Abstract
Rationale
It has been proposed that cocaine abuse results in skilled or “automatic” drug-taking behaviors. Brain regions important for skill learning are implicated in cocaine self-administration. However, the development of skill during self-administration has not been investigated.
Objectives
The present experiment investigated the development of skilled self-administration over extended drug use by employing a novel operant vertical head movement under discriminative stimulus (SD) control. In addition, the capacity of the head movement to serve as an operant was tested by manipulating drug levels above or below satiety drug levels via frequent microinfusions (0.2 sec) of cocaine delivered noncontingently.
Results
Animals acquired the vertical head movement operant, which increased in number over days. Task learning was demonstrated by reduced reaction time in response to the SD, increased propensity to self-administer upon SD presentation, and escalated drug consumption over days. Skill learning was demonstrated by 1) an increase over days in the velocity of operant vertical head movements, as a function of shorter duration but not altered distance, and 2) an increase over days in the probability of initiating the operant at the optimal starting position. Evidence that responding was specific to self-administration was revealed during periods of experimenter-manipulation of drug level: maintaining drug levels above satiety decreased responding while maintaining drug levels below satiety increased responding.
Conclusions
These results provide evidence that under the specific set of circumstances tested herein, cocaine self-administration becomes skilled over days of extended drug use. In addition, the vertical head movement can be used as an operant comparable to lever pressing with the additional benefit of quantifying skill learning.
Keywords: striatum, putamen, accumbens, pallidum, habit, abuse
Introduction
A seminal paper by Tiffany (1990) proposed that over extended drug use self-administration behavior becomes largely “automatic”, as demonstrated by skilled self-administration. The rodent dorsolateral striatum (DLS), homologous with the primate putamen (Carelli and West, 1991), is implicated in skill learning (Tang et al. 2007; 2009) as well as cocaine self-administration (Letchworth et al. 2001; Vanderschuren et al. 2005; Fuchs et al. 2006; Volkow et al. 2006; 2008). However, empirical tests of the existence of skilled drug self-administration behavior are lacking, and are necessary if these views are to advance the neurobiological understanding of drug abuse. In order to investigate whether self-administration becomes skilled over extended drug use, animals self-administered cocaine six hours every day for three weeks by using a novel vertical head movement as the operant. Instead of an all-or-none lever press, the vertical head movement enabled measurements of changes in distance, duration, velocity, start and end positions of operant responses as a function of training, as well as recording partial responses. Furthermore, given that animals will inhibit lever pressing when drug levels are maintained above satiety but increase lever pressing when drug levels are maintained below satiety (Pickens and Thompson 1968; Norman and Tsibulsky 2006), we tested whether animals’ use of the vertical head movement during cocaine self-administration was similar to that of the lever press.
Methods
Subjects and surgery
Male Long-Evans rats (n = 13; 325–335 g; Charles River, Wilmington, MA) were implanted with a catheter in the right jugular vein while deeply anesthetized (sodium pentobarbital, 50 mg/kg, i.p). Prior to surgery, subjects received injections of atropine methyl nitrate (10 mg/kg, i.p.) and penicillin G (75,000 U/0.25 ml, i.m.) in order to protect subjects from airway congestion and infection, respectively. Anesthesia was maintained with periodic injections of ketamine hydrochloride (60 mg/kg, i.m.). Following surgery and for the remainder of the experiment, animals were housed in Plexiglas self-administration chambers with the catheter connected to a fluid swivel. They were perfused with 0.2 ml heparinized bacteriostatic saline (0.9%) every twenty-five minutes to maintain catheter patency, except during self-administration. Rats started self-administration training ~8 days following surgery. Except during self-administration, water was available ad lib. Standard rat lab chow was provided following daily self-administration to maintain body weights approximately between 325 - 335 g. Protocols were performed in compliance with the Guide for the Care and Use of Laboratory Animals (NIH Publications 865–23) and were approved by the Institutional Animal Care and Use Committee, Rutgers University.
Procedure
Vertical head movements were tracked using six infrared-emitting diodes capable of transistor-transistor logic (HOA6299, Honeywell, Morristown NJ). The light of each photocell was contained within a 5.59 mm diameter beam at 880 nm wavelength, outside the visual spectrum of the rat retina under normal illuminated conditions (Green 1971; Pardue et al. 2001). Six photocells and the opposing six diodes were arranged along a 50 degree arc over 69 mm and attached outside the clear Plexiglas walls in one corner of the self-administration chamber. This arrangement enabled us to condition instrumental head movements that approximate the vertical head movements in a prior water self-administration task (Figure 1; Tang et al. 2007) in which operant head movements became skilled and habitual. Since the photocells were arranged vertically in the corner, horizontal or slanted movements were restricted. All photocell beam breaks were recorded for offline analysis of different movements using custom Matlab scripts. Except during self-administration or testing sessions, a rectangular opaque Plexiglas block (approximately 50 mm x 50 mm x 200 mm) was attached inside the chamber to block access to the “photocell corner” to prevent any possible extinction learning.
Fig 1.

Head-movement apparatus. Numbers refer to photocell number and are always located on the “receiving” photocell. Apparatus is shown with a front view (A), side view (B), and mounted to the rear corner of the chamber (“photocell corner”) in its operative position outside the chamber (C).
