Abstract
Notch signalling regulates a wide range of developmental processes. In the Drosophila peripheral nervous system, Notch regulates a series of binary fate decisions that lead to the formation of regularly spaced sensory organs. Each sensory organ is generated by single sensory organ precursor cell (SOP) via a series of asymmetric cell divisions. Starting from a SOP-specific Cis-Regulatory Module (CRM), we identified insensible (insb), a.k.a CG6520, as a SOP/neuron-specific gene encoding a nuclear factor that inhibits Notch signalling activity. First, over-expression of Insb led to the transcriptional repression of a Notch reporter and to phenotypes associated with the inhibition of Notch. Second, while the complete loss of insb activity had no significant phenotype, it enhanced the bristle phenotype associated with reduced levels of Hairless, a nuclear protein acting as a co-repressor for Suppressor of Hairless. In conclusion, our work identified Insb as a novel SOP/neuron-specific nuclear inhibitor of Notch activity in Drosophila.
Introduction
Cell fate decisions and patterning events during development are regulated by cell-cell interactions that are in part mediated by Notch receptors [1]. Trans-membrane receptors of the Notch family can be described as membrane-tethered transcriptional regulators [2]. Indeed, these receptors consist in a ligand-binding ectodomain linked via a trans-membrane domain to an intracellular domain that acts as a transcriptional regulator upon its ligand-dependent release from the membrane. A ligand-dependent conformational change in the ectodomain of Notch is thought to result in ectodomain shedding and intra-membrane processing of Notch. Following the release of the Notch Intra-Cellular Domain (NICD), the activated nuclear form of Notch [2], NICD forms a ternary complex with CSL (CBF1, Suppressor of Hairless, Lag-1), a sequence-specific DNA-binding protein known as Suppressor of Hairless [Su(H)] in flies, and a co-activator, known as Mastermind (Mam) in flies, to regulate the expression of Notch target genes. In the absence of NICD, CSL factors can bind the cis regulatory region and repress the expression of a subset of Notch target genes in both flies [3], [4], [5], [6] and mammals [7], [8], [9], [10]. Indeed, the human CSL factor CBF1 was initially identified as a transcriptional repressor [11] and several different CSL co-repressors have been identified in mammalian cells [7], [8], [12], [13]. NICD increases the occupancy of CSL binding sites, relieves the transcriptional repression mediated by CSL factors and promotes transcriptional activation [2], [3], [9], [14].
In Drosophila, repression by Su(H) is critical to prevent Notch target genes from being inappropriately activated in some developmental contexts [3], [4], [5]. Su(H) acts in part by recruiting the adaptor protein Hairless (H) and its co-repressors CtBP and Groucho [15]–[20]. While the activity of H appeared to be dispensable in most developmental contexts [17], including embryogenesis [21], repression by Su(H)-H complexes is required for cell fate decisions during adult peripheral neurogenesis [15], [17]–[20], [22]. During pupal development, the activity of H is first required in imaginal tissues for the stable determination of Sensory Organ Precursor cells (SOPs). SOP specification relies on Notch-mediated lateral inhibition such that Notch target genes are repressed in SOPs (Notch OFF) and activated in surrounding cells (Notch ON). The de-repression of Notch target genes in H mutant SOPs was shown to prevent their stable determination [5], [22]. Following their specification, each SOP undergoes a stereotyped series of asymmetric cell divisions to generate the four different cells forming a sensory bristle. The activity of H is also required for proper cell fate determination in the bristle lineage. A reduced level of H in heterozygous or hypomorphic mutant flies led to the transformation of shaft into a second socket, hence resulting into double-socket bristles [17].
Repression by Su(H)-H complexes may act in parallel to other regulatory mechanisms to inhibit the expression of Notch target genes in SOPs. For instance, the transcriptional repressor Longitudinal lacking (Lola) was shown to repress the expression of Notch target genes [23], and to genetically interact with H during adult peripheral neurogenesis [24]. Additionally, the nuclear BEN-solo family protein Insensitive (Insv) was recently shown to directly interact with Su(H) and to inhibit in a H-independent manner the expression of Notch target genes, both in embryos and in a cell-based assay [25]. This CSL co-repressor activity appears to be conserved in mammals since BEND6, a mouse homolog of Insv, binds CSL and antagonizes Notch-dependent gene expression in neural cells [26]. Of note, both Lola and Insv appear to be expressed at higher levels in SOPs, indicating that repression of Notch target genes is achieved by several mechanisms in this developmental context.
In this study, we characterized the genetic function of the CG6520/insb gene that encodes a novel SOP/neuron-specific nuclear protein involved in the repression of Notch target genes.
Results
Insensible is a SOP-specific Nuclear Protein
In a previous study, we used an in silico approach to identify cis-regulatory modules (CRM) regulating gene expression in sensory organ precursor cells (SOPs) in Drosophila [24]. This work led to the identification of a CRM just 5′ to the gene CG6520/insensible (insb) (Figure 1A) which was active in SOPs and other neural progenitor cells. The function of the gene insb is not known in D. melanogaster and orthologs of insb could only be identified in invertebrates. This gene encodes a novel small protein of 176 amino acids with no clear sequence similarities with previously described proteins and/or domains. Sequence analysis suggested the existence of a conserved bipartite nuclear localization signal and of two short motifs that are conserved amongst Drosophilidae orthologs (Figure S1).
