Abstract
Critical to the maintenance of circadian rhythmicity is the cyclic expression of at least some components of the central oscillator. High-amplitude cycling of mRNA and protein abundance, protein phosphorylation and nuclear/cytoplasmic shuttling have all been implicated in the maintenance of circadian period. Here we use a newly characterized Arabidopsis suspension cell culture to establish that the rhythmic changes in the levels of the clock-associated F-box protein, ZTL, are posttranscriptionally controlled through different circadian phase-specific degradation rates. This proteolysis is proteasome dependent, implicating ZTL itself as substrate for ubiquitination. This demonstration of circadian phase-regulated degradation of an F-box protein, which itself controls circadian period, suggests a novel regulatory feedback mechanism among known circadian systems.
Forward genetic screens in Arabidopsis for circadian period-altering loci have uncovered new types of components that are distinctly different from the factors that underlie circadian clock function in other systems. These include TOC1/APRR1, a pseudoresponse regulator (1–3), TEJ, which encodes a poly(ADP-ribose) glycohydrolase (PARG) (4), and ZEITLUPE (ZTL) which codes for a novel type of F-box protein that causes period lengthening when disrupted (ref. 5 and D.E.S., unpublished data) or hypomorphic (6).
F-box proteins are key elements of Skp-cullin-F-box protein (SCF) complexes, one class of E3 ubiquitin ligase complexes that facilitate ubiquitination of proteins targeted for proteasomal degradation. F-box proteins act as a bridge between the target substrates and the E3 ligase and confer substrate specificity to the complex (7). ZTL is the best characterized of a three-member gene family [ZTL/LKP1/ADO1 (5, 6, 8), FKF1 (9), and LKP2 (10)] that differ from other known F-box proteins in the unique assembly of three previously described domains within one class of polypeptide. N-terminal to the F-box region is a LOV domain, a special class of the PAS motif (11), which folds into a flavin-binding pocket (12) to bind flavin mononucleotide in the plant blue-light photoreceptor phototropin (13) and flavin adenine dinucleotide in the Neurospora blue-light photoreceptor WHITE COLLAR-1 (WC-1) (14, 15). Downstream of the F-box are six kelch repeats, domains previously shown to facilitate protein–protein interactions in a variety of proteins (16). Acting together, these domains may allow ZTL to function as a light-dependent regulator of proteolytic degradation of clock-associated proteins (6).
Cyclic expression of at least some of the components of the central oscillator is essential to maintain circadian rhythmicity. High-amplitude cycling of mRNA and protein abundance, protein phosphorylation and nuclear/cytoplasmic shuttling have all been implicated in the maintenance of circadian period (17, 18). Through the use of a newly characterized Arabidopsis suspension cell culture, we establish that the rhythmic changes in ZTL protein levels are posttranscriptionally controlled by way of different circadian phase-specific degradation rates, and that this degradation is proteasome-dependent. The phase-regulated degradation of an F-box protein, which itself controls circadian period, suggests a novel circadian regulatory feedback mechanism.
Materials and Methods
Plant and Cell Culture Growth and Maintenance.
Arabidopsis suspension-cultured cells were grown in 50 ml of Gamborg B5 medium (Sigma) supplemented with 1.1 mg/liter 2,4-D and 0.5 g/liter MES at 22°C under continuous fluorescent white light (60 μmol m−2·s−1). Cells were subcultured every 7 days at a 10-fold dilution with fresh medium. For circadian studies, 15 ml of 8-day-old cultures were diluted to 50 ml with fresh medium, grown in constant light for 1 day, then shifted to 12/12 h light/dark cycles for 2 or 3 days before onset of treatments. Sampled cells were frozen in liquid nitrogen. Seeds were surface sterilized and grown on solid Murashige and Skoog media (Sigma) (3).
RNA Gel Blot Analyses.
Cell culture total RNA was extracted and blotted according to standard methods, which are detailed in Supporting Materials and Methods, which is published as supporting information on the PNAS web site, www.pnas.org, together with primer sequences and PCR conditions.
Antisera Production and Sources.
Antisera to ZTL coding sequence were obtained by using techniques detailed in Supporting Materials and Methods.
Immunoblotting and Immunoprecipitation.
Cell and tissue extracts were immunoblotted and immunoprecipitated according to modifications of standard techniques, detailed in Supporting Materials and Methods.
Cell-Free Degradation Assay and Inhibitor Treatments.
Ten-day-old seedlings were ground in liquid nitrogen, resuspended in extraction buffer (25 mM Tris⋅HCl, pH 7.5/10 mM MgCl2/50 mM NaCl) (1 ml powder/1 ml) modified according to (19). Extracts were clarified by centrifugation at 16,000 × g (10 min; 4°C). Supernatant aliquots were transferred to individual tubes for each time point, DTT and ATP were added to 10 mM and incubated at 30°C for the appropriate time. For inhibitor studies, extracts were incubated with or without inhibitor at 30°C for 2h. Reactions were stopped (30 μl of 50% trichloroacetic acid), collected by centrifugation and resuspended in urea/SDS loading buffer. ZTL was detected by immunoblot analysis with anti-ZTL polyclonal antiserum 105. For determination of the in vivo degradation rate of ZTL in suspension cells, cycloheximide was added to 50 ml of entrained cells at time 0 to a final concentration of 20 μM. Proteins were extracted and subjected to immunoblot analysis.