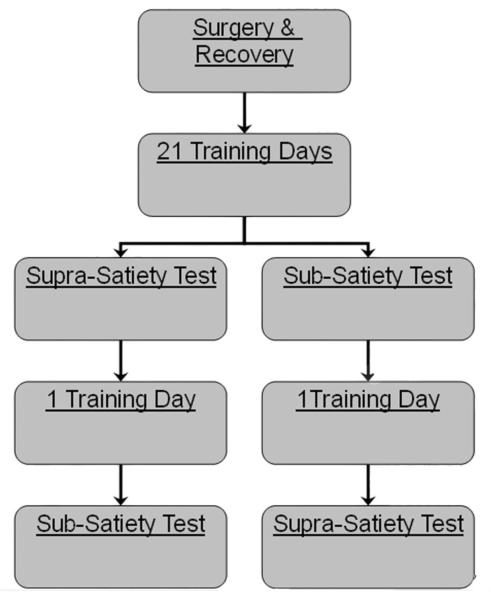
An experiment flow chart is displayed in figure 2. Self-administration began daily (7 days a week for three weeks) with the onset of the house light and removal of the Plexiglas block that prohibited access to the photocell corner. At the beginning of the first day of training, vertical head movements in the photocell corner were shaped via delivery of cocaine HCl infusion (0.24 mg/0.2 ml/inf) in the presence of the discriminative stimulus (SD) (3.5 kHz, 70 dB). The shaping process began with reinforcing a nose poke response into the photocell corner (breaking photocell 2) for several instances, then reinforcing an upward movement that consecutively breaks photocells 2 and 3 within one second for several instances. Shaping continued by reinforcing movements consecutively breaking photocells 2, 3, and 4 within one second, for several instances, and lastly reinforcing the final contingency of consecutively breaking photocells 2, 3, 4, and 5 within one second (termed “criterion head movement”). The criterion head movement required at least a 43.4 mm movement along the arc, starting at or below the 2nd photocell beam (13.5 mm from the floor) and crossing at least the fifth photocell. During shaping days, prior to the first self-infusion, the SD was continuously sounded until a single response (breaking photocell 2) was made, at which time the SD was terminated, cocaine was delivered, and a 40 second time out period began. Following the first self-infusion, the SD was sounded for 2 min or until a single response was made that satisfied the current shaping criterion, at which time the SD was terminated and cocaine was delivered. All time out periods following the first shaping self-infusion were 10 sec and the SD was presented again following the end of each time out. Shaping was considered complete following three self-infusions at the criterion head movement. Criterion movements within the time out period of training or testing sessions were recorded but had no programmed consequence. Shaping sessions were between 4 and 6 hours in duration.
Fig 2.
Experimental flow diagram.
Normal training began at the start of the subsequent training session, following acquisition of the criterion head movement (typically 1-2 days). Normal training consisted of SD presentations for 2 minutes (limited hold) or until an operant response was emitted. To allow for rapid “loading” infusions, until the tenth self-infusion, the time out period was a fixed 40 sec interval. All subsequent (maintenance) time out periods were pseudorandomly selected from a 30 item Fleshler-Hoffman (Fleshler and Hoffman 1962) distribution on a variable interval 1-6 min schedule. Regardless of “loading” or “maintenance” time out conditions, a criterion head movement during SD presentation produced an intravenous infusion of cocaine (0.24 mg/0.2 ml/inf), terminated the SD, and started the time out period. Given that the median inter-infusion-interval under free-access conditions at the present dose (approximately 0.77 mg/kg/inf) is 7.5 min (Fabbricatore et al. 2010), the 1-6 min time out was designed to allow animals the ability to attain drug satiety levels (see next paragraph). Specifically, the 1-6 minute time out schedule was used in an attempt to balance the animal’s suprasatiety and subsatiety experiences such that rats would experience both conditions prior to the suprasatiety and subsatiety tests. Self-administration sessions ended after 6 hours elapsed or 80 infusions were earned, whichever occurred first. Rats were never “primed”, i.e., noncontingently administered infusions of cocaine to initiate self-administration.
Following twenty-two days of self-administration, subjects were tested for sensitivity to high (n=12) or low drug levels (n = 11) with an ABA design termed the suprasatiety and subsatiety tests, respectively. All phases were two hours in duration, totaling six hours. During the initial A phase of the suprasatiety test, animals self-administered cocaine under normal training conditions for two hours. At the start of the third hour of self-administration (onset of B phase), the animal’s drug level was clamped by microinfusions (0.2 sec) administered at an inter-infusion-interval adjusted for each animal (average and SEM: 8.96 ± 0.54 sec) to maintain drug levels at 0.1 mg/kg above satiety. Drug satiety (Wise et al. 1995) was operationally defined as a drug level 0.1 mg/kg above the animal’s peak drug level during the initial A phase. SD presentations continued at the same rate as the A phase (1 to 6 min) with all contingencies programmed the same as the A phase. All contingencies were left intact during the B phase because the alternative, extinction conditions, could potentially inflate responding by an extinction burst, or potentially deflate responding by extinction learning. At the start of the fifth hour of self-administration (onset of the second A phase), microinfusions were terminated and all other contingencies were unchanged, i.e., a resumption of the same conditions as during the first two hours of the test (initial A phase).