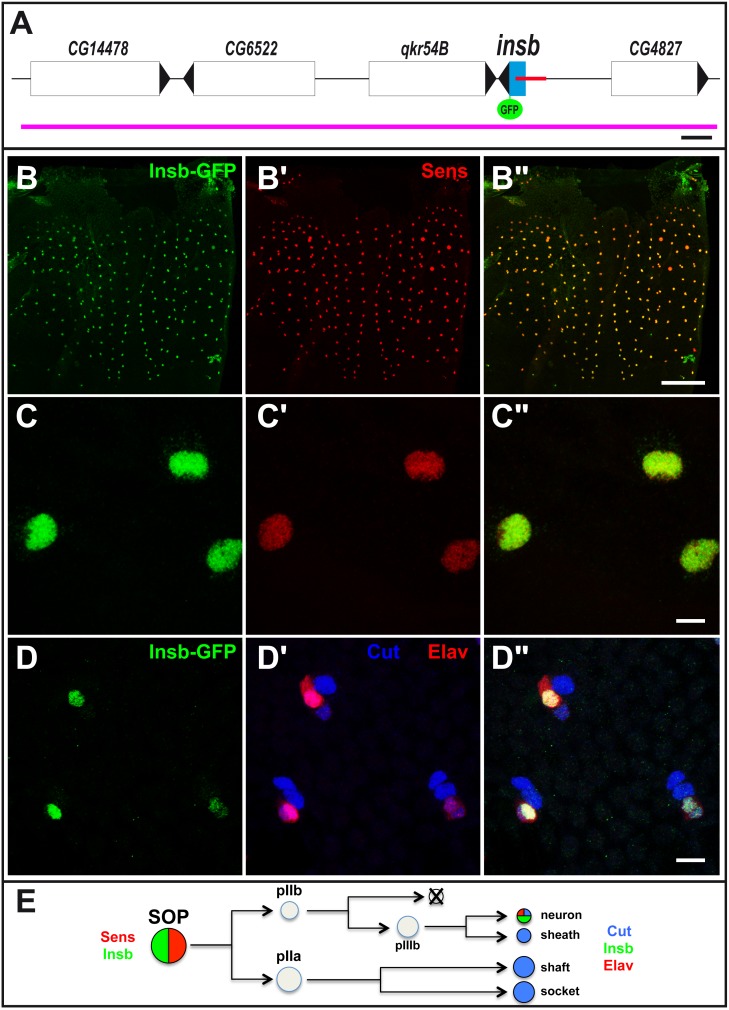
Figure 1. insb encodes a nuclear SOP-specific protein.
(A) Schematic representation of the insb genomic region: genes positions and orientations are shown in white with the exception of insb (blue). The SOP-specific CRM [24] is shown in red. The BAC used in this study is indicated in purple. GFP (green) was inserted 3′ to the insb ORF. Scale bar is 1 kb. (B–C”) Insb-GFP (GFP, green) was specifically detected in the nucleus of SOPs, marked by Senseless (Sens, red), in the notum of 16 hrs after puparium formation (APF) pupae. (D–D”) Insb-GFP (GFP, green) was detected in neurons (Elav, red) but not in other sensory organ cells (Cut, blue) at 24 hr APF. (E) Diagram of the bristle lineage with the markers used in this study. Scale bars are 100 µm. (B–B”) and 5 µm. (C–D”).
To test whether insb is expressed in SOP cells, we generated a GFP tagged-version of Insb expressed under its own regulatory sequences. Starting from a 22 kb genomic BAC covering the insb locus, we used recombineering in E. coli to generate an Insb-GFP BAC transgene (Figure 1A) [27], [28]. Analysis of endogenous Insb-GFP BAC expression in transgenic flies indicated that the insb gene was expressed in SOPs of the pupal notum (Figure 1B–B”). Additionally, we observed that the Insb-GFP protein was nuclear (Figure 1C–C”). Following the division of SOPs, Insb-GFP was detected in pIIa and pIIb cells and is later restricted to neurons (Figure 1D–E) (see [29] for a description of the bristle lineage). Consistent with these observations, RNA-seq data indicated that insb transcripts were specifically detected in the nervous system both during development and in the adult [30]. We conclude that insb is a SOP/neuron-specific gene that encodes a novel nuclear protein.
Insensible is not an Essential Gene
To study the function of the insb gene, we first used a loss of function approach. Since no mutation was available for this gene, we generated a deletion covering the insb gene. To generate a small deletion with precisely defined breakpoints, we took advantage of transposon insertion lines containing FRT sites [31]. Briefly, we used two FRT-containing transposons located 5′ (XP-d05000) and 3′ (WH-f07683) of the insb gene to select a trans-recombination event resulting in a 15 kb genomic deletion (insb Δ1; Figure 2A). This deletion removed the CG14478, CG6522, qkr54B and insb genes (Figure 2B). Homozygous insb Δ1 flies were viable and fertile. We therefore conclude that the activity of the insb gene is largely dispensable for fly development. Of note, the CG14478, CG6522, qkr54B genes similarly appeared to be dispensable for viability in the context of the laboratory.
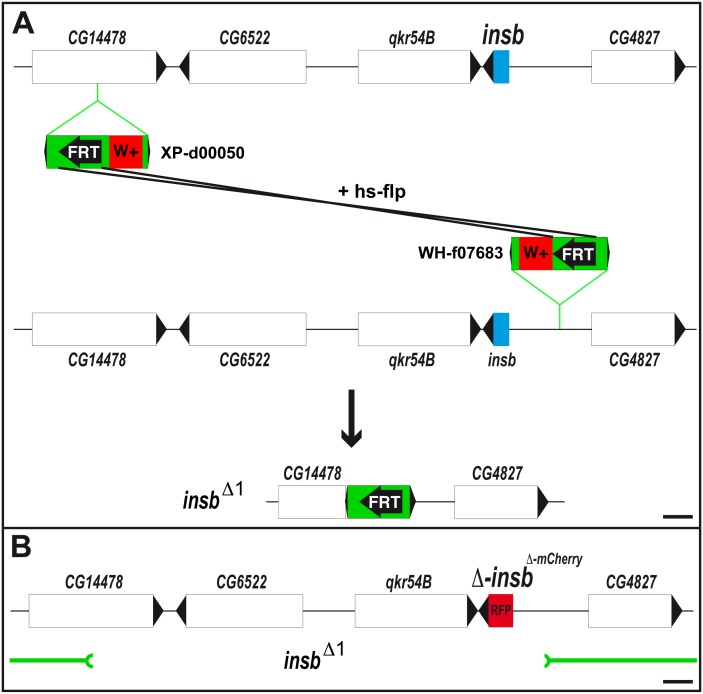
Figure 2. Generation of a synthetic insb null allele.
