Abstract
Mast cells are found abundant at sites of acupoints. Nerve cells share perivascular localization with mast cells. Acupuncture (mechanical stimuli) can activate mast cells to release adenosine triphosphate (ATP) which can activate nerve cells and modulates pain-processing pathways in response to acupuncture. In this paper, a mathematical model was constructed for describing intracellular Ca2+ signal and ATP release in a coupled mast cell and nerve cell system induced by mechanical stimuli. The results showed mechanical stimuli lead to a intracellular Ca2+ rise in the mast cell and ATP release, ATP diffuses in the extracellular space (ECS) and activates the nearby nerve cells, then induces electrical current in the nerve cell which spreads in the neural network. This study may facilitate our understanding of the mechanotransduction process induced by acupuncture and provide a methodology for quantitatively analyzing acupuncture treatment.
Keywords: Mast Cell, Nerve Cell, Ca2+ Signaling, ATP Release, Acupuncture.
1 Introduction
Mechanical stimuli have been applied to the skin for medical treatment in traditional Chinese medicine (TCM). As one basic technique in TCM, acupuncture is a method of applying mechanical stimuli by inserting needles into specific parts (acupoints) abundant of mast cells 1. When the needle is twirled, lifted and thrusted, the winding of collagen on the acupuncture needle changes the interstitial microenvironment 2-3. Although western medicine has treated acupuncture with considerable skepticism 4, emerging research implies acupuncture is effective for some conditions. In 2004, the NIH issued a Consensus Statement on Acupuncture, supporting the use of acupuncture for adult postoperative and chemotherapy nausea and vomiting, symptomatic control of pain, gastrointestinal disease treatment and stroke rehabilitation, etc 5. However, the biological basis of the acupuncture effects remains unknown. Recent emerging evidence suggests the possibility that mast cell-nerve cell interaction contributes to modulating signal transmission pathways 6.
Mast cells are common at sites that are in close contact with the external environment (skin, gastrointestinal tract and airways), they are distributed in virtually all organs and vascularized tissues 7. They are best known for their involvement in allergic reactions, in which IgE attaches to the Fc ε receptor on the mast cell surface and leads to a variety of mediators release after cross linking of surface-bound IgE by allergen. Nowadays, mast cells are found abundant at sites of acupoints 8. Our experiments showed acupuncture resulted in a remarkable increase in degranulation of the mast cells 9. Pretreatment of the acupuncture point with disodium chromoglycate (DSCG, mast cell stabilizer) not only counteracted the phenomenon of degranulation but also reduced analgesic effect of acupuncture 8. Further experiments showed mechanical stimuli can activate mast cells and allow Ca2+ inflow into the cell, which in turn induce mast-cell degranulation and mediators release 10-11. These evidences demonstrate the possible role of mast cells in acupuncture effects. Nerve cells share perivascular localization with mast cells 12. The specificity of mast cell-nerve cell spatial contacts has been demonstrated in vitro 13 and in vivo 14. Mast cell-nerve cell associations have been found in the myocardium 15, diaphragm 16, brain 17, gallbladder, ileum, mesentery, and skin of a variety of animals at both the anatomic and molecular levels, pointing to their fundamental interdependence 18. Mast cells are able to take up, store, and release a variety of biogenic amines through which they participate in physiological reactions. Mast cells' mediators can sensitize sensory neurons, which further activates mast cells by releasing neurotransmitters or neuropeptides. It is supposed purinergic receptors may be involved in mast cell-nerve cell interactions 19; e.g. ATP gates purinergic receptors, plays a key role in transmission of pain signals in the body 20, ATP releases from mast cells when activated, ATP activates membrane current in nerve cells then modulate body function.
Mathematical models have been developed for describing signal transmission pathways for mast cells and nerve cells respectively. Xu et al. has established a nociceptor model to study the underlying mechanisms in the process of skin thermal pain 21-22. Their model can contribute to the understanding of biothermomechanical- neurophysiological behavior of skin tissue 23. Bennet et al. proposed an astrocytes network model to investigate Ca2 + wave 24. In their models, activation of purinergic receptors by extracellular ATP is an important component of the response to pain in the neural system; Em depolarizations and intracellular Ca2 + waves propagation are the main signal transmission. Zhou et al. investigated action potential through a single branch point of a myelinated nerve fiber with a parent branch bifurcating into two identical daughter branches 25. To investigate the mechanism of mast cells' response to physical stimuli, Shi et al. has set up a mathematical model to simulate intracellular Ca2 + signals and degranulation to laser radiation 26. We have set up a mathematical model to explore signaling pathways in mast cells and clarified mast cells participating in acupuncture effect 27. However, there exist no mathematical models investigating signal transmission pathways modulated by acupuncture based on mast cell-nerve cell interaction.
In this paper, we construct a mathematical model for describing intracellular Ca2+ signal and ATP release in a coupled mast cell and nerve cell system induced by mechanical stimuli. We introduce methods of the coupled model in section 2 and present numerical simulations under a variety of conditions in section 3. This model may explain the mechanotransduction pathway induced by mechanical stimuli.
2 Methods
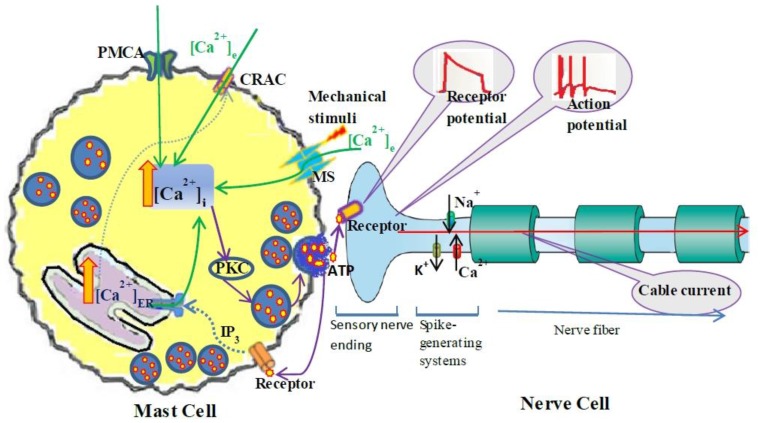
The dynamic process of mechanotransduction pathway induced by mechanical stimuli is simplified as following (Fig. 1): Acupuncture (mechanical stimuli) activates mechano-sensitive (MS) ion channels on mast cells membrane 10 and allow Ca2+ entry, local intracellular Ca2+ rise induces a cascade of intracellular signaling events including ATP release, therefore, increase interstitial adenosine and ATP concentrations at acupoint 28-29, ATP diffuses in ECS and activates adjacent nerve terminals and triggers action potential, which will spread in neural network (through branches of nerve fiber) and modulate the multiple pain-processing pathways in response to acupuncture.
Figure 1.
