Abstract
Our objective was to systematically review the data interrogating the association between gestational weight gain (GWG) and maternal and child health among women with twin gestations. We identified 15 articles of twin gestations that studied GWG in relation to a maternal, perinatal, or child health outcome and controlled for gestational age at delivery and prepregnancy body mass index. A positive association between GWG and fetal size was consistently found. Evidence on preterm birth and pregnancy complications was inconsistent. The existing studies suffer from serious methodological weaknesses, including not properly accounting for the strong correlation between gestational duration and GWG and not controlling for chorionicity. In addition, serious perinatal outcomes were not studied, and no research is available on the association between GWG and outcomes beyond birth. Our systematic review underscores that GWG in twin gestations is a neglected area of research. Rigorous studies are needed to inform future evidence-based guidelines.
Keywords: birth outcomes, maternal nutrition
Introduction
Maternal weight gain during pregnancy may have important implications for maternal and child health. Women with excessive gestational weight gain (GWG) are more likely to have high postpartum weight retention, which in turn can lead to prepregnancy obesity in future pregnancies and long-term maternal obesity (1–4). High GWG also increases the risk of gestational diabetes, cesarean delivery, macrosomia and childhood obesity (1, 2). Nevertheless, there are concerns that inadequate GWG increases the risk of infant mortality, preterm birth, and fetal growth restriction (1, 2). As maternal BMI modifies the association between GWG and health outcomes, determining the optimal range of GWG for women in each BMI category that balances the risks associated with low and high gain for maternal and child health is a public health priority (1).
There is a particularly urgent need to understand optimal GWG among women with twin gestations. The number of twin births in the United States has dramatically risen over the last 3 decades (5). This is a concern because twin pregnancies experience a disproportionate number of poor perinatal outcomes and obstetrical complications that are linked with low GWG in singletons (1), including a 2.7-fold increased risk of fetal death (6), 4-fold increased risk of death in the first month of life (7), and a 3.5-fold increased risk of small-for-gestational-age birth (8). Growth restriction in twins has been linked with long-term risks including higher adult fat mass (9). At the same time, mothers with twin gestations gain more weight during gestation than mothers of singletons (1), and more commonly experience diabetes, preeclampsia, and cesarean deliveries (10, 11)—outcomes typically associated with excessive weight gain. A greater accretion of maternal tissues and products of conception is expected in twin pregnancy, but, it is unknown whether the higher GWG in twin gestations is the result of enhanced physiologic and metabolic adaptations to pregnancy (12), and whether GWG patterns differ by chorionicity. For these reasons, singleton GWG guidelines are likely inappropriate for twin pregnancies.
Our objective was to systematically review the data interrogating the association between GWG and maternal and child health among women with twin gestations and to identify gaps in our understanding of this association.
Materials and Methods
Search strategy
We based the methodology of our systematic review on the PRISMA guidelines (13). In collaboration with a research librarian, we developed an electronic search strategy, which was then peer-reviewed by a second research librarian using an evidence based checklist (14). We searched Ovid MEDLINE, Embase, CINAHL and clinicaltrials.gov databases (Supplemental Digital Content). Advanced search criteria limited the search to humans and to English language papers. No restrictions were placed on study design. “Explosions” were used on the following MeSH terms: ‘weight gain’, ‘pregnancy’, ‘twins’, and ‘multiple pregnancy’. Keyword terms were: ‘maternal weight gain’, ‘gestational weight gain’, and ‘pregnancy’ within 2 words of ‘weight gain’. We also hand searched the bibliographies of selected studies.
Study selection
Two authors (L.M.B., S.J.P.) independently reviewed abstracts and titles for inclusion, classifying them as ‘relevant’, ‘potentially relevant’, and ‘not relevant.’ The same two authors reviewed the full text of ‘relevant’ and ‘potentially relevant’ articles for inclusion. Articles were included in the systematic review if they met the following criteria (1): studied GWG in twin pregnancies as an exposure variable (2) examined maternal, perinatal, or child health outcomes, such as birth weight (including small-for-gestational age), length of gestation (including preterm birth), cesarean section, preeclampsia, gestational diabetes mellitus, stillbirth, infant death, maternal postpartum weight retention, and offspring obesity, in relation to GWG in twin pregnancies. Disagreements were resolved through consultation with a third author (J.A.H.). Two authors (L.M.B., S.J.P.) then independently abstracted author names, publication year, sample size and characteristics, measurement of GWG and prepregnancy body mass index (BMI), outcomes, covariates, and results for all studies that met inclusion criteria. A third author (J.A.H.) evaluated the abstracted data for consistency.
Synthesis of study quality and findings
For each included article, we evaluated key components of study quality:
Did the measure of GWG account for length of gestation?
Women with longer pregnancies have more time to gain weight. To disentangle the impact of low GWG on maternal and offspring health outcomes from the impact of earlier gestational age at delivery (i.e. increased risks due to preterm birth), the measure of GWG must account for this built in correlation between gestational duration and total weight gain.
Did the study control for prepregnancy BMI?
BMI is a strong determinant of GWG, and extremes of prepregnancy BMI are risk factors for a number of adverse maternal and child health outcomes. As a result, it is critical that studies control for the potential confounding effects of prepregnancy BMI.
Given the importance of gestational age and prepregnancy BMI as confounders of GWG associations, we only summarized findings from studies that controlled for these covariates. These variables could be controlled for by a) restriction, b) matching, c) stratification, or d) adjustment. Controlling for gestational age through the use of a GWG measure that accounted for gestational age (e.g. average rate of GWG or adequacy of GWG in relation to the weight gain for gestational age recommended by the IOM) was accepted. The use of ideal body weight in lieu of BMI was also accepted.
We evaluated studies based on five additional elements of quality, but did not require these for studies to be included in our summary of results:
Were results presented separately for women in each prepregnancy BMI category? BMI modifies the impact of GWG on maternal and child health outcomes (1). For example, the relative risk of small-for-gestational age birth is greater with a low weight gain in a lean woman than low weight gain in an obese woman. Therefore, IOM weight gain guidelines differ by prepregnancy BMI category, with lower weight gains recommended as prepregnancy BMI increases.
Was GWG based on self-reported or measured weight at delivery? The IOM advocates for the use of measured weights instead of recalled weights in the study of gestational weight gain to decrease potential measurement error and misclassification bias (1).
Was pattern of GWG studied? While studying total GWG is useful, knowledge of optimal timing and rate of GWG in twin pregnancies will enable clinicians to provide effective prenatal GWG counseling.
Were chorionicity and assisted reproductive technologies examined as confounders or effect modifiers? Approximately 20% of all twin gestations have monochorionic placentation. Monochorionic twin are at greater risk for poor perinatal outcomes compared with dichorionic pregnancies in large part due to the risk of twin-twin transfusion syndrome and discordant fetal growth restriction (15). It is unknown whether chorionicity influences GWG in twin pregnancies, and thus its role as confounder and effect modifier needs to be evaluated. Likewise, little is known about the potential role of assisted reproductive technologies (16) as a counfounder or effect modifier in GWG research.
Did the study control for covariates other than gestational age and pregravid BMI that may confound GWG-adverse outcome associations? Factors such as maternal race/ethnicity, age, smoking, and socioeconomic indicators often act as confounders in studies of GWG in singleton pregnancies, and should be evaluated in twins as well.
Was gestational age confirmed by ultrasound? Accurate assessment of gestational age is needed not only for determining preterm birth, but also for controlling for length of pregnancy in the measurement of GWG, as noted above.
Given the heterogeneity of GWG measurements, populations and outcomes, we opted not to perform additional analyses such as meta-analysis or meta-regression.