(A) Generation of the insb Δ1 deficiency using Flp-FRT recombination between the two chromosomes carrying the P{XP}d00050 and PBac{WH}f07683 insertions. (B) Schematic representation the insbmCherry BAC used for the genomic rescue of the CG14478, CG6522 and qkr54B genes deleted by insb Δ1 deficiency (shown in green below). The ORF of insb was replaced by the mCherry encoding sequence (RFP, red). Scale bars are 1 kb.
To generate a single mutant background, i.e. mutant only for the insb gene, we rescued the insb Δ1 deletion allele by a BAC transgene encoding the CG14478, CG6522, qkr54B genes and in which the open reading frame (ORF) of insb was replaced by those of mCherry (Figure 2B). This mutant BAC is referred to here as insbmCherry and the synthetic combination of the insb Δ1 deletion with the insbmCherry BAC transgene as insbm.
Adult insbm flies showed no clear developmental defects with sensory bristles showing a normal pattern on the body surface (Figure 3A, B). This indicated that the specification of SOPs was largely unaffected by the complete loss of insb activity. Additionally, no significant bristle phenotype was observed at macrochaete positions in insbm mutant flies (Figure 3B; see also below the phenotype of flies heterozygous for insbm over a deficiency). Thus, this loss-of-function analysis showed that insb is not an essential gene.
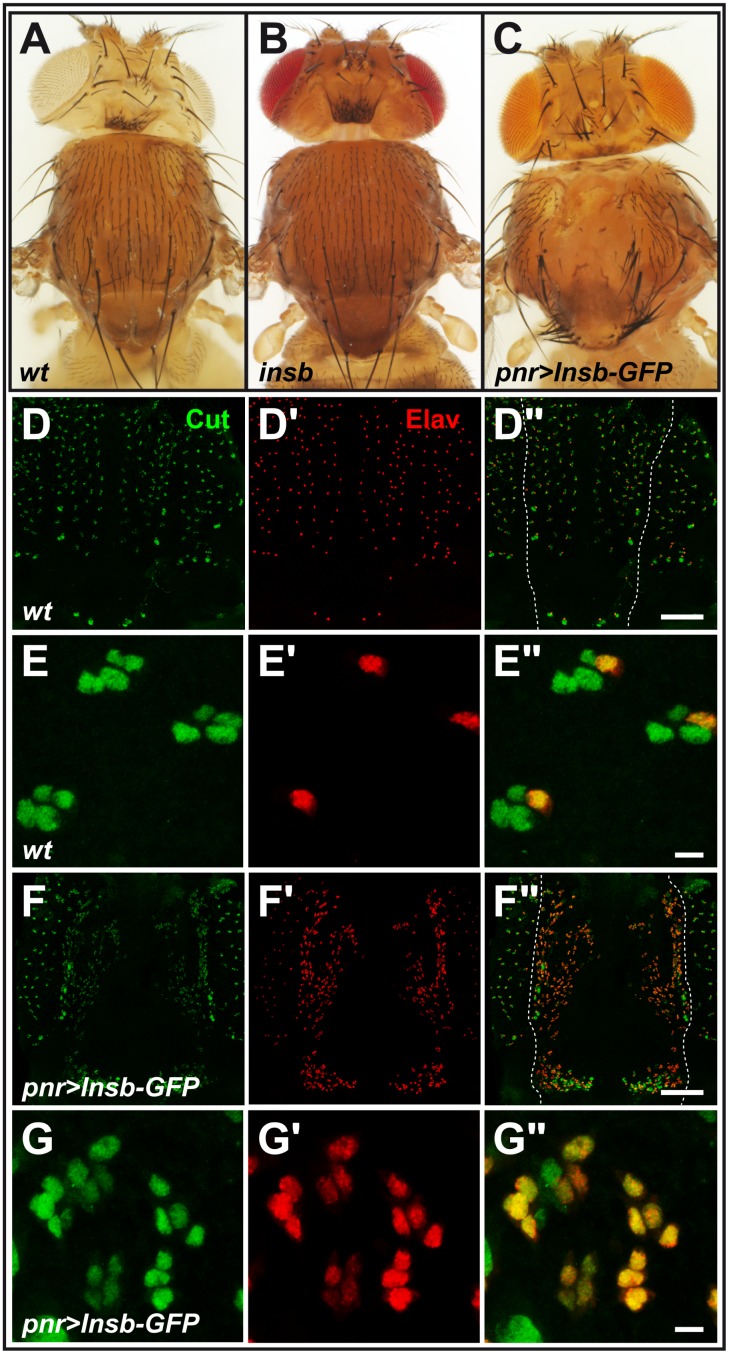
Figure 3. Overexpression of Insb led to Notch inhibition.
(A–C) micrographs showing the bristle pattern on the dorsal thorax of control (A), insbm (synthetic mutation resulting from combining the insb Δ1 deficiency with the insbmCherry BAC; B) and pnr>Insb-GFP (C) flies. Loss of insb had no significant effect on the bristle pattern whereas ectopic expression led to bristle loss (associated with a transformation of external cells into internal cells) and bristle tufts (due to an excess of SOPs). (D–G”) The bristle loss phenotype of pnr>Insb-GFP flies was associated with i) an increased density of sensory organs (Cut, green; Elav, red) in the dorso-central region (bracket) of the notum in 22 hrs APF pupae (compare pnr>Insb-GFP pupae in F–F” with wild-type (wt) pupae in D–D”) and ii) a transformation of sense organ cells into neurons (Elav, red). Both increased density of sensory organs and transformation of sensory cells into neurons are indicative of a strong loss of Notch signalling (see magnifications in E–E” and G–G”). The expression pattern of pnr-Gal4 is indicated with dashed lines in D” and F”. Scale bars are 100 µm. (D–D” and F–F”) and 5 µm. (E–E” and G–G”).