Schematic diagram of the coupled mast cell and nerve cell model. Mast Cell is activated by mechanical stimuli; the steps leading from MS channel activation to Ca2+ release from the calcium store (ER) into the cytosol and ATP release into ECS. ATP diffuses in ECS and binds to purinergic receptors of adjacent nerve terminals (sensory neuron) and triggers action potential which induces passive electrical flow spreading along branches of nerve fiber in neural networks.
2.1 Continuum model
Micro-tissue around acupoint is described as axisymmetric continuum and polar coordinates are employed. A mast cell (MC0) locates at r=0 and the tissue space occupied by other cells is considered as homogeneous. Nerve cells locate some distance (r=Dis, Dis=20, 40, 60, · · ·, 1000 µm) from MC0 and can be activated by neurotransmitter (ATP) released from mast cells. To make the problem computationally tractable, the specific geometries of mast cell and nerve cell are not considered. Since this is a continuum model, the concept of individual cell is not meaningful. Cellular properties refer to the average values at any location within the tissue. The model includes three compartments: mast cell cytosolic space, nerve cells (sensory neuron and its fibers) cytosolic space and the ECS. ECS is considered as one continuum space, the corresponding interstitial ions and neurotransmitters concentrations are described in polar coordinates by standard reaction-diffusion equations of the form
 |
(1) |
where DATP,e is the interstitial diffusion coefficient of ATP and [ATP]production is ATP release from cells.
We supposed ATP releases only from mast cell. It was proposed that PKC acts as the important agent that triggers bio-mediators release from mast cell 20, 27.
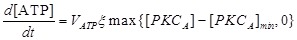 |
(2) |
where VATP is ATP production rate. [PKCA] is the active PKC concentration, [PKCA]min is the threshold concentration, which is necessary to prevent small amounts of [PKCA] from being amplified and thus leading to ATP release. ξ is a parameter that accounts for depletion of ATP inside the cell. ξ has initial value 1 and decreases according to 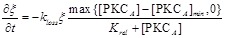 , where kloss is the depletion rate parameter, and Krel is the kinetic parameter 30.
, where kloss is the depletion rate parameter, and Krel is the kinetic parameter 30.
2.2 Ion currents in mast cell
The major stage in this process is Ca2+ entry through ion channels in membrane. Ca2+ currents between cells and ECS are described in Fig. 1. There are MS (mechano-sensitive) Ca2+ current (ICa, MS), CRAC (Ca2+ release-activated Ca2+) current (ICRAC) and PMCA (plasma membrane Ca2+-ATPase) Ca2+ current (IPMCA) in mast cells.
The second stage is the regulation of metabotropic receptor activities. It starts from the binding of ligand (ATP) to receptors and leads to, via a G-protein cascade, the production of IP3 and the release of Ca2+ from endoplasmic reticulum (ER) 17, 31. ER behaves as a Ca2+ store and exchanges Ca2+ with the cytosol via IP3 sensitive channels (JIp3), calcium pumps (Jpump) and leaks (Jleak), as described by Bennett et al 30.
The detailed processes are given in one of our paper online first 27.
2.3 Ion currents in nerve cell
The Hodgkin-Huxley (HH) model considered the long axon of a nerve cell with two ions, Na+ and K+, we add Ca2+ in addition. Besides following the same equations of HH model, we added ATP-activated channels according to recent research 32. Tan et al. found four types (F, I, S and VS) of ATP-activated cationic current and the VS type dominates the total current; therefore, we include VS current in the model. Including other three types of current makes no essential difference to the results of the model. We use GHK model to describe the VS current (Eq.3) which is suitable when there is a large difference in concentrations in the intracellular space (ICS) and ECS compartment 33.
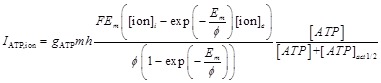 |
(3) |
Where gATP is the permeability of ATP-activated channel per second; F is the Faraday constant; Em is the membrane potential; ϕ=RT/zF is a parameter where R is the universal gas constant, T is the absolute temperature, z is the valence of ion; [ion]i and [ion]e are intracellular and extracellular Na+, K+ and Ca2+ concentration respectively; m and h are the activation and inactivation gating variables, respectively, satisfy the following equations when the channels are activated by ATP (10-5 M to 10-3 M) 32:
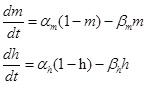 |
(4) |
We are interested in Ca2+ Dynamic, therefore, outward pump fluxes (IPump) and inward passive fluxes (IL,Ca) are included in cell membrane according to Friel 34. ER exchanges Ca2+ with the cytosol via JIp3, Jpump and Jleak following a same scheme as that in mast cell.
2.4 Ca2+ Dynamic in mast cell and nerve cell
The ER Ca2+ dynamics is governed by Ca2+ release from ER
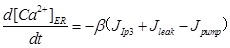 |
(5) |
Where β is the Ca2+ buffering factor 31.
Then cytoplasm Ca2+ dynamics is governed by cross-membrane Ca2+ flow and Ca2+ release from ER
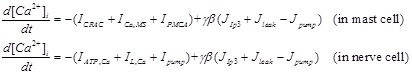 |
(6) |
Where γ is the ratio of ER volume to cytoplasm volume.
2.5 Passive electrical flow in nerve cell
We apply cable theory which describes the relationship between current and voltage in a one-dimension cable to study electrical signaling along nerve cells 35. We discretize the cable model by replacing the partial derivatives by difference formulas on equally distributed grid points 0 = x0<x1 < x2 < · · · <xN= L with size δx. The discretization uses centered difference formula
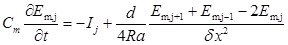 |
(7) |
Where Cm is the specific capacitance of the membrane, subscript j means the jth compartment, d is the diameter of the nerve cell and simplified as constant in this model, Ra is the axial resistance, Ij is the total membrane ionic current,
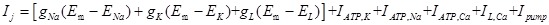 |
Where 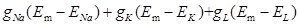 are Na+, K+ and leak current described by Hodgkin-Huxley 36, IATP, ion (ion= Na+, K+ and Ca2+) is the ATP current described by Eq. 3.
are Na+, K+ and leak current described by Hodgkin-Huxley 36, IATP, ion (ion= Na+, K+ and Ca2+) is the ATP current described by Eq. 3.
2.6 Calculation and Parameters
The differential equation (1) is solved on a spatial domain 0<r <L. The equation is first discretized by replacing the partial derivatives in r by difference formulas on equally distributed grid points 0 = r0<r1 < r2 < · · · <rN = L with size ∆r. The discretization using centered difference formula (notice ATP produced only at j=0)
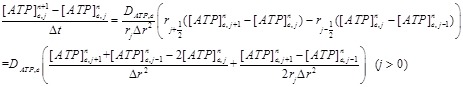 |
(8) |
Special care is needed to handle the coordinate singularity at r = 0. In this paper, we use the fact that we have radial symmetry at r =0. Thus, we can simply discretize the formula
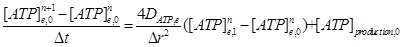 |
(9) |
Equations (1)-(7) are solved using a built-in Matlab solver ode15 with proper initial and boundary conditions. We have carried our simulations by applying mechanical stimuli at r = 0.