Results
Characterization of an Arabidopsis Cell Suspension Culture.
To further investigate the plant circadian system at the molecular and biochemical level we characterized a green photomixotrophic Arabidopsis cell culture system. We first tested to confirm the expression of the phytochrome and cryptochrome photoreceptors, by which entrainment of the central oscillator occurs in Arabidopsis (20). At least two of the five Arabidopsis phytochromes and both cryptochromes are expressed appropriately (see Fig. 7, which is published as supporting information on the PNAS web site).
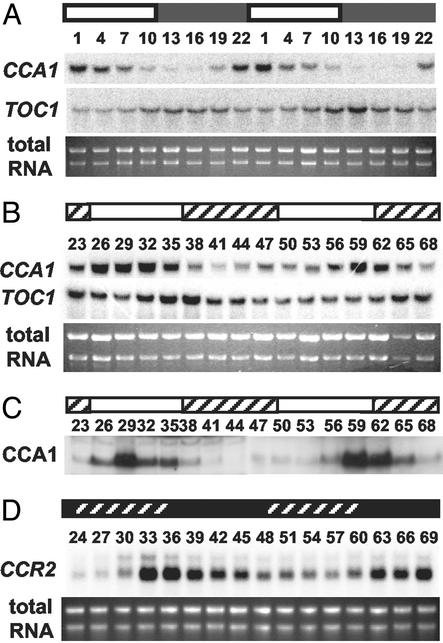
We next determined whether the suspension cell culture is light-entrainable. Fig. 1A shows the mRNA expression level of two clock-regulated genes (CCA1 and TOC1) that are likely components of the Arabidopsis circadian oscillator (21). CCA1 mRNA levels peaked early in the day and showed high-amplitude cycling similar to CCA1 expression patterns in Arabidopsis seedlings (22). Peak expression of TOC1 message levels occurred late in the day, nearly 12 h out of phase with CCA1 (Fig. 1A) and consistent with the reported expression pattern of TOC1 message in intact Arabidopsis (1, 2). These results show that the suspension culture cells can be entrained by light and that known clock-regulated genes are expressed with phases similar to that of intact plants.
Fig 1.
Rhythmic regulation of gene expression in Arabidopsis cell culture. Suspension cells entrained under a 12:12 LD cycle for 2 days were transferred and sampled under LD (A), LL (B and C), or DD (D) for the time indicated (h) as ZT (A) or hours in LL (B and C), or in DD (D). Northern blots (A, B, and D) were probed for CCA1 and TOC1 (A and B) or CCR2 expression (D). Immunoblots (C) were probed for CCA1 abundance by using anti-CCA1 antiserum (22). White and black bars indicate light and dark periods, respectively; crosshatches in B and C indicate subjective dark; crosshatch in D indicates subjective light. Total RNA was used as loading controls in A, B, and D.
We tested whether this entrained culture could sustain rhythmicity under constant conditions. After entrainment and release into constant light (LL) CCA1 message levels continued to oscillate robustly for up to 68 h after the last zeitgeber. TOC1 message was also rhythmic, with peak expression out of phase with CCA1 (Fig. 1B). CCA1 protein levels are also known to be rhythmic in seedlings (22) and a very robust oscillation was sustained in the cell suspension culture over the same LL time course (Fig. 1C). The phases of maximum CCA1 protein and message accumulation are identical (Fig. 1 B and C), as reported for Arabidopsis seedlings (22). Based on Fig. 1 B and C, we estimate the free-running period of this culture to be ≈30 h. This is significantly longer than the ≈24-h period reported for intact seedlings (22, 23). This may be caused by allele-specific differences in one or more oscillator components, or may be an inherent effect on circadian period of dispersed cell culture growth. We also tested the sustainability of circadian cycling in constant darkness (DD) by using CCR2, a clock-controlled evening-phased gene (1). CCR2 message continued to sustain a robust rhythmicity after 57 h in DD (Fig. 1D). Taken together these data support the presence of an intact phototransduction and circadian clock system in this Arabidopsis cell suspension culture.
Oscillation in ZTL Protein Abundance Is Posttranscriptionally Controlled in Light/Dark Cycles.
