MIP) images with CASCADE.
CAS was computed from the max WT and maximum LRNC percentage measurements, where max %LRNC represented the cross-sectional slice with the largest %LRNC value for the index carotid artery. CAS was determined as follows: CAS=1 if max WT ≤ 2 mm, otherwise if max WT > 2 mm, then CAS was set according to max %LRNC; CAS=2 if max %LRNC was <20%, CAS=3 if max %LRNC was between 20% and 40%, and CAS=4 if max %LRNC was >40%.
Statistical Analysis
Plaque variables were aggregated over the slices within each artery, where only slices present in scans at both time points were included. Arteries with less than 10 mm of common coverage were excluded. Patient demographics were summarized using mean ± SD and range for continuous measurements and count (percentages) for categorical variables. The Chi-Squared test for trend was used to test for an increasing association between CAS and new high-risk features.
Plaque progression was defined as the annualized wall volume change between time points. Subjects who developed ulcerations over the follow-up period were excluded from the analysis of plaque progression because morphology measurements (lumen and wall volumes) were confounded by the presence of ulceration. Plaque progression was summarized as mean ± SD for the whole group and for each subgroup defined by the baseline level of CAS. The progression rate was compared to 0 using the one-sample t-test. Linear regression was used to test for an increasing trend between the baseline CAS and plaque progression.
Receiver operating character (ROC) curves and the area-under-the-curve (AUC) statistic were used to summarize the strength of the association between new DLS or IPH and CAS, carotid stenosis and plaque burden—wall volume, mean NWI and max WT. Pearson's correlation coefficient was used similarly with the same predictors and plaque progression as the outcome. All statistical analyses were performed using R (version 2.11.0, R Foundation for Statistical Computing, Vienna, Austria). Throughout, two-tailed tests were used with p<0.05 denoting statistically significance.
Results
Out of the 120 subjects recruited, 20 were excluded: 12 with poor image quality at either time-point, seven with insufficient coverage, and one subject who underwent CEA during the study period. To evaluate the association between CAS and incident IPH and DLS, the 73 subjects out of 100 without these features at baseline were analyzed. The mean time interval between baseline and follow-up scans was 3.0 ± 0.2 years. Demographic information and baseline plaque measurements of these 73 subjects are shown in Table 1.
Incidence of New High-Risk Features
The incidence of new DLS or IPH amongst the 73 subjects without these features at baseline is summarized in Table 3. At follow up, 7 subjects developed DLS (including 2 ulcerations) and 4 developed IPH (two of which were coincident with DLS). All new DLS were found in those with CAS=3 or 4 at baseline. There was a significant increasing trend for new DLS with increasing baseline CAS (p<0.001) but not with new IPH (p=0.3).
Table 3.
Incidence of new disrupted luminal surface (DLS) or intraplaque hemorrhage (IPH) by baseline CAS (N=73 subjects without DLS or IPH at baseline).
| N (%†) |
||||
|---|---|---|---|---|
| Baseline CAS | N (%*) | DLS | IPH | DLS or IPH |
| 1 | 8 (11) | 0 (0) | 0 (0) | 0 (0) |
| 2 | 51 (70) | 0 (0) | 2 (4) | 2 (4) |
| 3 | 10 (14) | 4 (40) | 2 (20) | 4 (40) |
| 4 | 4 (5) | 3 (75) | 0 (0) | 3 (75) |
| All | 73 (100) | 7 (10) | 4 (5) | 9 (12) |
| p for trend test | < 0.001 | 0.264 | < 0.001 | |
Percent of total (N=73).
Percent of subjects counted in each row (see second column).
