Abstract
Objectives
Information about patients with primary immune deficiencies can be scarce because of the rarity of the disorders. Individual centers rarely have sufficient patients to educate trainees and garner collective wisdom. Registries for many diseases have proven their worth by providing essential information on disease spectrum, treatments and natural history. This study describes the construction and use of a registry for patients with primary immune deficiencies and other efforts to improve knowledge and care for affected patients and their families.
Methods
Registry demographics and data were extracted using proprietary reporting tools. Educational efforts and cell repository data were collected from centralized source material.
Results
The USIDNET Registry contains 3459 patients, with common variable immune deficiency being the most represented. Pilot studies identified strengths and weaknesses of the data. Visiting Professor and Visiting Scholar Programs have been successful, encouraging trainees at all levels to pursue a career in Immunology.
Conclusions
USIDNET’s comprehensive and integrated approach provides resources that strengthen the field of primary immune deficiencies, as shown by utilization by 312 distinct sites or individuals. The reach of USIDNET’s efforts is extended through the educational resources.
Keywords: Primary immune deficiency, registry, comparative effectiveness, immunoglobulin
Introduction
The Immune Deficiency Foundation (IDF) established one of the first patient registries on primary immune deficiency diseases (PIDDs) in 1992. It was funded by the NIH to collect data on Chronic Granulomatous Disease (CGD) (1). The information from this initial registry defined major health issues and mortality and provided background data necessary for judging effectiveness of emerging therapies for CGD, including interferon-γ (IFN-γ)(2) and antifungal prophylaxis (3). The collection of these data also spurred other efforts to improve therapy, based on hematopoietic cell transplantation (HCT) and later, gene therapy. These early efforts focused on natural history and presenting manifestations, with each survey focused on a single disease. CGD, severe combined immune deficiency (SCID), DiGeorge syndrome, Hyper IgM syndrome, Wiskott-Aldrich syndrome, X-linked agammaglobulinemia, and common variable immune deficiency were ultimately included. In 2008, the registry converted these patient entries to an electronic format and began to acquire additional new data using a standardized data collection system. Approximately 20% of the digital era patients have longitudinal data. In Europe, a similar registry-based effort to define the natural history of patients with CD40 ligand (CD40L) deficiency revealed a significant burden from liver disease and unusual malignancies that had not been previously appreciated (4). These early efforts spurred the expansion of registries for collections of PIDD patients on every continent (5–10). Collection of clinical, laboratory, and outcome data provide a real-world health survey of patients with rare PIDD disorders and form the foundation for evolving outcomes-based research (11). Overlaying prospective studies onto an existing registry infrastructure is likely to become a cost-effective approach to define outcomes and best treatment strategies (12). Questions about responses to therapy, genotype-specific management and comparisons of interventions for related disorders can be addressed through accurate registry data.
While collection of data on rare patient populations can provide essential information on the natural history of these diseases, aside from a few specialty centers, training programs for young physicians are not generally able to provide the necessary expertise for the diagnosis, evaluation and care of these patients. Recognizing this, medical societies, the IDF, the Jeffrey Modell Foundation and the NIH have supported a variety of programs to extend and enhance training opportunities in PIDD, laying the foundation for clinical work in this field. To fulfill this need, the IDF formed a new entity, the United States Immune Deficiency Network (USIDNET), which was awarded an NIH contract in 2003, with Hans D. Ochs as the Principal Investigator and Charlotte Cunningham Rundles as the co-Principal Investigator. The steering committee included Rebecca H. Buckley, Mary Ellen Conley, Thomas A. Fleisher, Ramsay Fuleihan, Raif Geha, Steven M. Holland, Luigi D. Notarangelo, Jennifer M. Puck, E. Richard Stiehm, and Kathleen E. Sullivan. A competitive renewal was funded in 2010, with Charlotte Cunningham Rundles as the Principal Investigator and a Steering Committee of Drs. Fuleihan, Notarangelo, Puck and Sullivan. This report describes the current status of the USIDNET with a specific focus on the patient Registry. Two additional goals of USIDNET are to develop the next generation of clinicians to provide expert care for patients with primary immune deficiencies and to provide a cell repository as a resource for investigations on primary immune deficiencies.
Methods
The USIDNET registry was surveyed at the end of 2013 to provide the data for this report. Data were extracted using Boolean queries for specific data. In addition, the status of the repository was defined by manually tabulating cell lines and requests for cell lines manually. Data on the educational programs was extracted from reports. The technical details of the registry are provided in the Supplement.