3 Results
3.1 Mast cell's response to mechanical stimuli
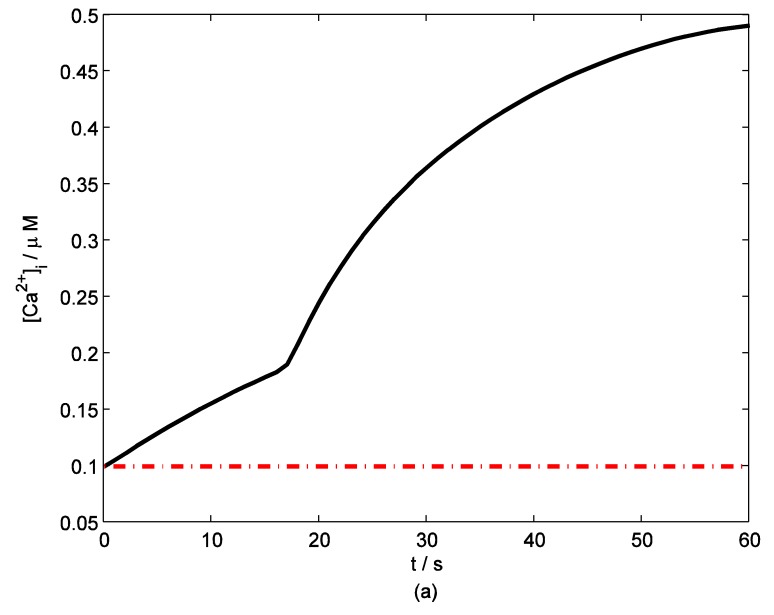
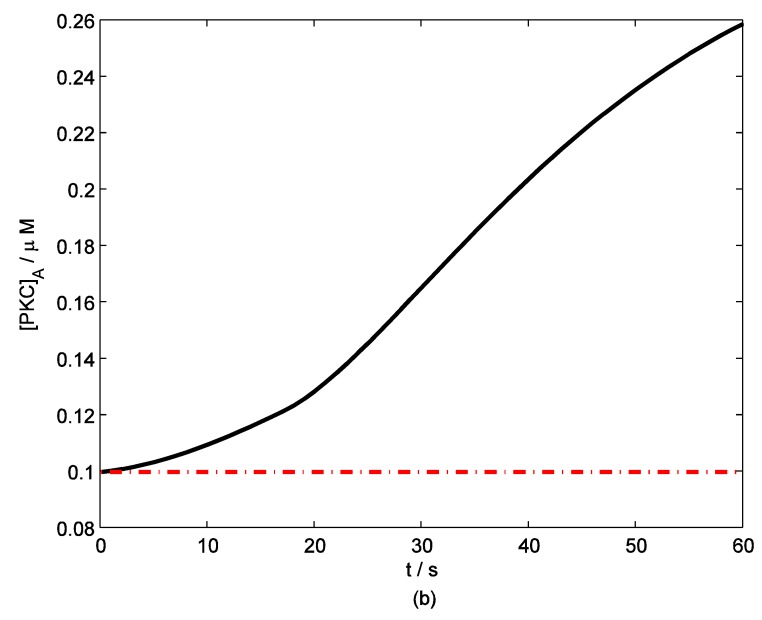
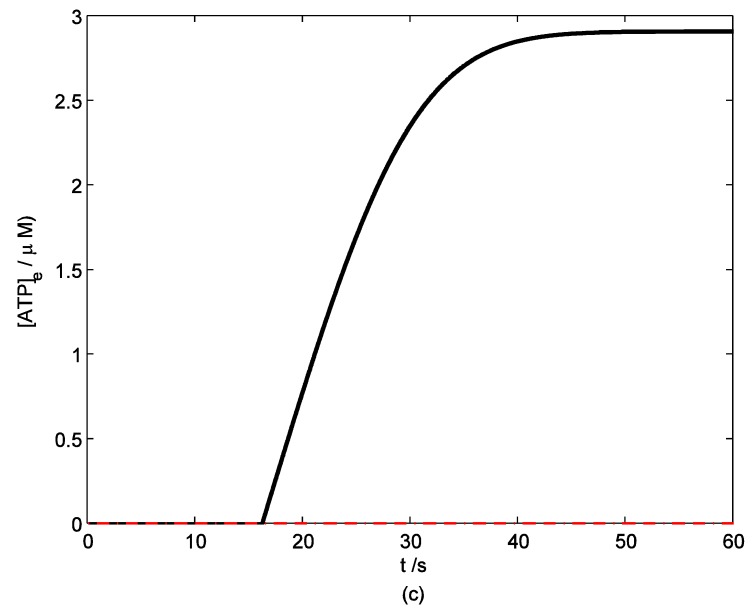
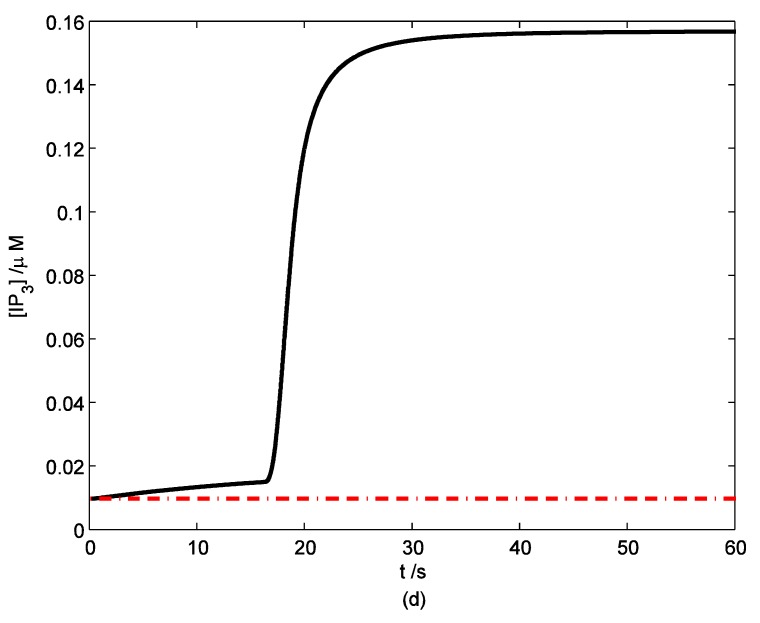
According to the model, mechanical stimuli to a single mast cell leads to both a [Ca2+]i rise as well as ATP release, which increases [ATP]e and then acts on P2-receptors of the cell in an autocrine manner. Fig. 2 shows the response of mast cell to mechanical stimuli. First is a fast [Ca2+]i rise (Fig. 2a) because of Ca2+ influx from ECS through the MS channels. In one hand, local intracellular Ca2+ rise activates PKC (Fig. 2b) and increase the sensitivity of secretory granules to Ca2+, thus driving ATP release (Fig. 2c) 8. In the other hand, [IP3] rise due to [Ca2+]i increasing (Fig. 2d); IP3 interacts with receptors (IP3R) on the endoplasmic reticulum (ER) leading the release of stored Ca2+ and the depletion of Ca2+ in ER triggers Ca2+ entry through CRAC channels, therefore leads to further [Ca2+]i rise. Fig.2a shows there is an obvious [Ca2+]i rise begin at t=18s which is accordance to [IP3] rise at t=18s or so (Fig.2d).