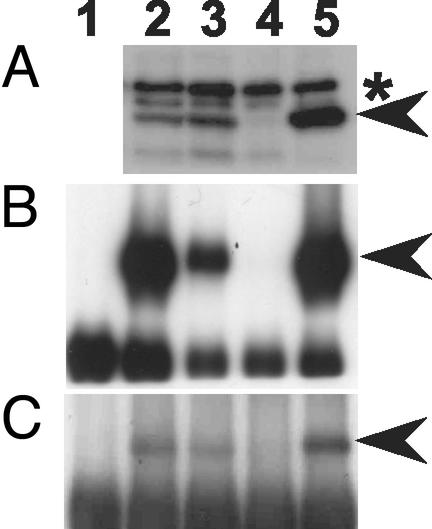
To test whether ZTL protein levels are under circadian or diurnal control, we developed two anti-ZTL antisera (105 and 93) that specifically discriminate ZTL protein from other family members (FKF1 and LKP2). ztl-3 (ado1) is a ZTL mRNA null line resulting from a T-DNA insertion in the C-terminal region (5). The predicted 66-kDa ZTL cross-reacting band present in the wild-type (WT) is absent from ztl-3 and more abundant in a ZTL overexpressing line (Fig. 2A). An identical band is present in cell suspension extracts, indicating the presence of ZTL in this culture. Neither this band nor the two slightly larger cross-reacting bands change in intensity in the fkf1 mutant, or in LKP2 overexpression lines (data not shown). These results were confirmed by using antiserum 105 to immunoprecipitate ZTL from protein extracts, followed by immunodetection with the same antiserum. A strongly reactive band was present at 66 kDa in all extracts except in ztl-3, and was also absent from immunoprecipitates obtained by using preimmune serum with cell culture extracts (Fig. 2B). Identical results were obtained from immunoprecipitates by using the anti-peptide antiserum 93, followed by immunodetection with antiserum 105 (Fig. 2C). These results establish the specificity of both antisera for ZTL protein and show the expression of ZTL in the suspension cell culture.
Fig 2.
Characterization of ZTL-specific antibodies. (A) Identification of ZTL in Arabidopsis seedlings and cell culture by using anti-ZTL polyclonal antiserum 105. Protein extracts from Arabidopsis cell culture (lane 2), WT (lane 3), ztl-3 (lane 4), and ZTL-overexpressing seedlings (lane 5) were separated by SDS-PAGE and immunoblotted. Asterisk indicates position of two nonspecific cross-reacting bands. (B) ZTL-specific immunoprecipitation with the anti-ZTL polyclonal antiserum 105. Extracts were immunoprecipitated with antiserum 105 (lanes 2–5) or preimmunue serum 105 (lane 1; cell culture), separated by SDS/PAGE and immunoblotted with antiserum 105. Lane identifications are as in A. (C) ZTL-specific immunoprecipitation with the anti-ZTL peptide antiserum 93. Extracts were immunoprecipitated with antiserum 93 (lanes 2–5) or preimmune serum 93 (lane 1) and probed with antiserum 105. Lane identifications are as in B. Arrowheads indicate ZTL position.
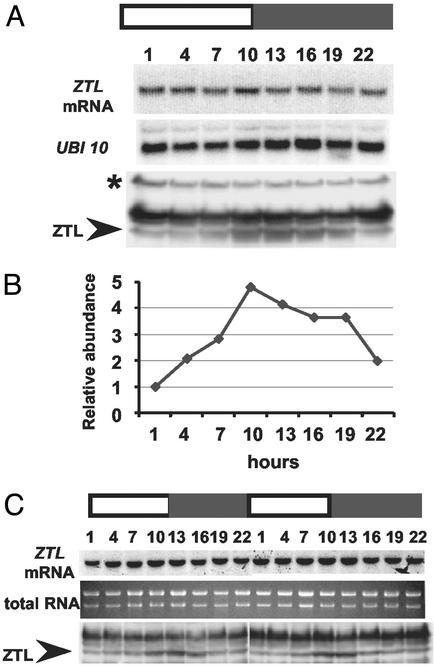
We next confirmed that ZTL message levels are unaffected by light/dark cycles in seedlings (6) and extended that observation to the Arabidopsis cell culture (Fig. 3 A and C). We used the 105 antiserum to determine the diurnal pattern of ZTL protein accumulation in seedlings. ZTL protein levels cycle with a 4-fold variation in amplitude, with peak expression near lights-off (ZT10–13) and lowest levels near dawn (ZT1) (Fig. 3 A and B). The timing and amplitude of ZTL expression in the suspension cell culture was similar to intact plants (Fig. 3C). In both tissues, the rise and fall of protein levels anticipates lights off and lights on, respectively, suggesting control by a circadian clock. Together with the constitutive expression of ZTL message, these results imply that the rhythmic variation in ZTL protein levels occurs posttranscriptionally. These data also strongly support the notion that the cell culture and whole plants maintain circadian systems by using the same components in a similar or identical manner.
Fig 3.
ZTL expression in LD cycles. Shown is RNA blot and immunoblot analysis of ZTL expression levels in seedlings (A and B) and cell culture (C) sampled at the indicated time (h) after last DL transition. ZTL protein levels (B) quantitated by using anti-ZTL polyclonal antiserum 105 and expressed relative to cross-reacting, constitutively expressed, nonspecific band (asterisk in A; not shown in C) used as an equal loading control; data are representative of three trials. White and black bars are as in Fig. 1. Arrowheads indicate ZTL position. Ubiquitin 10 (UBI 10) and total RNA were used as loading controls for A and C, respectively.
