Abstract
Purpose of review
The increasing prevalence of sarcopenic obesity in older adults has heightened interest in identifying the most effective treatment. This review highlights recent progress in management with an emphasis on lifestyle interventions and pharmacologic therapy aimed at reversing sarcopenic obesity.
Recent findings
While weight loss and exercise independently reverse sarcopenic obesity, they act synergistically in combination to improve body composition and physical function beyond which is observed with either intervention alone. Optimizing protein intake appears to have beneficial effects on net muscle protein accretion in older adults. Myostatin inhibition is associated with favorable changes in body composition in animal studies, though experience in humans is relatively limited. Testosterone and growth hormone offer improvements in body composition but the benefits must be weighed against potential risks of therapy. GHRH-analog therapy shows promise but further studies are needed in older adults.
Summary
At present, lifestyle interventions incorporating both diet-induced weight loss and regular exercise appear to be the optimal treatment for sarcopenic obesity. Maintenance of adequate protein intake is also advisable. Ongoing studies will determine whether pharmacologic therapy such as myostatin inhibitors or GHRH-analogs have a role in the treatment of sarcopenic obesity.
Keywords: sarcopenic obesity, myostatin inhibitors, exercise, weight loss, elderly, older adults
Introduction
Sarcopenic obesity has been appropriately characterized as a confluence of two epidemics, namely the aging of the population and the obesity epidemic [1]. It is characterized by obesity with decreased muscle mass and function [2], with a prevalence as high as 20% in older populations [3]. Indeed, older adults are particularly susceptible to the adverse effects of excess body fat on physical function because of 1) decreased muscle mass and strength that occurs with aging (sarcopenia) and 2) a need to carry greater body mass due to obesity. This increasingly prevalent phenotype has given rise to a population of older adults at increased risk for disability [2], institutionalization [4], and mortality [5]. While these sequelae are widely recognized as inherent to obesity in older adults, it is now accepted that the combination of obesity with sarcopenia, a change in body composition typical of aging, poses even greater risks for poor health-related outcomes and disability than either obesity or sarcopenia alone ([6–10]. The obvious public health implications in an aging society have underscored the importance of identifying the best approach for management of sarcopenic obesity. Unfortunately, the pathogenesis of sarcopenic obesity is multifactorial, such that the optimal treatment for this disorder is not well understood. Specifically, the excess adiposity owing to this condition has been attributed in part to a positive energy balance associated with aging, the consequence of decreases in all major components of total energy expenditure [11] as well as a reduction in physical activity [12•]. Concurrently, these aspects of aging affect the propensity for development of sarcopenia, which is further exacerbated by other age-related changes such as reduced protein intake [13], increased skeletal muscle fatty infiltration [14], impaired muscle energetics [15], altered skeletal muscle substrate metabolism [16], increased expression of myostatin [17], impaired sensitivity to the anabolic effects of insulin with associated mitochondrial dysfunction [18], and age-related reductions in growth hormone and testosterone secretion [10;17;19•–21]. Accordingly, a multifaceted approach to the management of sarcopenic obesity remains the most promising in terms of reducing the associated health care burden from both a personal and public health perspective. The current review provides a summary of recent advancements in therapies for sarcopenic obesity, encompassing a growing literature pertaining to lifestyle interventions and also pharmacologic therapies currently under investigation.
Lifestyle Interventions
The independent and combined effects of lifestyle interventions on sarcopenic obesity are well-described. We will review evidence pertaining to weight loss, exercise, and nutritional modification.
Weight loss
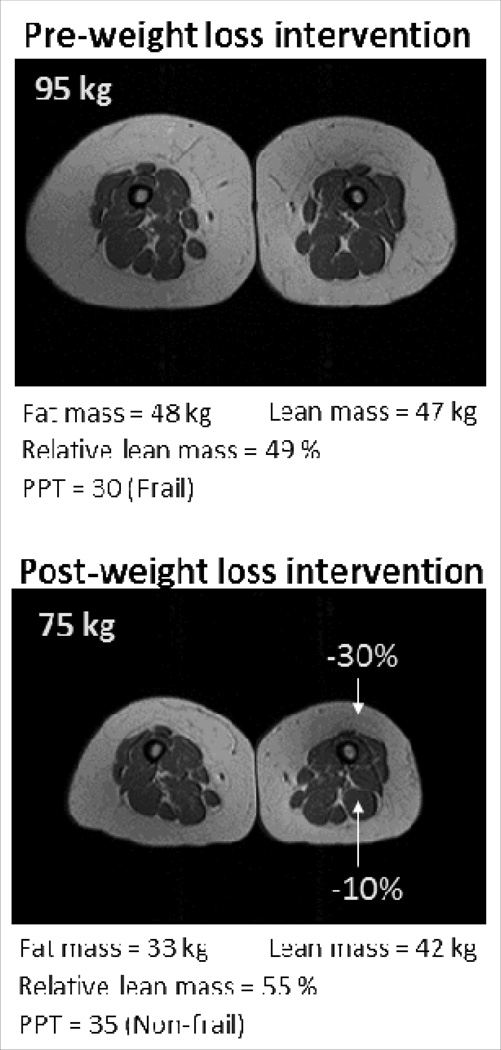
Excess adiposity is associated with a state of low-grade chronic inflammation which contributes to the decline in muscle mass and strength observed in older adults with sarcopenic obesity [22]. Moreover, ectopic fat accumulation in skeletal muscle is associated with impaired muscle strength [14], an important determinant of poor health in older age [9]. Intuitively, weight loss therapy would therefore appear an appropriate strategy for reversing sarcopenic obesity. However, weight loss in older adults remains controversial in part due to the associated loss in lean body mass associated with this intervention and concerns for exacerbating sarcopenia [23•]. Nonetheless, it has been demonstrated that weight loss therapy is not only feasible in frail, obese older adults [24], but that older adults may be more compliant with lifestyle interventions and achieve greater weight loss than younger adults [25;26•]. We investigated the effects of a diet-induced reduction in body weight (~10%) in obese older adults with frailty and found that, while there was some loss in lean body mass associated with this intervention, an even greater reduction in fat mass was observed such that the end result was an improvement in relative sarcopenia (percent body weight as lean body mass) and amelioration of frailty [27••]. As depicted in Figure 1 (MRI images of thigh), an approximate 20% reduction in body weight in this patient resulted in a greater reduction in fat mass than lean mass, leading to improvement of relative sarcopenia and resolution of frailty, as evaluated by the objective physical performance test [28]. Hence, despite a reduction in lean body mass, weight loss appears to be a suitable intervention for the treatment of sarcopenic obesity.