Figure 2.
Changes in [Ca2+]i, [PKC]A, [ATP]e and [IP3] in a single isolated mast cell model, as functions of time (t). Solid line represents simulation results after exposure to an initiating mechanical stimuli during time t=0-60s, dash line represents the stable state without stimuli. (a) immediately [Ca2+]i rise. (b) [Ca2+]i rise activates PKC ( [PKC]A rise). (c) ATP release after [PKC]A accumulate over threshold value. (d) [IP3] rise quickly when ATP release from mast cell and act on purinergic receptors of the cell.
3.2 Nerve cells responses to ATP stimuli
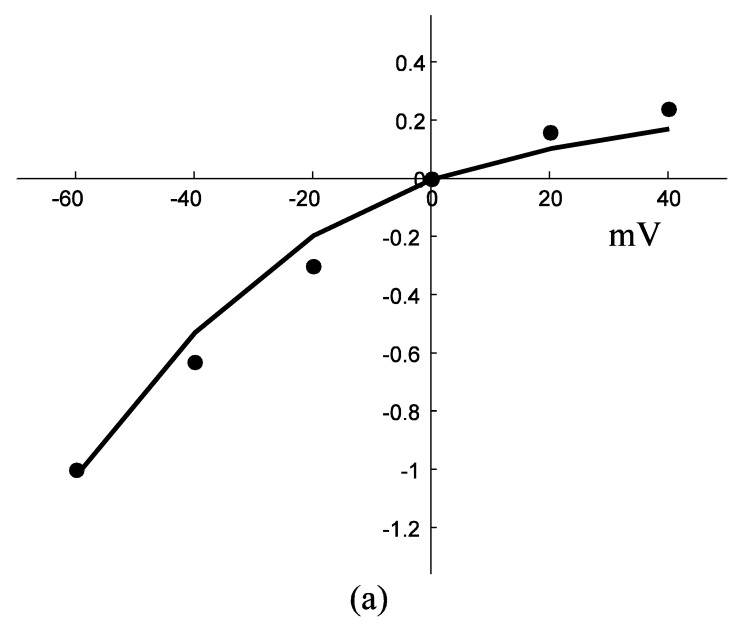
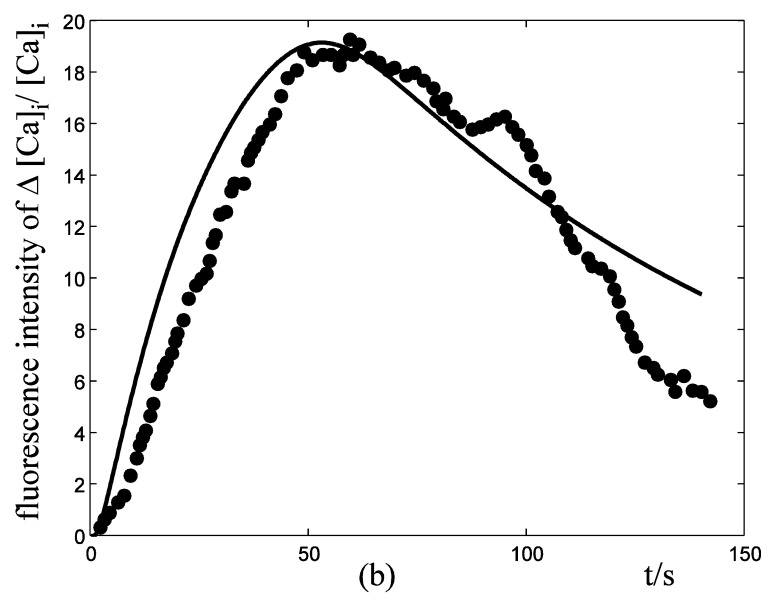
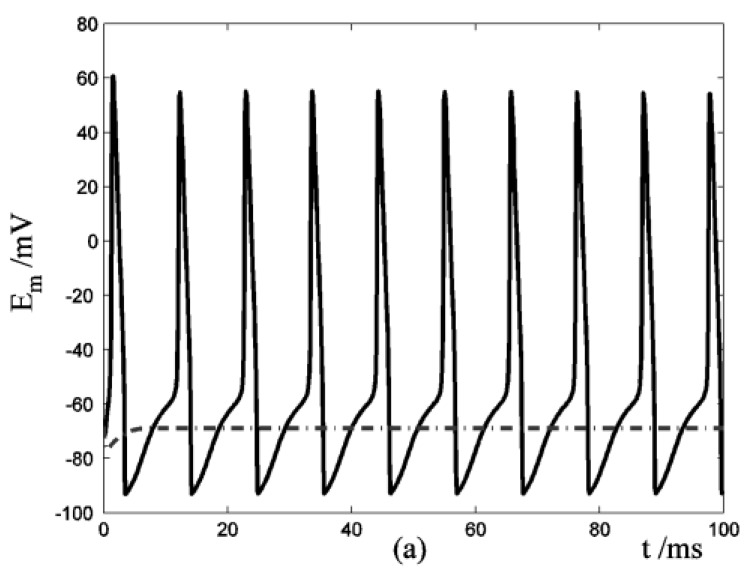
Application of ATP to nerve cells activates membrane currents IATP=IATP,K+IATP,Ca+IATP,Na and [Ca2+]i rise. By whole-cell patch-clamp, Tan et al. investigate the relationship of I-V for the VS type of IATP (at 10-4 M ATP) in rat nodose ganglion (NG) neurons 32. Fig. 3a showed our simulation results of IATP which are in accordance to Tan et al's experiment data. This comparison supports our hypothesis that there were cationic currents activated by ATP stimuli. Nunes et al. observed ATP (2.5-10×10-4 M) induced three temporal response patterns of [Ca2+]i rise in the petrosal ganglion of rat, and the slow rise and a slow decay type (R2) predominate [Ca2+]i in the younger animals 40. Fig. 3b showed the simulation results of [Ca2+]i in responses to ATP stimuli which are in accordance with the R2 response of Nunes et al. 's experiment data. IATP brings the membrane potential towards the threshold for triggering action potentials, and as the cable theory describing, local membrane potential induce electrical current along the axons and fibers of nerve cells which triggers action potentials propagation. Fig. 4a showed the local action potentials induced by IATP when block the cable effect. Fig. 4b showed the action potentials propagation along nerve cells induced by the electrical currents from its “upstream” neighbors.