Results
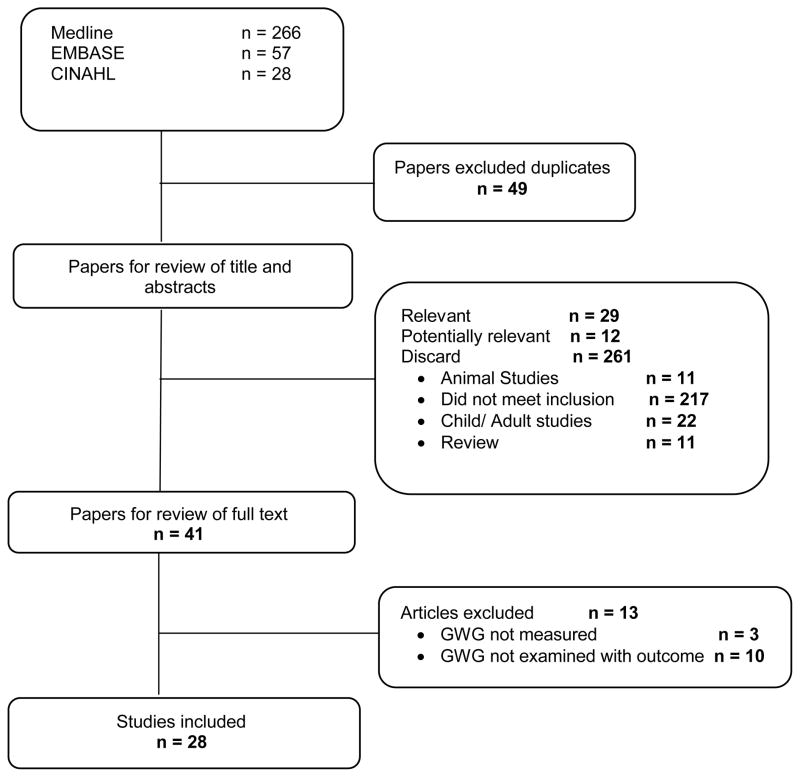
Of 351 articles retrieved using our search strategy, 28 articles met our final inclusion criteria (Figure 1). No additional studies were retrieved through the review of study bibliographies. All of these studies used cohort or case-control designs. There were no registered trials in clinicaltrials.gov. Two articles (17, 18) were excluded because subsequent studies were published examining the same study outcomes with an updated study population, leaving 26 studies (19–44).
Figure 1.
Study search flow chart
Table 1 presents the descriptions of the 25 articles and assessments of their study quality. Control for gestational age was performed in 18 of the 25 studies (19–22, 24–30, 32, 33, 35, 36, 38, 42, 45), for prepregnancy BMI in 17 studies (20–24, 26–28, 30, 32, 33, 35, 36, 38, 39, 42, 44, 45) (all of which defined BMI based on recalled prepregnancy weight), chorionicity and/or assisted reproductive technologies in 3 studies (27, 32, 33), and other covariates in 11 studies (20, 22, 24, 27, 32, 36–38, 41, 42, 44). Nine presented results stratified by prepregnancy BMI categories (21, 22, 26–28, 30, 35, 39) (including one which presented results only for normal weight women (27)). Seventeen studies based their measure of GWG on self-reported prepregnancy weight and a last measured prenatal weight (19, 20, 25–27, 30–33, 35, 38–43, 45), and 9 studied pattern of GWG (19, 25, 30, 32, 35, 37, 38, 41, 42). Half of the studies reported using ultrasound-confirmed gestational age (20, 25–27, 30–33, 37, 38, 43).
Table 1.
Description of studies retrieved through systematic search of gestational weight gain in twin pregnancies and evaluation of their quality
| Study | Sample and Setting | Controlled for | Results stratified by BMI | GWG based on measured weight 1 | Pattern of GWG | U/S conirmed GA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| GA | Pre-gravid BMI | Chorionicity | ART | other co-variates | ||||||
| Studies reviewed2 | ||||||||||
| Bohni et al. 2011 (20) | 173 gravidae without preexisting conditions or TTTS who delivered liveborn twins; Swiss hospital 2004–08 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Brown & Schloesser 1990 (21) | 503 gravidae delivering liveborn twins 38–42 wk; Kansas linked birth-death records 1980–86; 88% White, 8% Black | ✔ | ✔ | ✔ | ||||||
| Chu & D’Angelo 2009 (22) | 6345 gravidae delivering liveborn twins at ≥34 weeks; PRAMS data from 28 US states and New York City 2001–06; 69% White, 17% Black, 10% Hispanic | ✔ | ✔ | ✔ | ✔ | |||||
| Eller et al. 1993 (24) | 80 gravidae delivering twins at ≥28 weeks without TTTS; Charleston, SC 1988–93 | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Fox et al. 2010 (26) | 297 gravidae with pregravid BMI >18.5 who delivered twins at ≥37 weeks and did not transfer care; New York private maternal-fetal medicine practice 2005–09; >90% White | ✔ | ✔ | ✔ | ✔ | |||||
| Fox et al. 2011 (27) | 170 gravidae with pregravid BMI >18.5 who delivered twins at ≥37 weeks and did not transfer care; New York private maternal-fetal medicine practice 2005–09; >90% White | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ NL only | ✔ | ✔ | |
| Gónzalez-Quintero et al. 2012 (28) | 5123 gravidae with pregravid BMI ≥18.5 delivering twins at ≥24 wk; High-risk pregnancies from an Atlanta insurance plan 2004–10; 6.5% Black | ✔ | ✔ | ✔ | ||||||
| Lantz et al. 1996 (30) | 189 gravidae without preeclampsia or GDM who delivered liveborn twins; Tampa, Florida hospital 1981–93 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Luke & Leurgans 1996 (36) | 924 mothers attending Twins Days Festival in Ohio 1989–91, 1993; 100% White | ✔ | ✔ | ✔ | ||||||
| Luke et al. 1993a (38) | 163 gravidae delivering liveborn twins at 28–41 weeks without PE or GDM and BMI ≤29.6; Hospitals of Johns Hopkins 1979–89 and Northwestern 1983–89; 34% White, 66% Black | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Luke et al. 1993b (35) | 222 gravidae delivering liveborn twins at 28–41 weeks without PE or GDM and BMI ≤29.6; Hospitals of Johns Hopkins 1979–89 and Northwestern 1983–89; 34% White, 66% Black | ✔ | ✔ | ✔ | ✔ | ✔ | ||||
| Luke et al. 1998 (37) | 1564 gravidae delivering liveborn twins at ≥28 weeks; University hospitals of Johns Hopkins 1979–89, Miami 1990–99, Charleston 1988–99, and Michigan 1993–99; 37% White, 19% Hispanic, 43% Black | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Luke et al. 2003 (33) | 2324 gravidae delivering liveborn twins at ≥28 weeks; University hospitals of Johns Hopkins 1979–89, Miami 1990–99, Charleston 1988–99, and Michigan 1993–99; 37% White, 19% Hispanic, 43% Black | ✔ | ✔ | |||||||
| Luke et al. 2004 (32) | 218 gravidae delivering liveborn twins who did not transfer care; University of Michigan Health Systems 1996–2001 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Schwendemann et al. 2005 (42) | 11,827 gravidae with preterm labor symptoms delivering non-LGA twins; Kentucky database of women with preterm labor symptoms 1988–2002; 14% Black | ✔ | ✔ | ✔ | ✔ | |||||
| Studies not reviewed 2 | ||||||||||
| Ayustawati et al. 2003 (19) | 186 gravidae delivering twins at >35 weeks; Japan 1993–2000 | ✔ | ✔ | ✔ | ||||||
| Colletto & Segre 2005 (23) | 381 twin pregnancies; Brazil 1990–99 | |||||||||
| Fenton & Thirsk 1994 (25) | 100 gravidae aged 20–35 years with BMI 19–28 and delivering liveborn twins at ≥36 wk; Alberta, Canada 1980–89 | ✔ | ✔ | ✔ | ✔ | |||||
| Inde et al. 2010 (29) | 340 gravidae delivering twins Tokyo, Japan hospital 2003–08 | ✔ | ||||||||
| Lee et al. 2007 (31) | 174 gravidae without GDM or hypertension during pregnancy who delivered liveborn twins without congenital anomalies; Korean IVF center 2002–04 | ✔ | ✔ | |||||||
| Luke et al. 1991 (34) | 275 mothers attending Twins Days Festival, Ohio 1989–91, 1993; 100% White | |||||||||
| Pederson et al., 1989(39) | 217 gravidae delivering twins from a Swedish Hospital Medical Center, Seattle 1982–86; 84% White | ✔ | ✔ | ✔ | ||||||
| Riese et al. 2003 (40) | 199 gravidae delivering twins; Kentucky 1984–96 | ✔ | ||||||||
| Rydhstroem & Walles 1996 (41) | 144 gravidae delivering twins at ≥28 weeks; Swedish medical birth registry 1973–89 | ✔ | ✔ | ✔ | ||||||
| Soriano et al. 2002 (43) | 44 gravidae delivering twins; Israeli IVF pregnancies 1996 | ✔ | ✔ | |||||||
| Yeh & Shelton 2007 (44) | 1342 gravidae delivering liveborn twins; Upstate New York birth records 1999–2003; 84% White, 12% Black, 2% Hisp | ✔ | ✔ | |||||||
ART, assisted reproductive technologies; BMI, body mass index; GDM, gestational diabetes; GWG, gestational weight gain; LGA, large-for-gestational-age; PE, preeclampsia; TTTS, twin-twin transfusion syndrome; U/S, ultrasound
Based on the last measured weight during pregnancy and self-reported prepregnancy weight.
