Abstract
Racial/ethnic disparities in mortality among US breast cancer patients are well-documented. Our knowledge of the contribution of lifestyle factors to disease prognosis is based primarily on non-Latina Whites and is limited for Latina, African American and Asian American women. To address this knowledge gap, the California Breast Cancer Survivorship Consortium (CBCSC) harmonized and pooled interview information (e.g., demographics, family history of breast cancer, parity, smoking, alcohol consumption) from six California-based breast cancer studies and assembled corresponding cancer registry data (clinical characteristics, mortality), resulting in 12,210 patients (6,501 non-Latina Whites, 2,060 African Americans, 2,032 Latinas, 1,505 Asian Americans, 112 other race/ethnicity) diagnosed with primary invasive breast cancer between 1993 and 2007. In total, 3,047 deaths (1,570 breast cancer-specific) were observed with a mean (SD) follow-up of 8.3 (3.5) years. Cox-proportional hazards regression models were fit to data to estimate hazards ratios (HR) and 95% confidence intervals (CI) for overall and breast cancer-specific mortality. Compared with non-Latina Whites, the HR of breast cancer-specific mortality was 1.13 (95% CI, 0.97-1.33) for African Americans, 0.84 (95% CI, 0.70-1.00) for Latinas, and 0.60 (95% CI, 0.37-0.97) for Asian Americans after adjustment for age, tumor characteristics, and select lifestyle factors. The CBCSC represents a large and racially/ethnically diverse cohort of breast cancer patients from California. This cohort will enable analyses to jointly consider a variety of clinical, lifestyle, and contextual factors in attempting to explain the long-standing disparities in breast cancer outcomes.
Keywords: race/ethnicity, survival, tumor characteristics, lifestyle factors
Introduction
Differences in breast cancer mortality rates by race and ethnicity in the United States (US) have long been recognized. Mortality rates were similar for African Americans and non-Latina Whites until the late 1970s; in the early 1980s, these rates began to diverge with higher mortality in African Americans and have continued despite the lower breast cancer incidence in African Americans compared to non-Latina Whites (1-4). Based on Surveillance, Epidemiology and End Results (SEER) Registry data between 1993 and 1998, risk of death after breast cancer diagnosis was two-fold higher in African Americans, 30% higher in Latinas, and 10% lower in Asian Americans than in non-Latina Whites (5). The relative survival disparities by race/ethnicity have persisted in studies that used SEER data between 2000 and 2006 (6).
Racial/ethnic differences in survival after a breast cancer diagnosis can be attributed to multiple factors. They include differences in stage at diagnosis, which may be related to access to and utilization of mammography screening and health care, differences in the quality of care after diagnosis, factors that are related to socioeconomic status (SES) of patients and neighborhoods, and other neighborhood and medical institutional factors (7). Differences in tumor characteristics and aggressiveness, as well as treatment response, may also be related to racial/ethnic differences in genetic susceptibility and tumor biology. However, adjustment for stage at diagnosis, other tumor characteristics, first course treatment, and area-based SES have not completely explained the survival differences between African Americans and non-Latina Whites (6).
Lifestyle factors, including large body size (8-13), lack of regular exercise (14-16), a history of comorbid conditions (17-19), and low community-level SES (20) have also been independently and positively associated with the risk of death after breast cancer diagnosis. Given that these factors differ by race/ethnicity (17, 21-24), they may contribute to the long-standing racial/ethnic disparities in breast cancer survival. A common shortcoming of many previous studies is the inability to investigate multiple, interrelated factors that may exert independent, as well as combined, effects on survival, either due to limited availability of data as in cancer registry-based studies or small numbers of racial/ethnic minority populations in observational studies. Thus, much of our current knowledge of these prognostic factors is based on studies conducted predominantly in non-Latina White breast cancer patients, with some information available for African Americans (17, 25, 26), and sparse data on Latinas and Asian Americans (26).
The California Breast Cancer Survivorship Consortium (CBCSC) was established in 2011 as a collaborative effort that leverages data collected by six California-based studies of over 12,000 breast cancer patients. The inclusion of breast cancer cases from four racial/ethnic groups (African Americans, Asian Americans, Latinas, and non-Latina Whites) offers a unique opportunity to study the individual, clinical, and contextual factors as potential determinants of the observed survival disparities across racial/ethnic groups. Using harmonized questionnaire data on a variety of prognostic factors, enriched with commonly-derived clinical and follow-up information from the population-based, statewide California Cancer Registry (CCR), the CBCSC addresses many of the limitations of previous studies by having adequate sample size and information on individual lifestyle prognostic factors. In this paper, we describe the methods used to assemble the CBCSC and report on the risks of overall and breast cancer-specific mortality in African Americans, Latinas and Asian Americans relative to non-Latina Whites in this pooled cohort.
Materials and Methods
Structure and Composition of CBCSC
The CBCSC is comprised of six epidemiologic studies of breast cancer etiology and/or prognosis that were initiated in the 1990's and early 2000's. Details of these six studies (study design, age and racial/ethnic composition, study location, data collection methods) are described in Table 1. These six studies initially yielded 12,787 female breast cancer cases (6,695 non-Latina Whites, 2,223 African Americans, 2,120 Latinas, 1,636 Asian Americans, 113 other race/ethnicity). Individual study investigators received Institutional Review Board (IRB) approval from their respective institution(s) to participate in this collaboration, and IRB approval was also obtained from the State of California Committee for the Protection of Human Subjects for the use of CCR data.
Table 1. Characteristics of studies included in the CBCSC.
| Study | Design | Diagnosis years | Ages at diagnosis | Race/ethnicity | Location | Data collection | Language-data collection | Breast cancer cases included |
|---|---|---|---|---|---|---|---|---|
| Asian American Breast Cancer Study (AABCS)(50) | Population-based case-control | 1995-2001 | 25-74 y | Chinese Japanese Filipinas | Los Angeles County | In-person interview | English Chinese Tagalog | 1,138 |
| Women's Contraceptive and Reproductive Experiences Study (CARE) (51) | Population-based case-control | 1994-1998 | 35-64 y | Whites2 African Americans | Los Angeles County | In-person interview | English | 1,240 |
| San Francisco Bay Area Breast Cancer Study (SFBCS) (52) | Population-based case-control | 1995-2002 | 35-79 y | African Americans Latinas Non-Latina (NL)Whites | San Francisco Bay Area | In-person interview | English Spanish | 2,243 |
| Life After Cancer Epidemiology Study (LACE) (41) | Cancer survivor cohort, Kaiser Permanente Northern California (KPNC) | 2000-2002 | 18-70 y | African Americans Asians Latinas NL Whites | San Francisco Bay Area | Mail questionnaire | English | 1,735 |
| California Teachers Study (CTS) (53) | Prospective cohort of California teachers and administrators vested in the State Teachers Retirement System | 1995-2005 | 27-96 y | African Americans Asians Latinas NL Whites | California | Mail questionnaire | English | 3,855 |
| The Multiethnic Cohort (MEC) (54) | Prospective population-based cohort, general population | 1993-2007 | 45-75 y | African Americans Asians Latinas NL Whites | Primarily Southern California | Mail questionnaire | English Spanish | 1,999 |
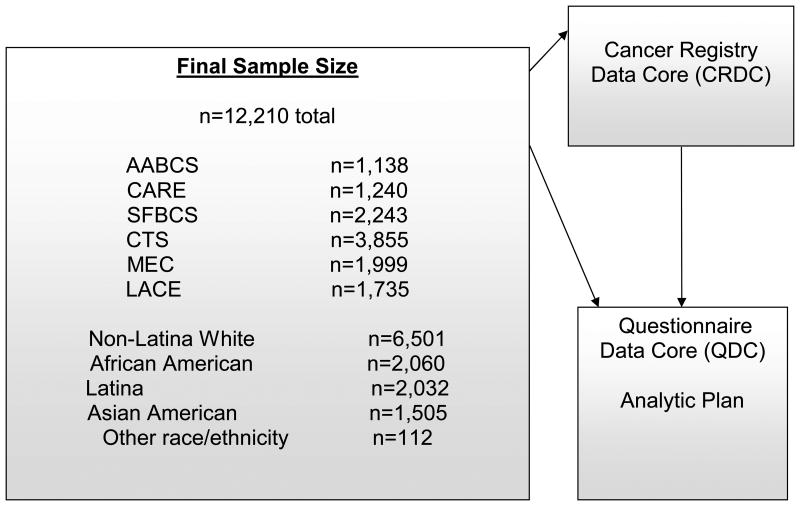
During a one-year pilot study, prognostic factors were identified that potentially could be harmonized across the CBCSC studies, given similar measurement across studies, and considerable variation in their distribution across racial/ethnic groups; these factors were body size, physical activity, and co-morbidities. Furthermore, linkage with geocoded patient records allowed us to identify institutional and neighborhood social and built environment factors that also showed racial/ethnic variation (24, 27). The study objectives and activities of the CBCSC were organized into four individual projects, each focused on a specific set of potential prognostic factors: Project 1: contextual factors (institutional and social and built environment, including distance to health care facility, walkability); Project 2: physical activity; Project 3: body size; and Project 4: co-morbidities. The three case-control studies contributed data to all four projects, whereas the cohort studies contributed data to a subset of the projects (Figure 1). For each project, working groups are organized that utilize research expertise among CBCSC investigators and includes representatives from all participating centers.
Figure 1. Eligibility Flow and Organization Structure of the California Breast Cancer Survivorship Consortium (CBCSC).
The systematic assembly of data for the four projects was facilitated by the Cancer Registry Data Core (CRDC) and the Questionnaire Data Core (QDC), and a common data dictionary and analytic plan were developed. The CBCSC received feedback from a team of external advisors with expertise in oncology, social sciences, health disparities, and patient advocacy.