Figure 1. Changes in body composition after weight loss therapy in a frail obese older adult.
Physical performance test (PPT) score 0–36 with higher scores indicating better performance (<32 indicates frailty)
Exercise
Sarcopenic obesity has been attributed in part to an age-related decline in physical activity [12], an observation which has prompted several studies on the effects of exercise on this disorder. Indeed, exercise has been shown to have beneficial effects on multiple aspects of sarcopenic obesity with a resultant increase in muscle protein synthesis [29], reduction in myostatin expression [30•], increase in intramuscular IGF-1 [31], restoration of skeletal muscle sensitivity to the anabolic effects of insulin [32], improvement of nutrient-stimulated vasodilation and nutrient delivery to muscle [33•], enhancement of mitochondrial function [18], and activation of skeletal muscle satellite cells felt to be protective against sarcopenic obesity [34]. Moreover, we have demonstrated that exercise, but not diet-induced weight loss, decreased skeletal muscle inflammatory gene expression in frail, obese older adults [35]. As with weight loss therapy, a regular multicomponent exercise intervention is associated with improvement in sarcopenia with a reduction in fat mass and increase in lean body mass, resulting in reversal of frailty [27]. We suggest an exercise intervention incorporating progressive resistance training (PRT), with three ~90-minute sessions per week consisting of 15 minutes of flexibility, 30 minutes of low-impact aerobic exercise, 30 minutes of high-intensity PRT, and 15 minutes of balance training [36]. PRT has also been studied in Asian Indian subjects, a population inherently prone to sarcopenic obesity with higher relative fat mass and lower relative lean mass than any other ethnic group [37], and was associated with improvements in muscle strength, waist circumference, and multiple metabolic outcomes [38].
Combined weight loss and exercise
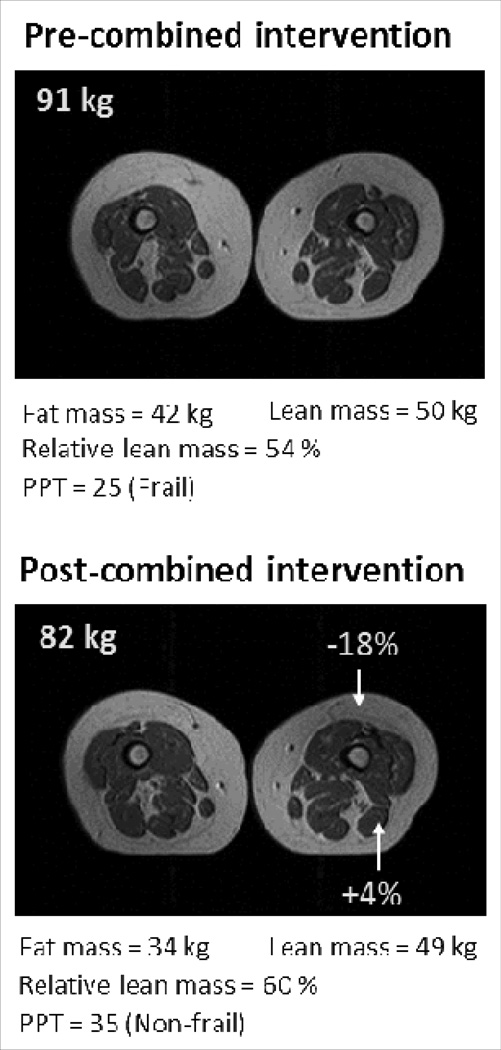
The most effective lifestyle intervention for treatment of sarcopenic obesity is one that includes both, diet-induced weight loss and regular exercise. We previously demonstrated that the combination of these interventions acted synergistically to improve sarcopenia and ameliorate frailty more so than either diet or exercise alone [27••]. Further, the reduction in lean body mass associated with weight loss therapy was attenuated, although not prevented, when combined with regular exercise. Muscle strength increased in the combined intervention despite the modest reduction in lean body mass, suggesting an improvement in muscle quality. Figure 2 illustrates this point further by demonstrating that a combined diet and exercise intervention was associated with an increase in relative lean mass and resolution of frailty despite the absolute reduction in lean body mass. These results lend support to the recommendation that lifestyle interventions targeting reversal of sarcopenic obesity incorporate both, diet-induced weight loss and regular exercise.