Plaque Progression During Follow-Up
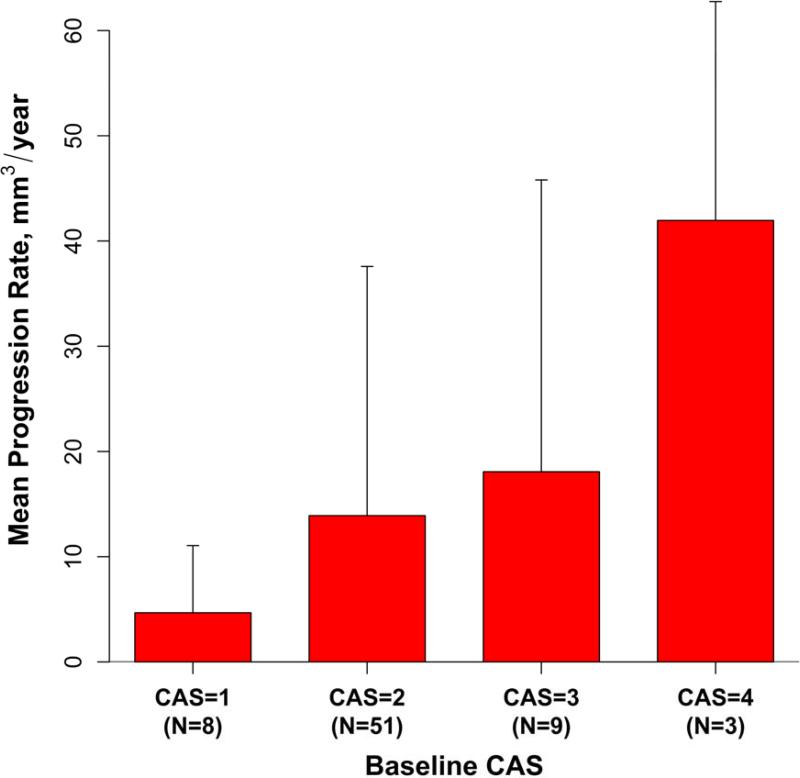
Two participants with evidence of new ulceration at follow-up were excluded from the plaque progression analysis (one with baseline CAS=3 and the other with CAS=4). The mean progression rate was 14.6 ± 23.5 mm3/year (p<0.001 for comparison to 0) in the remaining 71 subjects. Figure 2 shows the mean progression rates within the subgroups defined by baseline CAS. There was a significant increasing trend by linear regression (p=0.03) between the mean progression rates of 4.7, 13.9, 18.1, and 42.0 mm3/year and baseline CAS=1, 2, 3 and 4, respectively.
Figure 2. Mean annualized change in wall volume of subjects grouped by baseline CAS.
The vertical bars represent 1 standard deviation.
Comparison of CAS, Carotid Stenosis, and Plaque Burden Measurements in Predicting New DLS and IPH
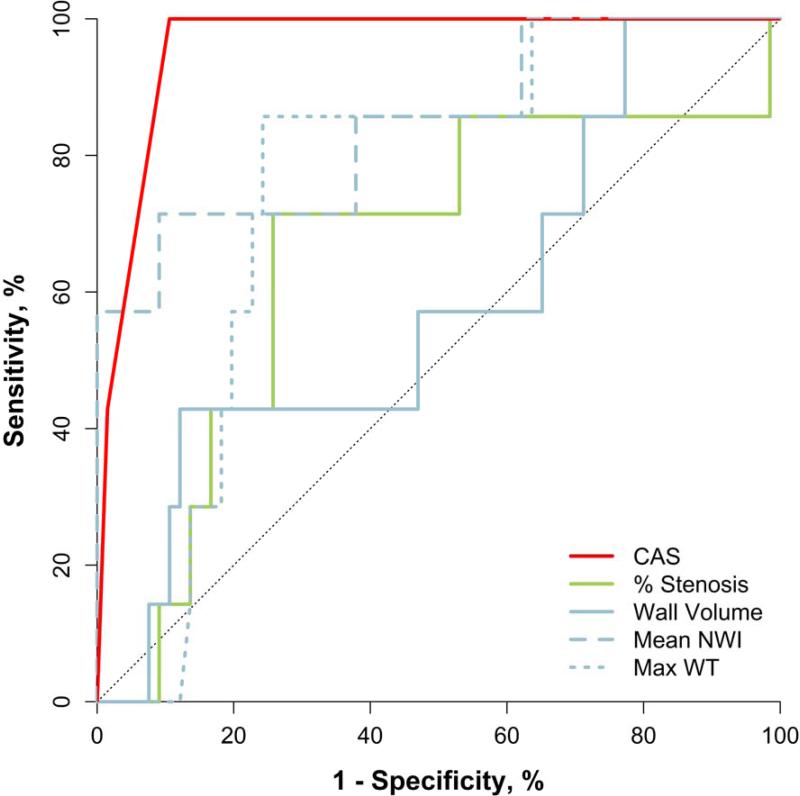
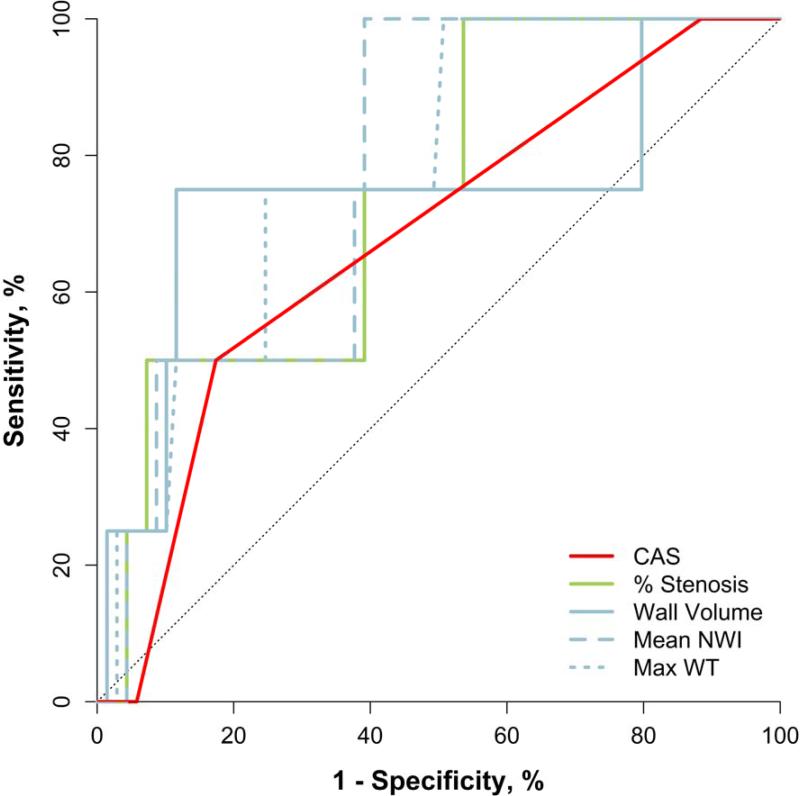
ROC analysis was conducted with new DLS and IPH as outcomes and baseline CAS, stenosis and plaque burden (wall volume, mean NWI and max WT) as predictors (Table 4). CAS had the highest AUC (AUC=0.96 [95% CI: 0.92, 1.0]) among predictors for new DLS while mean NWI and max WT had the highest AUC for predicting new IPH (AUC=0.78, p > 0.06 for both). The AUCs for