Table 1. Genotypes.
| Figures | Genotypes |
| Fig. 1B–D” | w/w;; PBac{y[+]-attP-9A. insbGFP}VK00019 |
| Fig. 3A, 3D–E” | w/w |
| Fig. 3B | w/w; FRT(w[hs])}G13, insb Δ1 /FRT(w[hs])}G13, insb Δ1 ; PBac{y[+]-attP-9A. insb ΔmCherry }VK00019/PBac{y[+]-attP-9A. insb ΔmCherry }VK00019 |
| Fig3C, 3F–3G” | w/w;; pnr-Gal4/UAS-Insb-GFP |
| Fig. 4A–A” | w/w; en-Gal4, Gal80ts/+ |
| Fig. 4B–B” | w/w; en-Gal4, Gal80ts/UAS-Insb-GFP |
| Fig. 5A, I | w/w |
| Fig. 5B, I | w/w; FRT(w[hs])}G13, insb Δ1 /Df(2R)BSC406; PBac{y[+]-attP-9A. insb ΔmCherry }VK00019/+ |
| Fig. 5C, I | w/w; insv23B/insv23B |
| Fig. 5D, I | w/w; FRT(w[hs])}G13, insb Δ1 , insv23B/FRT(w[hs])}G13, insb Δ1, insv23B; PBac{y[+]-attP-9A. insb ΔmCherry }VK00019/TM6B, Tb [1] |
| Fig. 5E, I | w/w;; H[E31], P[mw+], P[neo, FRT]82B/TM6B, Tb [1] |
| Fig. 5F | w/w; FRT(w[hs])}G13, insb Δ1 /FRT(w[hs])}G13, insb Δ1 ; PBac{y[+]-attP-9A. insb ΔmCherry }VK00019, H[E31]/TM6B, Tb [1] |
| Fig. 5G, I | w/w; insv23B/insv23B; H[E31], P[mw+], P[neo, FRT]82B/TM6B, Tb [1] |
| Fig. 5H, I | w/w; FRT(w[hs])}G13, insb Δ1 , insv23B/FRT(w[hs])}G13, insb Δ1, insv23B; PBac{y[+]-attP-9A. insb ΔmCherry }VK00019, H[E31]/TM6B, Tb [1] |
| Fig. 5I | w/w; FRT(w[hs])}G13, insb Δ1 /Df(2R)BSC406; PBac{y[+]-attP-9A. insb ΔmCherry }VK00019, H[E31]/+ |
| Fig. 5I | w/w; FRT(w[hs])}G13, insb Δ1 /Df(2R)BSC406; PBac{y[+]-attP-9A. insb ΔmCherry }VK00019, H[E31]/PBac{y[+]-attP-9A. insbGFP}VK00019 |
| Fig. 6A–B” | w/w; insv23B/insv23B; PBac{y[+]-attP-9A. insbGFP}VK00019/PBac{y[+]-attP-9A. insbGFP}VK00019 |
| Fig. 6C–C” | w/w; en-Gal4, Gal80ts/+; PBac{y[+]-attP-9A. insbGFP}VK00019/UAS-insv |
| Fig. 6D | w/w, sd-Gal4, Gal80ts |
| Fig. 6E | w/w, sd-Gal4, Gal80ts;; UAS-Insb-GFP/+ |
| Fig. 6F | w/w, sd-Gal4, Gal80ts; insv23B/insv23B; UAS-Insb-GFP/+ |
Insensible can Inhibit the Expression of Notch Target Genes
We next used a gain-of-function approach to further examine the function of the insb gene. To do so, we generated a UAS-Insb-GFP transgene. The ectopic expression of the Insb-GFP protein was then achieved using various Gal4 drivers. Using pannier-Gal4 (pnr-Gal4), we found that over-expression of Insb-GFP resulted in a bristle loss phenotype (Figure 3C). This balding phenotype was associated with both an increased density of sensory organs in the dorso-central region of the notum that expressed pnr-Gal4 (Figure 3D–D” and F–F”) and an increased number of Elav-positive neurons (Figure 3E–E” and G–G”). Hence, we propose that overexpression of Insb-GFP led first to the specification of too many SOPs, hence the increased number of external sensory organs, and second to the transformation of external cells into internal cells, notably neurons, leading to the balding phenotype seen in adult flies. Thus, this insb gain-of-function generated a lateral inhibition and cell fate transformation phenotypes similar to the ones observed upon loss of Notch activity [32]. We therefore propose that the insb gene encodes a nuclear antagonist of Notch.
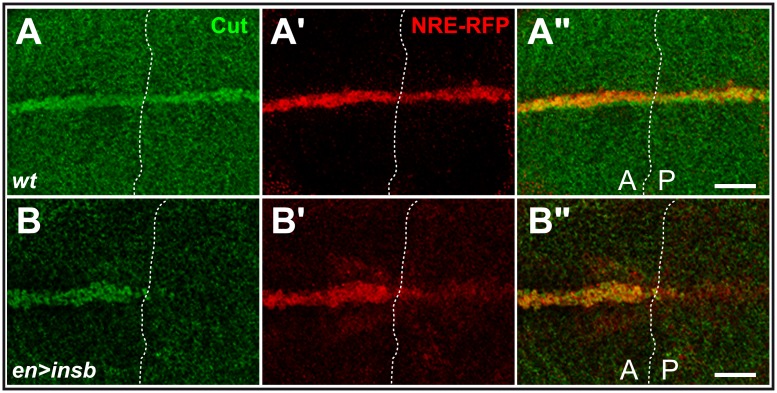
To further test this proposal, we analysed the effect of insb over-expression on another Notch-dependent process. The development of the wing involves the activation of Notch at the wing margin. We found that ectopic expression of Insv-GFP in posterior cells using the en-Gal4 driver inhibited the expression of the Notch target gene cut (Figure 4) [33] and resulted in posterior notches in adult wings (not shown). Moreover, the expression of an artificial Notch reporter construct, NRE-RFP [34], was also significantly reduced in posterior cells (Figure 4). We therefore conclude from these gain-of-function experiments that insb can inhibit the activity of Notch. We therefore propose that Insb is a nuclear antagonist of Notch that contributes to inhibit the expression of Notch target genes in SOPs (and more generally in neural cells).
Figure 4. Ectopic Insb inhibits the expression of Notch targets.
(A) Expression of Insb in posterior (P) cells led to the reduced expression of the Notch target gene cut (Cut, green) and of the NRE-RFP reporter (RFP, red) in third instar wing imaginal discs (B–B”; compare with a wt control disc showing the expression of Cut and NRE-RFP in both anterior (A) and P cells along the dorsal-ventral boundary in A–A”). Scale bars are 20 µm. (A–B”).