Two pilot studies were performed to demonstrate the robustness of the data. For these studies, the relevant data fields were queried using Boolean logic. The ages were converted to years manually and doses were manually converted to mg/kg/month from the frequency field and the mg/kg field. Data were then subsequently analyzed by a t-test. The distributions were displayed using either Excel (Microsoft Office) or Prism (Graphpad) programs.
Results
Registry demographics
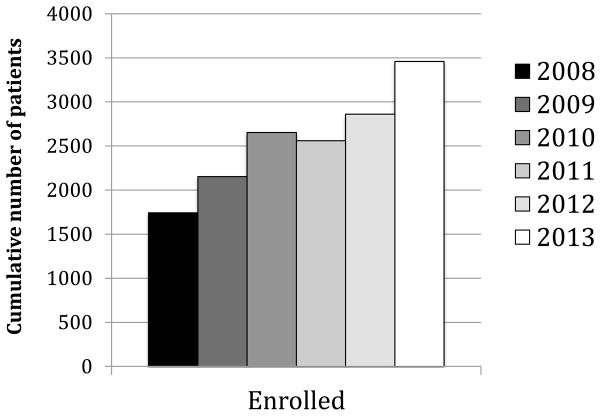
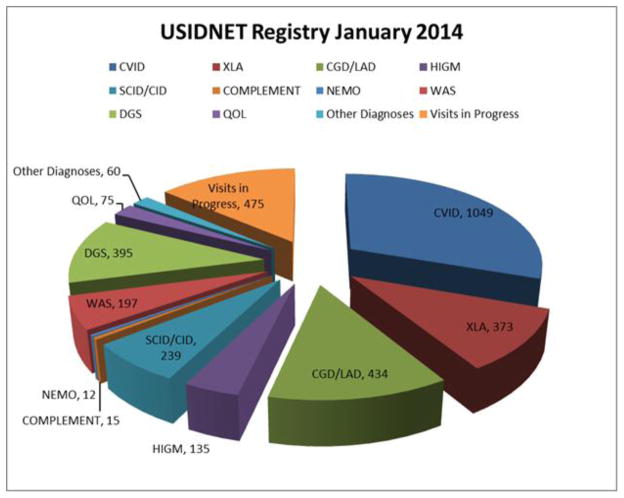
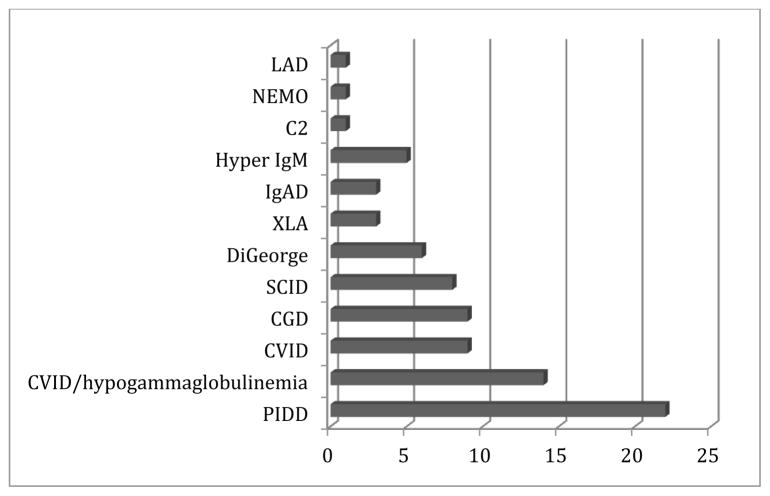
Figure 1 demonstrates the enrollment since 2008, when the USIDNET Registry entered the electronic era. There are currently 42 sites entering data: 37 academic institutions with protocols approved by their local IRB, and 5 medical offices that utilize the USIDNET IRB protocol. (Appendix 1) At the end of 2013, the number of unique patients in the registry was 3459. Patients’ records have come through three main pipelines: the majority of the data has been entered by physicians and their designees; the second route is for data to be entered by USIDNET staff who have visited medical centers to abstract data from charts; and a third route is for patients who have requested their own medical records to be sent to USIDNET for data entry by trained USIDNET staff. The last pathway is gaining importance as patients themselves desire to be participants in this ongoing endeavor. In 2013, for example, 77 patients had utilized the self-entry mode while 168 patient records had been entered from 16 of the academic sites. While data entered prior to 2008 contains more limited information in fewer fields, data since this time has been more comprehensive. In 2012, USIDNET instituted a requirement that records qualifying for Registry entry contain a minimal standard data set to enhance data collection and quality. A 2013 data audit revealed a data error rate of only 0.6%. Obvious outlier points have been either redacted or corrected after contact with the entering physician for clarification. The disorder with the highest entry was CVID with 1049 patients, followed by CGD/leukocyte adhesion deficiency (LAD) (n=434), DiGeorge syndrome (n=395), XLA (n=373), and SCID (n=239) (Figure 2).