Figure 2. Changes in body composition after combined (exercise plus weight loss) intervention in a frail obese older adult.
Physical performance test (PPT) score 0–36 with higher scores indicating better performance (<32 indicates frailty)
Nutritional modification
Aging is associated with both, a reduction in dietary protein intake [13] as well as a blunted muscle protein synthesis response to essential amino acid ingestion [39]. Moreover, the recommended dietary allowance for protein intake may not be adequate in older adults [40]. It has been demonstrated in older adults that ingestion of larger amounts of essential amino acids restores the muscle protein synthesis response similarly to that observed in younger adults, suggesting a threshold effect which can be overcome with increased protein intake [41]. Taking this into consideration, it has been suggested that 25–30 grams of high quality protein be ingested per meal in order to prevent sarcopenia in older adults [42]. This recommendation is based on evidence that ingestion of less than 25–30 grams of protein per meal is associated with suboptimal muscle protein synthesis in the elderly [43]. Likewise, ingestion in excess of 30 grams of protein per meal has not been shown to further improve the anabolic response [44]. While sufficient protein intake is paramount to optimizing the muscle protein synthetic response, a diet relatively low in carbohydrates may also be advisable as coingestion of carbohydrates has been shown to exert negative effects on muscle protein turnover in the elderly [45].
Supplementation with leucine, the most potent branched-chain amino acid for stimulation of protein synthesis, has also been proposed for the prevention of sarcopenia [46]. Indeed, leucine supplementation in older adults has been associated with enhanced muscle protein synthesis independently of ingestion of other amino acids [47]. While these data are promising with regard to nutritional modification and improvements in muscle protein synthesis, long-term studies are ongoing in older adults to determine whether these interventions are effective in preventing muscle loss in older adults.
Pharmacologic therapy
While lifestyle interventions are a cornerstone of management of sarcopenic obesity, it is appreciated that these measures are not always feasible in all patients, either due to physical limitations or poor adherence. Accordingly, there has been growing interest in pharmacologic therapies for this increasingly prevalent condition. We will specifically review recent advancements in the use of myostatin inhibitors for the prevention of sarcopenia as well as explore the benefits and limitations of other anabolic therapies which have been studied in this context such as testosterone and mediators of the IGF-1 system.
Myostatin inhibitors
The role of myostatin in sarcopenic obesity has received considerable attention in recent years and there is accumulating evidence that its inhibition may result in favorable changes in both adiposity and lean body mass. A member of the TGF-β superfamily of secreted growth factors, myostatin is produced by skeletal muscle and adipose tissue, functioning as a negative regulator of muscle mass [48]. Its clinical relevance has been confirmed in rodent models whereby myostatin infusion has resulted in marked muscle wasting [49]. Moreover, myostatin influences adipocyte differentiation with substantial evidence to suggest myostatin-mediated crosstalk between muscle and adipose tissue [30•]. In this sense, skeletal muscle may be considered an endocrine organ, contributing a role in the regulation of body composition. Indeed, myostatin has proven to be a biomarker of sarcopenia in the elderly, correlating inversely with muscle mass, with higher levels being observed in frail older adults compared to younger adults [50]. In contrast, observations of myostatin deficiency in nature have shed light on the implications of myostatin inhibition, with exceptional muscularity and scarce adiposity being well-described in myostatin-deficient cattle [51]. Similarly, a homozygous mutation in the myostatin gene has been described in a human child with increased muscle strength and phenotypic features overlapping those described in myostatin-deficient livestock [52]. These observations have given way to experimental models of myostatin deficiency aimed at determining whether myostatin inhibition is a suitable strategy for the treatment of sarcopenic obesity. Data in animal models have been promising with myostatin knockout mice demonstrating favorable changes in adipose tissue [53•], reduced inflammatory markers [54], increased muscle mass [55], and protection against age-related sarcopenia [56]. Further, inhibition of myostatin by the administration of myostatin antibodies or introduction of inhibitory propeptides in mice has been associated with improved muscle mass and function [57–61], increased intramuscular satellite cell function and IGF-1 signaling (55), enhanced thermogenesis [62•], and resistance to obesity [61;62].
While the prospect of myostatin inhibition for the treatment of sarcopenic obesity appears promising based on animal studies, data have been much more limited in humans with past studies focusing primarily on treatment in patients with muscular dystrophy. In one phase I/II trial of a myostatin antibody, no improvement in muscle strength or function was observed in muscular dystrophy patients, although the study was not powered for efficacy [63]. In another study, myostatin inhibition in muscular dystrophy patients was associated with improvements in muscle function at a cellular level; however, there were no quantitative improvements in muscle strength observed [64]. While it is possible that the lack of efficacy beyond the cellular level may have been related to the underlying pathologic process in muscular dystrophy, these results do raise some uncertainty. For instance, similar findings were described in rodent models wherein the absence of myostatin resulted in compromised muscle force production [55] and impaired muscle energetics [65••] despite an increase in muscle mass. Further uncertainties regarding the effects of myostatin inhibition on muscle function stem from observations in individuals with the K153R polymorphism in the myostatin gene, a variant reported to reduce the ability of myostatin to modulate muscle mass and strength [66]. While this variant may contribute to exceptional longevity [67], there are also reports of diminished muscle force in some [68], but not all [69] affected individuals. There are other unanswered questions regarding the long-term cardiovascular safety of myostatin inhibition given the evidence of myocardial expression of myostatin and its role in the development of heart failure [70]. For these reasons, long-term data are needed to elucidate the role myostatin inhibition may have in the prevention or treatment of sarcopenic obesity, with current studies ongoing in healthy adults.