Insensible Genetically Interacts with Hairless
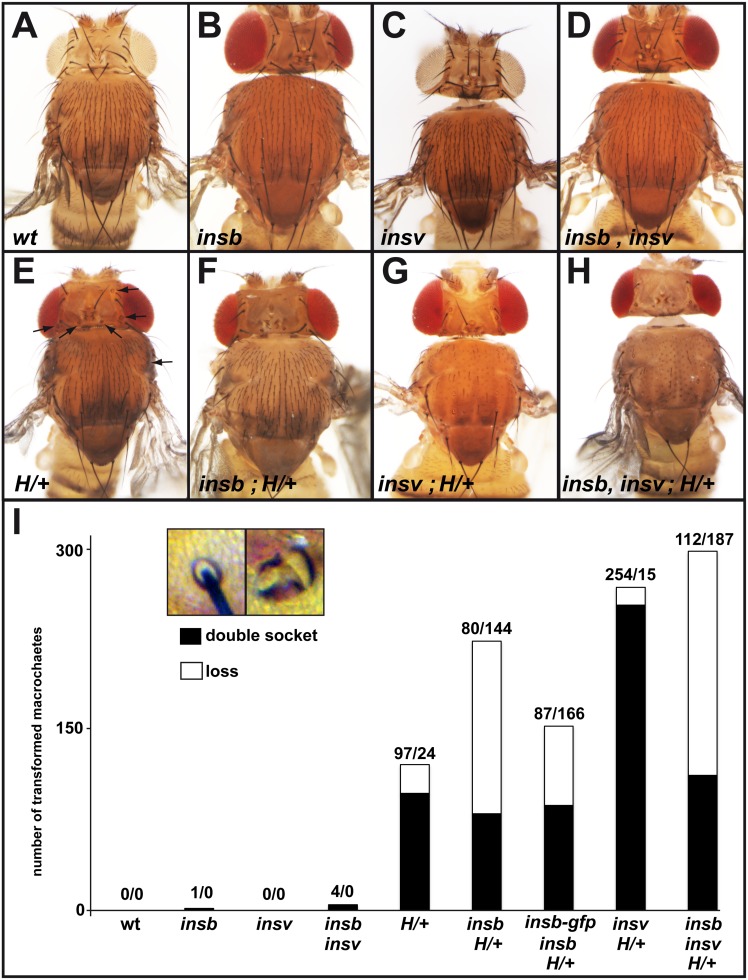
Several nuclear factors are known to contribute to the repression of Notch target genes in SOPs, including H [19], [35] [17] and Insv [25]. To further test the role of insb in the regulation of Notch target genes, we first studied genetic interaction between insb and H. To do so, we counted the number of missing and double-socket macrochaetes in flies mutant for insbm over a 55 kb deficiency deleting the insb locus, Df(2R)BSC406, in flies heterozygous for HE31, a null allele of H. As reported earlier, we found that heterozygous HE31 mutant flies exhibited a mild double-socket phenotype and a weak bristle loss phenotype (Figure 5E, I). Loss of insb in this context enhanced the bristle loss phenotype (Figure 5F, I). Moreover, the Insb-GFP BAC suppressed this genetic interaction. We conclude that insb genetically interacts with and that the BAC-encoded Insb-GFP is functional. Earlier studies had shown that the H bristle loss phenotype resulted from a failure of SOP determination due to a defect in the repression of Notch target genes in SOPs [35]. Thus, this genetic interaction was consistent with insb contributing to the repression of Notch target genes in SOPs. It further indicated that this proposed function of insb becomes essential when the activity of H is limiting. Consistent with a role of insb in antagonizing Notch signaling, we also found that the loss of insb activity suppressed the bristle density phenotype of Notch heterozygous flies (N55e11/+: 153+/−9 bristle in the dorso-central region; N55e11/+; insb Δ1 /Df(2R)BSC406: 133+/−7; n = 10 flies; wild-type and insb mutant flies had 120+/−4 and 128+/−6, respectively).
Figure 5. insb genetically interacts with H.
(A–H) Micrographs showing the bristle pattern on the dorsal thorax of control (A), insbm/ ,Df(2R)BSC406 (B), insv23B (C), insbm insv23B (D), HE31/+ (E) (affected macrochaetes are indicated with an arrow), and insbm, HE31/H+ (F), insv23B, HE31/+ (G), insbm insv23B HE31/+ (H) flies (see Table 1 for detailed genotypes). (I) Histogram showing the number of lost (no shaft, no socket) and double-socket (no shaft) macrochaetes in the genotypes shown in A–H (for each genotype, 10 animals were scored for a total of 400 macrochaetes). A strong genetic interaction was observed between insb and H. The inset shows a control and a double-socket bristle from an HE31/+ fly.
We next tested interaction between insbm and insv23B, a null allele of insv [25]. As reported earlier, insv mutant flies had no detectable macrochaete bristle phenotype (Figure 5C, I) [25]. We also confirmed that the loss of insv activity strongly enhanced the H double socket phenotype (Figure 5G, I) [25]. While no interaction was observed in insb insv double mutant flies (Figure 5D, I), the loss of insb enhanced the bristle loss phenotype of insv H+/− flies (Figure 5H, I). One possible interpretation for these genetic interaction data is that the nuclear factors Insb and Insv act together, in parallel with H, to inhibit Notch target gene expression in SOPs and its progeny cells.