Figure 1.
Overall enrollment into the USIDNET registry. The cumulative enrollment since 2008, when the registry went fully electronic is demonstrated in this figure. The slight decrease in 2011 was due to a concerted effort to eliminate duplicate entries.
Figure 2.
Representation of different disorders. The distribution of the frequencies of the main disorders enrolled in the registry are shown in this pie chart.
The gender distribution in the Registry was 37.4% female vs. 62.6% male (reflecting in part the X chromosome-linked genetic PIDD disorders that affect only males), and 25% of patients were less than 18 years old at the time of the most recent entry.
Pilot Studies
Two pilot studies were performed to demonstrate the robustness of the Registry data. For these studies, the relevant data fields were queried using Boolean logic. The ages were converted to years manually and doses were manually converted to mg/kg/month from the frequency field and the mg/kg field. Data were then subsequently analyzed by a t-test. The distributions were displayed using either Excel or Prism.
Racial composition
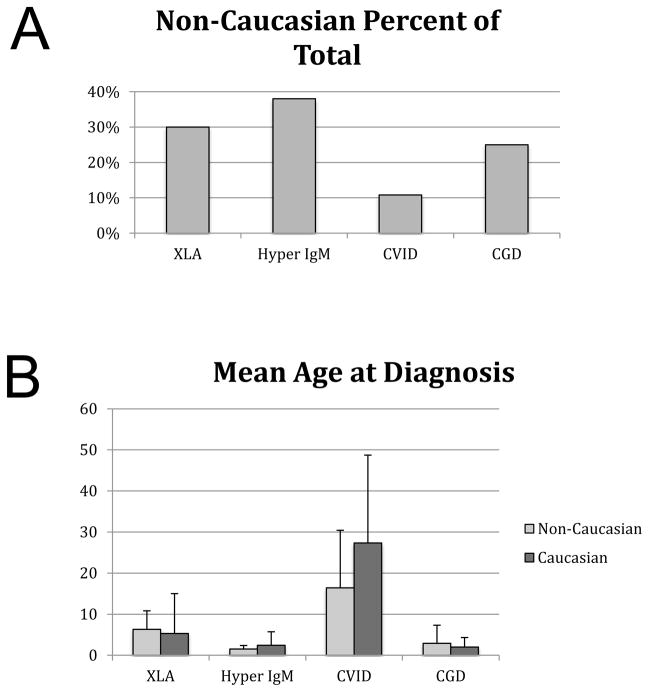
A goal of comparative effectiveness research is to identify and mitigate health care disparities due to gender, race, or socioeconomic class. The racial distribution of the overall USIDNET Registry was 88.3% Caucasian, 7.1% African American, 2.8 % Asian/Pacific Islander, 1.8% Native American. Sixteen patients had multiple races listed. To explore the utility of the registry as a tool for comparative effectiveness research, we investigated the age of diagnosis across racial groups. Some PIDDs are enriched among Caucasians, but most single gene disorders, and all X-linked disorders, are believed to occur in comparable frequencies across racial and ethnic groups. Unexpectedly, a relatively small percentage of patients had an age at diagnosis defined. Approximately 2/3 of the overall cohort had race indicated but only 366 had age at the time of diagnosis identified (although date of diagnosis was available on most patients). Therefore, this study was based on a smaller data set than anticipated. First, we analyzed the percentage of non-Caucasian patients in each disease subgroup. As expected from other literature resources, CVID was enriched for Caucasians. In the other disorders, minorities made up roughly a third of the patient cohort (Figure 3). The first pilot study was to examine racial differences in age at the time of diagnosis (Figure 3). We hypothesized that healthcare disparities would be reflected in the age at diagnosis due to differences in healthcare access, However, there were no significant differences in the age at diagnosis for Caucasians versus non-Caucasians in any diagnostic group. Furthermore, the ages at the time of diagnosis were concordant with what has been seen in other disease-specific studies, validating these results.