Testosterone
A predictable decline in testosterone with aging parallels both the loss in lean body mass and the gain in fat mass which lead to sarcopenic obesity [71]. The beneficial effects of testosterone replacement on body composition and muscle strength in hypogonadal men have been well documented in a recent review [72••]. The current review will focus rather on the effects of testosterone therapy in healthy men on age-related changes in body composition. While most studies have reported favorable changes in fat mass and lean body mass, the data pertaining to muscle strength have been mixed. Hildreth et al recently investigated the effects of 12 months of testosterone therapy or placebo in healthy older adults randomized to progressive resistance training versus no exercise [73••]. In those subjects randomized to exercise, testosterone therapy was associated with improvements in fat mass and fat-free mass; however, no differences were observed in physical function or muscle strength compared to placebo. In contrast, upper body strength improved in the non-exerciser subjects treated with testosterone although no improvements were noted in physical function. Changes in body composition paralleled those observed in the exercisers. Similarly, other studies in healthy older men have reported favorable effects of testosterone therapy on body composition [74;75]. In terms of muscle strength, a 36-month double-blind placebo-controlled trial identified no improvements in knee extension or flexion with testosterone therapy [74]. A 4-week study of the effects of testosterone therapy in 6 healthy older men demonstrated improvements in hamstring and quadriceps strength although there was no control group for comparison [76]. Likewise, Bhasin et al reported a dose-dependent increase in leg press strength in healthy older men after 20 weeks of testosterone therapy which did not differ from the effects observed in a cohort of young men [75]. Importantly, adverse events were observed more frequently with higher doses of testosterone. Thus, evidence suggests that testosterone therapy in healthy older men exerts beneficial effects on body composition which may be protective against sarcopenic obesity; however, there is need for careful monitoring for potential adverse events such as erythrocytosis, growth of subclinical prostate cancer, worsening of obstructive sleep apnea, and fluid retention.. The 2010 Endocrine Society Guidelines suggest treatment in older adults only if clinical and biochemical evidence of hypogonadism are present and after an informed discussion regarding the risks and benefits of therapy [77].
Other therapies
Aging is associated with a progressive decline in growth hormone (GH) secretion and IGF-1 production [72••, which is felt to be responsible in part for the decline in lean body mass and increase in fat mass that contribute to sarcopenic obesity [78]. Thus, GH therapy has been studied as an anti-aging agent and, in healthy older adults, reverses these changes in body composition [79]. Unfortunately, treatment has also been associated with significant adverse events such as arthralgias, edema, and glucose intolerance, and for this reason a systematic review in 2007 concluded that GH should not be used as anti-aging therapy [80]. Thus, more recent studies have employed novel techniques to augment endogenous pulsatile GH with the intention of minimizing the adverse events associated with exogenous GH therapy. The growth hormone secretagogue capromorelin improved body composition and physical function in healthy older adults but was associated with aggravation of glucose homeostasis [81]. On the other hand, Makimura et al recently reported the effects of a GHRH analog which reduced fat mass and increased lean body mass in obese individuals yet was not associated with abnormalities in glucose homeostasis or other adverse events compared to placebo [82••]. The subjects in this study were younger than those included in the capromorelin study. Nonetheless, the results are promising and future studies are needed to determine whether tesamorelin may be useful for the treatment of sarcopenic obesity in older adults.
The role for androgenic therapies aside from testosterone in improving body composition has also been evaluated. While there are conflicting data pertaining to the use of dehydroepiandrosterone (DHEA) alone on muscle mass and strength, we have demonstrated that DHEA supplementation potentiates the anabolic effects of heavy resistance exercise in older adults [83]. A recent meta-analysis of double blind placebo controlled trials in elderly men showed that DHEA supplementation can induce a small but significant positive effect on body composition, which is dependent on DHEA conversion to androgens or estrogens [84]. The use of anabolic steroids for reversal of the changes in body composition associated with aging has received less attention in recent years. Treatment of elderly men with the synthetic anabolic androgen, oxandrolone, was associated with improvements in lean body mass, fat mass, and muscle strength [85], but significant reductions in high-density lipoprotein (HDL) cholesterol were also observed [86]. Recently, a phase 2 trial in cancer cachexia, suggested that a non-steroidal selective androgen receptor modulator, enobasarm, might lead to improvements in lean body mass without the toxic effects associated with androgens.
Other treatments currently in development include inhibitors of transcription factor nuclear factor kappa B (NF-κB) for protection against cancer-related cachexia. Early studies demonstrate favorable effects of NF-κB inhibition on cancer-related cachexia and provide further insight into the pathogenesis of this disorder, offering promise for continued progress in the development of targeted therapies for muscle wasting disorders [87].
Conclusion
The rising prevalence of obesity in older adults coupled with the age-related decline in muscle mass and resulting relative sarcopenia act synergistically to maximize disability, morbidity, and mortality. Given the public health implications, an effective treatment strategy in an aging population is essential. We propose that efforts to treat sarcopenic obesity be based primarily on lifestyle interventions. While there is convincing evidence that weight loss and exercise independently result in reversal of sarcopenic obesity and frailty, an intervention strategy incorporating combined weight loss and exercise has proven to be the most effective treatment for this disorder. We agree with recommendations to ensure adequate high-quality protein intake in older adults. With regard to pharmacologic therapies for sarcopenic obesity, we do not believe that the data as of yet support testosterone therapy in the absence of symptomatic hypogonadism. On the other hand, there is promising, albeit limited data pertaining to the use of myostatin inhibitors and GHRH-analogs for sarcopenic obesity and we will look to future studies with cautious optimism.
