Abstract
Local persistence of plant species in the face of climate change is largely mediated by genetic adaptation and phenotypic plasticity. In species with a wide altitudinal range, population responses to global warming are likely to differ at contrasting elevations. In controlled climate chambers, we investigated the responses of low and high elevation populations (1200 and 1800 m a.s.l.) of three nutrient-poor grassland species, Trifolium montanum, Ranunculus bulbosus, and Briza media, to ambient and elevated temperature. We measured growth-related, reproductive and phenological traits, evaluated differences in trait plasticity and examined whether trait values or plasticities were positively related to approximate fitness and thus under selection. Elevated temperature induced plastic responses in several growth-related traits of all three species. Although flowering phenology was advanced in T. montanum and R. bulbosus, number of flowers and reproductive allocation were not increased under elevated temperature. Plasticity differed between low and high elevation populations only in leaf traits of T. montanum and B. media. Some growth-related and phenological traits were under selection. Moreover, plasticities were not correlated with approximate fitness indicating selectively neutral plastic responses to elevated temperature. The observed plasticity in growth-related and phenological traits, albeit variable among species, suggests that plasticity is an important mechanism in mediating plant responses to elevated temperature. However, the capacity of species to respond to climate change through phenotypic plasticity is limited suggesting that the species additionally need evolutionary adaptation to adjust to climate change. The observed selection on several growth-related and phenological traits indicates that the study species have the potential for future evolution in the context of a warming climate.
Introduction
Global annual mean temperature has risen by approximately 0.85°C over the past century and climate scenarios project a further warming of 2 to 4°C for the 21st century [1]. This rapid temperature increase and the concomitant alterations of other environmental factors, such as higher levels of atmospheric CO2 and changing precipitation regimes, will drastically alter living conditions for plant and animal species across the globe and affect individuals, populations and communities [2]–[5]. Temperature is one of the main factors determining plant physiology and performance, and as a consequence, strongly influences the geographic distribution of plants [6], [7]. Therefore, climate warming has caused latitudinal and altitudinal range shifts of many plant species worldwide and further shifts are expected [2], [3]. Along elevation gradients, a considerable increase in number of plant species on alpine summits indicates an upward shift of species’ ranges [8]–[12]. However, the migration potential might be inferior to the rapid rate of current climate change, which is expected to render many species unable to track the climate they are currently adapted to [13], [14]. Continuing habitat loss and fragmentation further exacerbate migration by impeding gene flow [15], [16] and threaten the persistence of many species [17], [18].
Alternatively to shifts in abundance and distribution, plants may persist in a changing climate through evolutionary adaptation and phenotypic plasticity [19]–[22]. Several studies provide evidence of climate driven population differentiation [23]–[26]. Thus, temperature and climate warming might be strong selective agents leading to the adaptive evolution of key plant traits [27], [28]. In contrast to evolutionary adaptation, which requires several generations, current phenotypic plasticity in plants, the ability of a genotype to change its phenotype in response to different environments [29], allows short-term responses to rapid warming [21], [30], [31]. Among the best documented plastic responses to climate change are shifts in phenology observed in many species across the globe [2], [3], [32]. Although these meta-analyses are mainly based on genetically unstructured samples, which could not disentangle genetic and plastic changes, studies in phenological garden networks on structured samples show similar results [33], [34]. Besides plastic shifts in phenology, several studies have documented morphological and physiological trait plasticity to temperature changes, reviewed in [35], and specifically plastic responses to elevated temperature, such as decreased seed dormancy [36], [37] or reduced seed longevity [38].
Adaptive plasticity is the ability of an individual genotype to express phenotypes, which enhance fitness in response to variation in selection [21], [31], [39]. This is likely facilitating species persistence under climate change and assisting rapid evolutionary adaptation [40]–[44]. While numerous studies reported adaptive plasticity to different environmental conditions, such as shade [45], drought [46], [47] and flooding [48], empirical evidence for adaptive plastic responses to climate change is still scarce, but see [49], [50]. The capacity for plastic responses is supposed to vary due to different physiological thresholds, such as temperature and precipitation tolerance. Consequently, trait plasticity generally differs among species, and might even vary among populations from contrasting habitats across a species’ range [50], [51]. Higher trait plasticity would be an advantage for plants experiencing greater spatial and temporal habitat heterogeneity because it allows them to maximize their fitness under different environmental conditions [39], [52], [53].
Mountain ecosystems are characterised by steep climate gradients and by increased environmental variation over short spatial and temporal scales at high as compared to nearby low elevation sites [54]. Because of the greater habitat heterogeneity we hypothesize that high elevation plants exhibit greater trait plasticity than their low elevation congeners. Varying climate conditions along altitudinal gradients might also result in different selection pressures that shape intraspecific trait adaptation to elevation. Little is known about how the adaptive potential of plants and their ability to respond to climate change through phenotypic plasticity shape phenotypic variation along altitudinal gradients. Transplant experiments in the Pyrenees and the Australian Alps revealed genetic differentiation but also trait plasticity [55], [56]. In the Swiss Alps Frei et al. [57] described plasticity in reproductive phenology as an important plant response to transplantation to warmer low elevation sites. However, since these experiments were performed along natural elevation gradients, they were not able to separate the effects of temperature and warming from the influence of concomitant climate factors.