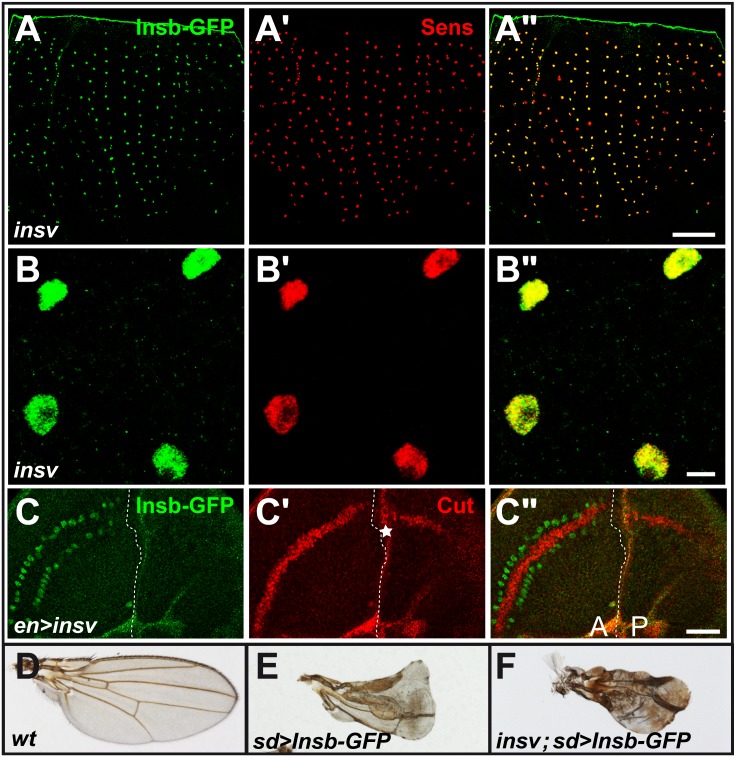
To begin testing whether insb and insv act within in a linear regulatory pathway, we examined the expression of the insb-GFP BAC transgene in insv mutant flies. We found that that the insb-GFP gene was normally expressed in SOPs (Figure 6A–A”) and that the Insb-GFP protein was still nuclear (Figure 6B–B”), We conclude that the SOP-specific expression of insb and the nuclear localization of Insb did not depend on insv activity. Additionally, ectopic expression of insv in wing imaginal discs using engrailed-Gal4 (en-Gal4) led to a loss of cut expression in posterior cells (Figure 6C–C”) and to a wing notching phenotype in adult flies (data not shown) but did not result in ectopic insv-GFP expression (Figure 6C). These data did not support a model whereby Insv regulates the expression of insb. It also suggested that repression of Notch target genes by Insv can take place without up-regulating the expression of insb.
Figure 6. Insv and Insb may act independently of one another.
(A–B”) Insb-GFP (GFP, green) was specifically detected in the nucleus of SOPs (Sens, red), in 16 hrs APF insv mutant pupae. (C–C”) Ectopic expression of Insv in posterior (P) cells inhibited the expression of the Notch target gene cut (Cut, red) in wing discs without inducing the expression of Insb-GFP (GFP, green). The background red signal (star in C’) was generated by image projections due to a fold along A/P boundary in wing discs over-expressing Insv. (D–F) adult wings: wild-type (D), overexpression of Insb-GFP in wild-type (E) and insv mutant background (F) using sd-Gal4 driver. Loss of insv function had no effect on the Insb-GFP induced phenotype. Scale bars are 100 µm (A–A”), 5 µm (B–B”) and 20 µm (C–C”).
We next tested whether Insb can inhibit the activity of Notch in the absence of insv. To do so, Insb-GFP was overexpressed using sd-Gal4 in wing imaginal discs of wild-type and insv mutant flies. We found that the Notch-like phenotypes induced by overexpressed Insb-GFP, i.e. loss of wing margin, was not suppressed by the loss of insv activity (Figure 6D–F). This result suggested that Insb can inhibit Notch independently of Insv. Together, our data suggest that repression of Notch targets by Insb is important when the level of H is limiting and that Insv may function independently of Insb to inhibit the expression of Notch target genes.
Discussion
Our study identified Insb as a novel SOP/neuron-specific nuclear factor that antagonizes Notch to regulate cell fate. First, we have shown that over-expression of Insb inhibited the activity of Notch during sensory organ formation and blocked the expression of a Notch reporter construct in wing discs. This indicated that Insb has the ability to inhibit the expression of Notch target genes. Since the Notch reporter construct used here responded directly to Notch via paired Su(H) binding sites [34], [36], [37], Insb likely acts via these binding sites, i.e. by modulating the activity of Su(H)-bound complexes. Second, while the activity of insb appeared to be largely dispensable during development, its activity became essential for the proper determination of sensory bristle cells when the activity of H becomes limiting, i.e. when Notch target genes are derepressed upon reduced H levels [22]. Thus, like Insv, Insb appears to function in a partly redundant manner with H. Additionally, while loss of insb and insv activities similarly enhanced the H haplo-insufficient phenotype, no genetic interaction was observed in double mutant flies. One possible interpretation for this lack of genetic interaction is that Insv and Insb act together to regulate the same process, so that the complete loss of one or both genes have similar phenotypic consequences. Since Insv did not regulate the expression of insb, one possibility is that Insb positively regulates the expression of the insv gene and that Insv antagonizes Notch. Alternatively, the two proteins may act together to repress the expression of Notch target genes via the Su(H) binding sites. Consistent with this, Insv was proposed to repress the expression of Notch target genes by two mechanisms: first in a Su(H)-dependent mechanims, Insv would act as a CSL co-repressor to promote repression through Su(H) binding sites; second, Insv may directly bind DNA via its BEN domain and regulate gene expression in a Su(H)-independent manner. Whether Insb physically interacts with Insv and regulates its transcriptional activities await biochemical studies. While a functional homolog of Insv has recently been characterized in the mouse, no clear homolog of Insb could be easily identified in vertebrates. Thus, deciphering how Insb regulates in flies the activities of Insv and other CSL associated co-repressors, such as H, may provide new insights into molecular mechanisms of co-repression by CSL-associated factors. Finally, while the expression and function of Insb was primarily studied here in the context of sensory organ development, this gene was also expressed at high levels in neuroblasts of the developing larval brain, suggesting that Insb may have a broader role as a Notch antagonist.
In conclusion, our study identified Insb as a nuclear SOP/neuron-specific antagonist of Notch signaling that may act together with Insv to repress the expression of Notch target genes.
Materials and Methods
Flies and Transgenes
The insb Δ1 deficiency was generated by Flp-FRT recombination as described in Parks et al, [31] using P{XP}d00050 and PBac{WH}f07683. The resulting 15,396 nt deletion (corresponding to deficiency FDD-0000787 in http://www.drosdel.org.uk/fdd/fdd_info.php) was selected based on eye color (loss of w+) and confirmed by PCR.