The within-subjects “subsatiety” control test was conducted to examine the effect of noncontingent infusions on behavioral measures with an ABA design. During the initial A phase of the subsatiety test, animals self-administered cocaine under normal training conditions for two hours. At the start of the third hour of self-administration (onset of B phase), the animal’s drug level was clamped by: i) presenting the SD at elongated intervals (between 21 and 29 min) derived from subjects’ performance in the initial A phase, which prevented the animal from achieving satiety; and ii) administering microinfusions (0.2 sec) at an inter-infusion-interval adjusted for each animal (average: 20.82 ± 0.56 sec; significantly longer than suprasatiety intervals, t(10) = −12.95, p < 10−6), which prevented drug levels from falling below 2 - 2.5 mg/kg. All operant contingencies were left intact during the B phase. At the start of the fifth hour (onset of the second A phase), microinfusions were terminated, SD presentations were returned to the same conditions (1-6 min) as in the initial A phase, and all other contingencies were unchanged. The order of suprasatiety and subsatiety tests was counterbalanced between animals and separated by a self-administration session under normal training conditions.
Drug level calculation
Assuming first-order pharmacokinetics, calculated drug level (mg/kg body weight) was determined over successive infusions by the equation:
where Tn = the time since the previous cocaine infusion (min), D = infusion dose/bodyweight (mg/kg), Bn-1 = cocaine level at time of last infusion (mg/kg) K = rate constant of 0.028875, reflecting a 0.4 hour metabolic half-life for cocaine (Nayak et al. 1976).
Using this formula, custom infusion intervals were determined for phase B during suprasatiety and subsatiety tests. For the suprasatiety test, the target satiety drug level was entered into both Bn and Bn-1 terms. For the subsatiety test, the target drug level between 2 - 2.5 mg/kg was entered into both Bn and Bn-1 terms. For both tests, D was defined as 0.0189 mg (dose per infusion at 0.2 sec pump duration) divided by bodyweight (kg). Given that the rate constant of 0.028875 was entered into term K, interval Tn was arithmetically solved.
Statistical analysis
Outcome variables, e.g., criterion head movements, self-administered mg/kg/day, etc., were analyzed as a function of training day or testing phase using repeated measures ANOVAs (PASW 18.0.0, SPSS, Chicago, IL). For analyzing test results, data were taken from the second hour of each two-hour phase in order to remove the influence of initially changing drug levels (e.g. loading behavior in the first A phase and the effects of the satiety clamp in the second A phase). Pairwise comparisons (least squares difference) were computed for significant main effects of testing phase to assess differences between phases. Alpha criterion for all tests was 0.05. Reaction time was defined as the daily average of all differences in time between the end of a criterion head movement and preceding SD presentation (onset). Hit probability was calculated by a daily fraction in which the numerator was number of self-infusions and the denominator was number of SD presentations. To determine escalation of intake, the time of the time of the tenth self-infusion of cocaine was determined each day. Four animals did not reach ten infusions on day 1. These data points were included as missing data points in the repeated measures ANOVA.
Results
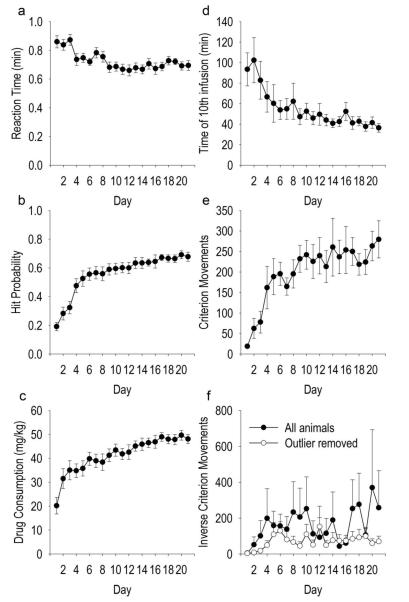
Animals self-administered cocaine using the discrete SD paradigm, in which SD presentation at 1-6 min intervals set the occasion for every cocaine infusion. In response to the SD, average daily reaction time (hits only) decreased over days of self-administration training (Figure 3A), F(2, 240) = 4.38, p < 10−7. The probability of self-administering cocaine upon SD presentation also increased over days (Figure 3B), F(2, 240) = 21.062, p < 10−41, to a value of 0.68 ± 0.03 (mean ± SEM) hit probability on the final training day. If one assumes that a miss reflects a suprasatiety drug level on that trial, this result suggests animals gained both subsatiety and suprasatiety experience during training. Furthermore, animals escalated their daily consumption of cocaine (Figure 3C) (F(2, 240) = 10.305, p < 10−21) as well as decreased their latency to load up to high drug levels (Figure 3D) (F(2, 160) = 2.150, p < 0.01 over days of training.
Fig 3.
Self-administration and cue learning. Reaction time in response to the SD (A), probability of self-administering cocaine upon SD presentation (B), cocaine consumption (C), latency to load up to the tenth infusion (D), number of criterion movements (E), and number of inverse criterion movements (F). Values are average ± SEM (y-axes) per day (x-axes).