In this study we grew plants in controlled climate chambers and investigated responses of low (1200 m a.s.l.) and high (1800 m a.s.l.) elevation populations of three montane grassland species, Trifolium montanum L., Ranunculus bulbosus L. and Briza media L. to experimental warming. Plants were grown under elevated temperature, corresponding to predicted future conditions under climate change, and in control treatments corresponding to current ambient conditions at the plant origin whereas all other environmental parameters were kept identical. We measured growth-related, reproductive and phenological traits of the experimental plants and assessed differences in trait plasticity of low and high elevation populations. Using phenotypic selection analyses [58], we examined whether trait values and plasticities are under selection. Specifically, we addressed the following questions: (i) How are plant traits and fitness affected by elevated temperature? (ii) Does the degree of trait plasticity vary between low and high elevation populations? (iii) Are traits under selection? Does the strength or direction of selection differ between ambient and elevated temperature conditions? (iv) Does variation in temperature impose selection on trait plasticity? Studying current levels of genetic differentiation and variation in trait plasticity in response to elevated temperature will lead to a better understanding of future plant responses to predicted climate change.
Materials and Methods
Study Species
The common perennial species Trifolium montanum L., Ranunculus bulbosus L. and Briza media L. were selected for this study based on their co-occurrence in semi-dry nutrient-poor calcareous grasslands and their similar altitudinal range from 400 to 2000 m a.s.l [59]. The three species differ in phenology, pollination syndromes and reproduction: Trifolium montanum is an insect-pollinated late-flowering herb, R. bulbosus an insect-pollinated early-flowering herb and B. media is a wind-pollinated grass.
The leguminous Trifolium montanum, which forms a symbiosis with soil rhizobia, produces 1–5 flowering shoots from June to July. A shoot bears 1–6 inflorescences with c. 150 white flowers each, which are pollinated by bumblebees and bees. Seeds are small (c. 0.7 mg), but dispersal distance is very limited as they are primarily gravity-dispersed [60].
Ranunculus bulbosus produces a corm that serves as nutrient storage and as summer perennating organ [61]. In late winter, R. bulbosus begins to mobilize corm reserves to add new leaves to the overwintering rosette and starts to form a new corm. From late April to July, the plant produces one or more flower stalks of c. 30 cm height bearing 8–15 yellow flowers [62]. The seeds lack dispersal aids and are therefore expected to be mainly gravity dispersed. After a period of aestivation, a new rosette is formed in early autumn [63], [64].
Briza media reproduces clonally and sexually with each ramet potentially forming one flower stalk [65]. The flowers emerge from June to July, have large feathery stigmas, and mature into indehiscent fruits that disperse next to the mother plant mainly by gravity [65]. Long-distance seed dispersal by grazing animals has also been observed [66].
Plant Material
In summer 2008, seeds were collected in seven to thirteen population pairs per species across the Swiss Alps (Table S1). For seed sampling, permits were obtained from the managers of the respective sites. Each population pair consisted of one population in the centre of the species range at c. 1200 m a.s.l. (1095–1275 m a.s.l.) and another close to the upper range limit at c. 1800 m a.s.l. (1720–1860 m a.s.l.), hereafter referred to as low and high elevation populations respectively. The vertical distance of 600 m between these elevations approximates a difference in annual mean temperature of 4°C [54] corresponding to the expected temperature rise of a typical climate change scenario for the year 2100 [1]. The horizontal distance between the two populations of a pair was 1–18 kilometres. Seeds were sampled from at least ten maternal plants in each population, air-dried and stored in paper bags at 4°C.
In spring 2009, seeds were germinated in a greenhouse. After four to six weeks, seedlings were planted into individual pots (800 cm3), filled with a 3∶2 mixture of nutrient-poor commercial soil and sand. Pot positions were randomized weekly. From October 2009 to April 2010, the plants were overwintered outdoors at Davos (1500 m a.s.l.) to support vernalisation processes.
Climate Chamber Experiment
In May 2010, a controlled climate chamber experiment was established to test the responses of plants to current ambient temperature, corresponding to the temperature at their altitude of origin, and to elevated temperature, corresponding to 4°C higher temperature as compared to current temperature at their altitude of origin. The lowest temperature treatment (hereafter ‘Tmin’) was ambient temperature for plants of high elevation origin (1800 m a.s.l.). Elevated temperature for high elevation plants was equivalent to ambient temperature for plants of low elevation origin (1200 m a.s.l.), and was designated as ‘T’. Elevated temperature for low elevation plants was denoted as ‘Tplus’ and corresponded to ambient temperature at 600 m a.s.l. Each temperature treatment was replicated in two climate chambers. To separate temperature from other climate effects, only temperature was varied in this experiment while humidity and illumination treatments were kept the same in all chambers. Temperature, humidity and light regime followed both a daily as well as a seasonal cycle. To simulate the daily temperature cycle with lower temperature, higher humidity and dark conditions at night and opposed conditions during the day, seven step cycles were programmed according to climate station measurements [67]. For example in July, temperatures, relative humidity and light were varied diurnally between 5°C/80%/0 klx as the night’s minimum and 18°C/48%/21 klx as the daily maximum (Table 1). To account for seasonal changes of the climate regime, program cycles were adapted monthly.