Studies were reviewed if they controlled for both gestational age and prepregnancy BMI.
We summarized the findings of the 16 studies that controlled for both gestational age and prepregnancy BMI in their evaluations of GWG in relation to adverse outcomes. Table 2 synthesizes these findings, while Table 3 (infant outcomes) and Table 4 (maternal outcomes) provide detailed descriptions of the results.
Table 2.
Synthesis of findings relating GWG to infant and maternal outcomes based on significance testing
| Study | n | Fetal growth | Gestational age | Optimal neonatal outcome | GDM | Gest HTN |
|---|---|---|---|---|---|---|
| Bohni et al. 2011 (20) | 173 | + | ||||
| Brown & Schloesser 1990 (21) | 503 | UW: + NL: + OV: null OB: + Sev OB: null |
||||
| Chu & D’Angelo 2009 (22) | 6345 | UW: + NL: + OV: + OB: + |
||||
| Eller et al. 1993 (24) | 80 | + | ||||
| Fox et al. 2010 (26) | 297 | NL: generally + OV: null OB: null |
NL: generally null OV: − OB: null |
NL: null OV: null OB: null |
NL: null OV: null OB: null |
|
| Fox et al. 2011 (27) | 170 | + NL: + |
null NL: null |
null NL: null |
||
| Gónzalez-Quintero et al. 2012 (28) | 5123 | NL: + OV: + OB: + |
NL: + OV: + OB: + |
NL: − OV: null OB: null |
NL: + OV: + OB: null |
|
| Lantz et al. 1996 (30) | 189 | UW: + NL: generally + OV: null |
||||
| Luke & Leurgans 1996 (36) | 924 | + | ||||
| Luke et al. 1993a (38) | 163 | + | + | + | ||
| Luke et al. 1993b (35) | 222 | + | ||||
| Luke et al. 1998 (37) | 1564 | + | ||||
| Luke et al. 2003 (33) | 2324 | ** | ||||
| Luke et al. 2004 (32) | 218 | + | ||||
| Schwendemann et al. 2005 (42) | 11,827 | + |
Data not provided to determine relationship
+, −, or null indicates a positive, negative, or null associations, respectively, between GWG and the outcome.
GDM, gestational diabetes; Gest HTN, gestational hypertension; UW, underweight; NL, normal weight; OB, obese; OV, overweight
Table 3.
Summary of findings on gestational weight gain in twin pregnancies and infant outcomes among studies that controlled for prepregnancy BMI and gestational age at delivery
| Study | GWG measure | Outcome defined | Control variables | Results |
|---|---|---|---|---|
| Fetal growth | ||||
| Bohni et al. 2011 (20) | Total GWG | Twin BW<10th %ile; Mean twin BW<2500g; placental weight | Pregravid BMI, GA, parity, smoking, maternal age | Total GWG was 2.1 kg greater in mothers with both twins’ BW>10th percentile than in mothers with one or both twins’ BW<10th percentile (p=0.02) and was 2.2 kg greater among mothers of twins with mean BW≥2500g than mothers with mean BW <2500g (p=0.03). Total GWG was not associated with placental weight (beta=0.003, p=.19). |
| Brown & Schloesser 1990 (21) | Total GWG | Twin BW | None | Total GWG was lower for term deliveries of infants <2501 g and 2501–3000 g vs. term deliveries of infants 3001–3500 g in UW (35.1 lb and 39.3 lb vs. 44.2 lb, p<0.05) and NL (31.2 lb and 37.9 lb vs. 40.9 lb, p<0.05). In OB total GWG was lower for deliveries of infants <2501 g vs. 3001–3500 g (29.5 lb vs. 37.2 lb, p<0.05). There were no differences for OW or severely OB. |
| Chu & D’Angelo 2009 (22) | Total GWG | LBW (BW<2500g) of 1 randomly selected twin | GA and maternal age | LBW decreased as total GWG increased from ≤14 lb to ≥65 lb for NL (61.2% to 35.6 lb, p<0.05); OV (61.4% to 44.0%, p<0.05); OB (52.7% vs. 48.0%, p<0.05) as well as from 15–24 lb to 55-64 lb for UW (87.2% vs. 54.9%, p<0.05). |
| Eller et al. 1993 (24) | Rate of total GWG | Mean twin BW centile, mean twin BW ≥25th percentile | Race, smoking, GA, % ideal body weight | The rate of total GWG was positively associated with mean twin BW centile (p<0.05). Weekly rate of gain was higher in mothers delivering twins with mean BW centile ≥25th %ile (1.01 lb/wk) than those with mean BW centile <25th %ile (0.81 lb/wk, p-value not shown). |
| Fox et al. 2010 (26) | Adequacy of GWG 1 | Both twins BW >1000g, >1500 g, 2500 g; Any twin <5% of birth weight standard | None | Normal total GWG vs. low total GWG % of both twins >1000 g NL: 97.5% vs. 91.2%, p<0.05 OV: 100% vs. 94.1%, p=0.15 OB: 100% vs. 100% % of both twins >1500 g NL: 92.6% vs. 87.5%, p=0.23 OV: 97.1% vs. 88.2%, p=0.21 OB: 100% vs. 100% % of both twins >2500 g NL: 38.8% vs. 22.5%, p=0.02 OV: 41.2% vs. 29.4%, p=0.41 OB: 47.6% vs. 50%, p=0.91. % of any twin <5% BW standard NL: 21.5% vs. 35.0%, p=0.03 OV: 23.5% vs. 17.6%, p=0.63 OB: 28.6% vs. 25%, p=0.85 Results were similar when limited to births ≥37 weeks |
| Fox et al. 2011 (27) | Adequacy of GWG 1 | Twin BW at ≥37 wk; Both twins BW >2500 g at ≥37 wk | Race, pregravid BMI, age, IVF, GA, chorionicity, fetal reduction | Poor total GWG vs. normal total GWG vs. excessive total GWG Larger twin BW at ≥37 wks: Overall: 2,699.1g vs. 2,887.2g vs. 3,011.2g, p<0.001 NL: 2,679.5g vs. 2,805.1g vs. 2,991.6g, p<0.001 Smaller twin BW at ≥37 wks: Overall: 2,475.2g vs. 2,578.8g vs. 2,711.9g, p=0.002 NL: 2,441.3g vs. 2,504.5g vs. 2,669.5g, p=0.004 % of both twins >2500 g at ≥37 wks: Overall: 40.0% vs. 60.5% vs. 79.5%, p<0.001 NL: 32.6% vs. 56.1% vs. 76%, p=0.001 |
| Gónzalez-Quintero et al. 2012 (28) | Total GWG compared with the 2009 IOM recs (<IOM vs. ≥IOM) | Both twins BW >1500g and >2500g | None | Total GWG <IOM vs. ≥IOM % both twins BW >1500g Overall: 85.3% vs. 92.4%, p<0.001 NL: 86.4% vs. 92.8%, p<0.001 OV: 86.1% vs. 91.7%, p<0.01 OB: 81.3% vs. 92.1%, p<0.001 % both twins BW >2500g Overall: 24.4% vs. 37.3%, p<0.0001 NL: 22.0% vs. 33.9%, p<0.001 OV: 28.6% vs. 40.7%, p<0.001 OB: 26.6% vs. 42.3%, p<0.001 |