Cancer Registry Data Core (CRDC)
The role of the CRDC was to centralize and streamline the ascertainment of cancer registry variables from the CCR for the four projects. The CCR is a state-mandated population-based cancer registry that is part of the SEER program. Through its regional registries, the CCR routinely collects patient data from medical records on age at diagnosis, sex, race/ethnicity, marital status, birthplace, and tumor characteristics (American Joint Committee on Cancer (AJCC) stage, tumor size, grade, nodal involvement, histology, estrogen receptor (ER) and progesterone receptor (PR) status, laterality, HER2 (although missing for most cases prior to 2004, and thus not further considered)), first course of treatment (extent of surgical resection, chemotherapy, radiation), and vital status (including cause of death for the deceased) through hospital follow-up and linkages to vital statistics, death records, and other databases. Given that multiple tumors of an individual are captured, data on cancer(s) prior to and subsequent to the qualifying tumors for CBCSC eligibility are also available insofar as the women remain a resident of California.
Each participating study provided either unique CCR identification numbers or personal patient identifiers to enable linkage to the CCR database. The CRDC then consolidated and created a dataset of clinical (tumor characteristics and treatment data), survival, and census block group SES variables. Missing clinical data from the CCR, such as chemotherapy, were not supplemented with data available from contributing study sources, given that these data were not collected systematically by all studies. Vital status as of December 31, 2009, the end of follow-up period of this study, was used to ensure, to the extent possible, that all cases in the pooled analysis had comparable opportunity for follow-up. Breast cancer-specific deaths were derived from the underlying cause of death on the death certificate based on ICD-9 (9174) or ICD-10 (C50) codes. Patients' addresses at diagnosis are routinely geocoded by the CCR to coordinates (latitude, longitude). Neighborhood SES at the block group level was assigned to cases with at least a zip code+4 digit postal extension, using a previously developed index that incorporates US census data on education, occupation, unemployment, household income, poverty, rent, and house values (28). Cases diagnosed prior to 1996 were assigned to the SES measure developed with the 1990 census data, and those diagnosed in 1996 or later were assigned to the measure using 2000 census data.
Questionnaire Data Core (QDC)
The role of the QDC was to harmonize a select set of demographic and lifestyle factors considered to be relevant covariates for adjustment in all of the project analyses. We considered in our base model reproductive variables including parity, number of births and timing of first birth, alcohol consumption, and smoking as these factors have been found to influence mortality in breast cancer patients in some studies and may also influence mortality via hormonally mediated pathways (29-34). Each of the six studies provided specific questionnaire variables that were then harmonized and merged into a common dataset. This pooled dataset included basic demographic variables (date of birth, race/ethnicity, education, birthplace, age at migration to the US if foreign-born, language of interview), and the major suggested breast cancer risk and prognostic factors, including pregnancies (number and outcome of pregnancies, ages at first and last birth), menopause (type of menopause, age at menopause), family history of breast cancer (number of affected first-degree relatives, age at diagnosis of the affected relatives), smoking pattern (never/former/ current, number of cigarettes smoked per day) and alcohol consumption before breast cancer diagnosis.
For some variables (race/ethnicity, birthplace, education, parity, age at first birth, time since last birth, family history of breast cancer, age at diagnosis of affected relatives), the questionnaire response categories were similar across the studies although the definitions were not always identical. For example, although all studies collected information on family history of breast cancer in first-degree female relatives, several studies asked about cancer history in full siblings (LACE, CTS, MEC), whereas two studies collected data separately for both full- and half-sisters (AABCS, CARE), and this information was not specified in another study (SFBCS). We left these data as they were originally coded, given that it is not possible to re-define the categories used by the contributing studies. The differences in classification caused by these inconsistencies are likely to be minimal. Other variables such as alcohol consumption and smoking (cigarettes per day among former and current smokers) were collected as continuous variables in some studies (AABCS, CARE, SFBCS, LACE) but as categorical variables in other studies (CTS, MEC); the midpoint of a category was assigned for the latter group. The questions that were used to assess menopausal status varied across the six studies. However, because each of the studies had carefully developed its own algorithm to determine menopausal status, we relied on each study's original classification of menopausal status.
Pooling Process
Of the initial 12,787 breast cancer case participants from the six studies submitted to the CRDC, 577 cases were excluded for various reasons (Figure 1, Study Exclusions). For the 492 cases who participated in more than one study, resulting in 506 duplicate records, we applied an inclusion rule to use data from the case-control studies first and then from the cohort studies in the order of LACE, CTS, and MEC. The final analytic dataset included 12,210 breast cancer cases (6,501 non-Latina Whites, 2,060 African Americans, 2,032 Latinas, 1,505 Asians Americans, 112 other race/ethnicity) (Figure 1). For a small group of cases (n=126) with multiple breast tumors diagnosed on the same day, we designated the study-qualifying tumor based on a combination of stage, grade, and histology, and considered the worst prognosis tumor as the qualifying tumor.
We evaluated the representativeness of the 12,210 breast cancer cases included in the CBCSC to all breast cancers identified by the CCR (excluding in situ cases and those with <30 days of follow-up) diagnosed in women between 1993 and 2007 in California. The CCR group included 208,542 non-Latina Whites, 17,099 African Americans, 38,459 Latinas, and 24,958 Asian Americans. We compared key tumor characteristics, with the goal of identifying any specific patterns of differences in distribution by race/ethnicity.
Common Analytic Plan
A common analytic approach was developed to facilitate the evaluation of the degree to which the residual race/ethnicity differences in overall and breast cancer-specific mortality could be explained by racial/ethnic differences in social and built environment and neighborhood factors, physical activity, body size, and co-morbidity variables after controlling for important tumor and lifestyle factors. We first developed a main effects stratified Cox regression model to estimate hazard ratios (HR) and associated 95% confidence intervals (CIs) by reverse stepwise selection. The starting model included ‘study’ as a stratification factor and all of the tumor characteristic and lifestyle variables (available in the CRDC and QDC). Age at diagnosis was included as a continuous variable on both the natural scale and the log10 scale in order to appropriately account for the ordered effect of age and allow for non-linearity in its effect in the Cox model. Two time scales, time from diagnosis and attained age (35, 36) were investigated in the development of the final baseline models for overall and breast cancer-specific mortality. All variables, except for age at diagnosis were subject to removal using backward stepwise regression. The order of removal was determined by the more significant (between the two time scales) of the Cox partial likelihood ratio test for that variable, and removal continued until all remaining variables had a likelihood ratio p-value of <0.20. No interactions were included in the model. Women in the case-control studies (AABCS, CARE, SFBCS) and the prospective survivor cohort (LACE) survived after diagnosis until the time of data collection; thus their follow-up was left censored since women who died or were lost to follow-up before data collection by the parent study were not included in this study. The mean (SD) years from diagnosis to data collection were 1.6 (0.8) for AABCS, 0.4 (0.3) for CARE, 1.4 (0.6) for SFBCS, and 1.8 (0.5) for LACE. Women in these four studies were admitted to the risk set at the time of data collection rather than at the time of diagnosis. Women in the prospective population cohorts (CTS, MEC) were included in the pooled analysis if their breast cancer diagnosis occurred during the study follow-up period. The mean (SD) number of years between data collection and cancer diagnosis was 6.9 (4.0) for MEC and 5.0 (2.8) for CTS. The percentages of women diagnosed within 1 year, 1-<2 years, 2 to <5 years, and 5 or more years after questionnaire completion were 8.1%, 6.4%, 21.0%, and 64.5%, respectively, for the MEC. The corresponding figures were 9.3%, 9.8%, 31.2%, and 49.7% in the CTS. Women were followed until death; for breast cancer-specific death, we censored women who died of other causes on their dates of death.
The HRs for overall and breast cancer-specific mortality in African Americans, Latinas, and Asian Americans relative to non-Latina Whites determined from the fully adjusted baseline models were compared to the corresponding HR estimates obtained in models adjusted for a limited number of clinical variables (age at diagnosis, race/ethnicity, registry region, AJCC stage, ER/PR status, surgery type, radiation therapy) and excluded lifestyle factors including parity, alcohol consumption, and smoking history (referred to as “Limited Model”). The limited models were used in previous SEER-based studies (5, 6, 37). To evaluate the potential confounding by study and race/ethnicity (e.g., AABCS contributed data only to the analysis on Asian Americans and over 70% of Latina data were from SFBCS), we repeated the analysis excluding ‘study’ as a stratification factor. We also compared the HR results in CBCSC women to all breast cancer patients in the CCR.
Results
Characteristics of the breast cancer cases included in the pooled analysis (n=12,210) are shown in Tables 2 and 3. Large proportions of Asian Americans (66.6%) and Latinas (43.5%) were foreign-born. Low neighborhood SES was most common among African Americans (27.6%), followed by Latinas (13.3%), Asian Americans (9.3%), and non-Latina Whites (3.2%). Similarly, the frequency of tumor characteristics associated with worse survival (i.e., Stage III/IV, grade III/IV, nodal involvement, tumor size ≥5 cm, and ER negative/PR negative) tended to be highest in African Americans, followed by Latinas, Asian Americans and non-Latina Whites. These distributions of tumor characteristics differed significantly between non-Latina Whites and each of the other three racial/ethnic groups. With the exception of tumor histology, most of the other tumor characteristics also differed significantly between African Americans, Latinas, and Asian Americans (Table 2).