Table 1. Daily cyles of the climate chamber program for the three temperature treatments Tmin, T and Tplus reflecting July outdoor temperatures at 1800 m, 1200 m and 600 m a.s.l.
| Interval | Running time (h) | Temperature (°C) | Relative humidity (%) | Illumination (klx) | ||
| Tmin | T | Tplus | ||||
| 1 | 2 | 8 | 12 | 16 | 72 | 0 |
| 2 | 3 | 6 | 10 | 14 | 81 | 0 |
| 3 | 5 | 5 | 9 | 13 | 84 | 0 |
| 4 | 6 | 18 | 22 | 26 | 55 | 21 |
| 5 | 2 | 19 | 23 | 27 | 51 | 21 |
| 6 | 2 | 18 | 22 | 26 | 51 | 16 |
| 7 | 4 | 10 | 14 | 18 | 63 | 1 |
Parameters were changed gradually to reach the set value at the end of the running time of each interval. Relative humidity and light regime were kept the same in all temperature treatments.
The plants of each species and population were assigned equally to ambient and elevated temperature treatments and to both climate chambers per treatment. They were rotated weekly within each chamber and monthly between the two chambers of the same treatment. The experiment included 174 plants from ten population pairs of T. montanum, 88 plants from seven population pairs of R. bulbosus and 312 plants from 13 population pairs of B. media.
Assessment of Plant Traits
In May 2010 and at the end of the growing season (October 2010), number of leaves (respectively ramets for B. media) and length of the longest leaf of each plant were measured. To obtain a measure for growth rate, the increment of number of leaves (respectively ramets for B. media) was calculated. In September 2010, one mature leaf per individual plant was sampled to assess specific leaf area (SLA). Immediately after sampling, the leaf blades were scanned (HP AllInOne colour Scanner, Hewlett-Packard GmbH, Dübendorf, Schweiz) and their areas were determined with lamina version 1.0.2 [68]. The scanned leaf blades were oven-dried at 60°C for 48 h and weighed. To determine SLA, we divided the leaf area by the dry weight of the leaf blade [69].
From May to July 2010, 75% of T. montanum and 84% of R. bulbosus individuals flowered. Due to the low flowering rate in B. media (8%), reproductive and phenological traits were only assessed for the two herbs. The phenological development was monitored twice a week to record the Julian Day (JD) of appearance of the first flower bud and the first open flower of each individual. In August, flowers of R. bulbosus and inflorescences of T. montanum were counted and the reproductive biomass (i.e. flower stalks and flowers) was harvested.
In October 2010, all plants were harvested and oven-dried. Above-ground (i.e. reproductive organs and leaves) and below-ground biomass of T. montanum and R. bulbosus were measured separately. Subsequently, biomass partitioning (i.e. above-ground biomass as a proportion of total biomass) and reproductive allocation (i.e. the proportion of reproductive biomass on total above-ground biomass) were calculated. In B. media, only above-ground biomass was assessed because we were not able to remove all soil sticking to the fine root system without considerable loss of root biomass.
Statistical Analyses
We first tested for effects of elevated temperature and genetic differentiation on phenotypic trait variation. Then, we conducted two sets of selection gradient analyses where we tested for selection on plant traits as well as for selection on trait plasticities in response to elevated temperature. All statistical analyses were performed with the statistical software R [70]. The residuals were checked for deviations from the model assumptions and the data was transformed if necessary [71]. To account for multiple tests we applied a Bonferroni correction within species whenever applicable.
We performed nested mixed-model analyses of covariance (Ancova) for each species separately to analyse the effects of elevated temperature and altitude of origin. The model consisted of the two fixed factors ‘altitude of origin’ and ‘temperature treatment’, the random factor ‘population’ and the two interaction terms ‘temperature treatment × altitude of origin’ and ‘temperature treatment × population’. A significant ‘altitude of origin’ effect (GAlt) indicates genetic differentiation between low and high elevation populations. A significant ‘temperature treatment’ effect (ETemp) indicates trait variation due to different environmental conditions i.e. phenotypic plasticity in a measured trait. A significant ‘temperature treatment × altitude of origin’ interaction (GAlt×ETemp) indicates differences in plasticity between the altitudes of origin. The ‘altitude of origin’ was tested on the population level and the ‘temperature treatment’ on the interaction of ‘temperature treatment × population’ while all other terms were tested on the residuals. Besides direct responses to the environment, our measurements of plasticity might also be influenced by ontogenetic drift since the analyses did not account for plant size [72]. By including initial plant size (length of the longest leaf) as a covariate in the models we account for possible maternal effects [73].