carotid % stenosis were 0.65 (p=0.2) and 0.74 (p=0.1) for new DLS and IPH, respectively. None of the variables considered were significantly associated with new IPH. When the combined outcome of new DLS or IPH was examined, CAS had the highest AUC (AUC=0.86 [95% CI: 0.71, 1.0]), followed closely by mean NWI (AUC=0.84 [95% CI: 0.70, 0.99]).
Table 4.
Predicting new disrupted luminal surface (DLS) or intraplaque hemorrhage (IPH) using a single baseline predictor: CAS, stenosis or a plaque burden measure (N=73 subjects without DLS or IPH at baseline).
| Outcome |
||||||
|---|---|---|---|---|---|---|
| DLS | IPH | DLS or IPH | ||||
| AUC (95% CI) | p* | AUC (95% CI) | p* | AUC (95% CI) | p* | |
| CAS | 0.96 (0.92, 1.00) | <0.001 | 0.68 (0.42, 0.94) | 0.142 | 0.86 (0.71, 1.00) | <0.001 |
| Stenosis | 0.65 (0.43, 0.88) | 0.183 | 0.74 (0.52, 096) | 0.110 | 0.69 (0.50, 0.89) | 0.063 |
| Wall volume | 0.58 (0.36, 0.81) | 0.465 | 0.74 (0.43, 1.00) | 0.104 | 0.59 (0.37, 0.81) | 0.383 |
| Mean NWI | 0.84 (0.67, 1.00) | 0.003 | 0.78 (0.60, 0.95) | 0.065 | 0.84 (0.70, 0.99) | 0.001 |
| Max WT | 0.75 (0.60, 0.90) | 0.030 | 0.78 (0.59, 0.97) | 0.062 | 0.76 (0.62, 0.90) | 0.013 |
AUC=area under the receiver operating characteristic curve, CI=confidence interval, NWI=normalized wall index, WT=wall thickness.