Figure 3.
Response of IATP and [Ca2+]i in a single isolated nerve cell model to ATP stimuli. solid line represents simulation results, filled circles represent the experimental data. (a) sequential current trace of IATP with the cell clamped at different holding potentials ranging from -60 to +40 mV. (b) [Ca2+]i responses after its exposure to an initiating ATP stimuli during time t=0-30s.
Figure 4.
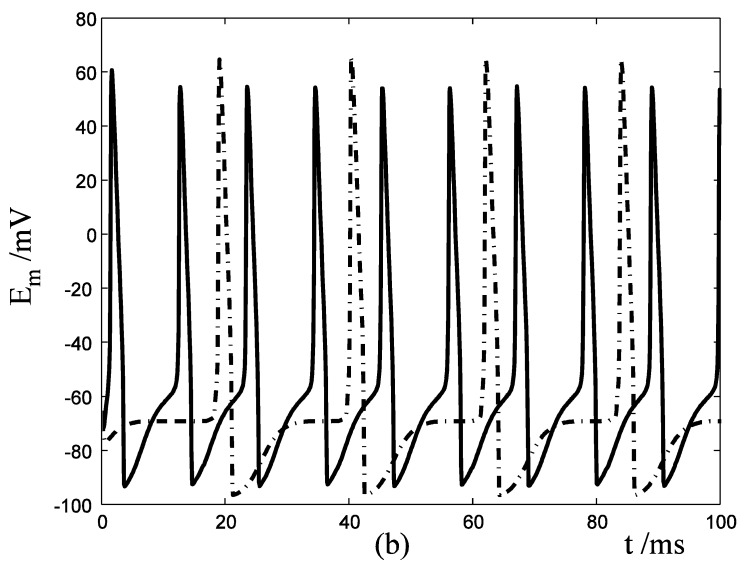
Response of Em in a single isolated nerve cell model to ATP stimuli. Solid line represents simulation results of the segment exposed to an initiating ATP stimuli, dash line represents simulation results of the segment without ATP stimuli. (a) block the cable current from the activated segment to other segment of the nerve cell. (b) include cable current from the activated segment to other segment of the nerve cell.
3.3 Mast cell-nerve cell interaction at acupoint
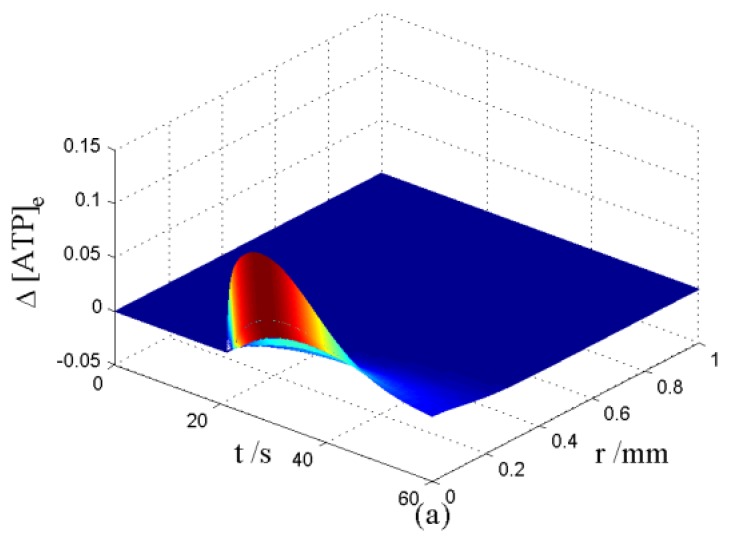
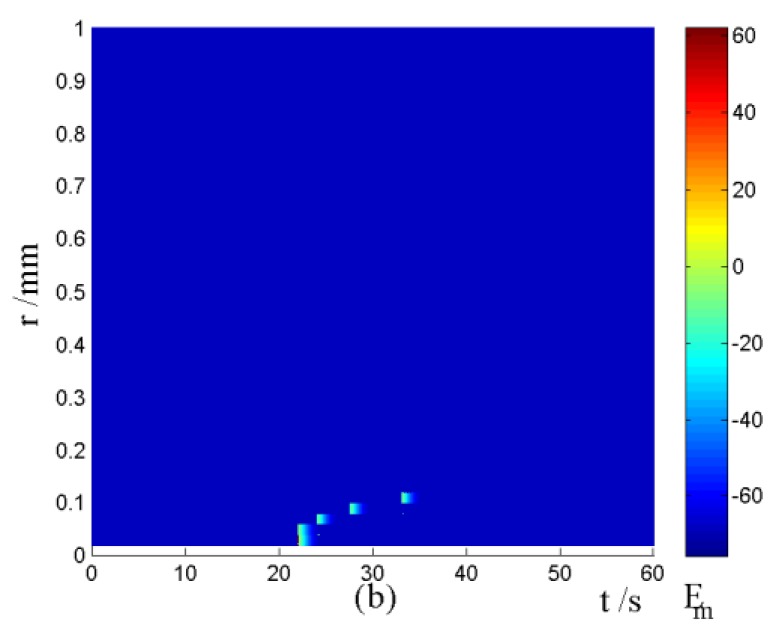
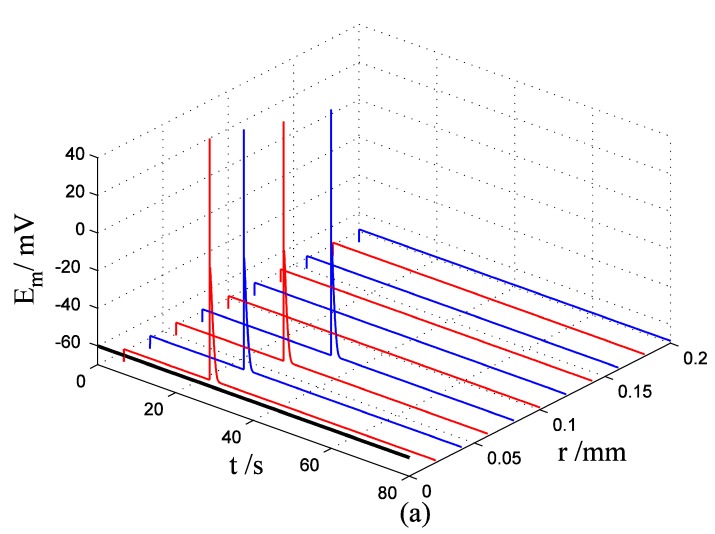
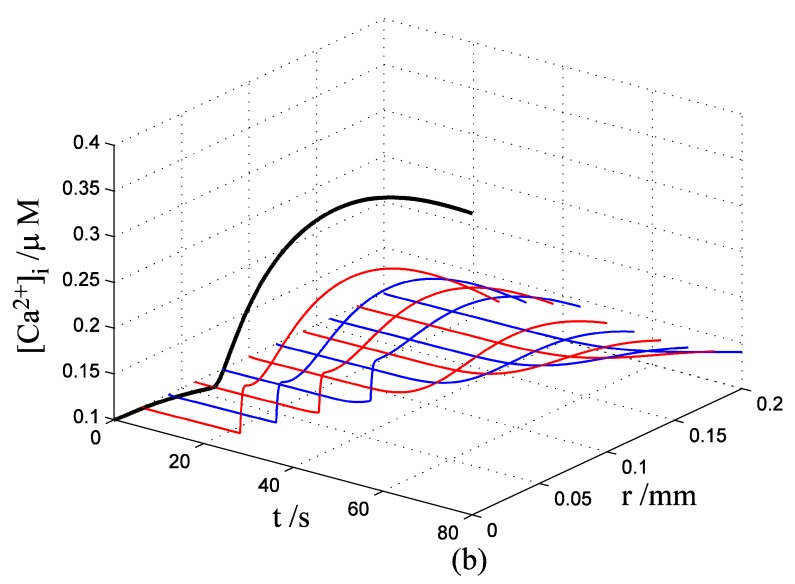
Nerve cells share perivascular localization with mast cells and can be activated by ATP release from mast cells. Figs. 5-6 shows the coupled responses of a mast cell (at r=0) and nerve cells (at r=Dists) to mechanical stimuli at r=0. Fig. 5a shows mechanical stimuli induce ATP release from mast cell, then increase [ATP]e (the peak near t=16 s & r=0). ATP diffuses in ECS and activate the nearby nerve cells. Fig. 5b shows nerve cells at Dist<100 µm (bright points present there are triggered action potentials) are activated by ATP released from mast cell, while nerve cells at Dist>100 µm are not activated because [ATP]e decreases with Dist increasing and doesn't reach the threshold for triggering action potentials. Fig. 6 shows Em and [Ca2+]i responses in nearby nerve cells. Fig. 6a shows action potentials are triggered in nerve cells at Dist=20~ 80 µm, we extend simulation time to 80 s to show there are no action potentials in nerve cells at Dist>=100 µm. Fig. 6b shows [Ca2+]i rise is independent of action potential, [Ca2+]i peak denpends on Dists which means it depends on [ATP]e. Table 2 summarized the results.
Figure 5.
Changes in [ATP]e and [Ca2+]i in the coupled mast cell and nerve cell model, as functions of time (t) and radius distance (r), after its exposure to an initiating mechanical stimuli at r=0 during time t=0-60s. (a) [ATP]e contour in ECS. (b) Em response in nerve cells.
Figure 6.
Responses in Em and [Ca2+]i in nerve cells at different Dists in the coupled mast cell and nerve cell model to ATP released from the activated mast cell at r=0 by initiating mechanical stimuli during time t=0-60s. (a) Em response. (b) [Ca2+]i response.
Table 2.
Response time of Em and [Ca2+]i peak of different Dists
| Dist | Response time of Em | [Ca2+]i peak (µM) |
|---|---|---|
| 2×10-5 m | 22 s | 0.33 |
| 4×10-5 m | 24 s | 0.31 |
| 6×10-5 m | 27 s | 0.29 |
| 8×10-5 m | 33.1s | 0.27 |
| 1×10-4 m | No | 0.24 |
4 Conclusion and discussion
4.1 Mechanical stimuli increase [Ca2+]i in mast cell and lead to ATP release