We conducted phenotypic selection analyses on trait values in each temperature treatment to assess whether traits were under selection [58], [74]. Selection gradients were calculated based on regressions of relative fitness, measured as total biomass (T. montanum and R. bulbosus) and above ground biomass (B. media), on standardized trait values of individual plants. A trait was under positive selection if trait values were positively correlated with genotype fitness. To test whether selection differed between treatments, we calculated Ancovas of relative fitness in which we included the trait as a covariate and its interaction with the temperature treatment [48].
Phenotypic selection analyses on trait plasticities were performed to test whether trait plasticity itself was under selection, i.e. if plasticity was positively correlated with overall fitness, indicating adaptive plasticity. Selection gradients on trait plasticity were determined by regressing average fitness of a population on population trait values averaged over both treatments (i.e. elevation of the reaction norm) and on population values of plasticity (i.e. steepness of the reaction norm) [75], [76]. The elevation of the reaction norm was included to disentangle the fitness effect of the average value from the fitness effect of plasticity [77]. As proxies for fitness, we used number of inflorescences (T. montanum and R. bulbosus), total biomass (T. montanum and R. bulbosus) and above ground biomass (B. media). For both sets of selection gradient analyses, regression coefficients were standardized by expressing them in units of 1 SD to allow comparisons between traits and fitness measures.
Results
Environmental and Genetic Effects on Plant Variation
The model analyses allowed us to explain trait variation due to environmental effects of elevated temperature (ETemp) and genetic differentiation (GAlt), as well as their interactions (GAlt×ETemp). The random term population nested within altitude of origin had essentially no influence on trait variation (P>0.165) except for a difference in the proportion of above-ground biomass in T. montanum and B. media (P<0.001), as well as a tendency for different budding starts in R. bulbosus (P = 0.056; Tables 2 and 3). The covariate had a significant influence on the majority of traits (Tables 2 and 3). However, omitting the covariate did not change the results qualitatively (details not shown).
Table 2. Nested mixed-model Ancova for growth-related traits of Trifolium montanum, Ranunculus bulbosus and Briza media grown under ambient and elevated temperature.
| Biomass | Leaf length | Growth rate | SLA | Above-ground/total biomass | ||||||||
| d.f. | MS | F | MS | F | MS | F | MS | F | MS | F | ||
| T. montanum | ||||||||||||
| Initial plant size | 1 | 19.31 | 76.26*** | 167.18 | 16.77*** | 4.74 | 14.36** | 105822.00 | 2.99 | 1.23 | 16.52*** | |
| Temp | 1 | 7.07 | 27.91** | 108.63 | 10.89** | 4.16 | 12.59* | 157334.00 | 4.45 | 0.73 | 9.83(*) | |
| Orig | 1 | 15.24 | 60.18*** | 198.74 | 19.93** | 0.07 | 0.22 | 76416.00 | 2.16 | 0.49 | 6.57 | |
| Pop[Orig] | 18 | 0.38 | 1.52 | 11.47 | 1.15 | 0.50 | 1.53 | 47855.00 | 1.35 | 0.28 | 3.75*** | |
| Temp × Orig | 1 | 0.49 | 1.95 | 243.42 | 24.41*** | 0.33 | 0.99 | 38816.00 | 1.10 | 0.06 | 0.84 | |
| Temp × Pop | 18 | 0.32 | 1.25 | 7.21 | 0.72 | 0.35 | 1.06 | 43784.00 | 1.24 | 0.08 | 1.04 | |
| Residuals | 128 | 0.25 | 9.97 | 0.33 | 35383.00 | 0.07 | ||||||
| R. bulbosus | ||||||||||||
| Initial plant size | 1 | 15.99 | 22.61*** | 0.26 | 0.21 | 394.70 | 12.96** | 6230.20 | 5.16 | 0.00 | 0.13 | |
| Temp | 1 | 1.06 | 1.50 | 10.56 | 8.49(*) | 378.12 | 12.41* | 2107.50 | 1.75 | 0.00 | 0.11 | |
| Orig | 1 | 0.05 | 0.07 | 26.08 | 20.96** | 504.64 | 16.56 | 30617.20 | 25.38*** | 0.03 | 0.77 | |
| Pop[Orig] | 12 | 0.88 | 1.24 | 1.18 | 0.95 | 66.72 | 2.19 | 863.10 | 0.72 | 0.04 | 1.31 | |
| Temp × Orig | 1 | 0.49 | 0.70 | 0.22 | 0.17 | 18.98 | 0.62 | 10155.70 | 8.42(*) | 0.01 | 0.28 | |
| Temp × Pop | 12 | 0.88 | 1.25 | 0.94 | 0.76 | 21.58 | 0.71 | 1267.10 | 1.05 | 0.02 | 0.46 | |
| Residuals | 47–59 | 0.71 | 1.24 | 30.47 | 1206.40 | 0.03 | ||||||
| B. media | ||||||||||||
| Initial plant size | 1 | 4.33 | 86.02*** | 54.40 | 14.45*** | 9.19 | 0.95 | 0.22 | 5.30(*) | |||
| Temp | 1 | 1.74 | 34.68*** | 14.84 | 3.94* | 132.64 | 13.71* | 0.04 | 0.87 | |||
| Orig | 1 | 1.29 | 25.66* | 0.05 | 0.01 | 24.62 | 2.54 | 0.09 | 2.14 | |||
| Pop[Orig] | 24 | 0.17 | 3.36*** | 5.56 | 1.48 | 15.46 | 1.60 | 0.04 | 0.89 | |||
| Temp × Orig | 1 | 0.11 | 2.13 | 333.25 | 88.50*** | 1.62 | 0.17 | 1.31 | 31.99*** | |||
| Temp × Pop | 24 | 0.03 | 0.67 | 1.91 | 0.51 | 12.45 | 1.29 | 0.04 | 0.99 | |||
| Residuals | 259 | 0.05 | 3.77 | 9.68 | 0.04 | |||||||
d.f., degrees of freedom; MS, mean squares; F, F-values; Initial plant size, covariate; Temp, temperature treatment; Orig, altitude of population origin; Pop, population. Residual d.f. vary per trait due to missingness. Bonferroni-corrected significance levels within species are indicated by asterisks: ***P Bonf <0.001, **P Bonf <0.01, *P Bonf <0.05, (*)P Bonf<0.08.