The number of criterion head movements increased over days (Figure 3E), F(2, 240) = 6.375, p < 10−12. The number of vertical head movements initiated at photocell 1 or photocell 2 that were less than the criterion head movement distance did not change over days. The number of inverse criterion movements (downward movements starting at or above photocell 5 and ending at or below photocell 2) did not change over days (Figure 3F, black circles). These results suggest that animals learned to discriminate the upward criterion head movement as the operant. One animal was an outlier with regard to inverse criterion movements. For this rat, stereotypical movements included a downward (but not upward criterion) movement within the photocell corner. The number of inverse criterion movements with the outlier animal removed (Figure 3F, white circles) did not change over days. All other animals exhibited a locus of stereotypy away from the photocell corner. Moreover, the stereotypy-inducing properties of cocaine did not interfere with criterion head movements: during week three a comparatively longer latency (mean 30.22 ± 8.09 sec) was observed between a criterion head movement and the subsequent head movement than would be expected from stereotypical head movements (e.g. “head-bobbing” at several head movements per sec).
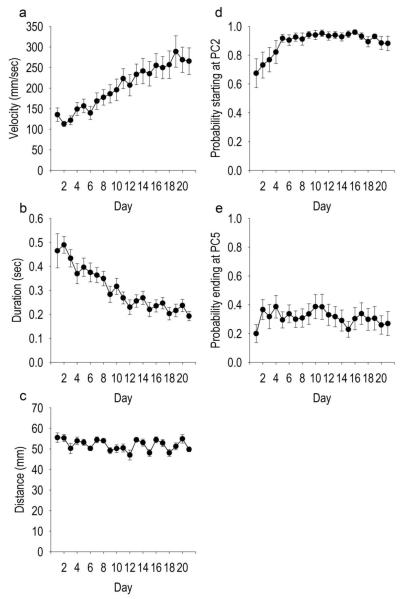
After shaping was complete, criterion head movements gradually became more efficient and skilled over training days, although this was not required (i.e., the programmed criterion movement never changed). Over days of training, the average daily criterion head movement increased in average velocity, i.e., distance/duration (Figure 4A) (F(2, 240) = 13.250, p < 10−27) via primarily a decrease in duration (Figure 4B) (F(2, 240) = 14.314, p < 10−29) rather than a change in total distance (Figure 4C). The most efficient criterion movement was one that began at photocell 2 and ended at photocell 5. The probability of a criterion movement starting at photocell 2 increased over days (Figure 4D) (F(2, 240) = 3.98, p < 10−6) but the probability of ending at photocell 5 did not change (Figure 4E).
Fig 4.
Skill learning: changes in criterion head movement characteristics with extensive repetition. Changes in velocity (A), duration (B), distance (C) , probability of starting at photocell two, the minimum required for a criterion movement (E), and the probability of ending at photocell 5. Values for A, B, and C are average median ± SEM per day for criterion head movements (y-axes). Values for D and E are average probability ± SEM. All x-axes refer to training day.
In order to test whether the frequency of criterion head movements was sensitive to drug level manipulations, animals underwent a suprasatiety test using an ABA design. The repeated measures ANOVA yielded a significant main effect of test phase for criterion head movements, F(2, 22) = 17.260, p < 10−4 (Figure 5; black circles). Pairwise comparisons revealed that the number of criterion head movements during the suprasatiety B phase significantly decreased below that observed during the initial A phase (p < 10−3) and significantly increased during the second A phase compared with the B phase (p < 10−5) but not the initial A phase. The average ± SEM drug levels during the suprasatiety phases were 4.90 ± 0.35 mg/kg during the initial A phase, 6.67 ± 0.37 mg/kg during the suprasatiety B phase, and 4.98 ± 0.33 mg/kg in the second A phase.
Fig 5.
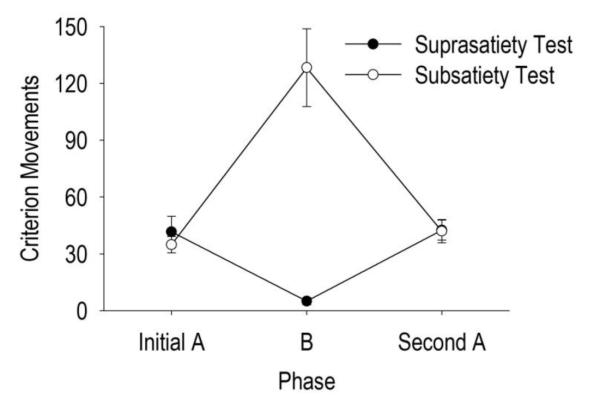
Drug level testing. Average criterion head movements during the suprasatiety test (black circles) or subsatiety test (white circles). Values are average ± SEM criterion movements per specified phase (x-axis, initial A phase, B phase, second A phase).
Animals underwent similar testing when drug level was clamped below satiety levels. A repeated measures ANOVA yielded a significant main effect of test phase for criterion head movements (Figure 5; white circles), F(2, 22) = 24.434, p < 10−5. In spite of noncontingent infusions of cocaine, experimentally maintaining drug levels below satiety significantly increased criterion head movements (pairwise comparison p < 10−3) during the subsatiety B phase, when compared with the initial A phase. Animals significantly decreased criterion head movements (p < 10−3) during the second A phase compared with the B phase but did not differ in comparison to the first A phase. The average ± SEM drug levels were 5.06 ± 0.29 mg/kg during the initial A phase, 3.66 ± 0.08 mg/kg during the subsatiety B phase, and 4.66 ± 0.20 mg/kg in the second A phase.