| Lantz et al. 1996 (30) | Rate of GWG <20 wk and ≥20 wk | Both twins ≥2500g | None | Both twins BW ≥2500g vs. both twins BW <2500g Rate of gain 0–20wk UW: 1.13 lb/wk vs. 0.70 lb/wk, p=0.02 NL: 0.61 lb/wk vs. 0.75 lb/wk, p=ns OV: 0.46 lb/wk vs. 0.38 lb/wk, p=ns Rate of gain 20wk to delivery UW: 1.92 lb/wk vs. 1.29 lb/wk, p=0.03 NL: 1.63 lb/wk vs.1.29 lb/wk, p=0.05 OV: 1.85 lb/wk vs. 1.74 lb/wk, p=ns Rate of total GWG UW: 1.42 lb/wk vs. 0.95 lb/wk, p<0.001 NL: 1.15 lb/wk vs. 0.88 lb/wk, p=0.04 OV: 1.07 lb/wk vs. 0.85 lb/wk, p=ns |
| Luke et al. 1993a (38) | Rate of GWG <24 wk and ≥24 wk | Twin BW; BW ratio (twin BW divided by singleton BW standard at 50th %ile; BW ≤10th% | Race, parity, smoking, pregravid BMI, GA, twin gender discordancy | Rate of total GWG that was low (≤0.85 lb/wk) at <24 weeks and low (<1 lb/wk) at ≥24 weeks was positively associated with sum of twins’ BW (beta=-0.20, p<0.001), BW of smaller twin (beta=-0.19, p<0.001), BW of larger twin (beta=-0.20, p<0.001), sum of twins BW ratio (beta=-0.24, p<0.001), smaller twin BW ratio (beta=-0.22, p<0.001), larger twin BW ratio (beta=-0.26, p<0.001), smaller twin BW ≤10th% (aOR 6.99 (95% CI: 2.1, 23.7) p<0.005), and larger twin BW ≤10th% (aOR 5.33 (95% CI: 1.9, 14.8), p<0.005). |
| Luke & Leurgans 1996 (36) | Total GWG | Mean twin BW; Mean twin z-score | Pregravid BMI, smoking | Total GWG associated with increased average twin BW (beta=4.6, p<0.001) and average twin BW z-score (beta=0.02, p<0.001). |
| Luke et al. 1998 (37) | Rate of GWG <20 wk, 20–28 wk, 28–36 wk | Rate of fetal growth, sum of twin BW | Race, parity, smoking, pregravid BMI, GA, maternal age |
Rate of the sum of fetal growth 20–28 wk GWG <20 wk (per lb gained): beta=0.55 g/wk, p<0.001 GWG 20–28 wk (per lb gained): beta=1.98 g/wk, p<0.001 Rate of the sum of fetal growth 28–36 wk GWG <20 wk (per lb gained): beta=0.84 g/wk, p<0.01 GWG 20–28 wk (per lb gained): beta=2.99 g/wk, p<0.001 Sum of twin pair BW GWG <20 wk (per lb gained): beta=14.15 g, p<0.001 GWG 20–28 wk (per lb gained): beta=20.45 g, p<0.001 GWG 28–36 wk (per lb gained): beta=8.72 g, p<0.05 if not preeclamptic; beta=1.38 if preeclamptic |
| Luke et al. 2004 (32) | Rate of GWG at <20 weeks | <10th %ile for fetal femur length, AC, HC; <10th %ile for any of the 3 measures | Race, short stature, parity, smoking, chorionicity, fetal gender, fetal reduction, insurance, ART, preeclampsia | GWG <0.65 lb/wk at <20 weeks was associated with an increased odds of fetal femur length <10th% at 28wks (aOR 3.39 95% CI: 1.34, 8.59, p<0.05) and having a fetus with a femur length, AC, or HC <10th% at 28 wks (aOR 2.62 95% CI: 1.14, 5.99, p<0.01). |
| Schwendemann et al. 2005 (42) | Rate of GWG after signs of preterm labor | At least 1 SGA infant | Prepregnancy BMI, smoking | The OR (95% CI) for delivering at least 1 SGA infant was 1.35 (1.16, 1.56) for GWG <0.5 lb/wk (referent group not identified) and 0.77 (0.68, 0.86) for GWG >1 lb/wk (referent group not identified). |
| Preterm birth | ||||
| Fox et al. 2010 (26) | Adequacy of total GWG1 | GA at delivery; PTB and sPTB <37, <35, <32, <28 weeks; indicated PTB (not defined) | None | Normal total GWG vs. low total GWG GA at delivery NL: 36.0 vs. 35.4 wk, p=0.14 OV: 36.2 vs. 34.4 wk, p=0.01 OB: 36.7 vs. 36.5 wk, p=0.78 % PTB <37 weeks; % sPTB <37 weeks NL: 57.9% vs. 62.5%, p=0.51; 46.9% vs. 55.2%, p=0.29; OV: 50.0% vs. 64.7%, p=0.32; 26.1% vs. 60.5%, p=0.03 OB: 47.6% vs. 50.0%, p=0.90; 33.3% vs. 42.9%, p=0.66 % PTB <35 weeks; % sPTB <35 weeks NL: 19.8% vs. 25%, p=0.39; 16.5% vs. 21.1%, p=0.43 OV: 20.6% vs. 52.9%, p=0.02; 13.3% vs. 46.7%, p=0.01 OB: 9.5% vs. 25%, p=0.28; 9.5% vs. 25%, p=0.28 % PTB <32 weeks; % sPTB <32 weeks NL: 5.0% vs. 13.8%, p=0.02; 3.4% vs. 11.5%, p=0.02 OV: 0% vs. 17.6%, p=0.01; 0% vs. 17.0%, p=0.01 OB: 4.8% vs. 0%, p=0.53; 4.8% vs. 0%, p=0.53 % PTB <28 weeks; % sPTB <28 weeks NL: 1.7% vs. 6.2%, p=0.08; 1.7% vs. 6.2%, p=0.08 OV: 0% vs. 5.9%, p=0.15; 0% vs. 5.9%, p=0.15 OB: 0% vs. 0%, p=na; 0% vs. 0%, p=na % Indicated PTB NL: 19.9% vs. 15.0%, p=0.49 OV: 32.4% vs. 11.4%, p=0.11 OB: 14.3% vs. 12.5%, p=0.9 |
| Gónzalez-Quintero et al. 2012 (28) | Rate of total GWG compared with the 2009 IOM recs (<IOM vs. ≥IOM) 1 | GA; sPTB <37, <35, <32, <28 wk | None | Normal total GWG vs. low total GWG % sPTB <37 weeks; % sPTB <35 weeks; % sPTB <32 weeks; % sPTB <28 weeks All: 33.3% vs. 41.4%; 16.4% vs. 24.0%; 4.4% vs. 9.0%; 1.1% vs. 3.5%, all p<0.001 NL: 34.5% vs. 44.2%; 16.7% vs. 24.9%; 4.3% vs. 8.6%; 1.0% vs. 3.1%; all p<0.0001 OV: 31.8% vs. 39.5%; 16.2% vs. 23.5%; 4.6% vs. 8.2%; 1.1% vs. 3.1%, all p<0.05 OB: 35.3% vs. 32.0% p=0.3; 16.2% vs. 21.9% p<0.05; 4.5% vs. 11.2%, p<0.001; 1.4% vs. 5.0%, p<0.01 |
| Luke et al. 1993a (38) | Rate of GWG <24 wk and ≥24 wk | PTB 28–32 wk, 33–34wk, 35–36 wk, 37–38 wk, 39–41 wk | None | The percent of mothers with low early (<24 weeks) rate of GWG (≤0.85 lb/wk) and low late (≥24 weeks) rate of GWG (<1 lb/wk) decreased as GA at delivery increased: 28–32 wk (29%) to 39–41 wk (10%, p<0.05). The % of mothers with low early rate of GWG (≤0.85 lb/wk) and high late rate of GWG (≥1 lb/wk) decreased as GA at delivery increased: 28–32 wk (3%) to 39–41 wk (24%, p<0.05). |
| “Optimal outcome” | ||||
| Luke et al. 1993a (38) | Rate of GWG <24 wk and ≥24 wk | A) BW of smaller twin >10th and ≤8 day hospital stay B) BW of smaller twin >10th, ≤8 day hospital stay, and GA 35–38 wk |
Race, parity, smoking, pregravid BMI, GA, twin gender discordancy | Having a low early (<24 weeks) rate of GWG (≤0.85 lb/wk) and a low late (≥24 weeks) rate of GWG (<1 lb/wk) was associated with an increased odds of an adverse neonatal outcome (defined as a pregnancy that did not meet the criteria for ideal outcome A or B) (aOR 4.26 95% CI:1.3, 14.3, p<0.05; aOR 3.35, 95% CI: 1.0, 10.9, p<0.05, respectively). Having a low early rate of GWG and high late rate of GWG (≥1 lb/wk) was associated with a reduced odds of an adverse neonatal outcome (defined as a pregnancy that did not meet the criteria for ideal outcome B): aOR 0.31, 95% CI: 0.13, 0.74, p<0.001. High early/high late rate of GWG and high early/low late rate of GWG were not associated with either outcome (adjusted results not shown). |