Table 3. Nested mixed-model Ancova for reproductive and phenological traits of Trifolium montanum and Ranunculus bulbosus grown under ambient and elevated temperature.
| Number of flowers | Reproductive allocation | Budding start | Flowering start | ||||||
| d.f. | MS | F | MS | F | MS | F | MS | F | |
| T. montanum | |||||||||
| Initial plant size | 1 | 64.76 | 47.07*** | 1.96 | 42.38*** | 24721.90 | 30.61*** | 17.43 | 36.37*** |
| Temp | 1 | 0.25 | 0.18 | 0.01 | 0.17 | 5641.00 | 6.98** | 11.24 | 23.46*** |
| Orig | 1 | 6.33 | 4.60 | 0.12 | 2.55 | 7049.00 | 8.73* | 10.79 | 22.51** |
| Pop[Orig] | 18 | 2.49 | 3 | 0.09 | 1.84 | 680.80 | 0.84 | 0.58 | 1.22 |
| Temp × Orig | 1 | 8.53 | 6.20 | 0.02 | 0.50 | 0.10 | 0.00 | 1.00 | 2.09 |
| Temp × Pop | 17–18 | 0.96 | 0.70 | 0.03 | 0.71 | 340.00 | 0.42 | 0.23 | 0.47 |
| Residuals | 77–128 | 1.38 | 0.05 | 807.80 | 0.48 | ||||
| R. bulbosus | |||||||||
| Initial plant size | 1 | 18.19 | 22.86*** | 0.12 | 8.82* | 3312.60 | 23.61*** | 3925.90 | 26.58*** |
| Temp | 1 | 3.08 | 3.87 | 0.02 | 1.73 | 1188.00 | 8.47*** | 2238.00 | 15.15*** |
| Orig | 1 | 1.88 | 2.36 | 0.14 | 10.12 | 627.10 | 4.47 | 2197.50 | 14.88(*) |
| Pop[Orig] | 12 | 0.97 | 1.23 | 0.02 | 1.27 | 391.80 | 2.79(*) | 219.70 | 1.49 |
| Temp × Orig | 1 | 0.69 | 0.86 | 0.01 | 0.43 | 16.90 | 0.12 | 3.30 | 0.02 |
| Temp × Pop | 12 | 0.77 | 0.97 | 0.02 | 1.67 | 30.30 | 0.22 | 29.00 | 0.20 |
| Residuals | 45–59 | 0.80 | 0.01 | 140.30 | 147.70 | ||||
d.f., degrees of freedom; MS, mean squares; F, F-values; Initial plant size, covariate; Temp, temperature treatment; Orig, altitude of population origin; Pop, population. Residual d.f. vary per trait due to missingness. Bonferroni-corrected significance levels within species are indicated by asterisks: ***P Bonf <0.001, **P Bonf <0.01, *P Bonf <0.05, (*)P Bonf<0.08.