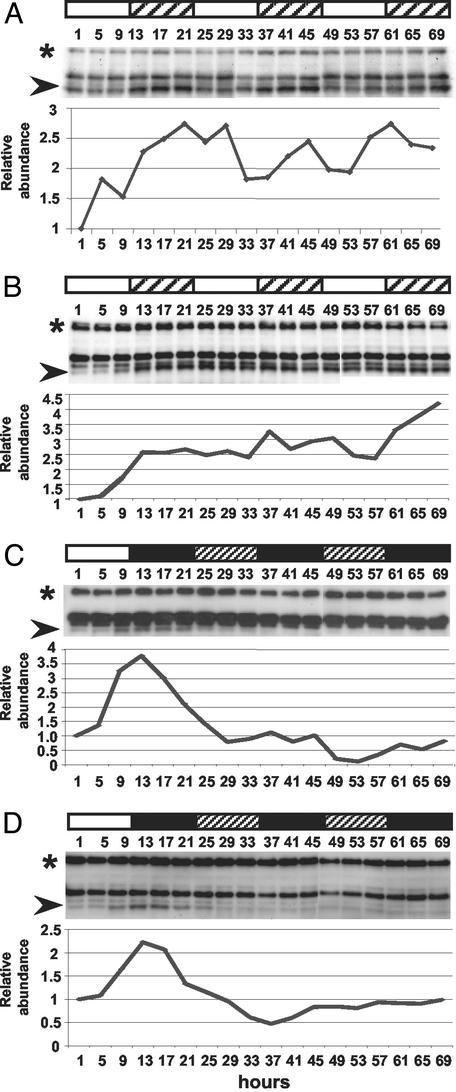
To determine whether this variation is clock-controlled whole plants and suspension cells were entrained in LD and allowed to free-run in LL. Fig. 4 A and B shows ZTL oscillations damp to high levels after extended time in LL in seedlings and cell culture, respectively. In both, the expression level at the expected trough during the first subjective photoperiod is markedly higher than during the first photoperiod (1–9 h) when ZTL expression is lowest. In contrast, ZTL damps to low levels in extended DD (Fig. 4 C and D). These results indicate that the cycling of ZTL protein levels cannot be sustained for long in the absence of LD, in contrast to the robust maintenance of a CCA1 protein rhythm under the same conditions (Fig. 1C).
Fig 4.
ZTL expression in constant conditions. Immunoblot analysis of ZTL levels in seedlings (A and C) and cell culture (B and D) sampled at the indicated time (h) after last DL transition by using anti-ZTL polyclonal antiserum 105. White and black bars indicate light and dark periods, respectively; crosshatch indicates subjective dark (A and B) and subjective light (C and D). Each trial was repeated at least once; quantitation of representative blots is shown. Arrowheads indicate ZTL position; asterisk indicates protein loading control.
ZTL Degradation Rate Is Circadian-Phase Dependent.
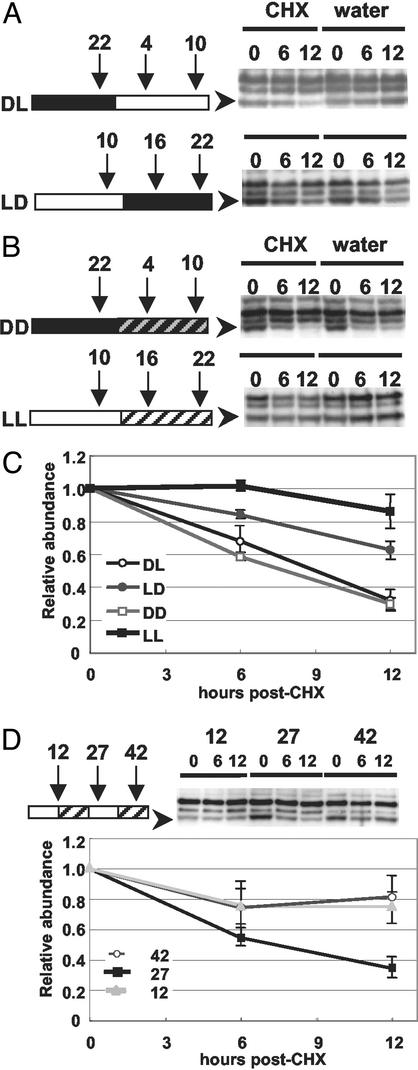
To better understand the regulation of ZTL protein abundance, we exploited the cell culture system to determine whether ZTL protein stability was a factor in the cycling ZTL protein levels. The cytoplasmic protein synthesis inhibitor, cycloheximide (CHX), was added to the entrained culture 3 h before the time of minimum ZTL accumulation [zeitgeber time (ZT) 22; time 0] and 3 h before the time of maximum ZTL accumulation (ZT 10; time 0). Control samples (water) were collected in parallel. Cultures were sampled 0, 6, and 12 h after CHX (or water) addition. ZTL protein depletion was consistently more rapid over the 12 h span of minimum protein accumulation (ZT22–10), than over the 12 h span of maximum ZTL accumulation (ZT10–22) (Fig. 5 A and C). For the former time period (ZT22–10), the estimated half-life of ZTL is 9 h and largely corresponds to the photoperiod (Fig. 5C). This contrasts the much longer 15 h half-life (extrapolated) during the dark period (ZT10–22) (Fig. 5C). To establish that this difference was not light dependent, we performed the same assay under DD (from ZT 22) and LL (from ZT10) (Fig. 5B). Very similar degradation rates were obtained for the respective phases in photoperiodic and constant conditions (Fig. 5C). These results show that phase-dependent protein degradation rates determine, in part, differences in ZTL abundance. The slightly greater stability of ZTL in LL implies that the protein is also stabilized by light. We further tested whether this apparent light-mediated stabilization affects the phase-dependent degradation rate of ZTL. Entrained cell culture was sampled after 12, 27, and 42 h in LL, which corresponds to the predicted peaks (12 and 42) and trough (27) of ZTL protein expression in extended free run, based on ZTL expression being out of phase with CCA1 (Fig. 1C) and a free-running period of 30 h. CHX was applied at these three time points and the ZTL degradation rates over the three 12-h time courses were determined. ZTL degradation rate at the predicted trough (hour 27) is significantly shorter (7–8 h half-life) than at the two predicted peaks (hours 12 and 42; half-life ≫ 12 h) (P < 0.02; Tukey's highly significant difference) (Fig. 5D). Taken together these results show that the ZTL degradation rate is circadian-phase dependent.