| Luke et al. 2003 (33) | Rate of GWG 0–20 wk, 21–28 wk, >28 wk | Optimal BW (2850–2928g at 36–38 wk), optimal fetal growth (105–110g/wk from 20–28 wk; 155–161g/wk from 28wk to term) | Smoking, parity, pregestational diabetes, GDM, PE, chorionicity, fetal growth <20 weeks | Authors’ conclusions on optimal rates of GWG at 0–20 wk, 21–28 wk, and 29 wk-delivery, respectively: UW: 0.57–0.79 kg/wk; 0.68–0.79 kg/wk; 0.57 kg/wk NL: 0.45–0.68 kg/wk; 0.57–0.79 kg/wk; 0.45 kg/wk OV: 0.45–0.57 kg/wk; 0.45–0.68 kg/wk; 0.45 kg/wk OB: 0.34–0.45 kg/wk; 0.34–0.57 kg/wk; 0.34 kg/wk |
AC, abdominal circumference; aOR, adjusted odds ratio; ART, assisted reproductive technologies; BMI, body mass index; BW, infant birth weight; CI, confidence interval; GA, gestational age; GDM, gestational diabetes; GWG, gestational weight gain; HC, head circumference; IOM, Institute of Medicine; IVF, in vitro fertilization; NL, normal weight before pregnancy; OB, obese before pregnancy; OV, overweight before pregnancy; PE, preeclampsia; PTB, preterm birth; sPTB, spontaneous preterm birth; UW, underweight before pregnancy
Rate of total GWG compared with the 2009 IOM recommendations divided by 37 weeks.
Table 4.
Summary of findings on gestational weight gain in twin pregnancies and maternal outcomes among studies that controlled for prepregnancy BMI and gestational age at delivery
| Study | GWG measure | Outcome defined | Control variables | Results |
|---|---|---|---|---|
| Gestational diabetes | ||||
| Fox et al. 2010 (A) (26) and 2011 (B) (27) | Adequacy of GWGa | GDM (definition not specified) | A: None B: Race, pregravid BMI, age, IVF, GA, chorionicity, fetal reduction |
A: No difference in GDM prevalence comparing low GWG vs. normal GWG in NL (6.2% vs. 4.1%, p=0.5), OV (11.8% vs. 8.8%, p=0.7) or OB (0% vs. 30%, p=0.08). B: No difference in GDM prevalence comparing low, normal, and excessive GWG overall (7.3%, 7.9%, 2.6%, p=0.15) or among NL (7.0%, 2.0%, 0%, p=0.9). |
| Gónzalez-Quintero et al. 2012 (28) | Rate of total GWG compared with the 2009 IOM recs (<IOM vs. ≥IOM) a | GDM (definition not specified) | None | Higher prevalence of GDM in those gaining below IOM guidelines vs. at or above guidelines overall (12.7% vs. 8.7%, p<0.0001) and in NL (11.6% vs. 7.2%, p<0.0001), but not in OV (11.6% vs. 8.7%, p=0.1) or OB (17.3% vs. 13.2%, p=0.09). |
| Hypertensive disorders of pregnancy | ||||
| Fox et al. 2010 (A) (26) and 2011 (B) (27) | Adequacy of GWGa | Gestational hypertension; preeclampsia (both defined using 2002 ACOG criteria) | A: None B: Race, pregravid BMI, age, IVF, GA, chorionicity, fetal reduction |
A: No difference in gestational hypertension prevalence comparing low GWG vs. normal GWG in NL (13.9% vs. 19.8%, p=0.2), OV (11.8% vs. 21.2%, p=0.4) or OB (25.0% vs. 20.0%, p=0.7). B: No difference in gestational hypertension prevalence comparing low, normal, and excessive GWG overall (12.7%, 9.2%, 13.2%, p=0.9) or among NL (7.0%, 8.2%, 12.0%, p=0.6). No difference in preeclampsia prevalence comparing low, normal, and excessive GWG overall (7.3%, 7.9%, 10.5%, p=0.8) or among NL (7.0%, 8.2%, 12.0%, p=0.8). |
| Gónzalez-Quintero et al. 2012 (28) | Rate of total GWG compared with the 2009 IOM recs (<IOM vs. ≥IOM) a | Pregnancy-related hypertension (definition not specified) | None | Lower prevalence of pregnancy-related hypertension in those gaining below IOM guidelines vs. at or above guidelines overall (16.3% vs. 22.5%, p<0.0001), in NL (12.2% vs. 17.4%, p<0.0001), and in OV (17.7% vs. 27.2%, p=0.001), but not OB (26.3% vs. 30.9, p=0.1) |
| Luke et al. 1993b (35) | Total GWG and rate of GWG at ≤24 wk and >24 wks | Preeclampsia (defined using the 1986 ACOG criteria) | None | Compared with controls, preeclamptic pregnancies had higher total GWG (21.1 kg vs. 17.2 kg, p<0.0001), higher rate of total GWG (0.61 kg/wk vs. 0.54 kg/wk, p<0.0001), and higher rate of GWG at >24 weeks (0.97 kg/wk vs. 0.66 kg/wk, p<0.0001), but there was no difference in rate of GWG ≤24 wk (0.47 kg/wk vs. 0.44 kg/wk, p=ns). |
ACOG, American Congress of Obstetricians and Gynecologists; ART, assisted reproductive technologies; BMI, body mass index; GA, gestational age; GDM, gestational diabetes; GWG, gestational weight gain; IOM, Institute of Medicine; IVF, in vitro fertilization; NL, normal weight before pregnancy; OB, obese before pregnancy; OV, overweight before pregnancy;
Rate of total GWG compared with the 2009 IOM recommendations divided by 37 weeks.
Fetal growth
Twelve articles reported on GWG and fetal growth, and they consistently demonstrated that after accounting for differences in gestational duration, mothers with twin pregnancies who gained less weight were more likely than those with higher weight gain to deliver infants that were lighter at birth and were more likely to be SGA (20, 24, 27, 28, 36) (Tables 2 and 3). Similar findings were observed in the studies that stratified by prepregnancy BMI (21, 22, 26–28, 30), but results were not always statistically significant due to reduced sample sizes. In general, a low rate of GWG in early and late pregnancy was associated with SGA or reduced birth weight in all women (38, 42) or only among lean women (30). A positive association between rate of GWG in early (<20 weeks), mid (20–28 weeks) and late (>28 weeks) pregnancy and ultrasound fetal measurements was noted in two studies (33, 45).