Test for AUC > 0.5.
P-values were computed using an exact method, so are more reliable than the confidence intervals, which were computed using an approximation.
Comparison of CAS and Severity of Carotid Stenosis in Predicting Plaque Burden Progression
The correlations between the plaque progression rate and baseline CAS, stenosis and plaque burden are summarized in Table 5. CAS had the highest correlation (r=0.26 [95% CI: 0.03, 0.47]) while % stenosis has the lowest (r=-0.06 [95% CI: −0.29, 0.18]). The three plaque burden measures had similar levels of correlation with progression of r=0.22 – 0.23, but there was only a trend towards significance (p<0.1 for all three measures).
Table 5.
Univariate correlations between plaque progression over three years and baseline CAS, stenosis and plaque burden (N=71 subjects who did not develop ulceration over follow up).
| r (95% CI) | p | |
|---|---|---|
| CAS | 0.26 (0.03, 0.47) | 0026 |
| Stenosis | −0.06 (−0.29, 0.18) | 0.641 |
| Wall volume | 0.22 (−0.02, 0.43) | 0.069 |
| Mean NWI | 0.23 (−0.01, 0.44) | 0.057 |
| Max WT | 0.22 (−0.01, 0.43) | 0.060 |
r = Pearson correlation coefficient, CI=confidence interval, NWI=normalized wall index, WT=wall thickness.
Discussion
The initial formulation and testing of CAS was based on data from a cross-sectional study (17). The serial carotid MRI study reported herein demonstrates that CAS measures are predictive of future carotid luminal surface disruption, thus providing prospective confirmation of the earlier single time-point study findings. Furthermore, a significant association was noted between CAS and plaque progression in asymptomatic patients with 50-79% stenosis and who do not have carotid DLS or IPH at baseline. We caution the reader against over-interpretation of the data presented given the relatively low number of events. Nevertheless, the data is promising for a simplification of carotid risk stratification using CAS and provides strong evidence for conducting future studies that utilize a larger study sample to validate these initial findings. Findings from randomized clinical trials indicate that the benefit of CEA or stenting in this group of individuals is marginal. However, the further risk stratification offered by CAS has the potential to target subgroups that may benefit from intervention, although this must be confirmed in a randomized clinical trial. CAS may also provide a means to identify individuals who would benefit from more intensive clinical follow-up. While reliable multivariate analysis could not be conducted due to the limited sample size and event rate, CAS appeared to be more strongly associated with both plaque progression and new DLS than percent stenosis. This suggests that CAS may provide a better assessment on risk of stroke, beyond the current clinical angiographic solution, for asymptomatic patients with 50-79% stenosis.
Since maximum percent LRNC is the major input variable to CAS, the finding that CAS is positively associated with the incidence of new DLS (p<0.001) is consistent with the relationship between percent LRNC volume and DLS previously reported by Underhill, et al. (26) In the previous study, IPH was present in nearly 30% of the carotid arteries at baseline. In this study, it was shown that the risk associated with LRNC, as represented by CAS, holds in the subgroup without IPH at baseline.
While baseline CAS predicted incident DLS during follow-up, its relationship with new IPH was inconclusive. There were only four incidences of new IPH, and a positive association between CAS and new IPH was not found. However, all the new intraplaque hemorrhages occurred in subjects with CAS=2 and 3 at baseline. For subjects with CAS=4, 78% of them had IPH already at baseline. While a conclusion cannot be drawn from the small number of events seen here, this may suggest that IPH tends to occur at an earlier stage of plaque development and leads to growth into the higher levels of CAS, which is consistent with the concept of IPH as a primary driver of plaque growth, as shown previously. (25,27)
The MR plaque burden measure, mean NWI, appeared to predict new DLS or plaque progression well though not better than CAS. During the original development of CAS, mean NWI was not considered, though max NWI was as well as max WT and other plaque burden measures. In that cross-sectional analysis, max % LRNC area was more strongly associated with DLS and IPH than any plaque burden measures and thus CAS was based primarily on LRNC rather than plaque burden. From a biological perspective, it is expected that CAS, which is based on the presence and size of the LRNC, will provide a more precise measure of risk for plaque disruption than mean NWI, which is based solely on plaque size. For large plaques that are predominantly fibrotic or calcified, the risk for disruption predicted by mean NWI may vary from CAS. Future prospective studies may consider mean NWI as an additional predictor to confirm that this finding holds longitudinally.
