Abstract
The orchestration of transcriptional programs depends on proper gene-enhancer pairing. While much remains to be learned about this process in normal development, two recent studies in Cell and Cancer Cell highlight how the genomic rearrangement of an enhancer plays a causal role in the onset of a leukemogenic program.
Genetic lesions are well-chronicled drivers of various malignancies, with evidence accumulating from early cytogenetic studies as well as modern molecular techniques. The majority of such techniques have placed an emphasis on the amplification, deletion, or rearrangement of coding sequences, and have successfully identified hundreds of important drivers in cancer (Garraway and Lander, 2013). However, the vast expanse of non-coding sequence in mammalian genomes is known to contain regulatory elements that contribute to the control of gene expression and could therefore influence gene expression via mutation, rearrangement, deletion, or amplification. Precedence for the involvement of such elements in oncogenesis has been set by the demonstration that expression of MYC and other oncogenes can be deregulated in lymphoid cells via juxtaposition of the immunoglobulin heavy chain regulatory regions, which drive aberrant expression (ar-Rushdi et al., 1983). A more recent demonstration of the importance of mutations in regulatory regions was the identification of mutations in telomerase promoter regions in melanoma (Huang et al., 2013). However, it remains unclear how and to what extent deregulation of oncogene expression as a result of mutations in gene regulatory regions drives cancer pathogenesis.
Enhancer elements are regions of DNA that function as distal, non-promoter, cis-acting regulators of gene expression that often operate in a tissue-specific manner. As an area that has not been queried by traditional technologies, such regulatory elements remain at the frontier in the study of both normal and aberrant gene expression, with the latter containing implications for the regulation of putative oncogenic drivers. Key challenges in the study of enhancers involve difficulties in their initial identification and the identification of the genes upon which they act. Advances in genome-wide measurements of transcription factor binding and chromatin state have begun to address the former, as enhancers can now be identified based on the presence of particular chromatin modifiers and histone modifications. With hundreds of thousands of putative enhancers identified in the human genome, it is now imperative that they be linked to their respective genes in the context of both normal development and pathogenesis. Enhancer function can be affected by factors that bind to the enhancer, chromatin modifications associated with enhancers, lineage specific signaling pathways, and mutations altering the enhancer sequence itself; the importance of these elements in cancer is only beginning to be explored (Herz et al., 2014).
EVI1 is a proto-oncogenic transcription factor involved in the regulation of hematopoietic stem cells, and its overexpression has been linked to acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) and carries a poor prognosis (Glass et al., 2014). EVI1 deregulation is often accompanied by a nearby inversion inv(3) or translocation t(3;3) of largely non-genic sequence, but mechanistic links between these rearrangements and EVI1 expression changes have remained poorly understood. Two new studies utilize orthogonal approaches to dissect the regulatory potential of these sequences and subsequently discover how the genomic rearrangement of a single enhancer element disrupts the regulation of two genes involved in the onset of AML (Figure 1).