Table 2. Select Clinical and Demographic Cancer Registry Characteristics of Breast Cancer Patients in the Final Pooled Dataset in the CBCSC, by Race/Ethnicity.
| Non-Latina White N=6,501 (53.2%) |
African American N=2,060 (16.9%) |
Latina N=2,032 (16.6%) |
Asian American N=1,505 (12.3%) |
Other race/ethnicity N=112 (0.9%) |
Total N=12,210(100%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| N | %a | N | %a | N | %a | N | %a | N | %a | N | %a | |
| Stage (AJCC)b | ||||||||||||
| I | 3,416 | 54.1 | 827 | 42.4 | 911 | 46.5 | 729 | 49.7g | 64 | 58.7 | 5,947 | 50.4 |
| II | 2,486 | 39.4 | 923 | 47.3 | 868 | 44.3 | 629 | 42.9 | 40 | 36.7 | 4,946 | 41.9 |
| III | 312 | 4.9 | 143 | 7.3 | 145 | 7.4 | 90 | 6.1 | 5 | 4.6 | 695 | 5.9 |
| IV | 102 | 1.6 | 58 | 3.0 | 34 | 1.7 | 19 | 1.3 | 0 | 0 | 213 | 1.8 |
| Unknown | 185 | 109 | 74 | 38 | <5 | 409 | ||||||
| Grade | ||||||||||||
| I | 1,522 | 26.1 | 280 | 15.6 | 306 | 16.9 | 215 | 15.6g | 28 | 27.2 | 2,351 | 21.6 |
| II | 2,537 | 43.5 | 639 | 35.6 | 780 | 43.2 | 594 | 43.1 | 41 | 39.8 | 4,591 | 42.1 |
| III/IV | 1,769 | 30.4 | 874 | 48.8 | 721 | 39.9 | 569 | 41.3 | 34 | 33.0 | 3,967 | 36.4 |
| Unknown | 673 | 267 | 225 | 127 | 9 | 1,301 | ||||||
| Nodal involvement | ||||||||||||
| No | 4,338 | 68.6 | 1,208 | 62.1 | 1,244 | 63.6 | 981 | 66.3 | 76 | 70.4 | 7,847 | 66.4 |
| Yes | 1,984 | 31.4 | 737 | 37.9 | 713 | 36.4 | 499 | 33.7 | 32 | 29.6 | 3,965 | 33.6 |
| Unknown | 179 | 115 | 75 | 25 | <5 | 398 | ||||||
| Tumor Size (cm) | ||||||||||||
| <1 | 1,135 | 18.3 | 235 | 12.2 | 300 | 15.7 | 267 | 18.5 d,g | 19 | 17.3 | 1,956 | 16.9 |
| 1-<5 | 4,730 | 76.2 | 1,512 | 78.6 | 1,495 | 78.1 | 1,084 | 75.1 | 85 | 77.3 | 8,906 | 76.8 |
| ≥5 | 346 | 5.6 | 176 | 9.2 | 120 | 6.3 | 92 | 6.4 | 6 | 5.4 | 740 | 6.4 |
| Unknown | 290 | 137 | 117 | 62 | <5 | 608 | ||||||
| ER/PR statusb | ||||||||||||
| ER+/PR+ | 4,019 | 70.3 | 957 | 57.7 | 1,099 | 62.6 | 830 | 69.5 | 73 | 72.3 | 6,978 | 66.9 |
| ER+/PR− | 739 | 12.9 | 184 | 11.1 | 206 | 11.7 | 126 | 10.6 | 15 | 14.9 | 1,270 | 12.2 |
| ER−/PR+ | 90 | 1.6 | 66 | 4.0 | 45 | 2.6 | 39 | 3.3 | <5 | 1.0 | 241 | 2.3 |
| ER-/PR- | 868 | 15.2 | 453 | 27.3 | 406 | 23.1 | 199 | 16.7 | 12 | 11.9 | 1,938 | 18.6 |
| Unknown | 785 | 400 | 276 | 311 | 11 | 1,783 | ||||||
| Chemotherapy | ||||||||||||
| No | 3,922 | 61.2 | 1,197 | 59.1d | 1,074 | 53.8 | 795 | 54.3 g | 62 | 55.4 | 7,050 | 58.7 |
| Yes | 2,490 | 38.8 | 828 | 40.9 | 923 | 46.2 | 670 | 45.7 | 50 | 44.6 | 4,961 | 41.3 |
| Unknown | 89 | 35 | 35 | 40 | 0 | 199 | ||||||
| Radiation therapy | ||||||||||||
| No | 2,860 | 44.0 | 1,120 | 54.4 | 985 | 48.5 | 883 | 58.7 | 45 | 40.2 | 5,893 | 48.3 |
| Yes | 3,641 | 56.0 | 940 | 45.6 | 1,047 | 51.5 | 622 | 41.3 | 67 | 59.8 | 6,317 | 51.7 |
| Hormone therapy | ||||||||||||
| No | 3,472 | 54.7 | 1,367 | 67.5 | 1,244 | 62.4 | 978 | 67.8 | 52 | 48.1 | 7,113 | 59.7 |
| Yes | 2,877 | 45.3 | 658 | 32.5 | 751 | 37.6 | 465 | 32.2 | 56 | 51.9 | 4,807 | 40.3 |
| Unknown | 152 | 35 | 37 | 62 | <5 | 290 | ||||||
| Surgery type | ||||||||||||
| No surgery | 115 | 1.8 | 98 | 4.8 | 36 | 1.8 | 18 | 1.2 | 0 | 0.0 | 267 | 2.2 |
| Mastectomy | 2,547 | 39.2 | 843 | 40.9 | 926 | 45.6 | 792 | 52.6 | 51 | 45.5 | 5,159 | 42.3 |
| Lumpectomy | 3,827 | 58.9 | 1,116 | 54.2 | 1,069 | 52.6 | 693 | 46.1 | 60 | 53.6 | 6,765 | 55.4 |
| Other | 12 | 0.2 | <5 | 0.1 | <5 | 0.0 | <5 | 0.1 | <5 | 0.9 | 19 | |
| Prior tumor | ||||||||||||
| No | 5,972 | 91.9 | 1,906 | 92.5d | 1,924 | 94.7 | 1,445 | 96.0g | 106 | 94.6 | 11,353 | 93.0 |
| Yes | 529 | 8.1 | 154 | 7.5 | 108 | 5.3 | 60 | 4.0 | 6 | 5.4 | 857 | 7.0 |
| Subsequent tumor | ||||||||||||
| No | 5,438 | 83.6 | 1,695 | 82.3d | 1,769 | 87.1 | 1,282 | 85.2d,g | 98 | 87.5 | 10,282 | 84.2 |
| Yes | 1,063 | 16.4 | 365 | 17.7 | 263 | 12.9 | 223 | 14.8 | 14 | 12.5 | 1,928 | 15.8 |
| Vital status | ||||||||||||
| Deceased | 1,512 | 23.3 | 786 | 38.2 | 457 | 22.5 | 270 | 17.9 | 22 | 19.6 | 3,047 | 25.0 |
| Alive | 4,989 | 76.7 | 1,274 | 61.8 | 1,575 | 77.5 | 1,235 | 82.1 | 90 | 80.4 | 9,163 | 75.0 |
| Histology | ||||||||||||
| Ductal | 4,572 | 70.3 | 1,535 | 74.5e | 1,513 | 74.5 f | 1,118 | 74.3g | 87 | 77.7 | 8,825 | 72.3 |
| Lobular | 1,393 | 21.4 | 280 | 13.6 | 314 | 15.4 | 218 | 14.5 | 20 | 17.9 | 2,225 | 18.2 |
| Other | 536 | 8.2 | 245 | 11.9 | 205 | 10.1 | 169 | 11.2 | 5 | 4.5 | 1,160 | 9.5 |
| Marital status | ||||||||||||
| Single | 677 | 10.6 | 391 | 19.6 | 267 | 13.5 | 192 | 13.0 | 16 | 14.7 | 1,543 | 12.9 |
| Married | 4,113 | 64.4 | 821 | 41.2 | 1,197 | 60.4 | 1,069 | 72.2 | 70 | 64.2 | 7,270 | 60.8 |
| Separated/divorced | 758 | 11.9 | 423 | 21.2 | 254 | 12.8 | 77 | 5.2 | 10 | 9.2 | 1,522 | 12.7 |
| Widowed | 838 | 13.1 | 357 | 17.9 | 263 | 13.3 | 142 | 9.6 | 13 | 11.9 | 1,613 | 13.5 |
| Unknown | 115 | 68 | 51 | 25 | <5 | 262 | ||||||
| Neighborhood SESc | ||||||||||||
| Lowest | 201 | 3.2 | 559 | 27.6 | 263 | 13.3 | 137 | 9.3 | <5 | 1.0 | 1,161 | 9.8 |
| Low-middle | 591 | 9.4 | 528 | 26.1 | 402 | 20.3 | 251 | 17.1 | 16 | 15.4 | 1,788 | 15.1 |
| Middle | 1,119 | 17.8 | 420 | 20.8 | 430 | 21.7 | 288 | 19.6 | 28 | 26.9 | 2,285 | 19.3 |
| High-middle | 1,717 | 27.3 | 338 | 16.7 | 447 | 22.6 | 376 | 25.5 | 31 | 29.8 | 2,909 | 24.5 |
| Highest | 2,657 | 42.3 | 177 | 8.8 | 440 | 22.2 | 420 | 28.5 | 28 | 26.9 | 3,722 | 31.4 |
| Unknown | 216 | 38 | 50 | 33 | 8 | 345 | ||||||
Percentages are calculated without the unknown category
American Joint Committee on Cancer (AJCC). The California Cancer Registry (CCR) mandated the collection of estrogen receptor (ER)/progesterone receptor (PR) for diagnoses starting in 1990.