Plant responses to elevated temperature
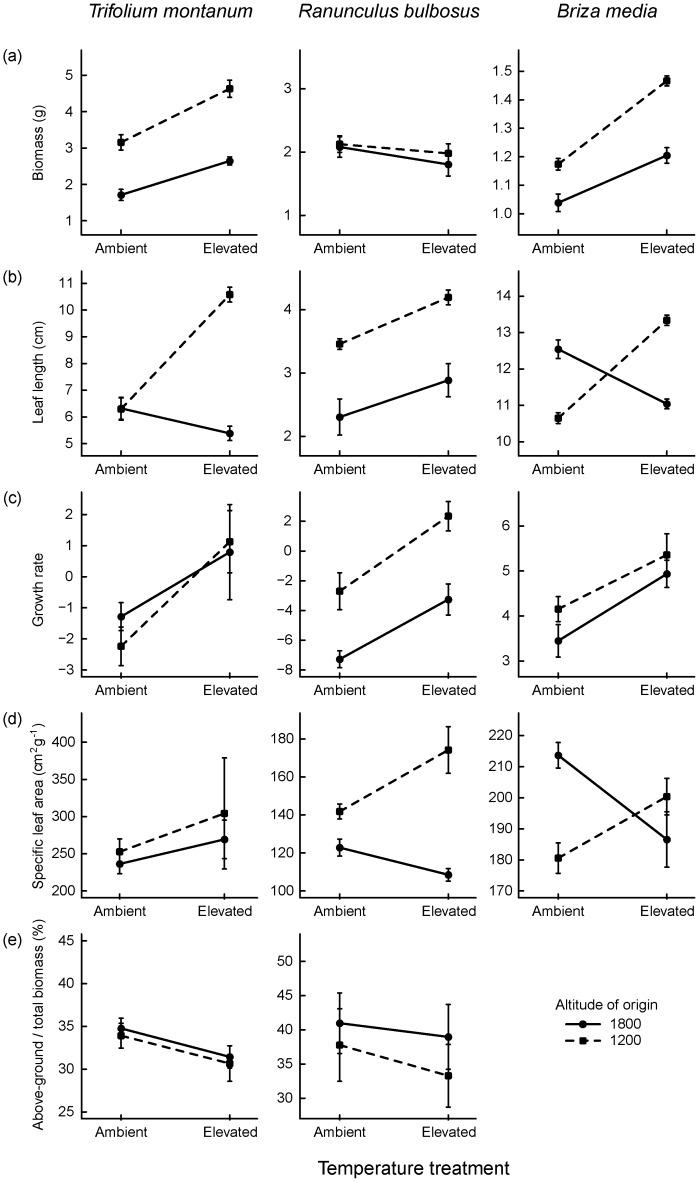
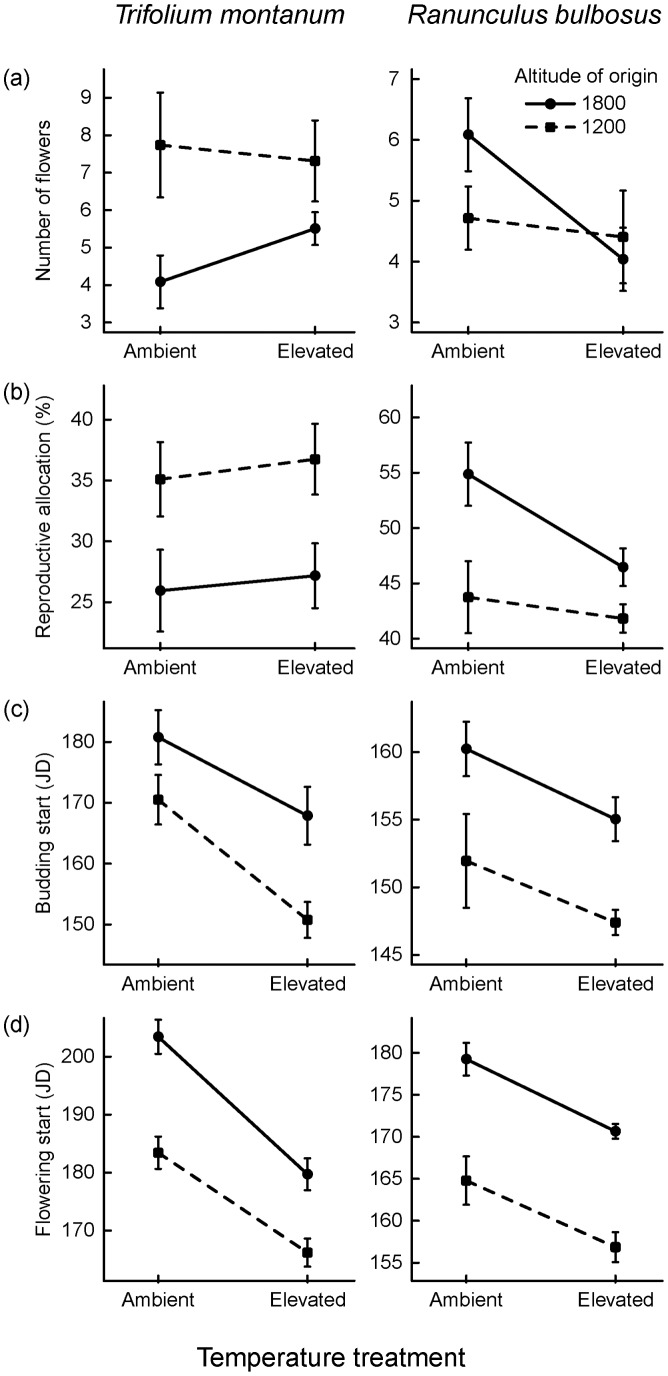
Trifolium montanum responded to elevated temperature with an increase in total biomass (ETemp: P = 0.002; Tables 2 and S2; Fig. 1) and growth rate (ETemp: P = 0.026), but a slightly reduced proportion of above-ground biomass (ETemp: P = 0.059). SLA was unaffected by temperature (ETemp: P = 0.668). Flower bud initiation and anthesis were advanced under elevated temperature (ETemp: P<0.007; Tables 3 and S3; Fig. 2) whereas number of flowers and reproductive allocation showed no temperature-induced plasticity (ETemp: P>0.9).