Study limitations: The size of the study sample was not large and subjects were recruited from only two related centers. Furthermore, the rate of new events and the prevalence of CAS=4 in subjects without DLS or IPH were small, which also prevented reliable multivariate analysis to statistically compare the different potential predictors. Additionally, the association between the development of neurologic symptoms and CAS could not be assessed due to the study design which required subjects to have completed both a baseline and 3 year follow up MRI of their plaques. Clinical guidelines at the recruitment centers indicate CEA for patients with 50-79% stenosis by US who become symptomatic and thus no follow up MRI was available in those cases. A larger, multi-center study is needed to have sufficient power to assess the ability of CAS for prediction of future ischemic events. Finally, 17% of patients were excluded in this study due to imaging issues. Recently developed 3-D MRI techniques such as 3D-MERGE and SNAP (29, 30) have been developed that will reduce the percentage of excluded subjects in future studies. Advantages of these new techniques include: 1) larger field of view and isotropic 3D scans which will improve co-registration of serially acquired scans and reduce the number of cases excluded due to insufficient coverage; 2) shorter scan time which may reduce exclusions due to motion artifact; 3) decreased artifact due to low-flow; and 4) higher contrast between IPH, surrounding vessel wall, and lumen.
Conclusions
CAS, based on two plaque parameters (maximum wall thickness and percent lipid-rich necrotic core), was found to have a significant increasing relationship with incident DLS and plaque progression in this prospective, longitudinal study of individuals with asymptomatic, 50-79% carotid stenosis. The CAS concept provides a method to further stratify risk beyond luminal stenosis and may allow more effective targeting of patients for specific treatment to prevent future stroke.
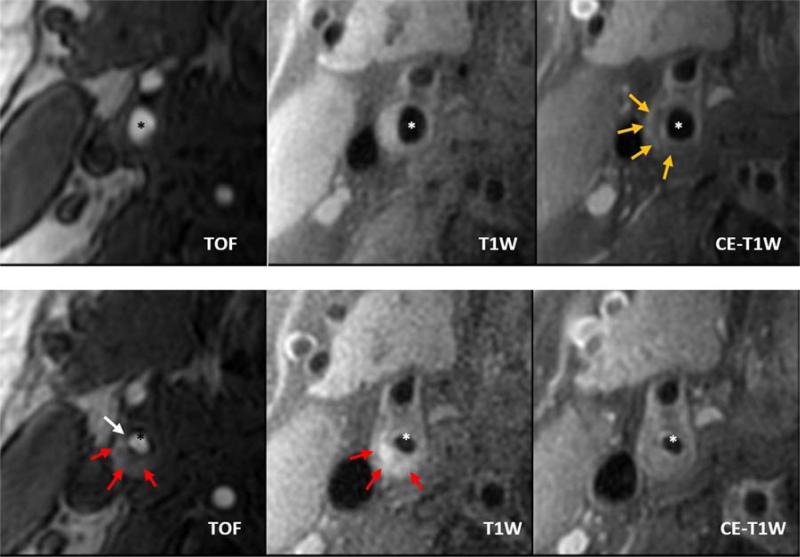
Figure 1. Baseline MRI (top row) demonstrating a CAS 4 internal carotid plaque with a large lipid rich necrotic core (yellow arrows).
The three-year follow-up MRI (bottom row) of the matched cross-section demonstrates fibrous cap rupture (white arrow) with intraplaque hemorrhage (red arrows). Also note the large increase in internal carotid wall area and decrease in lumen area. TOF = Time-of-Flight; T1W = pre-contrast T1-weighted image; CE-T1W = post-contrast enhanced T1-weighted image; * = lumen of the internal carotid artery.
Figure 3. Receiver-Operator Characteristic (ROC) curves for individual predictors of new disrupted luminal surface (DLS).
CAS=carotid atherosclerosis score, NWI=normalized wall index, WT=wall thickness.
Figure 4. ROC curves for individual predictors of new IPH.
CAS=carotid atherosclerosis score, NWI=normalized wall index, WT=wall thickness.
Table 2.