Figure 3.
Enrollment by race. A) Non-Caucasian race as a percent of the total enrollment is shown. B) The mean age at the time of diagnosis is shown for each disorder, comparing non-Caucasian entries with Caucasian entries.
Pilot study on immunoglobulin dosing
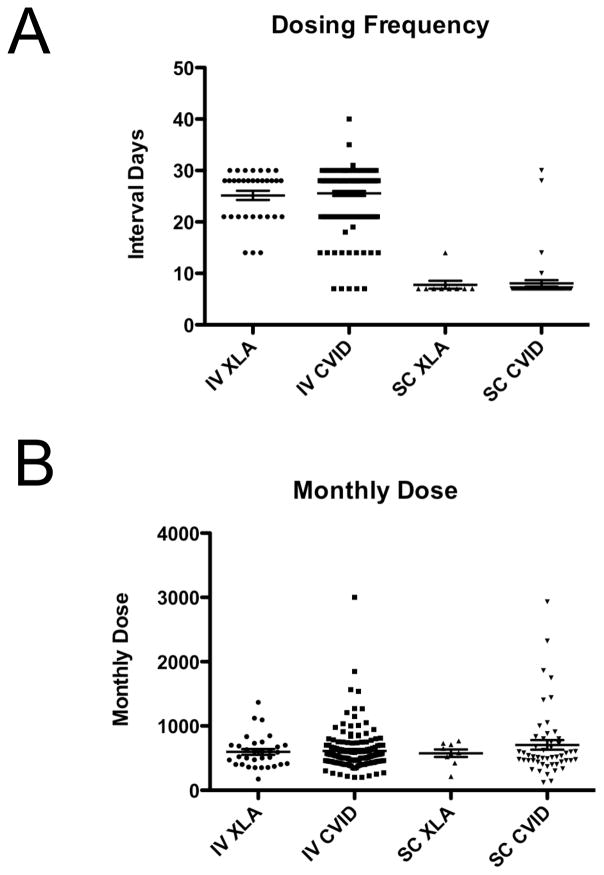
For several diseases, efforts to examine the diversity of therapeutic efforts and feedback regarding the treatment consensus has been helpful in improving patient outcomes (13–15). We therefore examined immunoglobulin dosing practices for XLA and CVID patients. We divided patients into those receiving subcutaneous immunoglobulin and those receiving intravenous immunoglobulin. We compared dosing frequency and dose calculated as mg/kg. As there have been no consensus standards, we wanted to examine the frequency of patients receiving either less than 400mg/kg/month or more than 800mg/kg/month, two typical targets for therapy. Three patients were receiving intramuscular injections and were not considered (1 XLA and 2 CVID). The intravenous dosing frequency ranged from every 4d to every 56d for CVID (Figure 4), and from every 7d to every 90 days for XLA. The subcutaneous dosing frequency ranged from every 3d to every 30d for CVID, and from every 3d to every 14d for XLA.
Figure 4.
Immunoglobulin replacement dosing. A) The interval between doses is shown for both XLA and common variable immune deficiency, dividing entries according to intravenous (IV) or subcutaneous (SC) entries. B) The monthly dose is shown for XLA and common variable immune deficiency, according to subcutaneous (SC) or intravenous (IV) administration.
To further define the spectrum of immunoglobulin replacement practices, we examined the dose as mg/kg/month for both XLA and CVID (Figure 4). To do this, the doses were all normalized to an every four week denominator. In CVID, the intravenous doses ranged from 200mg/kg/m to 3000mg/kg/m, whereas the subcutaneous doses ranged from 120mg/kg/m to 2900mg/kg/m. In XLA, the intravenous doses ranged from 175mg/kg/m to 1369mg/kg/m, and the subcutaneous doses ranged from 214mg/kg/m to 768mg/kg/m. While substantial variation in dosing of immunoglobulin replacement may be considered acceptable, these data suggested that some patients in both groups were receiving suboptimal replacement. Additional studies examining whether these diverse doses were appropriate for each patient based on infection patterns and serum IgG levels were beyond the scope of a registry that is not collecting standardized infection frequency reports, however, these data suggest that greater evidence is needed to either converge on a single appropriate dose or to stratify patients into different dosing targets.