Discussion
Animals acquired drug self-administration responding in the form of a vertical criterion head movement that increased in number over days. Animals discriminated the vertical head movement as the operant, as evidenced by the finding that inverse criterion movements (downward movements starting at photocell 5 or 6 and ending at photocell 2 or 1) did not change over days. Task learning was evidenced by the observed decreased reaction time in response to the SD, increased probability of self-infusion when animals were presented with the SD, and escalated drug consumption with a decreasing latency to load to high drug levels over days. These data are consistent with lever press experiments using an SD (Ghitza et al. 2003; Root et al. 2009; Barker et al. 2010). It is possible that tonic food restriction used in the present experiment played a role in self-administration behavior. However, Bongiovanni and See (2008) observed no differences in response rates or cocaine intake per bodyweight in animals food restricted or fed ad libitum, suggesting our food restriction protocol did not cause deviations from normal self-administration behavior.
Visual inspection of behavior clearly showed increased locomotor behavior during the earliest training days followed by predominant focused stereotypy over the remaining training days, suggesting that animals became sensitized to the psychomotor effects of cocaine. However, available measures automated by the photocell corner operant device, e.g., the number of inverse criterion movements and vertical head movements shorter in distance than the criterion head movement, did not change over training days. These observations suggest that this device, similar to a lever, because it is discriminated as the manipulandum where responding produces cocaine infusion, may not allow for adequate assessment of behavioral sensitization.
Stemming from the observations of Pickens and Thompson (1968), animals use a lever to self-administer cocaine when below a certain satiety threshold. This led to the observation that animals do not press the lever to self-administer while above the satiety threshold (Pickens and Thompson 1971; Yokel and Pickens 1973; 1974; Dougherty and Pickens 1974; Wise 1987; Wise et al. 1995; Markou et al. 1999; Tsibulsky and Norman 1999; Norman et al. 2003; Lynch and Carroll 2001; Norman and Tsibulsky 2006; Olmstead et al. 2000; Panlilio et al. 2006). Consistent with these observations, the vertical head movement was used by animals as an operant similar to the lever press, in that criterion head movements were increased when drug levels were clamped below satiety and decreased when drug levels were clamped above satiety.
The observed decrease in criterion head movements during supra-satiety was not the result of stereotypy interfering with criterion head movements. All indices from automated data analyses and video monitoring of self-administration behavior indicate that animals differentiate stereotypy from operant responding, whether the operant is a head movement or a lever press. The present animals (12/13 rats) exhibited a locus of stereotypy away from the photocell corner. They demonstrated clear approaches toward the photocell corner, vertical responses within the photocell corner, and retreats away from the photocell corner to return to the locus of stereotypy. The stereotypical movements induced by cocaine (e.g., head-bobbing) were not the operant movements that earned cocaine, given that the average latency for the next movement after a criterion movement was approximately 30 seconds, longer than stimulant-induced unconditioned, stereotypical head movements detailed in previous studies occurring at approximately 10 Hz (Fowler et al 2003; 2007). These results are consistent with previous research demonstrating that animals can intracranially self-stimulate by pressing one lever while self-administering with another lever (Wise et al. 1977). Indeed, most animals (75%) in the present study exhibited at least one criterion movement during the supra-satiety phase. Specifically, stimulants induced circling, and head bobbing in one place. Taken together these results indicate that i) stereotypy does not interfere with operant responding and ii) stereotypical head movements were not the operant responses in the present task.
Rats self-administered cocaine for six hours/day for nearly one month. The specific set of circumstances (the photocell corner device and the vertical head movement operant) allowed us to address whether skilled cocaine self-administration developed in the rat. Although programmed criterion movement requirements remained constant, parameters of the operant movement changed over days, indicative of motor skill learning. The velocity of the criterion head movement increased over days as a function of decreasing duration, but not as a function of changed overall distance. In addition, animals increased the probability of starting criterion movements at photocell 2, the optimal starting movement position. In contrast, the probability of ending at photocell 5, the optimal ending movement position, did not change. It appears that over time, rats learn several aspects regarding the self-administration task. First, animals learn to respond in reaction to the SD (Ghitza et al. 2003; Root et al. 2009). Second, animals learn to discriminate the operant response, which becomes skilled with respect to movement parameters (velocity, duration) and efficiency (starting position), supporting Tiffany’s (1990) prediction that self-administration of drugs of abuse becomes highly skilled over extended use. Third, animals learn to control responding such that responding is increased during subsatiety and decreased during suprasatiety. One limitation of the present design was the lack of an additional neutral cue in addition to the SD. Discrimination training with two tones of different frequencies serving as S+ and S− may result in stronger stimulus control than that in which the S− is the absence of the S+ tone (Jenkins and Harrison, 1960). However, this approach also introduces more complexity, unpredictability, and expectations/anticipations resulting from S+/S− sequences. These factors were specifically unwanted in the present design, whose purpose was to reduce variability while maximizing familiarity and predictability, to avoid complicating the measurement of skill acquisition.