Fig 5.
Phase-specific in vivo degradation of ZTL. Immunoblot analysis of ZTL levels in cell culture treated with cyclohexamide (CHX) or water at time 0 (ZT22 or ZT10) and sampled 6 and 12 h later. (A) Effects of CHX addition at ZT22 (DL; above) and ZT10 (LD; below) in LD cycles. Diagrams show CHX and water addition and sampling times (ZT) relative to time in light (white bar) or dark (black bar) beside representative immunoblots (right) showing ZTL levels after time (h) in the presence of CHX (first three lanes) or water (next three lanes). Arrowheads show ZTL position. (B) Effects of DD (Top) and LL (Bottom) on ZTL accumulation. Panel features are as in A. Darkened diagonals, subjective day; lightened diagonals, subjective dark. (C) Quantitation of endogenous ZTL levels in cell culture after CHX addition. LD, DL, DD, and LL are as in A and B. Values are relative to time 0 (mean of three trials ± SEM). (D) Effects of CHX addition at predicted peaks (12 and 42 h) and trough (27 h) of ZTL accumulation in constant light after LD entrainment. Cartoon shows CHX addition times relative to time (h) in LL beside representative immunoblots (Right) showing ZTL levels after time (h) in the presence of CHX. Samples were collected as in A and B. Quantitation of ZTL (arrowhead) is relative to time 0 (mean of three trials ± SD). Protein loading balanced as in Fig. 4 (band not shown).
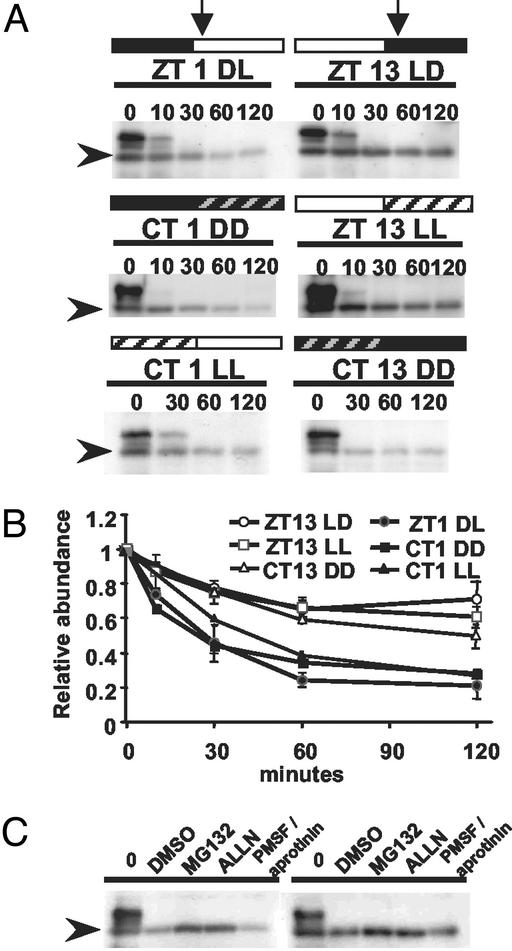
We next determined the in vitro degradation rate of ZTL in whole plant extracts taken at different circadian time points. This approach tested whether the potential for ZTL protein degradation is circadian phase dependent. At ZT1 (DL), ZTL protein level decreased 5-fold over a 2-h incubation period (Fig. 6 A and B). At ZT13 (LD), ZTL levels decreased <2-fold over the same time course (Fig. 6 A and B). Other polypeptides that cross-react with antiserum 105 were equally rapidly degraded at both samplings, indicating that the ZTL results are not caused by global differences in proteolytic capability at these two time points. When the dark period was extended from ZT24 into subjective light [circadian time (CT) 1 DD] the in vitro degradation rate remained unchanged (Fig. 6 A and B). Similarly, we observed that LD and LL (ZT13 LL) degradation rates were identical when the photoperiod was extended into subjective night (Fig. 6 A and B). We also tested the effect of a preceding light (CT1 LL) or dark (CT13 DD) period on the degradation rate of ZTL at CT1 and CT13, respectively. ZTL protein half-life was about three times shorter 1 h after subjective dawn (CT1 LL) than 1 h into subjective dark (CT13 DD) (Fig. 6 A and B). These results confirm in seedlings that ZTL protein degradation is circadian phase dependent. This difference is likely caused by phase-dependent modifications of ZTL, as other proteins that cross-react with the anti-ZTL antibody degraded equally rapidly at all time points.