Figure 1. Population average reaction norms for growth-related traits of Trifolium montanum, Ranunculus bulbosus and Briza media in response to ambient and elevated temperature in a climate chamber experiment.
(a) total biomass (above-ground biomass for Briza media), (b) leaf length, (c) growth rate, (d) specific leaf area and (e) proportion of above ground biomass. Solid lines indicate high elevation plants (1800 m a.s.l.) and dashed lines low elevation plants (1200 m a.s.l.). Error bars indicate standard errors of trait means.
Figure 2. Population average reaction norms for reproductive and phenological traits of Trifolium montanum and Ranunculus bulbosus in response to ambient and elevated temperature in a climate chamber experiment.
(a) number of flowers, (b) reproductive allocation, (c) budding start and (d) flowering start. Solid lines indicate high elevation plants (1800 m a.s.l.) and dashed lines low elevation plants (1200 m a.s.l.). Error bars indicate standard errors of trait means.
In R. bulbosus, growth rate increased (ETemp: P = 0.011; Table 2; Fig. 1) and leaf length tended to increase (ETemp: P = 0.053) under elevated temperature whereas total biomass, SLA and proportion of above ground biomass were not affected by temperature (ETemp: P>0.9). Flowering phenology was advanced under elevated temperature with buds and flowers appearing earlier (ETemp: P<0.001; Table 3; Fig. 2) whereas number of flowers and reproductive allocation were unaffected by temperature (ETemp: P>0.139).
In B. media, leaf length, above-ground biomass and growth rate increased under elevated temperature (ETemp: P<0.041; Table 2; Fig. 1) and SLA was unaffected by temperature (ETemp: P>0.9).
Genetic differentiation in traits and plasticity
In T. montanum, plants of low elevation origin produced almost twice as much biomass (GAlt: P<0.001; Table 2, Fig. 1) and longer leaves (GAlt: P = 0.005) than high elevation plants whereas growth rate, SLA and the proportion of above-ground biomass did not differ between altitudes of origin (P>0.9). Budding and flowering started earlier in low as compared to high elevation plants (GAlt: P<0.043; Table 3, Fig. 2). Number of flowers and reproductive allocation did not differ between low and high elevation plants (GAlt: P>0.9). Furthermore, plasticity differed between altitudes of origin only in leaf length, where trait means almost doubled in low elevation plants but remained nearly unchanged in high elevation plants under elevated temperature (GAlt×ETemp: P<0.001; Table 2, Fig. 1). Plasticity of total biomass, growth rate, SLA and proportion of above-ground biomass as well as reproductive and phenological traits did not differ between low and high elevation populations (GAlt×ETemp: P>0.1; Tables 2 and 3; Figs. 1 and 2).
In R. bulbosus, low elevation plants produced longer leaves (GAlt: P = 0.005; Table 2; Fig. 1) and larger SLA (GAlt: P<0.001) than high elevation plants whereas total biomass, growth rates and the proportion of above-ground biomass did not differ between low and high elevation plants (GAlt: P>0.158). Low elevation plants tended to flower earlier than high elevation plants (GAlt: P = 0.074; Table 3; Fig. 2). Number of flowers, reproductive allocation and budding dates did not differ between low and high elevation plants (GAlt: P>0.139). Furthermore, there were no significant differences in plasticity between low and high elevation populations neither in growth-related traits (GAlt×ETemp: P>0.07; Table 2; Fig. 1) nor in reproductive and phenological traits (GAlt×ETemp: P>0.9; Table 3; Fig. 2).
In B. media, low elevation plants produced more above ground biomass than high elevation plants (GAlt: P = 0.043; Table 2; Fig. 1). Leaf length, growth rate and SLA did not differ between low and high elevation plants (GAlt: P>0.534). Furthermore, plasticity of leaf traits differed between altitudes of origin: leaf length and SLA increased in low elevation but decreased in high elevation plants under elevated temperature (GAlt×ETemp: P<0.001; Table 2; Fig. 1). Plasticity of above ground biomass and growth rate did not differ between altitudes of origin (GAlt×ETemp: P>0.584).
Selection Gradient Analyses on Traits and Plasticities
The selection gradient analyses on trait values in T. montanum revealed negative selection gradients for flower bud initiation and the appearance of the first flower averaged over both temperature treatments, which was reflected in a significant effect of these traits on fitness measured as total biomass (P<0.008; Table 4). In R. bulbosus, the selection analyses indicated a negative effect of SLA on fitness measured as total biomass (P = 0.003; Table 4). In B. media, there was selection for higher growth rates averaged over both treatments (P<0.001). Selection gradient analyses on trait plasticities revealed no direct selection for plasticity in response to temperature in all traits and species (P>0.148; Table 5).