We have shown with our model that mechanical stimuli to a single mast cell leads to both an increase in [Ca2+]i as well as the release of ATP, which increases [ATP]e and then acts on P2-receptors of the cell in an autocrine manner. Osipchuk reported a wave-like spread of rise in [Ca2+]i could be elicited by mechanical perturbation of a single cell in a cluster of cells 41. Furthermore, both application of either a desensitizing concentration of ATP or P2-receptors antagonist (suramin) inhibited the spread rise initiated by mechanical stimulation of a single cell, suggesting that ATP release from mast cell when stimulated and lead to the spread of [Ca2+]i rise by acting on P2-receptors of the nearby cells.
4.2 ATP stimuli activate nerve cells and induce signal transporting in neural network
We have shown with our model that ATP activates membrane currents IATP and triggers action potentials in nerve cells. Because the structure of the neuron (Fig.1), local action potentials will induce electrical current spreading along the fibers or axons of neuron, thus transporting local information to remote regions in neural network. Afferent signal generation occurs at sensory nerve endings, in the mammalian somatosensory system, sensory neurons are responsible for the transduction of peripheral stimuli into action potentials that propagate to the center neural system (CNS) 42, These results can explain the signal spreading in neural system and the results are similar to the action potentials induced by mechanical stimuli in Delmas et al.'s experiment 43.
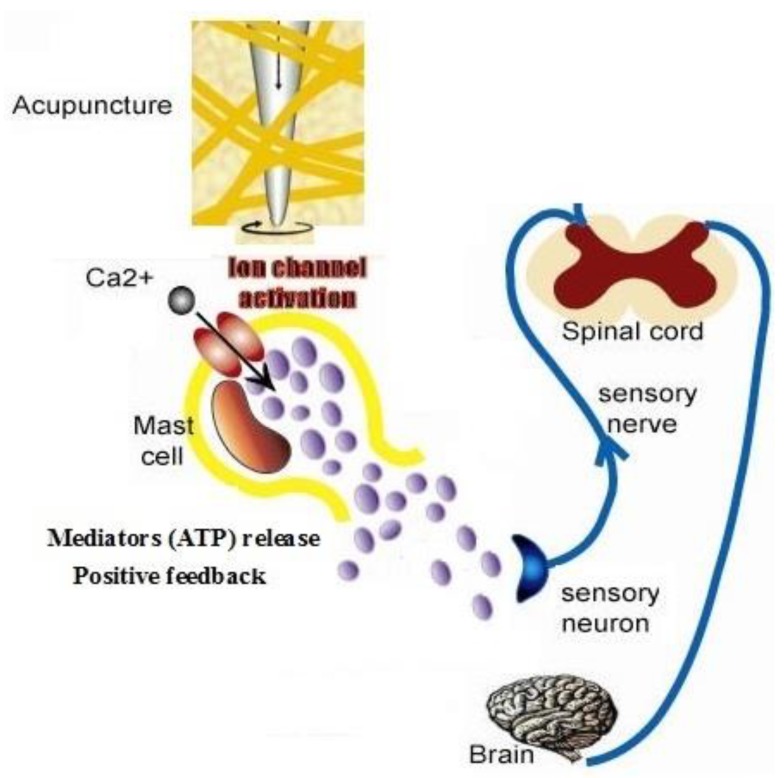
4.3 The interaction of mast cell and nerve cell plays a key role in response to mechanical treatment in TCM
Acupuncture is a method of applying mechanical stimuli into specific parts (acupoints) abundant of mast cells 4. Langevin et al. proposed that the deformation of connective tissue during acupuncture may act as mechanical stimulus on the connective tissue at the cellular level 2-3. Our experiment showed manual acupuncture stimulation of ST 36 can significantly potentiate the discharge activity of the sciatic nerve and induce degranulation of mast cells at the same time, suggesting an involvement of mast cells in initiating acupuncture signals by peripheral sensory nerve 44, Han CX et al. recorded the specific action potentials on dorsal spinal nerve root when acupuncture is made at Zusanli point with four different acupuncture manipulations 45. These results are accordance with our model's suggestion. Fig.7 shows the cellular mechanism of mast cell response to acupuncture in TCM. Mechanical stimuli during acupuncture activate mast cells through various receptors which leads to distinct signaling pathways causing the bio-mediators such as ATP release, which has a positive feedback effect to further release. ATP will diffuse in the ECS 46 and activate sensory nerve endings and form electronic signal, electrical current then spreads in neural network and transports local information to neural center. Our experiment indicates mast cells participate in the initiation of manual acupuncture signal in the acupoints 8 and conduct the effective information into centrum by activating the function of nerve cells 47.