Neighborhood socioeconomic status (SES) level based on a principal component analysis and the US Census-assigned variables on education, income, occupation, and housing costs at the census block level [Ref 26, Yost et al.,2001]
P>0.05 for comparisons between non-Latina Whites and each of the other three groups (African Americans, Latinas, and Asian Americans); otherwise they are P<0.05
P>0.05 for comparisons between African Americans and Latinas; otherwise they are P<0.05
P>0.05 for comparisons between African Americans and Asian Americans; otherwise they are P<0.05
P>0.05 for comparisons between Latinas and Asian Americans; otherwise they are P<0.05
Table 3. Select Sociodemographic and Lifestyle Characteristics of Breast Cancer Patients in the CBCSC, by Race/Ethnicity.
| Non-Latina Whites N=6,501 (53.2%) | African Americans N=2,060 (16.9%) | Latinas N=2,032 (16.6%) | Asian Americans N=1,505 (12.3%) | Other race/ethnicity N=112 (0.9%) | Total N=12,210(100%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| N | % a | N | % a | N | % a | N | % a | N | % a | N | % a | |
| Mean age (SD) | 62.0 (12.1) | 60.0 (12.6) | 58.6 (11.7) | 55.9 (11.8) | 60.9 (13.6) | 60.4 (12.3) | ||||||
| US Born | ||||||||||||
| Yes | 6,080 | 94.0 | 2,011 | 98.0 | 1,146 | 56.5 | 502 | 33.4 | 95 | 87.2 | 9,834 | 80.9 |
| No | 386 | 6.0 | 40 | 2.0 | 882 | 43.5 | 999 | 66.6 | 14 | 12.8 | 2,321 | 19.1 |
| Unknown | 35 | 9 | 4 | 4 | 3 | 55 | ||||||
| Education | ||||||||||||
| <high school | 155 | 2.4 | 281 | 13.7 | 749 | 37.1 | 115 | 7.7 | 6 | 5.4 | 1,306 | 10.7 |
| high school | 685 | 10.6 | 511 | 25.0 | 481 | 23.8 | 196 | 13.0 | 6 | 5.4 | 1,879 | 15.4 |
| some college | 1,074 | 16.5 | 761 | 37.2 | 471 | 23.3 | 345 | 23.0 | 26 | 23.2 | 2,677 | 22.0 |
| college graduate | 4,578 | 70.5 | 494 | 24.1 | 320 | 15.8 | 847 | 56.4 | 74 | 66.1 | 6,313 | 51.9 |
| Unknown | 9 | 13 | 11 | 2 | 0 | 35 | ||||||
| Parity | ||||||||||||
| Nulliparous | 1,395 | 21.7 | 314 | 15.4 | 254 | 12.6 | 347 | 23.2 | 24 | 21.8 | 2,334 | 19.3 |
| 1 birth | 886 | 13.7 | 381 | 18.7 | 234 | 11.6 | 251 | 16.8 | 17 | 15.5 | 1,769 | 14.6 |
| 2 births | 2,164 | 33.6 | 446 | 21.9 | 456 | 22.6 | 463 | 31.0 | 35 | 31.8 | 3,564 | 29.5 |
| 3 births | 1,228 | 19.1 | 375 | 18.4 | 407 | 20.2 | 254 | 17.0 | 10 | 9.1 | 2,274 | 18.8 |
| ≥4 births | 769 | 11.9 | 522 | 25.6 | 666 | 33.0 | 178 | 11.9 | 24 | 21.8 | 2,159 | 17.8 |
| Unknown | 59 | 22 | 15 | 12 | 2 | 110 | ||||||
| Age at first birth | ||||||||||||
| <20 | 549 | 10.9 | 761 | 44.7 | 481 | 27.6 | 66 | 5.8 | 14 | 16.5 | 1,871 | 19.3 |
| 20-24 | 1,795 | 35.6 | 586 | 34.5 | 710 | 40.8 | 297 | 26.1 | 34 | 40.0 | 3,422 | 35.3 |
| 25-29 | 1,710 | 33.9 | 218 | 12.8 | 338 | 19.4 | 474 | 41.6 | 22 | 25.9 | 2,762 | 28.5 |
| 30-34 | 733 | 14.5 | 98 | 5.8 | 143 | 8.2 | 200 | 17.5 | 11 | 12.9 | 1,185 | 12.2 |
| ≥35 | 251 | 5.0 | 38 | 2.2 | 69 | 4.0 | 103 | 9.0 | 4 | 4.7 | 465 | 4.8 |
| Nulliparous | 1,395 | 314 | 254 | 347 | 24 | 2,334 | ||||||
| Unknown | 68 | 45 | 37 | 18 | 3 | 171 | ||||||
| First-degree family history of breast cancer | ||||||||||||
| No | 5,139 | 80.6 | 1,723 | 84.9 | 1,731 | 85.6 | 1,288 | 86.2 | 87 | 81.3 | 9,968 | 82.9 |
| Yes | 1,233 | 19.4 | 307 | 15.1 | 292 | 14.4 | 207 | 13.8 | 20 | 18.7 | 2,059 | 17.1 |
| Unknown | 129 | 30 | 9 | 10 | 5 | 183 | ||||||
| Smoking status | ||||||||||||
| Never | 3,150 | 53.7 | 721 | 46.9 | 903 | 65.3 | 1,174 | 78.8 | 56 | 52.8 | 6,004 | 57.8 |
| Past ≤ pack/day | 1,704 | 29.1 | 465 | 30.3 | 313 | 22.6 | 217 | 14.6 | 30 | 28.3 | 2,729 | 26.3 |
| Past >1 pack/day | 504 | 8.6 | 39 | 2.5 | 20 | 1.4 | 16 | 1.1 | 12 | 11.3 | 591 | 5.7 |
| Current≤1pack/day | 379 | 6.5 | 283 | 18.4 | 139 | 10.1 | 77 | 5.2 | 7 | 6.6 | 885 | 8.5 |
| Current >1pack/day | 127 | 2.2 | 29 | 1.9 | 8 | 0.6 | 5 | 0.3 | 1 | 0.9 | 170 | 1.6 |
| Unknown | 637 | 523 | 649 | 16 | 6 | 1,831 | ||||||
| Alcohol consumption | ||||||||||||
| Non-drinker | 2,106 | 34.4 | 1,214 | 61.8 | 1,154 | 58.7 | 1,210 | 81.9 | 41 | 41.4 | 5,725 | 49.3 |
| ≤ 2 drink/week | 1,289 | 21.1 | 362 | 18.4 | 409 | 20.8 | 101 | 6.8 | 23 | 23.2 | 2,184 | 18.8 |
| >2 drinks/week | 2,719 | 44.5 | 387 | 19.7 | 402 | 20.5 | 166 | 11.2 | 35 | 35.4 | 3,709 | 31.9 |
| Unknown | 387 | 97 | 67 | 28 | 13 | 592 | ||||||
| Menopause status | ||||||||||||
| Premenopausal | 1,546 | 25.6 | 552 | 28.6 | 562 | 29.3 | 576 | 39.6 | 33 | 30.6 | 3,269 | 28.6 |
| Natural menopause | 3,566 | 59.1 | 888 | 46.0 | 1,006 | 52.5 | 664 | 45.7 | 57 | 52.8 | 6,181 | 54.0 |
| Bilateral Oophorectomy | 552 | 9.2 | 272 | 14.1 | 179 | 9.3 | 108 | 7.4 | 9 | 8.3 | 1,120 | 9.8 |
| Surgical/Medical menopause | 367 | 6.1 | 219 | 11.3 | 169 | 8.8 | 105 | 7.2 | 9 | 8.3 | 869 | 7.6 |
| Unknown | 470 | 129 | 116 | 52 | 4 | 771 | ||||||
Percentages are calculated without the unknown category
Most of the lifestyle factors also differed significantly between the four racial/ethnic groups. Low education (some high school or less) and ≥4 births were most frequent in Latinas (37.1% and 33.0%, respectively), and first birth at age <20 years (44.7%) and a history of smoking (53.1%) in African Americans; whereas a positive family history of breast cancer (19.4%) and a history of regular alcohol use (65.6%) was most frequent in non-Latina Whites (Table 3).
Table 4 shows characteristics of the breast cancer cases included in CBCSC compared to all invasive breast cancers diagnosed in California identified by the CCR during 1993-2007. In general, breast cancer cases in CBCSC had proportionally fewer stage III/IV cancers than those in the CCR; this pattern was consistently observed across all four racial/ethnic groups. The prevalence of ER negative tumors was also consistently lower in CBCSC breast cancer cases than in the CCR. However, the prevalence of poorly differentiated tumors (Grade III or IV) was comparable in CBCSC and the CCR. Differences in neighborhood SES also existed between breast cancer cases in CBCSC and in the CCR: a higher percentage of non-Latina Whites and Latinas in CBCSC lived in the highest SES neighborhoods (40.9% and 21.7%, respectively) compared to those in the CCR (31.5% and 11.4%, respectively). The percentage of African Americans living in the highest SES neighborhoods was comparable in CBCSC (8.9%) and the CCR (8.6%), but there was some underrepresentation of high SES Asian Americans in CBCSC (27.9%) compared with the CCR (31.0%).
Table 4. Characteristics of Breast Cancer Patients included in the CBCSC compared with those in the CCR and in SEER, by Race/Ethnicity.
| Risk factor | Source of Breast Cancer Case | Non-Latina Whites | African Americans | Latinas | Asian Americans |
|---|---|---|---|---|---|
| Total sample size | CBCSC | 6,501 | 2,060 | 2,032 | 1,505 |
| CCR | 208,542 | 17,099 | 38,459 | 24,298 | |
| SEER (2000-6) | 176,094 | 20,486 | 14,951 | 14,951 | |
| Stage III/IV a | CBCSC | 6.4 | 9.8 | 8.8 | 7.2 |
| CCR | 11.0 | 18.5 | 16.7 | 12.2 | |
| SEER (2000-6)a | 12.3 | 21.4 | 18.1 | 12.4 | |
| Grade III/IVb | CBCSC | 27.2 | 42.4 | 35.5 | 37.8 |
| CCR | 28.4 | 44.8 | 40.0 | 36.6 | |
| SEER (1992-8)b | 32.1 | 43.2 | 40.7 | 36.0 | |
| ER-c | CBCSC | 16.8 | 31.3 | 25.7 | 19.9 |
| CCR | 19.1 | 35.6 | 28.7 | 23.7 | |
| SEER (1992-8)d | 22.0 | 39.2 | 31.8 | 25.5 | |
| Neighborhood SES –highest quintile | CBCSC | 40.9 | 8.6 | 21.7 | 27.9 |
| CCR | 31.5 | 8.7 | 11.4 | 31.0 |
Separate baseline models for overall and breast cancer-specific mortality were built using time from diagnosis and attained age time scales. Tumor characteristics from Table 2 including stage, grade, nodal involvement, tumor size, ER/PR status, chemotherapy, prior tumor, and surgery type remained statistically significant in the final model (Table 5). Demographic factors (race/ethnicity, education, neighborhood SES, marital status), reproductive/lifestyle factors (age at first birth, alcohol consumption, smoking) and other factors (nativity, years of diagnosis, and region of residency) remained statistically significant in the final model for overall mortality using attained age time scales (Table 5). Results using time from diagnosis were very similar (data not shown) though the use of attained age appeared to provide a slightly better model fit for both overall and breast cancer-specific mortality. Most of the HRs were similar for overall and breast cancer-specific mortality, but there were somewhat stronger effects of stage, grade, nodal involvement, and tumor size on breast cancer-specific mortality (Table 5).