Preterm birth
Three studies examined the relation between GWG and preterm birth (Tables 2 and 3). Two studied total GWG relative to many preterm birth phenotypes. One found that rate of total GWG was positively associated with length of gestation in all BMI groups (28). The other reported generally null findings, but small sample size likely limited their power to detect effects (26). A third study (35) examined pattern of early (<24 weeks) and late GWG (≥24 weeks) relative to preterm birth. They found that women with low GWG in both periods were more likely to deliver preterm, while women with low early gain and high late gain were less likely to deliver early.
Optimal neonatal outcome
Two studies were available on GWG relative to ‘optimal’ twin neonatal outcome, and each defined ‘optimal’ differently (Tables 2 and 3). One study reported that having a low rate of GWG in early pregnancy (<24 weeks) and a high rate of gain in late (≥24 weeks) pregnancy was associated with delivering liveborn infants at 35–38 weeks who weighed ≥10th percentile and had ≤8 day hospital stays (38). A second study presented data on the range of GWG rates at 0–20, 21–28, and ≥29 weeks among mothers with ‘optimal’ neonatal outcomes (fetal growth 105–110 grams per week from 20–28 weeks, 155–161 grams per week from 28 weeks to term and birth weight 2850–2928g at 36–38 weeks), but did not present data to support these conclusions (33).
Adverse maternal outcomes
Three articles examined total GWG in relation to hypertensive disorders of pregnancy in women with twin gestations (26–28) and results were mixed (Tables 2 and 4). However, these authors examined GWG at the time of delivery rather than at the time of diagnosis, as recommended by the IOM (1). As a result, the data are difficult to interpret because the complications themselves are likely to influence total GWG. Only one study reported on rate of GWG before the diagnosis of the pregnancy complication (≤24 weeks). This article found no differences in the rate of GWG in the first half of pregnancy among women who subsequently went on to develop preeclampsia compared with those who did not develop preeclampsia (35).
Comment
We performed a systematic review of the evidence linking GWG to maternal and child health among women with twin pregnancies, and reviewed 15 observational studies that met our minimum criteria of controlling for gestational age in their measure of GWG and considering maternal BMI as a confounder. Most of this literature has focused on the outcome of fetal growth and provides consistent evidence to support a positive association between GWG and fetal size. However, relations were not always statistically significant when stratified by prepregnancy BMI, often due to small sample sizes. Only a few studies have examined other outcomes in twin pregnancies, such as preterm birth, gestational diabetes, or hypertensive disorders, and findings of these studies have been inconsistent.
The available evidence is limited by several major methodological concerns. First, all existing studies are observational. Although the findings from observational studies that we summarized all controlled for prepregnancy BMI and gestational age, other factors that may confound reported relations, such as use of assisted reproductive technologies, maternal race/ethnicity, smoking, parity, maternal age, and socioeconomic position were often not considered and confounding bias may result. Consequently, it is unknown whether weight causes a poor outcome or whether it may be a consequence of an underlying pathology that causes the poor outcome. Future studies must address the causality of GWG in twin pregnancies because the publishing of GWG guidelines by the IOM and other groups presupposes that modifying weight gain will improve health.
Second, none of the studies properly accounted for the strong correlation between gestational duration and GWG. Women with longer pregnancies have more time to gain weight. Studies linking total GWG to adverse pregnancy outcomes such as preterm birth or fetal growth may therefore be biased because the impact of low total GWG on adverse pregnancy outcomes cannot be disentangled from the impact of earlier gestational age at delivery (e.g., increased risks of poor fetal growth may be due to preterm birth, not low GWG). Calculating a rate of total GWG (GWG divided by gestational age) or a rate of weight gain within the first half of pregnancy (<20 weeks or <24 weeks) does not remove the correlation between gestational duration and total weight gain (46). This is because GWG is not linear throughout twin pregnancy (women gain less weight in the first trimester than in the second and third trimester (20)). Limiting to term pregnancies (e.g. 37–42 weeks) does not resolve the issue either, as women will continue to gain weight throughout the term period. This approach also results in a highly selective sample because more than half of twin pregnancies are born at <37 weeks (47). Methods recently developed to address the correlation between gestational duration and GWG in singletons, such as maternal weight gain for gestational age z-scores, should be explored in twins (20, 48).
Third, of the 12 articles that studied GWG in relation to fetal growth, absolute birth weight (e.g. low birth weight <2500g, very low birth weight <1500g, or mean birth weight) was the only outcome evaluated in four studies (21, 22, 28, 30) and was one of several other fetal growth measures in five other studies (20, 26, 36–38). Using absolute birth weight as an outcome is problematic because it cannot separate infants who are small due to preterm delivery from infants who are small due to intrauterine growth restriction (49). A better approach involves using birth weight for gestational age percentiles. Importantly, no studies used outcomes that reflect the complexity of fetal growth in twin pregnancies, such as twin growth discordance (50).
Fourth, only 3 studies accounted for chorionicity in their analysis. Twin gestations with monochorionic placentation are at higher perinatal health risk than dichorionic pregnancies (15). Although it is unknown whether GWG differs by chorionicity, it is critical that studies evaluate the potential for chorionicity to confound or modify effects on adverse maternal and child outcomes.
Fifth, all studies of gestational diabetes and 3 of the 4 studies on preeclampsia reported on total GWG at delivery, which fails to account for the temporality of the diagnosis. Gestational diabetes and preeclampsia themselves are likely to influence total GWG (1, 51, 52). Studies that examine gestational diabetes or preeclampsia require data on the pattern of GWG or GWG measured before the diagnosis of these conditions. Gestational diabetes and preeclampsia have been linked with GWG before diagnosis in singleton pregnancies (1), and this research question is important to explore in twins.
Finally, many studies were based on small sample sizes, and none of the studies provided sample size justifications to determine if they had sufficient statistical power. For example, one study of preterm birth in a sample of 297 women had only 29 obese women after stratifying by BMI categories (26). As this and other studies were likely underpowered to detect differences in rare outcomes such as preeclampsia, gestational diabetes, and early preterm birth in subgroups, future studies with adequate statistical power will be required. Increasing sample sizes either by taking advantage of vital records data, hospital databases with large delivery volumes, or extending the duration of the study period may be worthwhile pursuits.
To aid in the development of evidence-based clinical GWG guidelines for twin pregnancies, we recommend that more attention be paid to key elements of study design. As detailed in Table 5, the methodologic considerations to guide future research include accurate GWG and gestational age measurements; sufficient sample size; adequate control for relevant confounders; and stratification of results by prepregnancy BMI category. Moreover, studies are needed to fill the following critical knowledge gaps:
Table 5.
Methodologic considerations to guide the design of studies on gestational weight gain in twin pregnancies
|
What is the effect of GWG on serious adverse perinatal outcomes? Suboptimal GWG has been associated with increased risk of stillbirth and infant death in singleton pregnancies after controlling for gestational length (1), but no rigorous studies have been done in twins.
What are the consequences of high GWG in twin pregnancies on maternal postpartum weight retention and childhood obesity? Despite the links between high GWG and maternal and offspring obesity in singleton pregnancies (1, 3, 4, 53), not a single research study has been published on this topic in twin gestations. With the current epidemic rates of obesity in the U.S., and the higher weight gains observed in twin pregnancies, an understanding of the role of this modifiable risk factor is essential to ensure the health and well-being of mothers and offspring.
Is GWG in twins associated with other longer-term outcomes? Poor GWG has been associated with offspring neurocognitive impairment in singletons (54) and may be relevant to twins as well.