Use of the Registry: Data queries
Aside from targeted pilot studies, the USIDNET Registry has proven to be an excellent general resource for clinicians and investigators. Accordingly, use of the Registry has been tracked electronically since 2011. Since this time, users submitting queries have indicated that they are using the registry for preparing grant applications, guiding patient care, and spurring research, both basic and clinical. A total of 82 queries have been submitted, and the general topics of the queries are listed in Figure 5. In some cases, the query focused on a specific disease while other queries wanted to compare the frequency of a clinical feature across different PIDDs.
Figure 5.
Registry queries. The overall subject of the 82 queries is displayed graphically, ranked in order of frequency.
Educational efforts
On a continuing basis, the USIDNET has collaborated with the Clinical Immunology Society to support a yearly “Summer School” program in Primary Immune Deficiency, an intensive case-based program aimed at physicians and scientists who have a strong bent toward academic careers. The 12th Summer School was held in 2013, and at that time, there were 369 participants of this program. Of these, 215 were from US training programs. The majority were from Allergy Immunology Divisions, but some fellows in Infectious Diseases, Rheumatology, Hematology/Oncology and Gastroenterology had been accepted.
Other participants included 82 who were exceptional candidates chosen from medical centers in Central and South America and 7 additional participants were highly talented young physician-scientists from Europe. Examining the subsequent careers of these physicians revealed that of the candidates accepted from the US, 77% are now either Instructors, Assistant or Associate Professors at academic medical centers in the US. Their current positions include programs in Allergy Immunology, Infectious Diseases, Hematology/Oncology or Bone Marrow Transplantation. Nine graduates are now training directors at their own institutions. Some US graduates have also moved great distances to lead programs in Cambridge, England, the Karolinska Institute, Hamburg, Germany, Munich, Germany, Anchorage, Alaska, and Kuwait as examples. Of the Central and South American participants, 90% are now on the faculty of their home medical center. Of the European graduates, all are faculty members at academic institutions (Zurich, Leiden, Freiburg, Florence, Alberta, and Vancouver).
USIDNET, along with the IDF, supports a Visiting Professor program which has now sponsored 145 visits by PIDD experts to 133 different institutions, averaging 10 Visiting Professorships a year. This effort has directly impacted nearly 3000 people and every single host stated that the Visiting Professorship enhanced their understanding of primary immune deficiencies. After a Visiting Professorship, 94% of hosts said they would change their practice regarding PIDD as a result of the Visiting Professorship.
Similarly, the Visiting Scholar program, which supports trainees going to other institutions for advanced training, has had 64 applications and has sponsored 32 visits, averaging four every year. At this time, 16 participating institutions host these trainees. Feedback from the Visiting Scholar program of USIDNET has been overwhelmingly positive. On a scale of 1–5, with 5=strongly agree, the trainees felt that they had learned something that was important for their career (average=4.9) and they planned to seek additional training in PIDD (average=4.5). Additionally, trainees commented that they had a gained a better understanding or had learned a specific useful technique (average 4.6).
Cell Repository
The wide availability of scarce materials from subjects with rare PIDDs is of crucial importance to ongoing and future research in the basic sciences, where pathways and disease mechanisms may be worked out, as well as in development of new therapies, such as gene addition or gene editing. There were 100 distinct cell lines representing 15 PIDD categories available in the USIDNET Repository at the end of 2013. Vials of cells have been distributed to 67 scientists, allowing generation of novel information on disease pathophysiology (16).
Conclusions
USIDNET has been supported by NIH and private industry to enhance efforts to improve care for patients with PIDDs. Clinical immunology, including care of patients with rare primary defects of the immune system is a rapidly evolving specialty with over 200 specific disease genes now recognized and with increasing options for treatment (17). HCT, gene therapy, biological drugs and enzyme replacement therapy are available for specific indications, and more therapies are under development. Specific mutation testing is increasingly available and is now standard of care for many PIDDs (18). The rapidity of evolution of PIDD medicine and biology in the face of the rarity of the PIDD disorders will require nimble approaches to ensure that clinicians have access to the most recent, validated information as well as dynamic strategies to access that information. There is a knowledge gap between the grass roots care being delivered and optimal care with the few comparative studies defining best practices for PIDD (19). The expense and logistical difficulty of randomized trials to compare treatments across all the potential interventions indicates that easy answers from large trials will not be forthcoming for patients with PIDDs and their medical providers. Instead, best practices must evolve based on available evidence. Registries exist for many patient groups, both large and small, and have proven their potential to improve care (13–15). USIDNET has taken a multipronged approach to ensure that physicians caring for individuals with PIDD will have training, a network of support, and tools such as the registry and the repository of cell lines to ask the questions that arise in the daily care of patients.