Table 5. Hazard Ratios (HR) and Coefficient of Variation (CV)a for Final Models for All-Cause and Breast Cancer-Specific Mortality Models in the CBCSC.
| Overall Mortality | Breast Cancer-Specific Mortality | |||
|---|---|---|---|---|
| Variable | Attained Age HR a |
95% CI | Attained Age HRa |
95% CI |
| Age at diagnosisb | ||||
| 40 | 0.67 | 0.19-2.31 | 0.47 | 0.08-2.61 |
| 50 | 0.92 | 0.52-1.63 | 0.66 | 0.29-1.48 |
| 60 | 1.00 | 1.00 | ||
| 70 | 0.92 | 0.54-1.56 | 1.62 | 0.76-2.34 |
| 80 | 0.75 | 0.27-2.12 | 2.76 | 0.62-12.2 |
| Stage (AJCC)c | ||||
| I | 1.00 | 1.00 | ||
| II | 1.22 | 1.09-1.37 | 1.76 | 1.46-2.12 |
| III | 1.97 | 1.62-2.40 | 3.14 | 2.41-4.09 |
| IV | 4.79 | 3.82-6.00 | 7.98 | 5.94-10.7 |
| Unknown | 0.97 | 0.77-1.23 | 1.26 | 0.90-1.78 |
| Grade | ||||
| I | 1.00 | 1.00 | ||
| II | 1.16 | 1.03-1.31 | 1.61 | 1.29-2.02 |
| III/IV | 1.53 | 1.35-1.73 | 2.59 | 2.07-3.25 |
| Unknown | 1.14 | 0.99-1.33 | 1.60 | 1.23-2.09 |
| Nodal involvement | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.55 | 1.39-1.73 | 1.96 | 1.69-2.28 |
| Unknown | 1.80 | 1.53-2.12 | 2.30 | 1.81-2.91 |
| Tumor size (cm) | ||||
| <1 | 1.00 | 1.00 | ||
| 1-<5 | 1.24 | 1.09-1.41 | 1.88 | 1.46-2.43 |
| ≥5 | 1.41 | 1.16-1.71 | 2.22 | 1.64-3.01 |
| Unknown | 1.69 | 1.37-2.09 | 2.65 | 1.92-3.64 |
| Surgery type | ||||
| No surgery | 1.00 | 1.00 | ||
| Mastectomy | 0.58 | 0.48-0.71 | 0.50 | 0.39-0.64 |
| Lumpectomy | 0.50 | 0.41-0.61 | 0.41 | 0.32-0.53 |
| Other | 0.66 | 0.29-1.53 | 0.53 | 0.17-1.73 |
| ER/PR c status | ||||
| ER+/PR+ | 1.00 | 1.00 | ||
| ER+/PR- | 1.22 | 1.08-1.37 | 1.30 | 1.10-1.54 |
| ER-/PR+ | 0.99 | 0.76-1.28 | 1.08 | 0.78-1.49 |
| ER-/PR- | 1.35 | 1.21-1.50 | 1.46 | 1.28-1.67 |
| ER/PR Unknown | 1.10 | 0.99-1.22 | 1.21 | 1.04-1.40 |
| Chemotherapy | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.20 | 1.09-1.32 | 1.27 | 1.11-1.46 |
| Unknown | 0.92 | 0.68-1.24 | 1.00 | 0.68-1.48 |
| Radiation therapy | ||||
| No | NId | 1.00 | ||
| Yes | 1.11 | 0.98-1.25 | ||
| Prior tumor | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.45 | 1.28-1.64 | 1.23 | 1.01-1.50 |
| Neighborhood SES c | ||||
| Lowest | 1.00 | 1.00 | ||
| Lower-middle | 1.01 | 0.89-1.15 | 0.96 | 0.80-1.15 |
| Middle SES | 0.92 | 0.81-1.05 | 0.85 | 0.71-1.02 |
| Higher-middle | 0.78 | 0.68-0.90 | 0.78 | 0.65-0.94 |
| Highest | 0.84 | 0.72-0.97 | 0.77 | 0.64-0.94 |
| Unknown | 0.85 | 0.66-1.10 | 0.82 | 0.57-1.18 |
| Race/ethnicity | ||||
| Non-Latina White | 1.00 | 1.00 | ||
| African American | 1.02 | 0.91-1.15 | 1.13 | 0.97-1.33 |
| Latina | 0.75 | 0.65-0.87 | 0.84 | 0.70-1.00 |
| Asian American | 0.76 | 0.57-1.01 | 0.60 | 0.37-0.97 |
| Other | 0.96 | 0.62-1.47 | 1.00 | 0.51-1.95 |
| Marital status | ||||
| Single, never married | 1.00 | 1.00 | ||
| Married | 0.94 | 0.83-1.05 | 1.05 | 0.90-1.22 |
| Separated/Divorced | 1.20 | 1.04-1.37 | 1.25 | 1.04-1.51 |
| Widowed | 1.11 | 0.96-1.27 | 1.25 | 1.02-1.53 |
| Unknown | 0.89 | 0.68-1.16 | 0.86 | 0.58-1.27 |
| Educational level | ||||
| <high school | 1.00 | NId | ||
| high school | 0.78 | 0.68-0.90 | ||
| some college | 0.81 (0.07) | 0.71-0.93 | ||
| college graduate or more | 0.66 (0.08) | 0.57-0.77 | ||
| Unknown | 0.71 (0.36) | 0.39-1.29 | ||
| Age at first birth | ||||
| < 20 | 1.00 | NId | ||
| 20-24 | 0.96 | 0.86-1.07 | ||
| 25-29 | 0.93 | 0.81-1.06 | ||
| 30-34 | 1.05 | 0.90-1.24 | ||
| ≥35 | 0.95 | 0.76-1.18 | ||
| Nulliparous | 1.11 | 0.97-1.27 | ||
| Missing | 1.22 | 0.93-1.61 | ||
| Smoking status | ||||
| Never | 1.00 | 1.00 | ||
| Past ≤1 pack/day | 1.11 | 1.00-1.22 | 1.15 | 1.00-1.31 |
| Past >1 pack/day | 1.37 | 1.15-1.62 | 1.18 | 0.91-1.53 |
| Current ≤1 pack/day | 1.46 | 1.28-1.68 | 1.22 | 1.01-1.47 |
| Current >1 pack/day | 2.07 | 1.60-2.69 | 1.63 | 1.11-2.38 |
| Unknown | 1.39 | 1.16-1.65 | 1.61 | 1.25-2.07 |
| Alcohol consumption | ||||
| Non-drinker | 1.00 | NId | NId | |
| ≤ 2 drinks/week | 0.84 | 0.76-0.93 | ||
| >2 drinks/week | 0.88 | 0.81-0.97 | ||
| Unknown | 1.05 | 0.88-1.28 | ||
| US born | ||||
| Yes | 1.00 | NId | NId | |
| No | 0.87 | 0.77-0.99 | ||
| Unknown | 1.26 | 0.76-2.07 | ||
| Year of diagnosis | ||||
| 1993-1995 | 1.00 | 1.00 | ||
| 1996-1998 | 0.97 | 0.87-1.09 | 0.92 | 0.79-1.07 |
| 1999-2001 | 0.85 | 0.74-0.97 | 0.80 | 0.66-0.97 |
| 2001-2004 | 0.89 | 0.75-1.05 | 0.82 | 0.66-1.07 |
| 2005-2007 | 0.84 | 0.66-1.06 | 0.81 | 0.60-1.11 |
| Residency region | ||||
| Region 1 | 1.00 | 1.00 | ||
| CSP of Orange County | 0.71 | 0.53-0.94 | 0.63 | 0.41-0.98 |
| Central California | 1.08 | 0.83-1.40 | 0.97 | 0.66-1.45 |
| CSP/Sutter Cancer Center | 0.86 | 0.69-1.08 | 0.75 | 0.54-1.05 |
| Tri-Counties | 0.79 | 0.57-1.10 | 0.79 | 0.48-1.30 |
| Desert Sierra CSP | 0.94 | 0.72-1.24 | 0.87 | 0.58-1.29 |
| Cancer Registry of N California | 1.05 | 0.82-1.36 | 0.96 | 0.65-1.43 |
| San Diego/Imperial | 1.15 | 0.89-1.49 | 0.97 | 0.65-1.44 |
| Northern California Cancer Center | 1.09 | 0.94-1.27 | 1.04 | 0.84-1.27 |
| CSP of Los Angeles | 1.09 | 0.89-1.35 | 1.05 | 0.77-1.44 |
95% CI
HR estimates for specific ages at diagnosis are computed from the Cox model regression parameters of continue Age and log (Age).