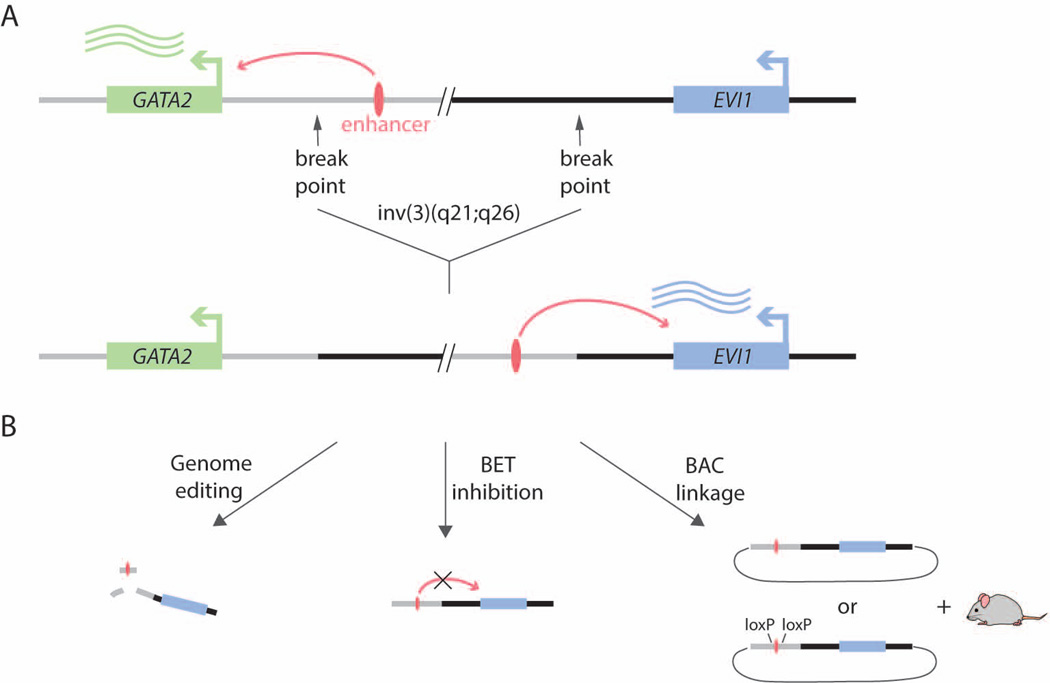
Figure 1. A Single Enhancer Rearrangement Deregulates Two Oncogenic Drivers.
A. The distal enhancer normally drives GATA2 expression in a myeloid-specific manner. Inversion or translocation of the locus simultaneously results in functional haploinsufficiency of GATA2 and upregulation of EVI1.
B. The enhancer can be deactivated through genome editing or pharmacologic BET inhibition. A novel BAC-linking strategy allowed a faithful recapitulation of the rearrangement and disease in a mouse model.
In a recent study published in Cell, Gröschel et al. (2014) utilized functional genomics and genome editing to characterize chromosome 3q-rearrangements in primary AML samples and human cell lines. Using an effective combination of chromosome conformation capture with ChIP-seq and RNA-seq, the authors isolated a 9 kb element within commonly translocated sequences which physically contacted the EVI1 promoter and stimulated its expression. Analysis of p300 binding further allowed the elucidation of a putative enhancer element, the excision of which was shown to reduce EVI1 expression and limit proliferation, with a marked skewing toward myelomonocytic differentiation. These changes were largely phenocopied by EVI1 knockdown. Further classification of the region surrounding the enhancer indicated that it may function as part of a so-called super-enhancer, with a characteristic H3K27ac pattern and BRD4 binding (Whyte et al., 2013). As such, cell lines containing the 3q-rearrangements were more responsive to the BET bromodomain inhibitor JQ1 and exhibited proliferation defects and reduced EVI1 expression.
In a complementary study in this issue of Cancer Cell, Yamazaki et al. (2014) took advantage of a bacterial artificial chromosome (BAC) system to recapitulate a human 3q-rearranged leukemia in a mouse model. To this end, they utilized a linked BAC strategy to precisely generate the inv(3)(q21;q26) inversion found in the human MOLM-1 leukemia cell line, with and without the putative enhancer element. They also generated a control BAC containing the truncated EVI1 gene alone. The resulting transgenic mice underwent molecular and phenotypic profiling. The human EVI1 gene was elevated in expression relative to the endogenous mouse copy in hematopoietic stem and progenitor cells (HSPCs) and related compartments. The contribution of the enhancer was best demonstrated by the onset of hematologic pathologies. Mice harboring BACs with the full inversion developed splenomegaly and transplantable leukemias, whereas those with enhancer-deleted BACs resembled control mice. The leukemias showed both myeloid and lymphoid properties, and while it is unknown how this arises, it is in agreement with a recent finding that EVI1 is expressed in a subset of pediatric acute lymphoblastic leukemias (Konantz et al., 2013).