What are optimal GWG ranges for women with extremes of prepregnancy BMI (i.e., underweight and severe obesity) who have twin pregnancies? Severe obesity in U.S. childbearing-aged women has tripled in the past 30 years (55), and the risk of adverse perinatal outcomes rise as obesity worsens (56, 57). Low GWG may attenuate these risks in women with twin gestations, as some research has suggested in singletons (58, 59). Yet, only one study in our review (21) evaluated severely obese mothers with twin pregnancies and was substantially underpowered to detect effects (n=33 mothers). Future studies should stratify results by severe obesity with adequate sample size to inform the short- and long-term safety of lower weight gain in this high-risk group. The IOM Committee had insufficient evidence to provide even provisional guidelines for underweight women. Although underweight is uncommon, these women are at risk of poor birth outcomes (1) and understanding how GWG may modify these risks is important.
Associations between GWG and outcomes in diverse, population-based cohorts. Research studies on twin GWG are primarily based on self-selected samples of white mothers. Reports based on GWG in diverse cohorts are needed to ensure that guidelines are generalizable and promote the health of all mothers and children in the U.S.
Causality of associations. Randomized trials of GWG interventions are needed to evaluate whether poor or excessive weight gains in twin gestations cause adverse outcomes for mothers and children. But first, careful observational studies must be conducted to inform the design of such trials. For instance, observational data in twin pregnancies can be used to identify the BMI-specific GWG ranges, diet and other lifestyle practices that are associated with optimal outcomes, which can then be the targets for lifestyle interventions.
Supplementary Material
Acknowledgments
Sources of support: This project was supported by NIH grant R01 NR014245 (PI: L Bodnar & J Hutcheon).
Footnotes
Reprints will not be available.
The authors declare no conflicts of interest.
LMB, BA, and JAH designed the research; LMB, SJP, JAH reviewed the literature; LMB and JAH wrote the paper; SJP, BA, and KPH assisted with the data interpretation and critically edited the paper; All authors read and approved the final manuscript; LMB had primary responsibility for final content.
References
- 1.IOM. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academies Press; 2009. [PubMed] [Google Scholar]
- 2.Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. Outcomes of Maternal Weight Gain, Evidence Report/Technology Assessment No. 168. AHRQ Publication No. 08-E-09. Rockville, MD: Agency for Healthcare Research and Quality; 2008. [PMC free article] [PubMed] [Google Scholar]
- 3.Olson CM. A call for intervention in pregnancy to prevent maternal and child obesity. Am J Prev Med. 2007;33(5):435–6. doi: 10.1016/j.amepre.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Oken E. Maternal and child obesity: the causal link. Obstet Gynecol Clin North Am. 2009;36(2):361–77. ix–x. doi: 10.1016/j.ogc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Martin JA, Hamilton BE, Osterman MJK. Three decades of twin births in the United States, 1980–2009. Hyattsville, MD: National Center for Health Statistics; 2012. [Google Scholar]
- 6.MacDorman MF, Kirmeyer S. The challenge of fetal mortality. NCHS data brief, no 16. Hyattsville, MD: National Center for Health Statistics; 2009. [PubMed] [Google Scholar]
- 7.Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Kirmeyer S, Mathews TJ, Wilson EC. Births: Final data for 2009. Hyattsville, MD: National Center for Health Statistics; 2011. [PubMed] [Google Scholar]
- 8.Alexander GR, Kogan M, Martin J, Papiernik E. What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin Obstet Gynecol. 1998;41(1):114–25. doi: 10.1097/00003081-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Skidmore PM, Cassidy A, Swaminathan R, Richards JB, Mangino M, Spector TD, MacGregor AJ. An obesogenic postnatal environment is more important than the fetal environment for the development of adult adiposity: a study of female twins. American Journal of Clinical Nutrition. 2009;90(2):401–6. doi: 10.3945/ajcn.2008.27269. [DOI] [PubMed] [Google Scholar]
- 10.Luke B, Brown MB. Contemporary risks of maternal morbidity and adverse outcomes with increasing maternal age and plurality. Fertility and Sterility. 2007;88(2):283–93. doi: 10.1016/j.fertnstert.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. QuickStats: Percentage of live births by cesarean delivery, by plurality--United States, 1996, 2000, 2006. MMWR. 2009;58(9):542. [Google Scholar]
- 12.IOM. Nutrition during Pregnancy. Washington, D.C: National Academy Press; 1990. [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson M, McGowan J, Cogo E, Grimshaw J, Moher D, Lefebvre C. An evidence-based practice guideline for the peer review of electronic search strategies. J Clin Epidemiol. 2009;62(9):944–52. doi: 10.1016/j.jclinepi.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham FG. Williams Obstetrics. 23. New York, USA: McGraw-Hill Professional; 2009. [Google Scholar]
- 16.Rossi AC, D’Addario V. Neonatal outcomes of assisted and naturally conceived twins: systematic review and meta-analysis. Journal of Perinatal Medicine. 2011;39(5):489–93. doi: 10.1515/jpm.2011.058. [DOI] [PubMed] [Google Scholar]
- 17.Luke B, Gillespie B, Min SJ, Avni M, Witter FR, O’Sullivan MJ. Critical periods of maternal weight gain: effect on twin birth weight. American Journal of Obstetrics & Gynecology. 1997;177(5):1055–62. doi: 10.1016/s0002-9378(97)70014-0. [DOI] [PubMed] [Google Scholar]
- 18.Luke B, Minogue J, Abbey H, Keith L, Witter FR, Feng TI, Johnson TRB. The association between maternal weight gain and the birth weight of twins. Journal of Maternal-Fetal Medicine. 1992;1(5):267–76. [Google Scholar]
- 19.Ayustawati, Matsubara S, Minakami H, Ohkuchi A, Izumi A, Sato I. Symphysis-fundus height and weight gain pattern in Japanese women with twin pregnancies. Journal of Reproductive Medicine. 2003;48(4):277–82. [PubMed] [Google Scholar]
- 20.Bohni SC, Roos M, Kurmanavicius J, Zimmermann R, Ochsenbein-Kolble N. New reference curves on maternal weight gain in twin pregnancy. Geburtshilfe und Frauenheilkunde. 2011;71(11):979–84. [Google Scholar]
- 21.Brown JE, Schloesser PT. Prepregnancy weight status, prenatal weight gain, and the outcome of term twin gestations. American Journal of Obstetrics & Gynecology. 1990;162(1):182–6. doi: 10.1016/0002-9378(90)90845-x. [DOI] [PubMed] [Google Scholar]
- 22.Chu SY, D’Angelo DV. Gestational weight gain among US women who deliver twins, 2001–2006. American Journal of Obstetrics & Gynecology. 2009;200(4):390, e1–6. doi: 10.1016/j.ajog.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Colletto GMDD, Segre CAM. Lack of effect of maternal body mass index on anthropometric characteristics of newborns in twin gestations. Genet Mol Res. 2005;4(1):47–54. [PubMed] [Google Scholar]
- 24.Eller DP, Newman RB, Ellings JM, Campbell BA, Miller MC., III Modifiable determinants of birth weight variability in twins. Journal of Maternal-Fetal Investigation. 1993;2(5):254–9. [Google Scholar]
- 25.Fenton TR, Thirsk JE. Twin pregnancy: the distribution of maternal weight gain of non-smoking normal weight women. Can J Public Health. 1994;85(1):37–40. [PubMed] [Google Scholar]
- 26.Fox NS, Rebarber A, Roman AS, Klauser CK, Peress D, Saltzman DH. Weight gain in twin pregnancies and adverse outcomes: examining the 2009 Institute of Medicine guidelines. Obstetrics & Gynecology. 2010;116(1):100–6. doi: 10.1097/AOG.0b013e3181e24afc. [DOI] [PubMed] [Google Scholar]