American Joint Committee on Cancer (AJCC), estrogen receptor(ER)/progesterone receptor (PR), socioeconomic status (SES)
HR estimates are not shown for variables that were not included (NI) in the final model.
A total of 3,047 deaths were observed after a mean (SD) follow-up of 8.3 (3.5) years. Compared with non-Latina Whites, the HR (95% CI) for overall mortality was 1.02 (0.91-1.15) for African Americans, 0.75 (0.65-0.87) for Latinas, and 0.76 (0.57-1.01) for Asian Americans in our fully adjusted baseline model that included all significant clinical characteristics and lifestyle factors with stratification by study. Larger racial/ethnic differences were observed when we restricted the analysis to the 1,570 breast cancer-specific deaths. Compared with non-Latina Whites, the HR (95% CI) was 1.13 (0.97-1.33) for African Americans, 0.84 (0.70-1.00) for Latinas, and 0.60 (0.37-0.97) for Asian Americans (Table 6, Base model). Next we conducted these analyses with adjustment for a limited number of clinical characteristics and without consideration of reproductive/lifestyle prognostic factors (parity, alcohol consumption, smoking) and other variables (differentiation, nodal involvement, tumor size, chemotherapy, prior tumor, SES, marital status, and year of diagnosis) as was usually done in SEER-based studies (5, 6) (Table 6, Limited Model 1). Relative to non-Latina Whites, the overall and breast cancer-specific mortality HR for African Americans increased (1.25 and 1.31, respectively); the overall HR in Latinas increased somewhat (0.82) but the breast cancer-specific HR remained unchanged, whereas the overall and breast cancer specific HR in Asian Americans decreased somewhat (0.71 and 0.56, respectively). When we repeated these analyses without stratification by study (Table 6, Limited Model 2), relative to non-Latina Whites, the HRs for overall and breast cancer-specific mortality in African Americans increased further (1.46 and 1.52, respectively), the HRs in Latinas approached unity (0.99 and 1.00, respectively), and the HRs in Asian Americans remained substantially lower than 1.0 (0.82 and 0.84, respectively).
Table 6. Hazard Ratios (HR) and 95% Confidence Intervals (CI)a for Overall and Breast Cancer-Specific Mortality in African Americans, Latinas, and Asian Americans Relative to Non-Latina Whites.
| Overall mortality | Breast cancer-specific mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-Latina Whites | African Americans | Latinas | Asian Americans | Non-Latina Whites | African Americans | Latinas | Asian Americans | |
|
| ||||||||
| HR | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Deaths | 1,561 | 739 | 470 | 266 | 703 | 439 | 261 | 161 |
| Base Modelb | 1.00 | 1.02 (0.91,1.15) | 0.75 (0.65, 0.87) | 0.76 (0.57, 1.01) | 1.00 | 1.13 (0.97, 1.33) | 0.84 (0.70, 1.00) | 0.60 (0.37, 0.97) |
| Limited Model1c | 1.00 | 1.25 (1.12,1.39) | 0.82 (0.72, 0.93) | 0.71 (0.53, 0.94) | 1.00 | 1.31 (1.13, 1.53) | 0.84 (0.71, 1.01) | 0.56 (0.35, 0.90) |
| Limited Model2d | 1.00 | 1.46 (1.33, 1.61) | 0.99 (0.89, 1.11) | 0.82 (0.71, 0.94) | 1.00 | 1.52 (1.33, 1.73) | 1.00 (0.86, 1.16) | 0.84 (0.70, 1.02) |
| Limited Model2-CCRe | 1.00 | 1.36 (1.32, 1.39) | 1.01 (0.98, 1.03) | 0.80 (0.78, 0.83) | 1.00 | 1.42 (1.37, 1.47) | 1.01 (0.99, 1.04) | 0.85 0.82, 0.89) |
95% confidence intervals for Hazard Ratios (HR) are obtained by dividing and multiplying HR by the factor M=(1+coefficient of variation)1.96
HR and 95% CI were estimated using multivariable Cox proportional hazards regression methods with attained age as the survival time and stratification by study (AABCS, CARE, SFBCS, LACE, CTS, MEC). The following variables were adjusted for in both the overall and breast cancer-specific mortality models unless otherwise specified: age at diagnosis, American Joint Committee on Cancer (AJCC) stage, grade, nodal involvement, tumor size, surgery type, estrogen receptor (ER)/progesterone receptor (PR) status, chemotherapy, radiation therapy (breast cancer-specific model only), prior tumor, neighborhood SES, race/ethnicity, marital status, education level (overall mortality model only), age at first birth (overall mortality model only) smoking, alcohol (overall model only), US born (overall model only), year of diagnosis, residence region (see Table 5)
As in footnote a, but including only main effects for race/ethnicity, age at diagnosis, registry region, AJCC stage, ER/PR status, surgery type, radiation therapy (breast cancer-specific mortality model only), and stratification by study (AABCS, CARE, SFBCS, LACE, CTS, MEC).
As in footnote b, but without stratification by study (AABCS, CARE, SFBCS, LACE, CTS, MEC).
As in footnote c, but the analysis was conducted in the entire CCR study population of 289,058 breast cancer patients diagnosed from 1993-2007.
Finally, we tested the Limited Model 2 using all 289,058 breast cancer patients in the CCR and found that the HRs for overall and breast cancer-specific mortality in Latinas were comparable to those in non-Latina Whites (1.01 and 1.01, respectively). In contrast, the HRs in African Americans were higher (1.36 and 1.42, respectively) and those in Asian Americans were lower (0.80 and 0.85, respectively) than the HRs in non-Latina Whites (Table 6, Limited model 2-CCR).
Discussion
Numerous observational studies have examined the role of diet, adiposity, physical activity, and other lifestyle factors in relation to the risk of breast cancer development. However, much less is known about the role of lifestyle factors and their interactions with contextual factors in relation to breast cancer prognosis, particularly in non-White populations. To our knowledge, the CBCSC is the largest study on breast cancer prognosis that includes lifestyle and contextual factors on a sizeable number of Latinas, African Americans, and Asian Americans, allowing for assessment of prognostic effects specific to each racial/ethnic group and comparison of the relative contribution of prognostic factors to disparities in survival across racial/ethnic groups.
The design and composition of the CBCSC study population differ from the study populations in three other large US studies on breast cancer prognosis (38-40). Our analysis primarily focuses on lifestyle factors before diagnosis of breast cancer, whereas the other three studies focused on lifestyle factors after breast cancer diagnosis. The Health, Eating, Activity, and Lifestyle (HEAL) Study (38) is a multicenter study of 1,183 breast cancer patients (615 Latinas from New Mexico, 202 Whites from Washington, 366 African Americans from LA County) diagnosed with in situ to stage IIIA breast cancer in the 1990s. Although the HEAL Study has been informative with regard to the relation between blood biomarkers and survival, this study is limited by the very small number of deaths to date (87 total deaths, 42 deaths from breast cancer) (38). The After Breast Cancer Pooling Project (ABCPP) (39) was established in 2009 and includes over 18,000 breast cancer survivors from four established studies to examine the role of physical activity, adiposity, supplement use, and quality of life in breast cancer prognosis. Although the number of non-Latina Whites in the ABCPP is almost twice as large as in our study, the numbers of African Americans (n=357), Latinas (n=387), and Asian Americans (n=292) are relatively small (39). The Pathways Study, a prospective cohort study of women diagnosed with breast cancer in Kaiser Permanente Northern California was initiated in 2006 (40). This study has now recruited nearly 4,500 women (8% African American, 12% Asian American, and 12% Latina) with an overarching goal to examine the associations of lifestyle and molecular factors with prognosis and survival (40). Thus, the CBCSC with its large numbers of non-Whites and relatively long follow-up represents a unique resource to study prognosis-related factors within and across multiple racial/ethnic groups. A limitation of our study is the lack of information on lifestyle factors after diagnosis in five of our studies. However, results from LACE, which collected information after diagnosis, suggest generally similar levels of health-related behaviors before and after diagnosis, with the exception of weight gain during the first 1-3 years post-diagnosis (41, 42). In the Pathways Study, habits such as use of complementary and alternative therapy also did not show substantial changes from the 5 years prior to diagnosis to after diagnosis (43). Similarly, in the HEAL Study, physical activity levels remained relatively stable up to 5 years postdiagnosis (44).
Various methodological issues were considered in the construction of the final baseline statistical model for our pooled analysis. A potential concern is the pooling of data from case-control and cohort studies. We conducted separate analysis by study design and did not find systematic differences across studies. This was tested formally in the context of our model as an interaction between race/ethnicity and study. The likelihood ratio tests for this effect on 11 degrees of freedom have p-values greater than 0.15 for both overall and breast cancer specific mortality. Our model included age at diagnosis as a continuous variable on both the natural scale and the log10 scale. Sensitivity analyses using categorized age confirmed that this approach captured the essential characteristics of the effect of age on overall and breast cancer-specific mortality. Missing or unknown values for relevant clinical characteristics and lifestyle prognostic factors were accounted for in the multivariate model by including a category for missing data. Although we considered using multiple imputation methods (45), we ultimately decided against this approach because the proportion of missing data is generally small in this cohort such that any bias in parameter estimates when compared with complete data is likely to be small. The multiple imputation approach also has some drawbacks as the appropriateness of assumptions required for its use may be impractical if imputation is needed for a large number of variables. We have accounted for competing risks in breast cancer-specific mortality analyses by censoring non-breast cancer causes of death. We evaluated the validity of this simpler approach by comparing it to a formal method for competing risks in Cox regression analysis proposed by Fine and colleagues (5, 46). This formal approach is computationally intensive compared to the simpler approach, but nevertheless yielded Cox regression parameter estimates that were essentially equivalent to those from the simpler approach (data not shown).