The nigrostriatal system, including the DLS, is important for skill learning. Compromised function results in motor deficits such as those observed in Parkinson’s patients (Marsden 1982; Ferro et al. 2005; Desmurget and Turner 2010), including deficits in acquiring motor programs (Flowers, 1975; Sheridan et al. 1987). Neurons in the DLS region receive topographic, convergent S1 and M1 innervation (Kunzle 1975; 1977; Selemon and Goldman-Rakic 1985; McGeorge and Faull 1989; West et al. 1990; Carelli and West 1991; Ebrahimi et al. 1992; Flaherty and Graybiel 1993; 1994; Kincaid and Wilson 1996), underlying their increased firing rates during somatosensory or motor manipulations of single body parts (Liles 1979; Crutcher and DeLong 1984a,b; Alexander and DeLong 1985; Liles and Updyke 1985; Carelli and West 1991; Mittler et al. 1994; Cho and West 1997) and no responsiveness to conditioned auditory cues (Root et al. 2010). It has recently been observed that DLS manipulations disrupt cocaine self-administration in rodents (Vanderschuren et al. 2005). Furthermore, drug-related video-induced cravings correlate with dopamine receptor availability in human DLS (Volkow et al. 2006; 2008). Thus, the demonstrated skill learning in the present experiment suggests the involvement of the DLS. However, this has not been directly tested using single unit recordings. The vertical head movement paradigm was developed to achieve compatibility between specific stimulant-induced movements and their representation in the DLS, namely, neurons related to vertical head movement. The present study represents the last in a series of studies validating the head movement paradigm as a model for studying DLS activity during extended stimulant self-administration (West et al, 1997; Pederson et al, 1997; Tang et al, 2007; Pawlak et al, 2010).
While not likely involved in the processing of skilled movements of specific body parts as observed in DLS, the ventral striatopallidal system is likely to process the criterion head movement as an operant. The accumbens is necessary for learning and performance of instrumental behavior (Atallah et al. 2007). Cells in the nucleus accumbens (Ghitza et al, 2004; Fabbricatore et al. 2010) and ventral pallidum (Root et al. 2010) exhibit changes in firing rate surrounding the seconds of cocaine-reinforced lever presses without exhibiting unconditioned forepaw-sensitive somatomotor firing properties. In contrast to the DLS with its discrete somatomotor firing properties, firing patterns exhibited following extensive cocaine self-administration in the ventral striatopallidal system might reflect approach, response, retreat, or other operant-related firing properties.
In conclusion, the present results demonstrate that cocaine self-administration can become skilled over extended drug use as previously hypothesized (Tiffany 1990). Furthermore, the vertical head movement appears to be used as an operant response similar to lever pressing with the additional benefits of enabling quantification of skill learning and analyses of simultaneously recorded DLS firing patterns.
Acknowledgements
We thank Linda King, Thomas Grace Sr., Jacqueline D. Thomas, and Jacquelin M. Mueller for technical assistance.
This research has been funded by grants from the National Institute on Drug Abuse; Contract grant numbers: DA 006886, DA 029873, DA 026252.
Abbreviations
- SD
discriminative stimulus
- DLS
dorsolateral striatum
Footnotes
There are no conflicts of interest.
References
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282(5387):298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J Neurophysiol. 1985;53:1417–1429. doi: 10.1152/jn.1985.53.6.1417. [DOI] [PubMed] [Google Scholar]
- Atallah HE, Lopez-Paniagua D, Rudy JW, O’Reilly RC. Separate neural substrates for skill learning and performance in the ventral and dorsal striatum. Nat Neurosci. 2007;10(1):126–131. doi: 10.1038/nn1817. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Root DH, Ma S, Jha S, Megehee L, Pawlak AP, West MO. Dose dependent differences in short ultrasonic vocalizations emitted by rats during cocaine self-administration. Psychopharmacology. 2010;211:435–442. doi: 10.1007/s00213-010-1913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongiovanni M, See RE. A comparison of the effects of different operant training experiences and dietary restriction on the reinstatement of cocaine-seeking in rats. Pharmacology, Biochemistry, and Behavior. 2008;89:227–233. doi: 10.1016/j.pbb.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carelli RM, West MO. Representation of the body by single neurons in the dorsolateral striatum of the awake, unrestrained rat. J Comp Neurol. 1991;309(2):231–249. doi: 10.1002/cne.903090205. [DOI] [PubMed] [Google Scholar]
- Cho J, West MO. Distributions of single neurons related to body parts in the lateral striatum of the rat. Brain Res. 1997;756(1-2):241–246. doi: 10.1016/s0006-8993(97)00143-1. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, DeLong MR. Single cell studies of the primate putamen. I. Functional organization. Exp Brain Res. 1984a;53(2):233–243. doi: 10.1007/BF00238153. [DOI] [PubMed] [Google Scholar]
- Crutcher MD, DeLong MR. Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res. 1984b;53(2):244–258. doi: 10.1007/BF00238154. [DOI] [PubMed] [Google Scholar]