- 27.Fox NS, Saltzman DH, Kurtz H, Rebarber A. Excessive weight gain in term twin pregnancies: examining the 2009 Institute of Medicine definitions. Obstetrics & Gynecology. 2011;118(5):1000–4. doi: 10.1097/AOG.0b013e318232125d. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Quintero VH, Kathiresan ASQ, Tudela FJ, Rhea D, Desch C, Istwan N. The association of gestational weight gain per institute of medicine guidelines and prepregnancy body mass index on outcomes of twin pregnancies. American Journal of Perinatology. 2012;29(6):435–40. doi: 10.1055/s-0032-1304824. [DOI] [PubMed] [Google Scholar]
- 29.Inde Y, Satomi M, Iwasaki N, Ono S, Yamashita E, Igarashi M, Hiraizumi Y, Murata T, Miyake H, Suzuki S. Maternal risk factors for small-for-gestational age newborns in Japanese dichorionic twins. Journal of Obstetrics & Gynaecology Research. 2011;37(1):24–31. doi: 10.1111/j.1447-0756.2010.01301.x. [DOI] [PubMed] [Google Scholar]
- 30.Lantz ME, Chez RA, Rodriguez A, Porter KB. Maternal weight gain patterns and birth weight outcome in twin gestation. Obstetrics & Gynecology. 1996;87(4):551–6. doi: 10.1016/0029-7844(95)00485-8. [DOI] [PubMed] [Google Scholar]
- 31.Lee KJ, Hur J, Yoo J. Twin weight discordance and maternal weight gain in twin pregnancies. International Journal of Gynaecology & Obstetrics. 2007;96(3):176–80. doi: 10.1016/j.ijgo.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Luke B, Brown MB, Hediger ML, Nugent C, Misiunas RB, Anderson E. Fetal phenotypes and neonatal and early childhood outcomes in twins. American Journal of Obstetrics & Gynecology. 2004;191(4):1270–6. doi: 10.1016/j.ajog.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Luke B, Hediger ML, Nugent C, Newman RB, Mauldin JG, Witter FR, O’Sullivan MJ. Body mass index--specific weight gains associated with optimal birth weights in twin pregnancies. Journal of Reproductive Medicine. 2003;48(4):217–24. [PubMed] [Google Scholar]
- 34.Luke B, Keith L, Johnson TR, Keith D. Pregravid weight, gestational weight gain and current weight of women delivered of twins. Journal of Perinatal Medicine. 1991;19(5):333–40. doi: 10.1515/jpme.1991.19.5.333. [DOI] [PubMed] [Google Scholar]
- 35.Luke B, Keith L, Lopez-Zeno JA, Witter FR, Saquil E. A case-control study of maternal gestational weight gain and newborn birth weight and birthlength in twin pregnancies complicated by preeclampsia. Acta Geneticae Medicae et Gemellologiae. 1993b;42(1):7–15. doi: 10.1017/s0515283600042232. [DOI] [PubMed] [Google Scholar]
- 36.Luke B, Leurgans S. Maternal weight gains in ideal twin outcomes. Journal of the American Dietetic Association. 1996;96(2):178–81. doi: 10.1016/S0002-8223(96)00050-8. [DOI] [PubMed] [Google Scholar]
- 37.Luke B, Min SJ, Gillespie B, Avni M, Witter FR, Newman RB, Mauldin JG, Salman FA, O’Sullivan MJ. The importance of early weight gain in the intrauterine growth and birth weight of twins. American Journal of Obstetrics & Gynecology. 1998;179(5):1155–61. doi: 10.1016/s0002-9378(98)70124-3. [DOI] [PubMed] [Google Scholar]
- 38.Luke B, Minogue J, Witter FR, Keith LG, Johnson TR. The ideal twin pregnancy: patterns of weight gain, discordancy, and length of gestation. American Journal of Obstetrics & Gynecology. 1993a;169(3):588–97. doi: 10.1016/0002-9378(93)90628-v. [DOI] [PubMed] [Google Scholar]
- 39.Pederson AL, Worthington-Roberts B, Hickok DE. Weight gain patterns during twin gestation. Journal of the American Dietetic Association. 1989;89(5):642–6. [PubMed] [Google Scholar]
- 40.Riese ML, Swift HM, Barnes SL. Newborn twin outcome predicted by maternal variables: differentiation by term and sex. Twin Research. 2003;6(1):12–8. doi: 10.1375/136905203762687843. [DOI] [PubMed] [Google Scholar]
- 41.Rydhstroem H, Walles B. Lack of correlation between maternal body weight or weight gain and stillbirth in twin pregnancy. Gynecol Obstet Invest. 1996;42(1):8–12. doi: 10.1159/000291879. [DOI] [PubMed] [Google Scholar]
- 42.Schwendemann WD, O’Brien JM, Barton JR, Milligan DA, Istwan N. Modifiable risk factors for growth restriction in twin pregnancies. American Journal of Obstetrics & Gynecology. 2005;192(5):1440–2. doi: 10.1016/j.ajog.2004.12.071. [DOI] [PubMed] [Google Scholar]
- 43.Soriano D, Weisz B, Seidman DS, Chetrit A, Schiff E, Lipitz S, Achiron R. The role of sonographic assessment of cervical length in the prediction of preterm birth in primigravidae with twin gestation conceived after infertility treatment. Acta Obstetricia et Gynecologica Scandinavica. 2002;81(1):39–43. doi: 10.1046/j.0001-6349.2001.00466.x. [DOI] [PubMed] [Google Scholar]
- 44.Yeh J, Shelton JA. Association of pre-pregnancy maternal body mass and maternal weight gain to newborn outcomes in twin pregnancies. Acta Obstetricia et Gynecologica Scandinavica. 2007;86(9):1051–7. doi: 10.1080/00016340701417026. [DOI] [PubMed] [Google Scholar]
- 45.Luke B. What is the influence of maternal weight gain on the fetal growth of twins? Clin Obstet Gynecol. 1998;41(1):56–64. doi: 10.1097/00003081-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Hutcheon JA, Bodnar LM, Joseph KS, Abrams B, Simhan HN, Platt RW. The bias in current measures of gestational weight gain. Ped Perinatal Epidem. 2012;26:109–16. doi: 10.1111/j.1365-3016.2011.01254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chauhan SP, Scardo JA, Hayes E, Abuhamad AZ, Berghella V. Twins: prevalence, problems, and preterm births. American journal of obstetrics and gynecology. 2010;203(4):305–15. doi: 10.1016/j.ajog.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 48.Hutcheon JA, Platt RW, Abrams B, Himes KP, Simhan HN, Bodnar LM. A weight-gain-for-gestational-age z score chart for the assessment of maternal weight gain in pregnancy. Am J Clin Nutr. 2013;97(5):1062–7. doi: 10.3945/ajcn.112.051706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. American Journal of Epidemiology. 1991;134(6):604–13. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 50.ACOG Practice Bulletin #56: Multiple gestation: complicated twin, triplet, and high-order multifetal pregnancy. Obstetrics and gynecology. 2004;104(4):869–83. doi: 10.1097/00006250-200410000-00046. [DOI] [PubMed] [Google Scholar]
- 51.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352(24):2477–86. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 52.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Jr, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361(14):1339–48. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birth weight, fetal growth, and postpartum weight retention. American Journal of Obstetrics and Gynecology. 2009;201(4):339, e1–14. doi: 10.1016/j.ajog.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Gage SH, Lawlor DA, Tilling K, Fraser A. Associations of Maternal Weight Gain in Pregnancy With Offspring Cognition in Childhood and Adolescence: Findings From the Avon Longitudinal Study of Parents and Children. American journal of epidemiology. 2013 doi: 10.1093/aje/kws239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 56.Siega-Riz AM, King JC. Position of the American Dietetic Association and American Society for Nutrition: obesity, reproduction, and pregnancy outcomes. J Am Diet Assoc. 2009;109(5):918–27. doi: 10.1016/j.jada.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 57.Salihu HM, Lynch O, Alio AP, Liu J. Obesity subtypes and risk of spontaneous versus medically indicated preterm births in singletons and twins. American journal of epidemiology. 2008;168(1):13–20. doi: 10.1093/aje/kwn092. [DOI] [PubMed] [Google Scholar]
- 58.Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010;91(6):1642–8. doi: 10.3945/ajcn.2009.29008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstet Gynecol. 2007;110(4):752–8. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.