Summary of patient demographics and baseline plaque burden at baseline (N=73).
| Variable | Mean ± SD or N (%) | Range |
|---|---|---|
| Age, years | 70.3 ± 9.6 | 45 – 89 |
| Body mass index, kg/m2* | 27.7 ± 5.4 | 18.9 – 54.3 |
| Systolic blood pressure, mmHg* | 144 ± 21 | 99 – 194 |
| Male sex | 62 (85) | - |
| Smoking status* | - | - |
| Never smoked | 10 (14) | - |
| Quit | 37 (51) | - |
| Active | 25 (35) | - |
| Diabetes mellitus* | 19 (26) | - |
| History of coronary artery disease* | 31 (46) | - |
| History of hypertension | 62 (85) | - |
| History of hypercholesterolemia | 61 (84) | - |
| Statin therapy | 51 (70) | - |
| Carotid stenosis, %† | 29 ± 21 | 0 – 77 |
| Wall volume, mm3 | 660 ± 228 | 249 – 1327 |
| Mean normalized wall index | 0.52 ± 8 | 0.36 – 0.74 |
| Max wall thickness | 3.4 ± 1.2 | 1.3 – 7.7 |
Excluding subjects missing measurement: 7 for body mass index, 1 for smoking status, and 5 for history of coronary artery disease.
Measured from 3D TOF MRA using NASCET criteria.
Acknowledgments
Sources of Funding
The study was supported by the National Heart, Lung and Blood Institute of the National Institutes of Health under award number R01HL061851, R01HL073401, R01HL67406 and T32HL7838. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Abbreviations
- CAS
carotid atherosclerosis score
- MRI
magnetic resonance imaging
- DLS
disrupted luminal surface
- FCR
fibrous cap rupture
- IPH
intraplaque hemorrhage
- WT
wall thickness
- LRNC
lipid rich necrotic core
- MRA
magnetic resonance angiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Xu is a consultant of VP Diagnostics. Dr. Yuan has received research grant support from the NIH, Philips Healthcare and VP Diagnostics, and has been a consultant for Bristol Myers Squibb, Imagepace, Merck, Boehringer Ingelheim and Pfizer. Dr. Hatsukami has a research grant support from the NIH and Philips Healthcare. The other authors have no relevant relationships to disclose.
References
- 1.Nagai Y, Kitagawa K, Sakaguchi M, et al. Significance of earlier carotid atherosclerosis for stroke subtypes. Stroke. 2001;32:1780–5. doi: 10.1161/01.str.32.8.1780. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 3.Moore WS, Barnett HJ, Beebe HG, et al. Guidelines for carotid endarterectomy. A multidisciplinary consensus statement from the Ad Hoc Committee, American Heart Association. Circulation. 1995;91:566–79. doi: 10.1161/01.cir.91.2.566. [DOI] [PubMed] [Google Scholar]
- 4.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 5.Endarterectomy for moderate symptomatic carotid stenosis: interim results from the MRC European Carotid Surgery Trial. Lancet. 1996;347:1591–3. [PubMed] [Google Scholar]
- 6.Rothwell PM, Slattery J, Warlow CP. A systematic comparison of the risks of stroke and death due to carotid endarterectomy for symptomatic and asymptomatic stenosis. Stroke. 1996;27:266–9. doi: 10.1161/01.str.27.2.266. [DOI] [PubMed] [Google Scholar]
- 7.Saam T, Underhill HR, Chu B, et al. Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol. 2008;51:1014–21. doi: 10.1016/j.jacc.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 8.Cheung HM, Moody AR, Singh N, Bitar R, Zhan J, Leung G. Late stage complicated atheroma in low-grade stenotic carotid disease: MR imaging depiction--prevalence and risk factors. Radiology. 2011;260:841–7. doi: 10.1148/radiol.11101652. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida K, Sadamasa N, Narumi O, Chin M, Yamagata S, Miyamoto S. Symptomatic low-grade carotid stenosis with intraplaque hemorrhage and expansive arterial remodeling is associated with a high relapse rate refractory to medical treatment. Neurosurgery. 2012;70:1143–50. doi: 10.1227/NEU.0b013e31823fe50b. discussion 1150-1. [DOI] [PubMed] [Google Scholar]