The USIDNET Registry has been built to ensure the high quality of the data and the diversity of representation. In the two pilot studies outlined here, we demonstrated the power of the Registry to identify targets for interventions to improve care. Our data suggest limited health care disparities depending on race, perhaps because this registry is strongly enriched for patients at academic centers. While many patients experienced delays in diagnosis, other reasons such as unfamiliarity with the diagnosis or insurance issues may have influenced the age of diagnosis more than race. This study cannot identify children who failed to achieve any PIDD diagnosis which might reflect issues related to subspecialty healthcare access. An unexpected finding was a paucity of information on the age at diagnosis. This could reflect the inability of abstractors to discern that information, the effect of patients moving from one physician to another, who may not know the distant history, or an indistinct time of diagnosis, with multiple evaluations spaced out over time. This paucity of data on the age at the time of diagnosis does point to a common weakness in all registry-based studies. While the overall quality of the data was high when audited, the enterers only can enter the available data. Certain data points may be vulnerable to this issue. The second pilot study examining real life dosing of immunoglobulin also revealed surprises. Both the frequencies and cumulative monthly doses reflected unexpected diversity. It is possible that clinical heterogeneity contributed to the wide dose range, as doses may be tailored to each patient’s need. This is more likely to be the explanation in CVID, where patient heterogeneity is widely recognized (20, 21). The XLA data, although limited, also suggested a high diversity in treatment regimens. This may reflect physician preferences or perceived effectiveness. This observation suggests that further focus on this important topic is warranted to better define the effectiveness of the different dosing strategies. The low end of the dose ranges would be expected to compromise protection against infections. A prospective study using the Registry as a data collection tool could address this question. The current data set has information on infections and IgG trough levels but longitudinal data entry has not been standardized. Using the current data as a springboard for further data collection could lead to evidence based standardization of treatment, as has been beneficial in cystic fibrosis and inflammatory bowel disease (22–24). These pilot studies demonstrate the strengths of the registry approach but also point to limitations. Registries can only report on the patients enrolled and therefore cannot address lack of diagnosis, they can be subject to bias related to ascertainment, and bias related to incomplete data capture.
The USIDNET patient count was 3459 at the end of 2013. The data quality was high but this number is clearly not a complete accounting of all US patients. The IDF in 2007 estimated that 230,000 people in the USA had a PIDD (25). The Jeffrey Modell Foundation identified 23,107 US patients in their survey and 79,764 patients worldwide (26). In comparison, the European registry contains ≈19,000 patients and the Latin American Registry contains ≈4,700 patients. Therefore, additional efforts will be required to improve ascertainment. Four new initiatives should help to improve enrollment: 1) Direct patient entry, 2) Incentivizing enrolling centers, 3) Better education regarding the benefits of registry participation, 4) Interfaces with electronic medical records. Until a broader spectrum of patients and a higher enrollment is achieved, analyses could be biased. A second limitation relates to genotype data. A key question for many diseases is whether a genotype-phenotype relationship exists. USIDNET collects mutation data on patients but clinicians are often unfamiliar with the nomenclature and greater education or the ability to mine electronic medical records will be required to maximize this aspect of the registry. Nevertheless, in spite of these limitations, USIDNET still represents a pivotal resource for questions regarding care of patients with PIDDs.
USIDNET has demonstrated the potential of the Registry to improve outcomes, however, the educational efforts have already borne fruit. Through an aggressive program to ensure that providers likely to see patients with PIDDs have access to teaching from recognized experts, USIDNET and the IDF have reached large numbers of trainees. Partnering with the Clinical Immunology Summer School has also motivated trainees and has developed a network of highly trained Immunologists. Additional efforts to provide research tools have also been appreciated by the community striving to elucidate mechanisms of disease in PIDDs.
Collectively, the efforts of USIDNET have moved the field of PIDD forward through education and resources. These tools provide a powerful foundation for the study and care of patients with PIDDs. Evolving efforts to incorporate the voice of patients to drive both research questions and to answer the subjective aspects of patient outcomes will ensure continuous improvement in patient care. By improving enrollment and continuing to reach out to clinicians, the registry will be a valued resource for many years to come. Advancing information technology efforts will be enabling and improve data entry. The network of clinicians and investigators through collaborative efforts and resource-sharing will ensure continuing advances in patient care.