Key points.
The most effective treatment strategy for sarcopenic obesity is one that incorporates both diet-induced weight loss and a regular multicomponent exercise program incorporating progressive resistance training
In the absence of hypogonadism, current evidence does not justify the use of testosterone therapy for the treatment of sarcopenic obesity.
Further studies are needed to determine whether there is a role for myostatin inhibitors or GHRH-analogs for the treatment of sarcopenic obesity.
Acknowledgements
Supported by grants RO10AG025501, RO1 AG31176 and resources at the New Mexico VA Health Care System.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Reference List
- 1.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–888. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 2.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 3.Kim YS, Lee Y, Chung YS, Lee DJ, Joo NS, Hong D, Song G, Kim HJ, Choi YJ, Kim KM. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J.Gerontol.A Biol.Sci.Med.Sci. 2012;67:1107–1113. doi: 10.1093/gerona/gls071. [DOI] [PubMed] [Google Scholar]
- 4.Zizza CA, Herring A, Stevens J, Popkin BM. Obesity affects nursing-care facility admission among whites but not blacks. Obes.Res. 2002;10:816–823. doi: 10.1038/oby.2002.110. [DOI] [PubMed] [Google Scholar]
- 5.Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J.Gerontol.A Biol.Sci.Med.Sci. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez LJ, Barbagallo M. The cardiometabolic syndrome and sarcopenic obesity in older persons. J.Cardiometab.Syndr. 2007;2:183–189. doi: 10.1111/j.1559-4564.2007.06673.x. [DOI] [PubMed] [Google Scholar]
- 7.Rolland Y, Lauwers-Cances V, Cristini C, Abellan van KG, Janssen I, Morley JE, Vellas B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l'OSteoporose) Study. Am.J.Clin.Nutr. 2009;89:1895–1900. doi: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes.Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 9.Stenholm S, Alley D, Bandinelli S, Griswold ME, Koskinen S, Rantanen T, Guralnik JM, Ferrucci L. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int.J.Obes.(Lond) 2009;33:635–644. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenholm S, Rantanen T, Heliovaara M, Koskinen S. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J.Am.Geriatr.Soc. 2008;56:462–469. doi: 10.1111/j.1532-5415.2007.01567.x. [DOI] [PubMed] [Google Scholar]
- 11.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [also published in: Obes Res. 2005: 13:1849–1863]. [DOI] [PubMed] [Google Scholar]
- 12. Milanovic Z, Pantelic S, Trajkovic N, Sporis G, Kostic R, James N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin.Interv.Aging. 2013;8:549–556. doi: 10.2147/CIA.S44112. This study provides evidence of the factors contributing to the reduction in physical activity observed with aging.
- 13.Sarti S, Ruggiero E, Coin A, Toffanello ED, Perissinotto E, Miotto F, Pintore G, Inelmen EM, Manzato E, Sergi G. Dietary intake and physical performance in healthy elderly women: a 3-year follow-up. Exp.Gerontol. 2013;48:250–254. doi: 10.1016/j.exger.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Marcus RL, Addison O, Dibble LE, Foreman KB, Morrell G, Lastayo P. Intramuscular adipose tissue, sarcopenia, and mobility function in older individuals. J.Aging Res. 2012;2012:629–637. doi: 10.1155/2012/629637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel MP, Wilbur T, Mathis M, Shankland EG, Trieu A, Harper ME, Marcinek DJ. Impaired adaptability of in vivo mitochondrial energetics to acute oxidative insult in aged skeletal muscle. Mech.Ageing Dev. 2012;133:620–628. doi: 10.1016/j.mad.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Proctor DN, Sinning WE, Walro JM, Sieck GC, Lemon PW. Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl.Physiol. 1995;78:2033–2038. doi: 10.1152/jappl.1995.78.6.2033. [DOI] [PubMed] [Google Scholar]
- 17.Leger B, Derave W, De BK, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation.Res. 2008;11:163–175B. doi: 10.1089/rej.2007.0588. [DOI] [PubMed] [Google Scholar]
- 18.Lanza IR, Nair KS. Muscle mitochondrial changes with aging and exercise. Am.J.Clin.Nutr. 2009;89:467S–471S. doi: 10.3945/ajcn.2008.26717D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeap BB, Paul Chubb SA, Lopez D, Ho KK, Hankey GJ, Flicker L. Associations of insulin-like growth factor-I and its binding proteins and testosterone with frailty in older men. Clin.Endocrinol.(Oxf) 2013;78:752–759. doi: 10.1111/cen.12052. This observational study emphasizes the relationship between anabolic hormones in aging men. In particular, components of the IGF-1 system are independent predictors of frailty.
- 20.Eichholzer M, Barbir A, Basaria S, Dobs AS, Feinleib M, Guallar E, Menke A, Nelson WG, Rifai N, Platz EA, Rohrmann S. Serum sex steroid hormones and frailty in older American men of the Third National Health and Nutrition Examination Survey (NHANES III) Aging Male. 2012;15:208–215. doi: 10.3109/13685538.2012.705366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nass R. Growth hormone axis and aging. Endocrinol.Metab Clin.North Am. 2013;42:187–199. doi: 10.1016/j.ecl.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, Ferrucci L. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl.Physiol. 2006 doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: A review of the controversy. Exp Gerontol. 2013 doi: 10.1016/j.exger.2013.02.005. This article summarizes the controversies of weight loss therapy in obese older adults and provides a comprehensive overview of the effects of lifestyle interventions in this population.