Our baseline overall and breast cancer-specific models were intended to capture racial/ethnic differences by adjusting for all relevant clinical characteristics and lifestyle prognostic factors, allowing us to evaluate whether the putative prognostic factors- contextual factors, physical activity, body size, and co-morbidities- have additional effects on racial/ethnic differences in survival experience. Using this fully-adjusted baseline model, African Americans showed higher breast cancer-specific mortality than non-Latina Whites (HR=1.13), but their overall mortality was similar. In contrast, the overall and breast cancer-specific mortality in Latinas and Asian Americans was considerably lower than in non-Latina Whites. In SEER-based studies of overall mortality and breast cancer-specific mortality by race/ethnicity that adjusted for a limited number of clinical characteristics and were unable to adjust for individual lifestyle prognostic factors (5, 6, 37), the risks of overall and breast cancer-specific mortality were 1.5-1.8 fold higher among African Americans, and 1.1-1.4 fold higher among Latinas, relative to non-Latina Whites, whereas the overall and breast cancer-specific mortality among Asian Americans was lower. To provide a comparison with SEER-based studies, we conducted analyses with CBCSC subjects that adjusted for a more limited number of clinical characteristics and did not adjust for individual lifestyle prognostic factors (Table 6, Limited Model 1). Results for CBCSC African Americans and Asian Americans were comparable to those reported in SEER-based studies showing respectively higher (HR= 1.2-1.3) and lower (HR=0.54-0.66) overall and breast cancer-specific mortality risks than was observed in CBCSC non-Latina Whites. However, contrary to the results from SEER-based studies, CBCSC Latinas did not have higher risk of overall and breast cancer-specific mortality than CBCSC non-Latina Whites. HRs in CBCSC and the CCR were comparable when we repeated the analyses using the Limited Model 2 and excluded ‘study’ as a stratification variable.
There are some possible explanations for these somewhat divergent results, particularly in CBCSC Latina breast cancer cases compared to those included in the SEER-based analyses. CBCSC cases represent women who were able and willing to complete detailed questionnaires or interviews and thus may not be representative of all breast cancer patients. In fact, we found that CBCSC participants had less advanced stage cancer (III/IV) than breast cancer patients in the CCR (Table 4) who, in turn, had somewhat better survival than women from a combination of SEER registries. Similarly, the prevalence of ER negative tumors was lowest among breast cancer patients in CBCSC, intermediate in the CCR, and highest in SEER. Interestingly, CBCSC non-Latina Whites and Latinas were more likely to live in high-SES neighborhoods than their counterparts in the CCR, but this was not observed for African American and Asian American women (Table 4). This difference in neighborhood SES may explain, in part, the better survival experience in CBCSC Latinas and this question will be investigated in future analyses as part of CBCSC Project 1 on contextual factors. Heterogeneity and regional differences within Latina and Asian American populations may also contribute to these differences in results. Latinas from Mexico, Puerto Rico, and South or Central America showed considerable differences in their breast cancer mortality (5, 6) such that the survival of Latinas in California, most (>80%) of whom are from Mexico (47), may not be representative of the survival of Latinas in the entire US. Similarly, our results for Asian Americans relate primarily to Chinese, Japanese, and Filipina women, who accounted for over 95% of the Asian women in CBCSC, but other Asian ethnic groups (e.g., Korean, Vietnamese) experience somewhat worse mortality than Chinese and Japanese women and also worse mortality than non-Latina Whites (5, 48).
In summary, the CBCSC is well-positioned to study breast cancer outcomes across the major US racial/ethnic groups using the combined resources of six well-established studies covering multiethnic populations in California. We found racial/ethnic disparities in survival after breast cancer diagnosis that remained after adjustment for differences in several clinical, lifestyle, and neighborhood factors. However, there are some study limitations. While we successfully harmonized data on lifestyle prognostic factors, the questions were not identical across all the studies and some assumptions were made. Despite statistical adjustment for ‘study’, we cannot exclude the possibility of potential effect modification by study and race/ethnicity. One limitation of all studies of cause of death may be the variation in attributing deaths to a particular cause by the person completing the death certificate. If this were to vary by race/ethnicity, it could potentially bias the risk estimates but this would affect not only our results but those of all other studies. We were also limited by the fact that only information on first course of treatment is available from the CCR and uncontrolled confounding by treatment may exist although we have previously shown that registry data on treatment including chemotherapy and surgery are of high quality (49). There are also important study strengths, including a large number of deaths due to breast cancer in African American, Latina, and Asian American breast cancer patients, which allowed us to examine the separate and combined effects of various lifestyle prognostic factors and tumor characteristics on survival. In addition, differential reporting of clinical and follow-up data by race/ethnicity was minimized given that these data were uniformly obtained from the CCR. The establishment of this rich resource provides the potential for unique insights regarding the roles of socioeconomic, medical, biological, cultural, and other determinants of racial/ethnic differences in breast cancer survival and opportunities for future studies of racial/ethnic disparities in breast cancer outcomes.
Acknowledgments
We are grateful to all the study participants for their contributions in the six California-based studies. We thank Juan Yang and Rita Leung at CPIC and Chiu-Chen Tseng at USC for their analytic support. This work was supported by grants (16ZB-8001 (USC, Wu), 16ZB-8002 (CPIC, Gomez), 16ZB-8003 (COH, Bernstein), 16ZB-8004 (KPNC, Kwan), 16ZB-8005 (USC, Monroe) from the California Breast Cancer Research Program. The Asian American Breast Cancer Study was supported by the California Breast Research Program (CBCRP) grants 1RB-0287, 3PB-0120, and 5PB-0018. The San Francisco Bay Area Breast Cancer Study was supported by National Cancer Institute grants R01 CA63446 and R01 CA77305; by the U.S. Department of Defense (DOD) grant DAMD17-96-1-6071; and by the CBCRP grants 4JB-1106 and 7PB-0068. The Women's CARE Study was funded by the National Institute of Child Health and Human Development (NICHD), through a contract with USC (N01-HD-3-3175), and the California Teachers Study was funded by the California Breast Cancer Act of 1993; National Cancer Institute grants (R01 CA77398 and K05 CA136967 to LB); and the California Breast Cancer Research Fund (contract 97-10500). The Multiethnic Cohort Study was supported by National Cancer Institute grants R01 CA54281, R37CA54281, and UM1 CA164973. The Life After Cancer Epidemiology Study is supported by National Cancer Institute grant R01 CA129059. Clinical and tumor characteristics and mortality data were obtained from the California Cancer Registry (CCR), also part of the National Cancer Institute's Division of Cancer Prevention and Control Surveillance, Epidemiology, and End Results Program, under contract number N01CN25403. The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN26120100035C awarded to the University of Southern California, and contract HHSN26120100034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, the California Department of Health Services, the National Cancer Institute, or the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Conflict of interest: None
Contributor Information
Anna H. Wu, University of Southern California, Los Angeles, CA
Scarlett Lin Gomez, Cancer Prevention Institute of California, Fremont, CA; Stanford University School of Medicine, Stanford, CA.
Cheryl Vigen, University of Southern California, Los Angeles, CA.
Marilyn L. Kwan, Division of Research, Kaiser Permanente Northern California, Oakland, CA
Theresa H.M. Keegan, Cancer Prevention Institute of California, Fremont, CA; Stanford University School of Medicine, Stanford, CA
Yani Lu, City of Hope, Duarte, CA.
Salma Shariff-Marco, Cancer Prevention Institute of California, Fremont, CA; Stanford University School of Medicine, Stanford, CA.
Kristine R. Monroe, University of Southern California, Los Angeles, CA
Allison W. Kurian, Stanford University School of Medicine, Stanford, CA
Iona Cheng, Cancer Prevention Institute of California, Fremont, CA.
Bette J. Caan, Division of Research, Kaiser Permanente Northern California, Oakland, CA
Valerie S. Lee, Division of Research, Kaiser Permanente Northern California, Oakland, CA
Janise M. Roh, Division of Research, Kaiser Permanente Northern California, Oakland, CA
Jane Sullivan-Halley, City of Hope, Duarte, CA.
Brian E. Henderson, University of Southern California, Los Angeles, CA
Leslie Bernstein, City of Hope, Duarte, CA.
Esther M. John, Cancer Prevention Institute of California, Fremont, CA; Stanford University School of Medicine, Stanford, CA
Richard Sposto, University of Southern California, Los Angeles, CA.