- Desmerget M, Turner RS. Motor sequences and the basal ganglia: kinematics, not habits. JNeurosci. 2010;30(22):7685–7690. doi: 10.1523/JNEUROSCI.0163-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J, Pickens R. Effects of phenobarbital and SKF 525A on cocaine self-administration in rats. Drug Addiction. 1974;3:135–143. [Google Scholar]
- Ebrahimi A, Pochet R, Roger M. Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neurosci Res. 1992;14(1):39–60. doi: 10.1016/s0168-0102(05)80005-7. [DOI] [PubMed] [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat accumbens during cocaine self-administration: tonic firing patterns. Eur J Neurosci. 2009;30:2387–2400. doi: 10.1111/j.1460-9568.2009.07033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbricatore AT, Ghitza UE, Prokopenko VF, West MO. Electrophysiological evidence of mediolateral functional dichotomy in the rat accumbens during cocaine self-administration: phasic firing patterns. Eur J Neurosci. 2010;31:1671–1682. doi: 10.1111/j.1460-9568.2010.07230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci ME, Canteras NS, Da Cunha C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: histological, neurochemical, motor and memory alterations. J Neurosci Methods. 2005;148(1):78–87. doi: 10.1016/j.jneumeth.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Input-output organization of the sensorimotor striatum in the squirrel monkey. J Neurosci. 1994;14(2):599–610. doi: 10.1523/JNEUROSCI.14-02-00599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty AW, Graybiel AM. Two input systems for body representations in the primate striatal matrix: experimental evidence in the squirrel monkey. J Neurosci. 1993;13(3):1120–37. doi: 10.1523/JNEUROSCI.13-03-01120.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshler M, Hoffman HS. A Progression for generating variable interval schedules. Journal of the Experimental Analysis of Behavior. 1962;5:529–530. doi: 10.1901/jeab.1962.5-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers K. Ballistic and corrective movements on an aiming task. Intention tremor and parkinsonian movement disorders compared. Neurol. 1975;25:413–421. doi: 10.1212/wnl.25.5.413. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Birkenstrand B, Chen R, Vorontsova E, Zarcone T. Behavioral sensitization toamphetamine in rats: changes in the rhythm of head movements during focused stereotypies. Psychopharmacology. 2003;170(2):167–177. doi: 10.1007/s00213-003-1528-5. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Coviiczekngton HE, Miczek KA. Stereotyped and complex motor routines expressed during cocaine self-administration: results from a 24-h binge of unlimited cocaine access in rats. Psychopharmacology. 2007;192(4):465–478. doi: 10.1007/s00213-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26(13):3584–8. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO. Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci. 2003;23(19):7239–7245. doi: 10.1523/JNEUROSCI.23-19-07239.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Fabbricatore AT, Prokopenko VF, West MO. Differences between accumbens core and shell neurons exhibiting phasic firing patterns related to drug-seeking behavior during a discriminitive-stimulus task. J. Neurophysiol. 2004;92(3):1608–1614. doi: 10.1152/jn.00268.2004. [DOI] [PubMed] [Google Scholar]
- Green DG. Light adaptation in the rat retina: evidence for two receptor mechanisms. Science. 1971;174(9):598–600. doi: 10.1126/science.174.4009.598. [DOI] [PubMed] [Google Scholar]
- Jenkins HM, Harrison RH. Effect of discrimination training on auditory generalization. J Exp Psychol. 1960;59:246–253. doi: 10.1037/h0041661. [DOI] [PubMed] [Google Scholar]
- Kincaid AE, Wilson CJ. Corticostriatal innervation of the patch and matrix in the rat neostriatum. J Comp Neurol. 1996;374(4):578–592. doi: 10.1002/(SICI)1096-9861(19961028)374:4<578::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Künzle H. Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res. 1975;88(2):195–209. doi: 10.1016/0006-8993(75)90384-4. [DOI] [PubMed] [Google Scholar]
- Künzle H. Projections from the primary somatosensory cortex to basal ganglia and thalamus in the monkey. Exp Brain Res. 1977;30(4):481–492. doi: 10.1007/BF00237639. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21(8):2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles SL, Updyke BV. Projection of the digit and wrist area of precentral gyrus to the putamen: relation between topography and physiological properties of neurons in the putamen. Brain Res. 1985;339:245–255. doi: 10.1016/0006-8993(85)90089-7. [DOI] [PubMed] [Google Scholar]
- Liles SL. Topographic organization of neurons related to arm movement in the putamen. In: Chase TN, editor. Advances in Neurology. Raven Press; New York: 1979. [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Experimental and Clinical Psychopharmacology. 2001;9(2):131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- Markou A, Arroyo M, Everitt BJ. Effects of contingent and non-contingent cocaine on drug-seeking behavior measured using a second-order schedule of cocaine reinforcement in rats. Neuropsychopharmacology. 1999;20(6):542–555. doi: 10.1016/S0893-133X(98)00080-3. [DOI] [PubMed] [Google Scholar]
- Marsden CD. The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology. 1982;32(5):514–39. doi: 10.1212/wnl.32.5.514. [DOI] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29(3):503–37. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mittler T, Cho J, Peoples LL, West MO. Representation of the body in the lateral striatum of the freely moving rat: Single neurons related to licking. Exp Brain Res. 1994;98:163–167. doi: 10.1007/BF00229122. [DOI] [PubMed] [Google Scholar]