Fig 6.
Phase-specific and proteasome pathway-dependent degradation of ZTL in vitro. Shown is immunoblot analysis of ZTL levels in seedlings after cell-free protein degradation assay. (A) Cell-free degradation of ZTL. Extracts were prepared from different circadian time points (vertical arrows), ZT 1 DL and ZT 13 LD in LD cycles, CT 1 and CT13 in DD and CT1 and ZT 13 in LL, and incubated in an in vitro degradation buffer for the indicated time (min). (B) Quantitation of ZTL level changes in cell-free protein degradation system. ZT and CT time points are as in A. Values are relative to time 0 and are means of two to three trials ± SEM. (C) Cell-free degradation of ZTL. Extracts from ZT1 (Left) and ZT13 (Right) in LD cycles were incubated for 2 h with 2% DMSO (solvent control), 40 μM MG132 or ALLN, or protease inhibitor mixture (2 mM PMSF and 10 μg/ml aprotinin). Arrowheads indicate ZTL position.
Some F-box proteins are degraded autocatalytically via an ubiquitin-dependent mechanism (24). Two proteasome-specific inhibitors were tested for their ability to retard ZTL degradation during in vitro incubation of extracts taken at ZT1 and ZT13. Both MG132 and ALLN were effective in stabilizing ZTL levels at both time points (Fig. 6C). MG132 was most effective, maintaining ZTL to levels near input levels. Other protease inhibitors diminished degradation but were not as effective as MG132 and ALLN under the same conditions. These results demonstrate that ZTL is degraded by the 26S proteasome.
Discussion
Circadian Cycling in an Arabidopsis Cell Suspension Culture.
We have identified and characterized an Arabidopsis suspension cell culture that can be entrained by light and can sustain circadian rhythms in LL and DD conditions. The appropriate phasing and robustness of CCA1 and TOC1 gene expression rhythms in LD shows that dispersed plant cells can be synchronized by light. Because the culture consists of individual cells and microcalli, our results indicate that physical connections between cells are not required for coordination of cyclic gene expression in plant cell populations. The sustained oscillations in constant conditions further support the notion that a fully functional circadian system is contained within each plant cell. This implies the presence of the photoreceptors and signal transduction system of the light input pathway, of all of the essential elements of the central oscillator, and of at least those components of the output pathway that regulate gene expression. Our results support a previous report of cell autonomous circadian activity in LL in intact plants (25), and extend this finding to cell culture in LL and DD (Fig. 2D). An alternate interpretation is that the cells condition the media and effect synchronization through chemical signaling. We consider this unlikely, as only two days of LD cycles are necessary to entrain the culture (data not shown).
The free-running period in the Arabidopsis cell culture (≈30 h) is significantly longer than previously reported in WT Arabidopsis plants under similar light conditions (23–25 h) (3, 20, 26, 27). This is likely not caused by the absence of PHYD or E, as loss of function mutations in either gene have no discernible effect on period in whole plants (28), and loss of PHYB (which reduces PHYC protein; ref. 29) has little effect on period in white light (D.E.S. and S. A. Kay, unpublished observations). Because plant cells passaged through tissue or cell culture can accrue genetic and epigenetic genomic modifications (somaclonal variation) (30), the longer period could be caused by one or more accumulated mutations in oscillator components. Nonetheless, this cell culture will be a useful new system for the study of the plant circadian clock that is complementary to the current molecular genetic approaches using whole plant Arabidopsis. Pharmacological approaches to probing clock mechanism can be revisited now that chemical manipulation in Arabidopsis culture can be corroborated in whole plants.
ZTL and the Gating of Light Input.
By using both cell culture and whole plants, we have shown that ZTL protein levels cycle in LD and that regulation is posttranscriptional. Peak accumulation occurs at ZT13 and minimum levels occur 12 h later (ZT1). The rise in levels before lights-off and the decline before lights-on suggests regulation by a circadian clock. This is confirmed in LL, yet in extended LL ZTL levels damp high. Conversely, ZTL protein levels peak during the skotoperiod in LD, but then fall and remain low in extended DD. These results indicate an additional influence of light on ZTL protein abundance. Well after ZTL protein has damped to high levels in LL and low levels in DD, robust circadian cycling persists in LL for CCA1 and in DD for CCR2, indicating that circadian regulation still operates. This finding suggests that the long period phenotype seen in extended LL in ztl mutants (5, 6) reflects an overall loss in functional ZTL protein levels, rather than in a deficiency at a particular circadian phase. Together these data suggest that there are fundamental differences between the operation of the circadian system under constant conditions and its regulation in LD.