- 24.Villareal DT, Banks M, Sinacore DR, Siener C, Klein S. Effect of weight loss and exercise on frailty in obese older adults. Arch.Intern.Med. 2006;166:860–866. doi: 10.1001/archinte.166.8.860. [DOI] [PubMed] [Google Scholar]
- 25.Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, Horton ES, Hoskin MA, Kriska A, Lachin J, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner JG, Venditti B, Wylie-Rosett J. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes.Res. 2004;12:1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Svetkey LP, Clark JM, Funk K, Corsino L, Batch BC, Hollis JF, Appel LJ, Brantley PJ, Loria CM, Champagne CM, Vollmer WM, Stevens VJ. Greater weight loss with increasing age in the weight loss maintenance trial. Obesity.(Silver.Spring) 2013 doi: 10.1002/oby.20506. This secondary analysis of a randomized controlled weight loss trial provides evidence supporting the feasibility of long-term weight loss maintenance in obese older adults.
- 27. Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. This is the single randomized controlled 1 year trial in obese older persons on the role of different lifestyle interventions to reverse frailty.
- 28.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J.Gerontol.A Biol.Sci.Med.Sci. 2000;55:M350–M355. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 29.Villareal DT, Smith GI, Sinacore DR, Shah K, Mittendorfer B. Regular multicomponent exercise increases physical fitness and muscle protein anabolism in frail, obese, older adults. Obesity.(Silver.Spring) 2011;19:312–318. doi: 10.1038/oby.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Argiles JM, Orpi M, Busquets S, Lopez-Soriano FJ. Myostatin: more than just a regulator of muscle mass. Drug Discov.Today. 2012;17:702–709. doi: 10.1016/j.drudis.2012.02.001. This review article provides a comprehensive summary of the physiologic role of myostatin with particular emphasis on the interplay between skeletal muscle and adipose tissue.
- 31.McMahon G, Morse CI, Burden A, Winwood K, Onambele GL. Muscular adaptations and insulin-like growth factor-I (IGF-I) responses to resistance training are stretch-mediated. Muscle Nerve. 2013 doi: 10.1002/mus.23884. [DOI] [PubMed] [Google Scholar]
- 32.Fujita S, Rasmussen BB, Cadenas JG, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Timmerman KL, Dhanani S, Glynn EL, Fry CS, Drummond MJ, Jennings K, Rasmussen BB, Volpi E. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am.J.Clin.Nutr. 2012;95:1403–1412. doi: 10.3945/ajcn.111.020800. This is the first evidence of an acute increase in aerobic exercise enhancing the skeletal muscle anabolic response to amino acid intake. The authors suggest this finding is the result of enhanced skeletal muscle nutrient delivery.
- 34.Thornell LE. Sarcopenic obesity: satellite cells in the aging muscle. Curr.Opin.Clin.Nutr.Metab Care. 2011;14:22–27. doi: 10.1097/MCO.0b013e3283412260. [DOI] [PubMed] [Google Scholar]
- 35.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J.Appl.Physiol. 2008;105:473–478. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frimel TN, Sinacore DR, Villareal DT. Exercise attenuates the weight-loss-induced reduction in muscle mass in frail obese older adults. Med Sci.Sports Exerc. 2008;40:1213–1219. doi: 10.1249/MSS.0b013e31816a85ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anbalagan VP, Venkataraman V, Pradeepa R, Deepa M, Anjana RM, Mohan V. The Prevalence of Presarcopenia in Asian Indian Individuals With and Without Type 2 Diabetes. Diabetes Technol.Ther. 2013 doi: 10.1089/dia.2013.0068. [DOI] [PubMed] [Google Scholar]
- 38.Hameed UA, Manzar D, Raza S, Shareef MY, Hussain ME. Resistance Training Leads to Clinically Meaningful Improvements in Control of Glycemia and Muscular Strength in Untrained Middle-aged Patients with type 2 Diabetes Mellitus. N.Am.J.Med.Sci. 2012;4:336–343. doi: 10.4103/1947-2714.99507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillon EL. Nutritionally essential amino acids and metabolic signaling in aging. Amino.Acids. 2012 doi: 10.1007/s00726-012-1438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volpi E, Campbell WW, Dwyer JT, Johnson MA, Jensen GL, Morley JE, Wolfe RR. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J.Gerontol.A Biol.Sci.Med.Sci. 2013;68:677–681. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am.J.Physiol. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 42.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr.Opin.Clin.Nutr.Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuthbertson DJ, Babraj J, Smith K, Wilkes E, Fedele MJ, Esser K, Rennie M. Anabolic signaling and protein synthesis in human skeletal muscle after dynamic shortening or lengthening exercise. Am J Physiol Endocrinol Metab. 2006;290:E731–E738. doi: 10.1152/ajpendo.00415.2005. [DOI] [PubMed] [Google Scholar]
- 44.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am.J.Clin.Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 45.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J.Clin.Endocrinol.Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leenders M, van Loon LJ. Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr.Rev. 2011;69:675–689. doi: 10.1111/j.1753-4887.2011.00443.x. [DOI] [PubMed] [Google Scholar]
- 47.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J.Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schirwis E, Agbulut O, Vadrot N, Mouisel E, Hourde C, Bonnieu A, Butler-Browne G, Amthor H, Ferry A. The beneficial effect of myostatin deficiency on maximal muscle force and power is attenuated with age. Exp.Gerontol. 2013;48:183–190. doi: 10.1016/j.exger.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296:1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 50.Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr.Health Aging. 2002;6:343–348. [PubMed] [Google Scholar]
- 51.Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat.Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. [DOI] [PubMed] [Google Scholar]
- 52.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N.Engl.J.Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 53. Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J. 2013;27:1981–1989. doi: 10.1096/fj.12-225755. This study provides evidence of the favorable metabolic effects of myostatin inhibition, with promising implications beyond those related to the treatment of sarcopenia.