Figure 7.
Schematic diagram of cellular mechanism of acupuncture effect.
In this paper, we developed a mathematical model to study Ca2+ signaling and ATP release in mast cell and nerve cell, and applied it to investigate the coupled response of mast cell and nerve cell system to mechanical stimuli. This study facilitates our understanding of the mechanotransduction process in acupoint region (abundant of mast cells and nerve cells) induced by mechanical stimuli, provides a methodology for quantitative analyze acupuncture treatment.
Table 1.
Model parameters and initial resting values.
| Parameter | Value | Source |
|---|---|---|
| DATP | 3×10-10 m2 s-1 | 31 |
| VATP | 0.1 s-1 | evaluated from 31 |
| [PKCA]min | 1.2×10-7 M | |
| kloss | 30 s-1 | 31 |
| Krel | 1×10-5 M | 31 |
| gATP | 0.15 mS cm-2 | evaluated from 32 |
| αm | 1000 s-1 | evaluated from 32 |
| βm | 5s-1 | 32 |
| αh | 0 | 32 |
| βh | 0.01s-1 | 32 |
| [ATP]act1/2 | 20 | |
| γ | 0.0244 | 37 |
| λ | 0.08 | 38 |
| Ra | 100 Ω cm | 39 |
| Cm | 0.75 µF cm-2 | 39 |
| initial resting values: | ||
| [PKCA] | 1×10-7 M s-1 | evaluated from 17 |
| m0 (initial value of m) | 0 | |
| h0 (initial value of h) | 1 | |
| [Ca2+]i | 1×10-7 M | 31 |
| [Ca2+]e | 2×10-3 M | 31 |
| [Ca2+]ER | 5×10-4 M | 37 |
| Em | -65 mV | 39 |
Acknowledgments
This work was supported by National natural science foundation of China (11202053), Shanghai Science Foundation (12ZR1401100) and 973 project (2012CB518502)
References
- 1.Schwarz W, Gu QB. Cellular Mechanisms in Acupuncture Points and Affected Sites. In: Xia Y, Ding DH, Schwarz W, editors. Current research in acupuncture, 1st ed. New York: Springer; 2013. pp. 37–51. [Google Scholar]
- 2.Langevin HM, Yandow JA. Relationship of acupuncture points and meridians to connective tissue planes. Anat Rec. 2002;269:257–265. doi: 10.1002/ar.10185. [DOI] [PubMed] [Google Scholar]
- 3.Yu XJ, Ding GH, Huang H. et al. Role of collagen fibers in acupuncture analgesia therapy on rats. Connect Tissue Res. 2009;50:110–120. doi: 10.1080/03008200802471856. [DOI] [PubMed] [Google Scholar]
- 4.Culliton BJ. Acupuncture: fertile ground for faddists and serious NIH research. Science. 1972;177:592–594. doi: 10.1126/science.177.4049.592. [DOI] [PubMed] [Google Scholar]
- 5.Bonafede M, Dick A, Noyes K. et al. The effect of acupuncture utilization on healthcare utilization. Med Care. 2008;46:41–48. doi: 10.1097/MLR.0b013e3181589b7d. [DOI] [PubMed] [Google Scholar]
- 6.Skaper SD, Facci L. Mast cell-glia axis in neuroinflammation and therapeutic potential of the anandamide congener palmitoylethanolamide. Philos T R Soc B. 2012;367:3312–3325. doi: 10.1098/rstb.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilfillan AM, Austin SJ, Metcalfe DD. Mast cell biology: introduction and overview. Adv Exp Med Biol. 2011;716:2–12. doi: 10.1007/978-1-4419-9533-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding GH, Zhang D, Huang M, Wang LN, Yao W. Xia Y, Ding GH, Schwarz W. Current research in acupuncture, 1st ed. New York: Springer; 2013. Function of Collagen and Mast Cells in Acupuncture Points. [Google Scholar]
- 9.Zhang D, Ding GH, Shen XY. et al. Role of mast cells in acupuncture effect: a pilot study. Explore-NY. 2008;4:170–177. doi: 10.1016/j.explore.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Wang LN, Ding GH, Gu QB. et al. Single-channel properties of a stretch-sensitive chloride channel in the human mast cell line HMC-1. Eur Biophys J Biophy. 2010;39:757–767. doi: 10.1007/s00249-009-0542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang D, Spielmann A, Ding GH. et al. Activation of mast-cell degranulation by different physical stimuli involves activation of the transient-receptor-potential channel TRPV2. Physiol Res. 2012;61:113–124. doi: 10.33549/physiolres.932053. [DOI] [PubMed] [Google Scholar]
- 12.Burger MM, Sordat B, Zinkernagel RM. Cell to cell interaction. Basel: Karger; 1990. [Google Scholar]
- 13.Blennerhassett MG, Tomioka M, Bienenstock J. Formation of contacts between mast cells and sympathetic neurons in vitro. Cell Tissue Res. 1991;265:121. doi: 10.1007/BF00318146. [DOI] [PubMed] [Google Scholar]
- 14.Arizono N, Matsuda S, Hattori T. et al. Anatomical variation in mast cell nerve associations in the rat small intestine, heart, lung, and skin. Similarities of distances between neural processes and mast cells, eosinophils, or plasma cells in the jejunal lamina propria. Lab Invest. 1990;62:626. [PubMed] [Google Scholar]
- 15.Göthert M, Garbarg M, Hey JA. et al. New aspects of the role of histamine in cardiovascular function: identification, characterization, and potential pathophysiological importance of H3 receptors. Can J Physiol Pharmacol. 1995;73:558–564. doi: 10.1139/y95-071. [DOI] [PubMed] [Google Scholar]
- 16.Artico M, Iannetti G, Tranquilli Leali FM. et al. Nerve fibers-mast cells correlation in the rat parietal pleura. Resp Physiol. 1998;113:181–188. doi: 10.1016/s0034-5687(98)00053-x. [DOI] [PubMed] [Google Scholar]
- 17.Theoharides TC. The mast cell: a neuroimmunoendocrine master player. Int J Tissue React. 1996;18:1–21. [PubMed] [Google Scholar]