These data also support the view that ZTL is not an essential component of the central oscillator, functioning instead as a parameter of the circadian system (6). ZTL may provide a link between the circadian phototransduction pathway and the control of circadian period. Phytochromes and cryptochromes are involved in the fluence-dependent control of period (20, 28), and both have been implicated as ZTL interaction partner (5). Hence, ZTL may lie at the conjunction between light input and core oscillator components. Although promoter activity and mRNA abundance of some PHY and CRY genes do cycle, evidence for cyclic variation in photoreceptor abundance is still lacking (31). Cyclic changes in ZTL protein levels could be, in part, the source of the gating of light input to the oscillator, as rhythmic changes in photoreceptor partners (e.g., ZTL) would effectively impart gating of light signaling on the central oscillator if the degradation target(s) of ZTL are oscillator components.
Phase-Dependent Degradation of ZTL as Novel Regulatory Mechanism.
Two different and independent approaches indicate that cyclic changes in ZTL protein levels are caused by circadian phase-specific differences in ZTL protein stability. First, CHX experiments showed that ZTL protein stability in vivo is 2-fold or more greater at times of maximum protein accumulation than at the times of minimum accumulation (Fig. 5). Second, an in vitro cell-free degradation assay using whole plant extracts showed more rapid degradation of ZTL at ZT1 than at ZT13. Taken together, these results imply a circadian phase-specific regulation of proteolysis. We have established further that this proteolysis occurs via the proteasome. Such proteasome-dependent degradation of F-box proteins has been shown previously for three yeast F-box proteins (Cdc4p, Grr1p, and Met30p) involved in cell cycle regulation (24, 32).
Proteasome-mediated proteolysis of ZTL could be clock-regulated at a number of levels. One would be a phase-specific ubiquitination resulting from circadian regulation of the ubiquitin-ligase complex, either in the abundance of one or more components, or in its activity. Recent microarray analyses of circadian-regulated gene expression in Arabidopsis show that an E2 ubiquitin conjugating enzyme and some RING-H2 E3 ligases cycle in constant conditions (33). Alternatively, the ubiquitination state of ZTL might vary over the circadian cycle, possibly caused by rhythmic variation in phosphorylation state. To date, all known F-box protein targets require phosphorylation for ubiquitination by the SCF complex (34). However, though all F-box proteins known to be ubquitinated are degraded autocatalytically via an SCF complex (24, 32, 35), there is no direct evidence that phosphorylation of F-box proteins is required for their proteasome-dependent degradation. Additionally, by using P32-orthophosphate feeding experiments, we found no evidence for phosphorylation of immunoprecipitated ZTL at either ZT1 or ZT13 (data not shown).
Recently, mPER2 has been shown to be ubiquitinated and degraded via the proteasome (36). In Drosophila, both timeless and period levels are degraded via a proteasome-dependent pathway (37, 38). Similarly, in Neurospora, FRQ protein phosphorylation status determines the FRQ protein degradation rate. These examples implicate proteasome-mediated control of clock-associated proteins in a number of circadian systems. ZTL is the first example of such in the plant circadian clock. Furthermore, as a component of an SCF complex (L. Han, M. Mason, and D.E.S., unpublished data), and as the ztl mutant phenotypes imply, ZTL is likely involved in the degradation of a period-controlling component of the circadian clock. This effect on circadian period will feedback to affect the phase of ZTL abundance. Hence, the phase-dependent degradation of ZTL itself now defines a novel regulatory mechanism by which the amplitude of the cycling of clock components may be controlled.
Supplementary Material
Acknowledgments
We thank E. Tobin and P. Quail for the CCA1 and phytochrome antibodies, respectively; J. Ecker and J. Alonso for prepublication access to ztl-3; and S. A. Kay for support early in this work. This work was supported by grants from the National Science Foundation (MCB-0080090) and U.S. Department of Agriculture/CREES (CRIS 2002-01374).
Abbreviations
LD, light-dark cycle
LL, constant light
DD, constant dark
ZT, zeitgeber time
CT, circadian time
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Strayer C., Oyama, T., Schultz, T. F., Raman, R., Somers, D. E., Mas, P., Panda, S., Kreps, J. A. & Kay, S. A. (2000) Science 289, 768-771. [DOI] [PubMed] [Google Scholar]