- Nayak PK, Misra AL, Mulé SJ. Physiological disposition and biotransformation of (3H) cocaine in acutely and chronically treated rats. J Pharmacol Exp Ther. 1976;196(3):556–69. [PubMed] [Google Scholar]
- Norman AB, Buesing WR, Norman MK, Tabet MR, Tsibulsky VL. The self-administration of WIN 35,428 and cocacine: comparisons of satiety threshold and elimination half-life in rats. Eur J of Pharmacology. 2003;483:281–287. doi: 10.1016/j.ejphar.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Norman AB, Tsibulsky VL. The compulsion zone: a pharmacological theory of acquired cocaine self-administration. Brain Res. 2006;1116(1):143–152. doi: 10.1016/j.brainres.2006.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Parkinson JA, Miles FJ, Everitt BJ, Dickinson A. Cocaine-seeking by rats: regulation, reinforcement, and activation. Psychpharmacology (Berl) 2000;152(2):123–131. doi: 10.1007/s002130000498. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Thorndike EB, Schindler CW. Cocaine self-administration under variable-dose schedules in squirrel monkeys. Pharmacol Biochem Behav. 2006;84(2):235–243. doi: 10.1016/j.pbb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Pardue MT, Ball SL, Hetling JR, Chow VY, Chow AY, Peachey NS. Visual evoked potentials to infrared stimulation in normal cats and rats. Doc Ophthalmol. 2001;103(2):155–162. doi: 10.1023/a:1012202410144. [DOI] [PubMed] [Google Scholar]
- Pederson CL, Wolske M, Peoples LL, West MO. Firing rate dependent effect of cocaine on firing of single neurons in the rat lateral striatum. Brain Research. 1997;760:261–265. doi: 10.1016/s0006-8993(97)00395-8. [DOI] [PubMed] [Google Scholar]
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: Effects of reinforcement magnitude and fixed-ratio size. J Pharmacol Exp Ther. 1968;161:122–129. [PubMed] [Google Scholar]
- Pickens R, Thompson T. Characteristics of stimulant drug reinforcement. In: Thompson T, Pickens R, editors. Stimulus properties of drugs. Appleton, Century, Croft, New York: 1971. pp. 177–192. [Google Scholar]
- Root DH, Fabbricatore AT, Barker DJ, Ma S, Pawlak AP, West MO. Evidence for Habitual and Goal-Directed Behavior Following Devaluation of Cocaine: A Multifaceted Interpretation of Relapse. PLoS ONE. 2009;4(9):e7170. doi: 10.1371/journal.pone.0007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DH, Tang CC, Ma S, Pawlak AP, West MO. Absence of cue-evoked firing in rat dorsolateral striatum neurons. Behavioural Brain Research. 2010;211(1):23–32. doi: 10.1016/j.bbr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MR, Flowers KA, Hurrell J. Programming and execution of movement in Parkinson’s disease. Brain. 1987;110(5):1247–71. doi: 10.1093/brain/110.5.1247. [DOI] [PubMed] [Google Scholar]
- Tang C, Pawlak AP, Prokopenko V, West MO. Changes in activity of the striatum during formation of a motor habit. Eur J Neurocsi. 2007;25(4):1212–1227. doi: 10.1111/j.1460-9568.2007.05353.x. [DOI] [PubMed] [Google Scholar]
- Tang CC, Root DH, Duke DC, Zhu Y, Teixeria K, Ma S, Barker DJ, West MO. Decreased firing of striatal neurons related to licking during acquisition and overtraining of a licking task. J Neurosci. 2009;29(44):13952–13961. doi: 10.1523/JNEUROSCI.2824-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97(2):147–68. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL, Norman AB. Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res. 1999;839:85–93. doi: 10.1016/s0006-8993(99)01717-5. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25(38):8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26(24):6583–6388. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage. 2008;39(3):1266–1273. doi: 10.1016/j.neuroimage.2007.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MO, Carelli RM, Gardner JP, Pomerantz M, Chapin JK, Woodward DJ. A region in the dorsolateral striatum of the rat exhibiting single-unit correlations with specific locomotor limb movements. J Neurophysiol. 1990;64:1233–1246. doi: 10.1152/jn.1990.64.4.1233. [DOI] [PubMed] [Google Scholar]
- West MO, Peoples LL, Michael AJ, Chapin JK, Woodward DJ. Low-dose amphetamine elevates movement-related firing of rat striatal neurons. Brain Research. 1997;745:331–335. doi: 10.1016/s0006-8993(96)01215-2. [DOI] [PubMed] [Google Scholar]
- Wise RA, Yokel RA, Hansson PA, Gerber GJ. Concurrent intracranial self-stimulation and amphetamine self-administration in rats. Pharmacol Biochem Behav. 1977;7(5):459–461. doi: 10.1016/0091-3057(77)90214-3. [DOI] [PubMed] [Google Scholar]
- Wise RA. Intravenous drug self-administration: A special case of positive reinforcement. In: Bozarth MA, editor. Methods of assessing the reinforcing properties of abused drugs. Springer-Verlag; New York: 1987. pp. 117–141. [Google Scholar]
- Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB., Jr Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 1995;120(1):10–20. doi: 10.1007/BF02246140. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Pickens R. Self-administration of optical isomers of amphetamine and methylamphetamine by rats. J Pharmacol Exp Ther. 1973;187(1):27–33. [PubMed] [Google Scholar]
- Yokel AR, Pickens R. Drug level of d- and l-amphetamine during intravenous self-administration. Psychopharmacologia. 1974;34:255–264. doi: 10.1007/BF00421966. [DOI] [PubMed] [Google Scholar]