- 54.Wilkes JJ, Lloyd DJ, Gekakis N. Loss-of-function mutation in myostatin reduces tumor necrosis factor alpha production and protects liver against obesity-induced insulin resistance. Diabetes. 2009;58:1133–1143. doi: 10.2337/db08-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc.Natl.Acad.Sci.U.S.A. 2007;104:1835–1840. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jackson MF, Luong D, Vang DD, Garikipati DK, Stanton JB, Nelson OL, Rodgers BD. The aging myostatin null phenotype: reduced adiposity, cardiac hypertrophy, enhanced cardiac stress response, and sexual dimorphism. J.Endocrinol. 2012;213:263–275. doi: 10.1530/JOE-11-0455. [DOI] [PubMed] [Google Scholar]
- 57.Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, Song Y, Min H, Wang X, Du J, Mitch WE. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011;25:1653–1663. doi: 10.1096/fj.10-176917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang K, Li Z, Li Y, Zeng J, He C, Yang J, Liu D, Wu Z. Muscle-specific transgenic expression of porcine myostatin propeptide enhances muscle growth in mice. Transgenic Res. 2013 doi: 10.1007/s11248-013-9709-4. [DOI] [PubMed] [Google Scholar]
- 59.Busquets S, Toledo M, Orpi M, Massa D, Porta M, Capdevila E, Padilla N, Frailis V, Lopez-Soriano FJ, Han HQ, Argiles JM. Myostatin blockage using actRIIB antagonism in mice bearing the Lewis lung carcinoma results in the improvement of muscle wasting and physical performance. J.Cachexia.Sarcopenia.Muscle. 2012;3:37–43. doi: 10.1007/s13539-011-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chiu CS, Peekhaus N, Weber H, Adamski S, Murray EM, Zhang HZ, Zhao JZ, Ernst R, Lineberger J, Huang L, Hampton R, Arnold BA, Vitelli S, Hamuro L, Wang WR, Wei N, Dillon GM, Miao J, Alves SE, Glantschnig H, Wang F, Wilkinson HA. Increased Muscle Force Production and Bone Mineral Density in ActRIIB-Fc-Treated Mature Rodents. J.Gerontol.A Biol.Sci.Med.Sci. 2013 doi: 10.1093/gerona/glt030. [DOI] [PubMed] [Google Scholar]
- 61.Nakatani M, Kokubo M, Ohsawa Y, Sunada Y, Tsuchida K. Follistatin-derived peptide expression in muscle decreases adipose tissue mass and prevents hepatic steatosis. Am.J.Physiol Endocrinol.Metab. 2011;300:E543–E553. doi: 10.1152/ajpendo.00430.2010. [DOI] [PubMed] [Google Scholar]
- 62. Zhang C, McFarlane C, Lokireddy S, Masuda S, Ge X, Gluckman PD, Sharma M, Kambadur R. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia. 2012;55:183–193. doi: 10.1007/s00125-011-2304-4. This study provides evidence of a protective role of myostatin inhibition in the development of obesity.
- 63.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, Eagle M, Florence JM, King WM, Pandya S, Straub V, Juneau P, Meyers K, Csimma C, Araujo T, Allen R, Parsons SA, Wozney JM, Lavallie ER, Mendell JR. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann.Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 64.Krivickas LS, Walsh R, Amato AA. Single muscle fiber contractile properties in adults with muscular dystrophy treated with MYO-029. Muscle Nerve. 2009;39:3–9. doi: 10.1002/mus.21200. [DOI] [PubMed] [Google Scholar]
- 65. Giannesini B, Vilmen C, Amthor H, Bernard M, Bendahan D. Lack of myostatin impairs mechanical performance and ATP cost of contraction in exercising mouse gastrocnemius muscle in vivo. Am.J.Physiol Endocrinol.Metab. 2013;305:E33–E40. doi: 10.1152/ajpendo.00651.2012. This important study explores the controversy pertaining to skeletal muscle function in the absence of myostatin and the results must be considered if myostatin inhibition is pursued for the treatment or prevention of sarcopenia.