- 2.Matsushika A., Makino, S., Kojima, M. & Mizuno, T. (2000) Plant Cell Physiol. 41, 1002-1012. [DOI] [PubMed] [Google Scholar]
- 3.Somers D. E., Webb, A. A. R., Pearson, M. & Kay, S. (1998) Development (Cambridge, U.K.) 125, 485-494. [DOI] [PubMed] [Google Scholar]
- 4.Panda S., Poirier, G. G. & Kay, S. A. (2002) Dev. Cell. 3, 51-61. [DOI] [PubMed] [Google Scholar]
- 5.Jarillo J. A., Capel, J., Tang, R. H., Yang, H. Q., Alonso, J. M., Ecker, J. R. & Cashmore, A. R. (2001) Nature 410, 487-490. [DOI] [PubMed] [Google Scholar]
- 6.Somers D. E., Schultz, T. F., Milnamow, M. & Kay, S. A. (2000) Cell 101, 319-329. [DOI] [PubMed] [Google Scholar]
- 7.Deshaies R. J. (1999) Annu. Rev. Cell. Dev. Biol. 15, 435-467. [DOI] [PubMed] [Google Scholar]
- 8.Kiyosue T. & Wada, M. (2000) Plant J. 23, 807-815. [DOI] [PubMed] [Google Scholar]
- 9.Nelson D. C., Lasswell, J., Rogg, L. E., Cohen, M. A. & Bartel, B. (2000) Cell 101, 331-340. [DOI] [PubMed] [Google Scholar]
- 10.Schultz T. F., Kiyosue, T., Yanovsky, M., Wada, M. & Kay, S. A. (2001) Plant Cell 13, 2659-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briggs W. R. & Christie, J. M. (2002) Trends Plant Sci. 7, 204-210. [DOI] [PubMed] [Google Scholar]
- 12.Crosson S. & Moffat, K. (2001) Proc. Natl. Acad. Sci. USA 98, 2995-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie J. M., Salomon, M., Nozue, K., Wada, M. & Briggs, W. R. (1999) Proc. Natl. Acad. Sci. USA 96, 8779-8783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Froehlich A. C., Liu, Y., Loros, J. J. & Dunlap, J. C. (2002) Science 297, 815-819. [DOI] [PubMed] [Google Scholar]
- 15.Cheng P., Yang, Y., Gardner, K. H. & Liu, Y. (2002) Mol. Cell. Biol. 22, 517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams J., Kelso, R. & Cooley, L. (2000) Trends Cell Biol. 10, 17-24. [DOI] [PubMed] [Google Scholar]
- 17.Lee C., Etchegaray, J. P., Cagampang, F. R., Loudon, A. S. & Reppert, S. M. (2001) Cell 107, 855-867. [DOI] [PubMed] [Google Scholar]
- 18.Young M. W. & Kay, S. A. (2001) Nat. Rev. Genet. 2, 702-715. [DOI] [PubMed] [Google Scholar]
- 19.Osterlund M. T., Hardtke, C. S., Wei, N. & Deng, X. W. (2000) Nature 405, 462-466. [DOI] [PubMed] [Google Scholar]
- 20.Somers D. E., Devlin, P. F. & Kay, S. A. (1998) Science 282, 1488-1490. [DOI] [PubMed] [Google Scholar]
- 21.Alabadi D., Oyama, T., Yanovsky, M. J., Harmon, F. G., Mas, P. & Kay, S. A. (2001) Science 293, 880-883. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z. Y. & Tobin, E. M. (1998) Cell 93, 1207-1217. [DOI] [PubMed] [Google Scholar]
- 23.Mizoguchi T., Wheatley, K., Hanzawa, Y., Wright, L., Mizoguchi, M., Song, H. R., Carre, I. A. & Coupland, G. (2002) Dev. Cell 2, 629-641. [DOI] [PubMed] [Google Scholar]
- 24.Galan J. M. & Peter, M. (1999) Proc. Natl. Acad. Sci. USA 96, 9124-9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thain S. C., Hall, A. & Millar, A. J. (2000) Curr. Biol. 10, 951-956. [DOI] [PubMed] [Google Scholar]
- 26.Millar A. J., Carré, I. A., Strayer, C. A., Chua, N.-H. & Kay, S. A. (1995) Science 267, 1161-1163. [DOI] [PubMed] [Google Scholar]
- 27.Swarup K., Alonso-Blanco, C., Lynn, J. R., Michaels, S. D., Amasino, R. M., Koornneef, M. & Millar, A. J. (1999) Plant J. 20, 67-77. [DOI] [PubMed] [Google Scholar]
- 28.Devlin P. F. & Kay, S. A. (2000) Plant Cell 12, 2499-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirschfeld M., Tepperman, J. M., Clack, T., Quail, P. H. & Sharrock, R. A. (1998) Genetics 149, 523-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaeppler S. M., Kaeppler, H. F. & Rhee, Y. (2000) Plant Mol. Biol. 43, 179-188. [DOI] [PubMed] [Google Scholar]
- 31.Toth R., Kevei, E., Hall, A., Millar, A. J., Nagy, F. & Kozma-Bognar, L. (2001) Plant Physiol. 127, 1607-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou P. & Howley, P. M. (1998) Mol. Cell 2, 571-580. [DOI] [PubMed] [Google Scholar]
- 33.Harmer S. L., Hogenesch, J. B., Straume, M., Chang, H. S., Han, B., Zhu, T., Wang, X., Kreps, J. A. & Kay, S. A. (2000) Science 290, 2110-2113. [DOI] [PubMed] [Google Scholar]
- 34.Craig K. L. & Tyers, M. (1999) Prog. Biophys. Mol. Biol. 72, 299-328. [DOI] [PubMed] [Google Scholar]
- 35.Wirbelauer C., Sutterluty, H., Blondel, M., Gstaiger, M., Peter, M., Reymond, F. & Krek, W. (2000) EMBO J. 19, 5362-5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagita K., Tamanini, F., Yasuda, M., Hoeijmakers, J. H., van der Horst, G. T. & Okamura, H. (2002) EMBO J. 21, 1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naidoo N., Song, W., Hunter-Ensor, M. & Sehgal, A. (1999) Science 285, 1737-1741. [DOI] [PubMed] [Google Scholar]
- 38.Grima G., Rouyer, F., Chelot, E., Papain, C. & Limbourg-Bouchon, B. (2002) Nature 420, 178-182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.