The CH322-168B11 BAC covering the insb locus (from 6,148 nt 5′ to the transcription start site to 15,129 nt downstream of the 3′UTR) was obtained from BACPAC (http://bacpac.chori.org) [28] and used to generate the insb-GFP, and insb ΔmCherry transgenes by BAC recombineering in E. coli as described in [27]. The 5′ and 3′ homology arms were produced using the following primers:
For the insb-GFP allele;
CG6520_5F: GCAACCGACTGAAGCGGTTCCGGA
CG6520_Rgfp: CAAAAACACCCCGCCCTAACAACA
CG6520_Fgfp: CTGTACAAGTAAGAGCAAACCGGAGGGCAG
CG6520_3R: CTTGCTCACCATGGCGTGCAGAAGTCCATC
The GFP cassette was amplified using the following primers:
CG6520_GfpF: CTTCTGCACGCCATGGTGAGCAAGGGCGAG
CG6520_GfpR: TCCGGTTTGCTCTTACTTGTACAGCTCGTC
For the insb ΔmCherry allele;
CG6520_5Fch: GACGGATGCACGGAGGAAGGGA
CG6520_Rch: CTCCTCGCCCTTGCTCACCATGCTTAGAGGATGAT
CG6520_Fch: CTGTACAAGTAAGAGCAAACCGGAGGGCAGGA
CG6520_3Rch: CTTGCTCACCATGGCGTGCAGAAGTCCATC
The mCherry cassette was amplified using the following primers:
CG6520_chF: CCTCTAAGCATGGTGAGCAAGGGCGAGGA
CG6520_chR: TCCGGTTTGCTCTTACTTGTACAGCTCGTC
Constructs were verified by sequencing of the recombined regions prior to phiC31-mediated integration at the PBac{y[+]-attP-9A}VK00019 site [27].
The UAS-Insb-GFP transgene was generated by PCR amplifying the ORF of CG6520 from wild-type genomic DNA using the primers;
5′-CG6520: CGGGATCCATGTCGGGCAAACTGATCATGA
3′-CG6520: GGGGTACCGGGGCGTGCAGAAGTCCATCGCT
The PCR product was cloned as a BglII/KpnI fragment into pUAST-EGFP. After verification by sequencing, the transgene was inserted into the fly genome by P-element transformation.
All injections were performed by BestGene Inc. (Chino Hills, USA).
The Df(2R)BSC406, HE31 [21], insv23B and UAS-insv flies [25] have been described previously. Conditional overexpression was achieved using the binary UAS/Gal4 system in combination with the thermo-sensitive Gal80ts inhibitor. The pannier-Gal4 driver (pnr-Gal4), engrailed-Gal4 (en-Gal4) and scalloped-Gal4 (sd-Gal4) drivers were used.
Immunostainings and Microscopy
Drosophila nota and wing imaginal discs were dissected from staged pupae and larvae and stained using standard techniques. Primary antibodies were: goat anti-GFP (1∶500; ab5450 from abcam), guinea-pig anti-Senseless (1∶3000; kind gift from H. Bellen), mouse anti-Cut 2B10 mAb (1∶500; DSHB), rat anti-Elav 7E8A10 mAb (1∶100; DSHB). Secondary antibodies were from Jackson ImmunoResearch Laboratories and coupled to Cy2, Cy3 and Cy5. Wing discs and nota were mounted in Mowiol 4–88 (Sigma) containing 2,5% DABCO (Sigma). Images were acquired using a Leica SPE confocal microscope using 20x (HCX PL APO CS, NA 0.6) and 63x (HCX PL APO CS, N.A. 1.3) objectives. Adult flies were imaged using a Zeiss Discovery V20 stereo-macroscope using a 1.0X (PlanApo S FWD 60 mm) objective. Images were processed using ImageJ and Adobe Photoshop softwares.
Supporting Information
Sequence alignment of Insb proteins. Multiple sequence alignment analysis revealed two regions conserved between different Drosophila species and an amino-terminal nuclear localization signal. Identical (red), similar (blue) and variable (black) amino acids are color-coded.
(TIF)
Acknowledgments
We thank S. Artavanis-Tsakonas, E. Lai and the Bloomington Drosophila Stock Center for flies. The 9F8A9 and 2B10 mAbs developed by G. M. Rubin, C. Goodman and S. Artavanis-Tsakonas were obtained from the Developmental Studies Hybridoma Bank (DSHB) developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242. We thank F. Bernard and all lab members for discussion.