Supplementary Material
Acknowledgments
The authors thank the patients and many physicians whose time and effort made the tools provided by USIDNET possible. This work was supported by U24 AI086037, Baxter, Talecris, CSL Behring, Sigma Tau, and AxelaCare.
Appendix
Medical Centers
Advocate Hope Children’s Hospital
Ann & Robert H. Lurie Children’s Hospital of Chicago
Boston Children’s Hospital
Children’s Hospital of Pennsylvania
CHU Sainte-Justine Montreal, Canada
Cincinnati Children’s Hospital Medical Center
Cincinnati Children’s Hospital Medical Center
Cleveland Clinic
Duke University Medical Center
Emory University
Levine Children’s Hospital
Louisiana State University
Mattel Children’s Hospital Univ. of CA, LA Mayo Clinic
Medical College of Wisconsin
Miami Children’s Hospital
Mt. Sinai School of Medicine
National Institute of Allergy and Infectious Diseases
Rainbow Babies and Children’s Hospital Ohio
Seattle Children’s Hospital
Texas Children’s Hospital
University of California, San Francisco
University of Colorado, Denver
University of Iowa
University Texas (UT) Southwestern Medical Center
University of Utah
Wake Forest University
Wayne State University
Women and Children’s Hospital of Buffalo
Yale University
Children’s Hospital Orange County
Joe DiMaggio Children’s Hospital
John Hopkins Hospital
Massachusetts’s General Hospital
NIH – NHGRI
University of Wisconsin (New 2013)
Hackensack University Medical Center (New 2013)
Enrolling Institutions under USIDNET IIRB
Allergy Associate of the Palm Beaches
Daniel Suez Allergy, Asthma, & Immunology
Midwest Immunology Clinic
United States Immunodeficiency Network (USIDNET)
References
- 1.Winkelstein JA, Marino MC, Johnston RB, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine. 2000;79:155–69. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 2.A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. The International Chronic Granulomatous Disease Cooperative Study Group. N Engl J Med. 1991;324(8):509–16. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 3.Gallin JI, Alling DW, Malech HL, Wesley R, Koziol D, Marciano B, et al. Itraconazole to prevent fungal infections in chronic granulomatous disease. N Engl J Med. 2003 Jun 12;348(24):2416–22. doi: 10.1056/NEJMoa021931. [DOI] [PubMed] [Google Scholar]
- 4.Levy J, Espanol-Boren T, Thomas C, Fischer A, Tovo P, Bordigoni P, et al. Clinical spectrum of X-linked hyper-IgM syndrome. J Pediatr. 1997 Jul;131(1 Pt 1):47–54. doi: 10.1016/s0022-3476(97)70123-9. [DOI] [PubMed] [Google Scholar]
- 5.Guzman D, Veit D, Knerr V, Kindle G, Gathmann B, Eades-Perner AM, et al. The ESID Online Database network. Bioinformatics. 2007 Mar 1;23(5):654–5. doi: 10.1093/bioinformatics/btl675. [DOI] [PubMed] [Google Scholar]
- 6.Ishimura M, Takada H, Doi T, Imai K, Sasahara Y, Kanegane H, et al. Nationwide survey of patients with primary immunodeficiency diseases in Japan. Journal of Clinical Immunology. 2011 Dec;31(6):968–76. doi: 10.1007/s10875-011-9594-7. [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick P, Riminton S. Primary immunodeficiency diseases in Australia and New Zealand. Journal of Clinical Immunology. 2007 Sep;27(5):517–24. doi: 10.1007/s10875-007-9105-z. [DOI] [PubMed] [Google Scholar]
- 8.Nozaki T, Takada H, Ishimura M, Ihara K, Imai K, Morio T, et al. Endocrine complications in primary immunodeficiency diseases in Japan. Clinical endocrinology. 2012 Oct;77(4):628–34. doi: 10.1111/j.1365-2265.2012.04390.x. [DOI] [PubMed] [Google Scholar]
- 9.Mir Saeid Ghazi B, Aghamohammadi A, Kouhi A, Farhoudi A, Moin M, Rezaei N, et al. Mortality in primary immunodeficient patients, registered in Iranian primary immunodeficiency registry. Iran J Allergy Asthma Immunol. 2004 Mar;3(1):31–6. [PubMed] [Google Scholar]
- 10.Aghamohammadi A, Moein M, Farhoudi A, Pourpak Z, Rezaei N, Abolmaali K, et al. Primary immunodeficiency in Iran: first report of the National Registry of PID in Children and Adults. Journal of Clinical Immunology. 2002 Nov;22(6):375–80. doi: 10.1023/a:1020660416865. [DOI] [PubMed] [Google Scholar]