References
- 1.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–13. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 2.Curtis E, Quale C, Haggstrom D, Smith-Bindman R. Racial and ethnic differences in breast cancer survival: how much is explained by screening, tumor severity, biology, treatment, comorbidities, and demographics? Cancer. 2008;112:171–80. doi: 10.1002/cncr.23131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jatoi I, Anderson WF, Rao SR, Devesa SS. Breast cancer trends among black and white women in the United States. J Clin Oncol. 2005;23:7836–41. doi: 10.1200/JCO.2004.01.0421. [DOI] [PubMed] [Google Scholar]
- 4.Jatoi I, Miller AB. Why is breast-cancer mortality declining? Lancet Oncol. 2003;4:251–4. doi: 10.1016/s1470-2045(03)01037-4. [DOI] [PubMed] [Google Scholar]
- 5.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 6.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2011;127:729–38. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKenzie F, Jeffreys M. Do lifestyle or social factors explain ethnic/racial inequalities in breast cancer survival? Epidemiol Rev. 2009;31:52–66. doi: 10.1093/epirev/mxp007. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin PJ, Boyd NF. Body size and breast cancer prognosis: a critical review of the evidence. Breast Cancer Res Treat. 1990;16:205–14. doi: 10.1007/BF01806329. [DOI] [PubMed] [Google Scholar]
- 9.Abrahamson PE, Gammon MD, Lund MJ, et al. General and abdominal obesity and survival among young women with breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:1871–7. doi: 10.1158/1055-9965.EPI-06-0356. [DOI] [PubMed] [Google Scholar]
- 10.Protani M, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–35. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 11.Ewertz M, Jensen MB, Gunnarsdottir KA, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2010;29:25–31. doi: 10.1200/JCO.2010.29.7614. [DOI] [PubMed] [Google Scholar]
- 12.Kwan ML, Chen WY, Kroenke CH, et al. Pre-diagnosis body mass index and survival after breast cancer in the After Breast Cancer Pooling Project. Breast Cancer Res Treat. 2012;132:729–39. doi: 10.1007/s10549-011-1914-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleveland RJ, Eng SM, Abrahamson PE, et al. Weight gain prior to diagnosis and survival from breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1803–11. doi: 10.1158/1055-9965.EPI-06-0889. [DOI] [PubMed] [Google Scholar]
- 14.Keegan TH, Milne RL, Andrulis IL, et al. Past recreational physical activity, body size, and all-cause mortality following breast cancer diagnosis: results from the Breast Cancer Family Registry. Breast Cancer Res Treat. 2010;123:531–42. doi: 10.1007/s10549-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sternfeld B, Weltzien E, Quesenberry CP, Jr, et al. Physical activity and risk of recurrence and mortality in breast cancer survivors: findings from the LACE study. Cancer Epidemiol Biomarkers Prev. 2009;18:87–95. doi: 10.1158/1055-9965.EPI-08-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.West-Wright CN, Henderson KD, Sullivan-Halley J, et al. Long-term and recent recreational physical activity and survival after breast cancer: the California Teachers Study. Cancer Epidemiol Biomarkers Prev. 2009;18:2851–9. doi: 10.1158/1055-9965.EPI-09-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braithwaite D, Tammemagi CM, Moore DH, et al. Hypertension is an independent predictor of survival disparity between African-American and white breast cancer patients. Int J Cancer. 2009;124:1213–9. doi: 10.1002/ijc.24054. [DOI] [PubMed] [Google Scholar]
- 18.Tammemagi CM. Racial/ethnic disparities in breast and gynecologic cancer treatment and outcomes. Curr Opin Obstet Gynecol. 2007;19:31–6. doi: 10.1097/GCO.0b013e3280117cf8. [DOI] [PubMed] [Google Scholar]
- 19.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285:885–92. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 20.Sprague BL, Trentham-Dietz A, Gangnon RE, et al. Socioeconomic status and survival after an invasive breast cancer diagnosis. Cancer. 2011;117:1542–51. doi: 10.1002/cncr.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pike MC, Kolonel LN, Henderson BE, et al. Breast cancer in a multiethnic cohort in Hawaii and Los Angeles: risk factor-adjusted incidence in Japanese equals and in Hawaiians exceeds that in whites. Cancer Epidemiol Biomarkers Prev. 2002;11:795–800. [PubMed] [Google Scholar]
- 22.Berstad P, Coates RJ, Bernstein L, et al. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev. 2010;19:1532–44. doi: 10.1158/1055-9965.EPI-10-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle P, Boniol M, Koechlin A, et al. Diabetes and breast cancer risk: a meta-analysis. Br J Cancer. 2012;107:1608–17. doi: 10.1038/bjc.2012.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keegan TH, Quach T, Shema S, Glaser SL, Gomez SL. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer. 2010;10:603. doi: 10.1186/1471-2407-10-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu Y, Ma H, Malone KE, et al. Obesity and survival among black women and white women 35 to 64 years of age at diagnosis with invasive breast cancer. J Clin Oncol. 2011;29:3358–65. doi: 10.1200/JCO.2010.34.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conroy SM, Maskarinec G, Wilkens LR, White KK, Henderson BE, Kolonel LN. Obesity and breast cancer survival in ethnically diverse postmenopausal women: the Multiethnic Cohort Study. Breast Cancer Res Treat. 2011;129:565–74. doi: 10.1007/s10549-011-1468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez SL, Glaser SL, McClure LA, et al. The California Neighborhoods Data System: a new resource for examining the impact of neighborhood characteristics on cancer incidence and outcomes in populations. Cancer Causes Control. 2011;22:631–47. doi: 10.1007/s10552-011-9736-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–11. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 29.Phillips KA, Milne RL, West DW, et al. Prediagnosis reproductive factors and all-cause mortality for women with breast cancer in the breast cancer family registry. Cancer Epidemiol Biomarkers Prev. 2009;18:1792–7. doi: 10.1158/1055-9965.EPI-08-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barnett GC, Shah M, Redman K, Easton DF, Ponder BA, Pharoah PD. Risk factors for the incidence of breast cancer: do they affect survival from the disease? J Clin Oncol. 2008;26:3310–6. doi: 10.1200/JCO.2006.10.3168. [DOI] [PubMed] [Google Scholar]
- 31.Daling JR, Malone KE, Doody DR, Anderson BO, Porter PL. The relation of reproductive factors to mortality from breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:235–41. [PubMed] [Google Scholar]
- 32.Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Reproductive history and mortality after breast cancer diagnosis. Obstet Gynecol. 2004;104:146–54. doi: 10.1097/01.AOG.0000128173.01611.ff. [DOI] [PubMed] [Google Scholar]
- 33.Alsaker MD, Opdahl S, Asvold BO, Romundstad PR, Vatten LJ. The association of reproductive factors and breastfeeding with long term survival from breast cancer. Breast Cancer Res Treat. 2011;130:175–82. doi: 10.1007/s10549-011-1566-3. [DOI] [PubMed] [Google Scholar]
- 34.Vrieling A, Buck K, Heinz J, et al. Pre-diagnostic alcohol consumption and postmenopausal breast cancer survival: a prospective patient cohort study. Breast Cancer Res Treat. 2012;136:195–207. doi: 10.1007/s10549-012-2230-2. [DOI] [PubMed] [Google Scholar]
- 35.Korn EL, Graubard BI, Midthune D. Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 36.Pencina MJ, Larson MG, D'Agostino RB. Choice of time scale and its effect on significance of predictors in longitudinal studies. Stat Med. 2007;26:1343–59. doi: 10.1002/sim.2699. [DOI] [PubMed] [Google Scholar]
- 37.Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162:1985–93. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 38.Duggan C, Wang CY, Neuhouser ML, et al. Associations of insulin-like growth factor and insulin-like growth factor binding protein-3 with mortality in women with breast cancer. Int J Cancer. 2013;132:1191–200. doi: 10.1002/ijc.27753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nechuta SJ, Caan BJ, Chen WY, et al. The After Breast Cancer Pooling Project: rationale, methodology, and breast cancer survivor characteristics. Cancer Causes Control. 2011;22:1319–31. doi: 10.1007/s10552-011-9805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kushi LH, Kwan ML, Lee MM, Ambrosone CB. Lifestyle factors and survival in women with breast cancer. J Nutr. 2007;137:236S–42S. doi: 10.1093/jn/137.1.236S. [DOI] [PubMed] [Google Scholar]
- 41.Caan B, Sternfeld B, Gunderson E, Coates A, Quesenberry C, Slattery ML. Life After Cancer Epidemiology (LACE) Study: a cohort of early stage breast cancer survivors (United States) Cancer Causes Control. 2005;16:545–56. doi: 10.1007/s10552-004-8340-3. [DOI] [PubMed] [Google Scholar]
- 42.Caan BJ, Kwan ML, Hartzell G, et al. Pre-diagnosis body mass index, post-diagnosis weight change, and prognosis among women with early stage breast cancer. Cancer Causes Control. 2008;19:1319–28. doi: 10.1007/s10552-008-9203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenlee H, Kwan ML, Ergas IJ, et al. Complementary and alternative therapy use before and after breast cancer diagnosis: the Pathways Study. Breast Cancer Res Treat. 2009;117:653–65. doi: 10.1007/s10549-009-0315-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mason C, Alfano CM, Smith AW, et al. Long-Term Physical Activity Trends in Breast Cancer Survivors. Cancer Epidemiol Biomarkers Prev. 2013;22:1153–61. doi: 10.1158/1055-9965.EPI-13-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 46.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk Journal of the. American Statistical Association: Taylor & Francis; 1999. pp. 496–509. [Google Scholar]
- 47.Center PH. Demographic profile of Hispanics in California. 2009 http://pewhispanic.org/states/?stateid=CA.
- 48.Gomez SL, Clarke CA, Shema SJ, Chang ET, Keegan TH, Glaser SL. Disparities in breast cancer survival among Asian women by ethnicity and immigrant status: a population-based study. Am J Public Health. 2010;100:861–9. doi: 10.2105/AJPH.2009.176651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurian AW, Lichtensztajn DY, Keegan TH, et al. Patterns and predictors of breast cancer chemotherapy use in Kaiser Permanente Northern California, 2004-2007. Breast Cancer Res Treat. 2012;137:247–60. doi: 10.1007/s10549-012-2329-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu AH, Yu MC, Tseng CC, Pike MC. Body size, hormone therapy and risk of breast cancer in Asian-American women. Int J Cancer. 2007;120:844–52. doi: 10.1002/ijc.22387. [DOI] [PubMed] [Google Scholar]
- 51.Marchbanks PA, McDonald JA, Wilson HG, et al. Oral contraceptives and the risk of breast cancer. N Engl J Med. 2002;346:2025–32. doi: 10.1056/NEJMoa013202. [DOI] [PubMed] [Google Scholar]
- 52.John EM, Phipps AI, Davis A, Koo J. Migration history, acculturation, and breast cancer risk in Hispanic women. Cancer Epidemiol Biomarkers Prev. 2005;14:2905–13. doi: 10.1158/1055-9965.EPI-05-0483. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein L, Allen M, Anton-Culver H, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States) Cancer Causes Control. 2002;13:625–35. doi: 10.1023/a:1019552126105. [DOI] [PubMed] [Google Scholar]
- 54.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151:346–57. doi: 10.1093/oxfordjournals.aje.a010213. [DOI] [PMC free article] [PubMed] [Google Scholar]