- 18.Bauer O, Razin E. Mast cell-nerve interactions. News Physiol Sci. 2000;15:213–218. doi: 10.1152/physiologyonline.2000.15.5.213. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki R, Furuno T, Okamoto K. et al. ATP plays a role in neurite stimulation with activated mast cells. J Neuroimmunol. 2007;192:49–56. doi: 10.1016/j.jneuroim.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2010;6:3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu F, Lu TJ, Seffen KA. Skin thermal pain modeling—A holistic method. J Therm Bio. 2008;33:223–237. [Google Scholar]
- 22.Xu F, Wen T, Seffen KA. Modeling of skin thermal pain: A preliminary study. Appl Math Comput. 2008;205:37–46. [Google Scholar]
- 23.Xu F, Lu TJ. Skin biothermomechanics: modeling and experimental characterization. Adv Appl Mech. 2009;43:147–248. [Google Scholar]
- 24.Bennett MR, Farnell L, Gibson WG. A quantitative model of cortical spreading depression due to purinergic and gap-junction transmission in astrocyte networks. Biophy J. 2008;95:5648–5660. doi: 10.1529/biophysj.108.137190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou L, Chui SY. Computer model for action potential propagation through branch point in myelinated nerves. J Neurophysiol. 2001;85:197–210. doi: 10.1152/jn.2001.85.1.197. [DOI] [PubMed] [Google Scholar]
- 26.Shi XM, Zheng YF, Liu ZR. A model of calcium signaling and degranulation dynamics induced by laser irradiation in mast cells. Chin Sci Bull. 2008;53:2315–2325. [Google Scholar]
- 27.Yao W, Huang HX, Ding GH. A dynamic model of calcium signaling in mast cells and LTC4 release induced by mechanical stimuli. Chin Sci Bull. 2013;59:956–963. [Google Scholar]
- 28.Zylka MJ. Needling adenosine receptors for pain relief. Nat Neurosci. 2010;13:783–784. doi: 10.1038/nn0710-783. [DOI] [PubMed] [Google Scholar]
- 29.Goldman N, Chen M, Fujita T. et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett MR, Farnell L, Gibson WG. A quantitative model of purinergic junctional transmission of calcium waves in astrocyte networks. Biophys J. 2005;89:2235–2250. doi: 10.1529/biophysj.105.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemon G, Gibson WG, Bennett MR. Metabotropic receptor activation, desensitization and sequestration-I: modelling calcium and inositol 1,4,5 -trisphosphate dynamics following receptor activation. J Theor Biol. 2003;223:93–111. doi: 10.1016/s0022-5193(03)00079-1. [DOI] [PubMed] [Google Scholar]
- 32.Tan Y, Zhao B, Zeng QC. et al. Characteristics of ATP-activated current in nodose ganglion neurons of rats. Neurosci Lett. 2009;459:25–29. doi: 10.1016/j.neulet.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 33.Koch C, Segev I, Methods in neuronal modeling: from ions to networks, 2nd ed. Cambridge: MIT Press; 1998. [Google Scholar]
- 34.Friel DD. [Ca2+]i oscillations in sympathetic neurons: an experimental test of a theoretical model. Biophys J. 1995;68:1752–1766. doi: 10.1016/S0006-3495(95)80352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hines ML, Carnevale NT. The neuron simulation environment. Neural Comput. 1997;9:1179–1209. doi: 10.1162/neco.1997.9.6.1179. [DOI] [PubMed] [Google Scholar]
- 36.Rinzel J. Electrical excitability of cells, theory and experiment: Review of the Hodgkin-Huxley foundation and an update. B Math Bio. 1990;52:3–23. [Google Scholar]
- 37.Fink CC, Slepchenko B, Loew LM. Determination of time-dependent inositol-1,4,5-trisphosphate concentrations during calcium release in a smooth muscle cell. Biophys J. 1999;77:617–628. doi: 10.1016/S0006-3495(99)76918-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazel T, Raymond R, Raymond-Stintz M. et al. Stochastic modeling of calcium in 3D geometry. Biophys J. 2009;96:1691–1706. doi: 10.1016/j.bpj.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kager H, Wadman WJ, Somjen GG. Simulated seizures and spreading depression in a neuron model incorporating interstitial space and ion concentrations. J Neurophysiol. 2000;84:495–512. doi: 10.1152/jn.2000.84.1.495. [DOI] [PubMed] [Google Scholar]
- 40.Nunes AR, Chavez-Valdez R, Ezell T. et al. Effect of development on [Ca2+]i transients to ATP in petrosal ganglion neurons: a pharmacological approach using optical recording. J Appl Physiol. 2012;112:1393–1402. doi: 10.1152/japplphysiol.00511.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 42.Delmas P, Hao JZ, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nat Rev Neurosci. 2011;12:139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 43.Hao JZ, Delmas P. Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. J Neurosci. 2010;30:13384–13395. doi: 10.1523/JNEUROSCI.2926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.SA ZY, Huang M, Zhang D. et al. Relationship between Regional Mast Cell Activity and Peripheral Nerve Discharges during Manual Acupuncture Stimulation of “Zusanli”(ST36) Acupuncture Research. 2013;38:118–122. [PubMed] [Google Scholar]
- 45.Han CX, Wang J, Che YQ. et al. Nonlinear characteristics extraction from electrical signals of dorsal spinal nerve root evoked by acupuncture at Zusanli point. Acta physica sinica. 2010;59:5881–5888. [Google Scholar]
- 46.Yao W, Li YB, Ding GH. Interstitial fluid flow: the mechanical environment of cells and foundation of meridians. Evid-Based Compl Alt. 2012. ArticleID853516. doi:10.1155/2012/853516. [DOI] [PMC free article] [PubMed]
- 47.Huang M, Zhang D, Sa ZY, In adjuvatn-induced arthritic rats, acupuncture analgesic effects are histamine dependent: potential reasons for acupoint preference in clinical practice. Evid-Based Compl Alt. 2012. Article ID 810512, doi:10.1155/2012/810512. [DOI] [PMC free article] [PubMed]