- 66.Gonzalez-Freire M, Rodriguez-Romo G, Santiago C, Bustamante-Ara N, Yvert T, Gomez-Gallego F, Serra Rexach JA, Ruiz JR, Lucia A. The K153R variant in the myostatin gene and sarcopenia at the end of the human lifespan. Age (Dordr.) 2010;32:405–409. doi: 10.1007/s11357-010-9139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garatachea N, Pinos T, Camara Y, Rodriguez-Romo G, Emanuele E, Ricevuti G, Venturini L, Santos-Lozano A, Santiago-Dorrego C, Fiuza-Luces C, Yvert T, Andreu AL, Lucia A. Association of the K153R polymorphism in the myostatin gene and extreme longevity. Age (Dordr.) 2013 doi: 10.1007/s11357-013-9513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santiago C, Ruiz JR, Rodriguez-Romo G, Fiuza-Luces C, Yvert T, Gonzalez-Freire M, Gomez-Gallego F, Moran M, Lucia A. The K153R polymorphism in the myostatin gene and muscle power phenotypes in young, non-athletic men. PLoS.ONE. 2011;6:e16323. doi: 10.1371/journal.pone.0016323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferrell RE, Conte V, Lawrence EC, Roth SM, Hagberg JM, Hurley BF. Frequent sequence variation in the human myostatin (GDF8) gene as a marker for analysis of muscle-related phenotypes. Genomics. 1999;62:203–207. doi: 10.1006/geno.1999.5984. [DOI] [PubMed] [Google Scholar]
- 70.Gruson D, Ginion A, Lause P, Ketelslegers JM, Thissen JP, Bertrand L. Urotensin II and urocortin trigger the expression of myostatin, a negative regulator of cardiac growth, in cardiomyocytes. Peptides. 2012;33:351–353. doi: 10.1016/j.peptides.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 71.Buvat J, Maggi M, Guay A, Torres LO. Testosterone deficiency in men: systematic review and standard operating procedures for diagnosis and treatment. J.Sex Med. 2013;10:245–284. doi: 10.1111/j.1743-6109.2012.02783.x. [DOI] [PubMed] [Google Scholar]
- 72. Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr.Rev. 2012;33:314–377. doi: 10.1210/er.2012-1002. This is a comprehensive review of the literature pertaining to anabolic hormone therapy and sarcopenia in healthy older men with emphasis on the efficacy of combination therapy and the need for more long-term studies.
- 73. Hildreth KL, Barry DW, Moreau KL, Vande GJ, Meacham RB, Nakamura T, Wolfe P, Kohrt WM, Ruscin JM, Kittelson J, Cress ME, Ballard R, Schwartz RS. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. J.Clin.Endocrinol.Metab. 2013;98:1891–1900. doi: 10.1210/jc.2012-3695. This is the first evidence of the interaction of testosterone therapy and progressive resistance training in a randomized controlled trial in healthy older men
- 74.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J.Clin.Endocrinol.Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 75.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer TW. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J.Clin.Endocrinol.Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 76.Urban RJ, Bodenburg YH, Gilkison C, Foxworth J, Coggan AR, Wolfe RR, Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am.J.Physiol. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- 77.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J.Clin.Endocrinol.Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 78.Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE. Effects of human growth hormone in men over 60 years old. N.Engl.J.Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 79.Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, Jayme J, O'Connor KG, Christmas C, Tobin JD, Stewart KJ, Cottrell E, St CC, Pabst KM, Harman SM. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–2292. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- 80.Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann.Intern.Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 81.White HK, Petrie CD, Landschulz W, MacLean D, Taylor A, Lyles K, Wei JY, Hoffman AR, Salvatori R, Ettinger MP, Morey MC, Blackman MR, Merriam GR. Effects of an oral growth hormone secretagogue in older adults. J.Clin.Endocrinol.Metab. 2009;94:1198–1206. doi: 10.1210/jc.2008-0632. [DOI] [PubMed] [Google Scholar]
- 82. Makimura H, Feldpausch MN, Rope AM, Hemphill LC, Torriani M, Lee H, Grinspoon SK. Metabolic effects of a growth hormone-releasing factor in obese subjects with reduced growth hormone secretion: a randomized controlled trial. J.Clin.Endocrinol.Metab. 2012;97:4769–4779. doi: 10.1210/jc.2012-2794. This is the first randomized controlled trial of a GH-secretagogue demonstrating favorable effects on body composition without negatively impacting glucose homeostasis.
- 83.Villareal D, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006:00100. doi: 10.1152/ajpendo.00100.2006. [DOI] [PubMed] [Google Scholar]
- 84.Corona G, Rastrelli G, Giagulli VA, Sila A, Forti G, Mannucci E, Maggi M. Dehydroepiandrosterone supplementation in elderly men: a meta-analysis study of placebo controlled trials. J Clin Endocrin Metab. 2013 doi: 10.1210/jc.2013-1358. [DOI] [PubMed] [Google Scholar]
- 85.Schroeder ET, Zheng L, Yarasheski KE, Qian D, Stewart Y, Flores C, Martinez C, Terk M, Sattler FR. Treatment with oxandrolone and the durability of effects in older men. J Appl.Physiol. 2004;96:1055–1062. doi: 10.1152/japplphysiol.00808.2003. [DOI] [PubMed] [Google Scholar]
- 86.Schroeder ET, Zheng L, Ong MD, Martinez C, Flores C, Stewart Y, Azen C, Sattler FR. Effects of androgen therapy on adipose tissue and metabolism in older men. J Clin Endocrinol.Metab. 2004;89:4863–4872. doi: 10.1210/jc.2004-0784. [DOI] [PubMed] [Google Scholar]
- 87.Der-Torossian H, Wysong A, Shadfar S, Willis MS, McDunn J, Couch ME. Metabolic derangements in the gastrocnemius and the effect of Compound A therapy in a murine model of cancer cachexia. J Cachexia.Sarcopenia.Muscle. 2013;4:145–155. doi: 10.1007/s13539-012-0101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]