Funding Statement
This work was funded by the CNRS and Institut Pasteur. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Artavanis-Tsakonas S, Rand MD, Lake RJ (1999) Notch signaling: cell fate control and signal integration in development. Science 284: 770–776. [DOI] [PubMed] [Google Scholar]
- 2. Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morel V, Schweisguth F (2000) Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev 14: 377–388. [PMC free article] [PubMed] [Google Scholar]
- 4. Koelzer S, Klein T (2003) A Notch-independent function of Suppressor of Hairless during the development of the bristle sensory organ precursor cell of Drosophila. Development 130: 1973–1988. [DOI] [PubMed] [Google Scholar]
- 5. Castro B, Barolo S, Bailey AM, Posakony JW (2005) Lateral inhibition in proneural clusters: cis-regulatory logic and default repression by Suppressor of Hairless. Development 132: 3333–3344. [DOI] [PubMed] [Google Scholar]
- 6. Furriols M, Bray S (2000) Dissecting the mechanisms of suppressor of hairless function. Dev Biol 227: 520–532. [DOI] [PubMed] [Google Scholar]
- 7. Kao HY, Ordentlich P, Koyano-Nakagawa N, Tang Z, Downes M, et al. (1998) A histone deacetylase corepressor complex regulates the Notch signal transduction pathway. Genes Dev 12: 2269–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oswald F, Winkler M, Cao Y, Astrahantseff K, Bourteele S, et al. (2005) RBP-Jkappa/SHARP recruits CtIP/CtBP corepressors to silence Notch target genes. Mol Cell Biol 25: 10379–10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castel D, Mourikis P, Bartels SJ, Brinkman AB, Tajbakhsh S, et al. (2013) Dynamic binding of RBPJ is determined by Notch signaling status. Genes Dev 27: 1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulligan P, Yang F, Di Stefano L, Ji JY, Ouyang J, et al. (2011) A SIRT1-LSD1 corepressor complex regulates Notch target gene expression and development. Mol Cell 42: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dou S, Zeng X, Cortes P, Erdjument-Bromage H, Tempst P, et al. (1994) The recombination signal sequence-binding protein RBP-2N functions as a transcriptional repressor. Mol Cell Biol 14: 3310–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsieh JJ, Zhou S, Chen L, Young DB, Hayward SD (1999) CIR, a corepressor linking the DNA binding factor CBF1 to the histone deacetylase complex. Proc Natl Acad Sci U S A 96: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yatim A, Benne C, Sobhian B, Laurent-Chabalier S, Deas O, et al. (2012) NOTCH1 nuclear interactome reveals key regulators of its transcriptional activity and oncogenic function. Mol Cell 48: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krejci A, Bray S (2007) Notch activation stimulates transient and selective binding of Su(H)/CSL to target enhancers. Genes Dev 21: 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schweisguth F, Posakony JW (1992) Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell 69: 1199–1212. [DOI] [PubMed] [Google Scholar]
- 16. Nagel AC, Krejci A, Tenin G, Bravo-Patino A, Bray S, et al. (2005) Hairless-mediated repression of notch target genes requires the combined activity of Groucho and CtBP corepressors. Mol Cell Biol 25: 10433–10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bang AG, Hartenstein V, Posakony JW (1991) Hairless is required for the development of adult sensory organ precursor cells in Drosophila. Development 111: 89–104. [DOI] [PubMed] [Google Scholar]
- 18. Brou C, Logeat F, Lecourtois M, Vandekerckhove J, Kourilsky P, et al. (1994) Inhibition of the DNA-binding activity of Drosophila suppressor of hairless and of its human homolog, KBF2/RBP-J kappa, by direct protein-protein interaction with Drosophila hairless. Genes Dev 8: 2491–2503. [DOI] [PubMed] [Google Scholar]
- 19. Bang AG, Posakony JW (1992) The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev 6: 1752–1769. [DOI] [PubMed] [Google Scholar]
- 20. Morel V, Lecourtois M, Massiani O, Maier D, Preiss A, et al. (2001) Transcriptional repression by suppressor of hairless involves the binding of a hairless-dCtBP complex in Drosophila. Curr Biol 11: 789–792. [DOI] [PubMed] [Google Scholar]
- 21. Schweisguth F, Lecourtois M (1998) The activity of Drosophila Hairless is required in pupae but not in embryos to inhibit Notch signal transduction. Dev Genes Evol 208: 19–27. [DOI] [PubMed] [Google Scholar]
- 22. Bailey AM, Posakony JW (1995) Suppressor of hairless directly activates transcription of enhancer of split complex genes in response to Notch receptor activity. Genes Dev 9: 2609–2622. [DOI] [PubMed] [Google Scholar]
- 23. Zheng L, Carthew RW (2008) Lola regulates cell fate by antagonizing Notch induction in the Drosophila eye. Mech Dev 125: 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rouault H, Mazouni K, Couturier L, Hakim V, Schweisguth F (2010) Genome-wide identification of cis-regulatory motifs and modules underlying gene coregulation using statistics and phylogeny. Proc Natl Acad Sci U S A 107: 14615–14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duan H, Dai Q, Kavaler J, Bejarano F, Medranda G, et al. (2011) Insensitive is a corepressor for Suppressor of Hairless and regulates Notch signalling during neural development. EMBO J 30: 3120–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai Q, Andreu-Agullo C, Insolera R, Wong LC, Shi SH, et al. (2013) BEND6 is a nuclear antagonist of Notch signaling during self-renewal of neural stem cells. Development 140: 1892–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Venken KJ, He Y, Hoskins RA, Bellen HJ (2006) P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science 314: 1747–1751. [DOI] [PubMed] [Google Scholar]
- 28. Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, et al. (2009) Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods 6: 431–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gho M, Bellaiche Y, Schweisguth F (1999) Revisiting the Drosophila microchaete lineage: a novel intrinsically asymmetric cell division generates a glial cell. Development 126: 3573–3584. [DOI] [PubMed] [Google Scholar]
- 30. Graveley BR, Brooks AN, Carlson JW, Duff MO, Landolin JM, et al. (2011) The developmental transcriptome of Drosophila melanogaster. Nature 471: 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Parks AL, Cook KR, Belvin M, Dompe NA, Fawcett R, et al. (2004) Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet 36: 288–292. [DOI] [PubMed] [Google Scholar]
- 32. Hartenstein V, Posakony JW (1990) A dual function of the Notch gene in Drosophila sensillum development. Dev Biol 142: 13–30. [DOI] [PubMed] [Google Scholar]
- 33. Micchelli CA, Rulifson EJ, Blair SS (1997) The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124: 1485–1495. [DOI] [PubMed] [Google Scholar]
- 34. Housden BE, Millen K, Bray SJ (2012) Drosophila Reporter Vectors Compatible with PhiC31 Integrase Transgenesis Techniques and Their Use to Generate New Notch Reporter Fly Lines. G3 (Bethesda) 2: 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bang AG, Bailey AM, Posakony JW (1995) Hairless promotes stable commitment to the sensory organ precursor cell fate by negatively regulating the activity of the Notch signaling pathway. Dev Biol 172: 479–494. [DOI] [PubMed] [Google Scholar]
- 36. Furriols M, Bray S (2001) A model Notch response element detects Suppressor of Hairless-dependent molecular switch. Curr Biol 11: 60–64. [DOI] [PubMed] [Google Scholar]
- 37. Lecourtois M, Schweisguth F (1995) The neurogenic suppressor of hairless DNA-binding protein mediates the transcriptional activation of the enhancer of split complex genes triggered by Notch signaling. Genes Dev 9: 2598–2608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of Insb proteins. Multiple sequence alignment analysis revealed two regions conserved between different Drosophila species and an amino-terminal nuclear localization signal. Identical (red), similar (blue) and variable (black) amino acids are color-coded.
(TIF)