Both studies establish that the regulatory element in question is a distal enhancer of GATA2 in the context of the wild-type allele. In Gröschel et al. (2014), the enhancer-EVI1 reporter assays established a pattern of cell type-specific activity, indicating enhancer dependence on the repertoire of available trans factors in the myeloid lineage, which was their first hint that the enhancer does not belong to the ubiquitous housekeeping gene RPN1 as previously hypothesized. Indeed, further allele-specific analysis confirmed that the enhancer normally acts on GATA2, and that the 3q rearrangement results in the functional haploinsufficiency of GATA2. Similarly, Yamazaki et al. (2014) began their study by demonstrating that a conserved homologous sequence in mouse is acting as a hematopoietic-specific distal enhancer of GATA2. Since GATA2 depletion has been linked to AML, MDS, and Emberger/MonoMAC syndromes (Bresnick et al., 2012), this highlights the possibility of a single enhancer rearrangement resulting in the simultaneous upregulation of a proto-oncogene and down-regulation of a tumor suppressor gene in a spatiotemporal specific manner.
Taken together, these two studies emphasize the importance of non-coding regulatory sequence rearrangements as a driving mechanism for leukemogenesis. While one paper takes advantage of genomic tools to characterize human samples and at least one potential therapeutic outlet, the other study establishes a technique for the precise recapitulation of genomic rearrangements for full in vivo characterization in mouse models. However, it should be noted that there may be important ways in which the mouse models differ from the human leukemias. For example, when using human BACs to express the translocated enhancer, the mice do not have the haploinsufficiency of GATA2 as seen in the human cells. Such a difference may lend insight into the contribution of decreased GATA2 expression in leukemogenesis and requires further study. Regardless, these findings illuminate a path to better characterize the mechanistic and therapeutic implications of non-genic rearrangements and further highlight the fact that a next frontier for cancer genomics will focus on the importance of mutations residing outside the coding exome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ar-Rushdi A, Nishikura K, Erikson J, Watt R, Rovera G, Croce CM. Differential expression of the translocated and the untranslocated c-myc oncogene in Burkitt lymphoma. Science. 1983;222:390–393. doi: 10.1126/science.6414084. [DOI] [PubMed] [Google Scholar]
- Bresnick EH, Katsumura KR, Lee HY, Johnson KD, Perkins AS. Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res. 2012;40:5819–5831. doi: 10.1093/nar/gks281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway LA, Lander ES. Lessons from the cancer genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Glass C, Wilson M, Gonzalez R, Zhang Y, Perkins AS. The role of EVI1 in myeloid malignancies. Blood Cells Mol Dis. 2014 doi: 10.1016/j.bcmd.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Herz HM, Hu D, Shilatifard A. Enhancer Malfunction in Cancer. Mol Cell. 2014;53(6):859–866. doi: 10.1016/j.molcel.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konantz M, Andre MC, Ebinger M, Grauer M, Wang H, Grzywna S, Rothfuss OC, Lehle S, Kustikova OS, Salih HR, Handgretinger R, Fend F, Baum C, Kanz L, Quintanilla-Martinez L, Schulze-Osthoff K, Essmann F, Lengerke C. EVI-1 modulates leukemogenic potential and apoptosis sensitivity in human acute lymphoblastic leukemia. Leukemia. 2013;27:56–65. doi: 10.1038/leu.2012.211. [DOI] [PubMed] [Google Scholar]
- Whyte WA, Orlando DA, Hnisz D, Abraham BJ, Lin CY, Kagey MH, Rahl PB, Lee TI, Young RA. Master transcription factors and mediator establish super-enhancers at key cell identity genes. Cell. 2013;153:307–319. doi: 10.1016/j.cell.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
Plus
- Gröschel S, Sanders MA, Hoogenboezem R, de Wit E, Bouwman BAM, Erpelinck C, van der Velden VHJ, Havermans M, Avellino R, van Lom K. A single oncogenic enhancer-rearrangement causes concomitant deregulation of EVI1 and GATA2 in leukemia. Cell. 2014 doi: 10.1016/j.cell.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Suzuki M, Otsuki A, Shimizu R, Bresnick EH, Engel JD, Yamamoto MA. A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21;q26) by activating EVI1 expression. Cancer Cell. doi: 10.1016/j.ccr.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]