- 10.Freilinger TM, Schindler A, Schmidt C, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. 2012;5:397–405. doi: 10.1016/j.jcmg.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Demarco JK, Ota H, Underhill HR, et al. MR carotid plaque imaging and contrast-enhanced MR angiography identifies lesions associated with recent ipsilateral thromboembolic symptoms: an in vivo study at 3T. AJNR Am J Neuroradiol. 2010;31:1395–402. doi: 10.3174/ajnr.A2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong L, Underhill HR, Yu W, et al. Geometric and compositional appearance of atheroma in an angiographically normal carotid artery in patients with atherosclerosis. AJNR Am J Neuroradiol. 2010;31:311–6. doi: 10.3174/ajnr.A1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saam T, Cai J, Ma L, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology. 2006;240:464–72. doi: 10.1148/radiol.2402050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan C, Zhang SX, Polissar NL, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation. 2002;105:181–5. doi: 10.1161/hc0202.102121. [DOI] [PubMed] [Google Scholar]
- 15.Takaya N, Yuan C, Chu B, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke. 2006;37:818–23. doi: 10.1161/01.STR.0000204638.91099.91. [DOI] [PubMed] [Google Scholar]
- 16.Singh N, Moody AR, Gladstone DJ, et al. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology. 2009;252:502–8. doi: 10.1148/radiol.2522080792. [DOI] [PubMed] [Google Scholar]
- 17.Underhill HR, Hatsukami TS, Cai J, et al. A noninvasive imaging approach to assess plaque severity: the carotid atherosclerosis score. AJNR Am J Neuroradiol. 2010;31:1068–75. doi: 10.3174/ajnr.A2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roederer GO, Langlois YE, Jager KA, et al. A simple spectral parameter for accurate classification of severe carotid disease. Bruit. 1984:174–178. [Google Scholar]
- 19.Yuan C, Kerwin WS, Yarnykh VL, et al. MRI of atherosclerosis in clinical trials. NMR Biomed. 2006;19:636–54. doi: 10.1002/nbm.1065. [DOI] [PubMed] [Google Scholar]
- 20.Kerwin W, Xu D, Liu F, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging. 2007;18:371–8. doi: 10.1097/rmr.0b013e3181598d9d. [DOI] [PubMed] [Google Scholar]
- 21.Saam T, Hatsukami TS, Takaya N, et al. The vulnerable, or high-risk, atherosclerotic plaque: noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64–77. doi: 10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 22.Saam T, Ferguson MS, Yarnykh VL, et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 2005;25:234–9. doi: 10.1161/01.ATV.0000149867.61851.31. [DOI] [PubMed] [Google Scholar]
- 23.Saam T, Hatsukami TS, Yarnykh VL, et al. Reader and platform reproducibility for quantitative assessment of carotid atherosclerotic plaque using 1.5T Siemens, Philips, and General Electric scanners. J Magn Reson Imaging. 2007;26:344–52. doi: 10.1002/jmri.21004. [DOI] [PubMed] [Google Scholar]
- 24.Takaya N, Cai J, Ferguson MS, et al. Intra- and interreader reproducibility of magnetic resonance imaging for quantifying the lipid-rich necrotic core is improved with gadolinium contrast enhancement. J Magn Reson Imaging. 2006;24:203–10. doi: 10.1002/jmri.20599. [DOI] [PubMed] [Google Scholar]
- 25.Underhill HR, Yuan C, Yarnykh VL, et al. Arterial remodeling in [corrected] subclinical carotid artery disease. JACC Cardiovasc Imaging. 2009;2:1381–9. doi: 10.1016/j.jcmg.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Underhill HR, Yuan C, Yarnykh VL, et al. Predictors of surface disruption with MR imaging in asymptomatic carotid artery stenosis. AJNR Am J Neuroradiol. 2010;31:487–93. doi: 10.3174/ajnr.A1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takaya N, Yuan C, Chu B, et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–75. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 28.Yu W, Underhill HR, Ferguson MS, et al. The added value of longitudinal black-blood cardiovascular magnetic resonance angiography in the cross sectional identification of carotid atherosclerotic ulceration. J Cardiovasc Magn Reson. 2009;11:31. doi: 10.1186/1532-429X-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balu N, Yarnykh VL, Chu B, Wang J, Hatsukami T, Yuan C. Carotid plaque assessment using fast 3D isotropic resolution black-blood MRI. Magn Reson Med. 2011;65:627–37. doi: 10.1002/mrm.22642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, Bornert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med. 2013;69:337–45. doi: 10.1002/mrm.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]