- 11.Shah BR, Drozda J, Peterson ED. Leveraging observational registries to inform comparative effectiveness research. Am Heart J. 2010 Jul;160(1):8–15. doi: 10.1016/j.ahj.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, et al. Thrombus aspiration during ST-segment elevation myocardial infarction. N Engl J Med. 2013 Oct 24;369(17):1587–97. doi: 10.1056/NEJMoa1308789. [DOI] [PubMed] [Google Scholar]
- 13.Pollard C, Bailey KA, Petitte T, Baus A, Swim M, Hendryx M. Electronic patient registries improve diabetes care and clinical outcomes in rural community health centers. The Journal of rural health: official journal of the American Rural Health Association and the National Rural Health Care Association. 2009 Winter;25(1):77–84. doi: 10.1111/j.1748-0361.2009.00202.x. [DOI] [PubMed] [Google Scholar]
- 14.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF) Circulation. 2010 Aug 10;122(6):585–96. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 15.Holmes JA, Carpenter WR, Wu Y, Hendrix LH, Peacock S, Massing M, et al. Impact of distance to a urologist on early diagnosis of prostate cancer among black and white patients. The Journal of urology. 2012 Mar;187(3):883–8. doi: 10.1016/j.juro.2011.10.156. [DOI] [PubMed] [Google Scholar]
- 16.Rogler LE, Kosmyna B, Moskowitz D, Bebawee R, Rahimzadeh J, Kutchko K, et al. Small RNAs derived from lncRNA RNase MRP have gene-silencing activity relevant to human cartilage-hair hypoplasia. Hum Mol Genet. 2014 Jan 15;23(2):368–82. doi: 10.1093/hmg/ddt427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Herz W, Bousfiha A, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, et al. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Frontiers in immunology. 2011;2:54. doi: 10.3389/fimmu.2011.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bousfiha AA, Jeddane L, Ailal F, Al Herz W, Conley ME, Cunningham-Rundles C, et al. A phenotypic approach for IUIS PID classification and diagnosis: guidelines for clinicians at the bedside. Journal of Clinical Immunology. 2013 Aug;33(6):1078–87. doi: 10.1007/s10875-013-9901-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teutsch SM, Fielding JE. Comparative effectiveness--looking under the lamppost. Jama. 2011 Jun 1;305(21):2225–6. doi: 10.1001/jama.2011.730. [DOI] [PubMed] [Google Scholar]
- 20.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008 Jan 1;111(1):77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 21.Rakhmanov M, Keller B, Gutenberger S, Foerster C, Hoenig M, Driessen G, et al. Circulating CD21low B cells in common variable immunodeficiency resemble tissue homing, innate-like B cells. Proc Natl Acad Sci U S A. 2009 Aug 11;106(32):13451–6. doi: 10.1073/pnas.0901984106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kappelman MD, Crandall WV, Colletti RB, Goudie A, Leibowitz IH, Duffy L, et al. Short pediatric Crohn’s disease activity index for quality improvement and observational research. Inflammatory bowel diseases. 2011 Jan;17(1):112–7. doi: 10.1002/ibd.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crandall WV, Margolis PA, Kappelman MD, King EC, Pratt JM, Boyle BM, et al. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics. 2012 Apr;129(4):e1030–41. doi: 10.1542/peds.2011-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuchman L, Schwartz M. Health outcomes associated with transition from pediatric to adult cystic fibrosis care. Pediatrics. 2013 Nov;132(5):847–53. doi: 10.1542/peds.2013-1463. [DOI] [PubMed] [Google Scholar]
- 25.Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007 Sep;27(5):497–502. doi: 10.1007/s10875-007-9103-1. [DOI] [PubMed] [Google Scholar]
- 26.Modell V, Gee B, Lewis DB, Orange JS, Roifman CM, Routes JM, et al. Global study of primary immunodeficiency diseases (PI)--diagnosis, treatment, and economic impact: an updated report from the Jeffrey Modell Foundation. Immunol Res. 2011 Oct;51(1):61–70. doi: 10.1007/s12026-011-8241-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.