Table 4. Selection gradients of growth-related and phenological traits of Trifolium montanum, Ranunculus bulbosus and Briza media under ambient and elevated temperature and F-values of ANCOVA testing for overall selection (Cov) and differences in selection between temperature treatments (Cov × Temp).
| Selection gradients | F-Values of ANCOVA | |||
| ambient | elevated | Cov | Cov × Temp | |
| T. montanum | ||||
| Growth rate | −0.320 | 0.009 | 0.15 | 4.57 |
| SLA | −0.332 | 0.009 | 0.85 | 0.17 |
| Budding start | −0.543 | −0.170 | 10.07** | 0.80 |
| Flowering start | −0.804 | −0.297 | 19.58*** | 1.40 |
| R. bulbosus | ||||
| Growth rate | −0.279 | −0.071 | 4.88 | 1.35 |
| SLA | −0.365 | −0.309 | 12.68** | 0.70 |
| Budding start | −0.119 | −0.145 | 0.94 | 0.04 |
| Flowering start | −0.236 | −0.309 | 4.50 | 0.23 |
| B. media | ||||
| Growth rate | 0.044 | 0.079 | 20.70*** | 0.02 |
| SLA | −0.040 | 0.025 | 0.38 | 3.38 |
Selection gradients are expressed as standardized regression coefficients after regression of the fitness measures total biomass (T. montanum, R. bulbosus) and above ground biomass (B. media) on the respective trait. All calculations are based on individual plant values. Bonferroni-corrected significance levels are indicated by asterisks: ***P<0.001, **P<0.01, *P<0.05.
Table 5. Selection gradients of trait plasticities in response to temperature in Trifolium montanum, Ranunculus bulbosus and Briza media.
| T. montanum | R. bulbosus | B. media | |||
| Total biomass | Number of flowers | Total biomass | Number of flowers | Above ground biomass | |
| Growth rate | 0.500 | 0.455 | 0.064 | 0.258 | 0.366 |
| SLA | 0.423 | 0.541 | −1.380 | −0.418 | −0.577 |
| Above-ground/total biomass | −0.497 | −0.189 | −1.334 | −0.450 | – |
| Budding start | 0.289 | 0.480 | −0.171 | 0.315 | – |
| Flowering start | 0.307 | 0.380 | 0.039 | 1.156 | – |
Selection gradients are expressed as standardized regression coefficients. All values are based on population means. Fitness measures are total biomass and number of flowers (T. montanum, R. bulbosus) and above ground biomass (B. media). Non of the selection gradients was significant.
Discussion
In the present study, we evaluated the effects of elevated temperature on phenotypic variation of the three perennial grassland species T. montanum, R. bulbosus and B. media. We investigated if trait plasticity varies between low and high elevation populations and if trait values and plasticities were under selection.
Plastic Responses to Elevated Temperature
Positive warming effects on several growth-related traits in all three species (Table 2) indicate that plant growth is constrained by low temperature conditions, which is common in temperate species [6]. In arctic and temperate zones plant species spend the majority of their life at mean temperatures below the growth optimum [78]. Thus, a moderate warming could enhance plant performance. Meta-analyses of in-situ warming experiments with arctic and alpine tundra species revealed biome-wide trends of increased vegetative growth, albeit variable among species [79], [80]. The variable responses to elevated temperature of the three study species might be associated with differences in characteristics of below-ground organs. Besides the positive responses in the other growth-related traits, the nitrogen-fixing T. montanum showed a slight decrease in the proportion of above ground biomass. The observed greater investment in roots under elevated temperature is contrary to the general trend that allocation to root biomass increases with decreasing temperature at higher elevation [54]. However, it is in line with the findings of Roughley and Dart [81] who described a positive effect of soil temperature on the formation of rhizobia in Trifolium subterraneum. The increased number of rhizobia enhances nitrogen availability, which stimulates root growth. Fabaceae, such as T. montanum, might therefore gain a competitive advantage over non-nitrogen-fixing species under warmer climate conditions. Competition experiments with elevated CO2, which has a similar positive effect on nitrogen fixation as elevated temperature, provide evidence for such a competitive advantage, reviewed in [82]. The less pronounced warming effect on growth-related traits in R. bulbosus could be related to the species-specific life-form. The nutrient-storing corm might allow a temperature independent formation of new above ground tissue after the period of summer aestivation and buffer against short-term climatic fluctuations as shown for R. nivalis [83].
Preformation of flower buds might lead to a time lag in the reproductive response to warming potentially explaining why the experimental warming did not affect reproductive traits of T. montanum and R. bulbosus (Table 3). This phenomenon is relatively common among temperate herbaceous perennials [84] and has been documented for tundra species in in-situ warming experiments [79]. Thus, it might have required at least two growing seasons to provide a strong estimate of the effects of increased temperature on reproduction of the perennial study species. Although these species did not respond plastically to elevated temperature, they are likely to be affected by changes in other environmental factors (e.g. reduced precipitation and higher evapotranspiration) co-occurring in natural systems. Alternatively, the lack of reproductive responses could indicate that reproductive traits are genetically fixed suggesting that these species need to adjust to climate change through the relatively slow process of evolutionary adaptation [20], [85]. In the context of rapid climate change, this process might be too slow to secure plant persistence [86]–[88]. Evidence for evolutionary adaptation to climate change is still scarce, and rapid evolution has been documented mainly in fast-growing annuals [27], [89] but also in perennials [90].
Reproductive phenology of T. montanum and R. bulbosus was advanced under elevated temperature (Table 3) confirming the findings of other studies that these traits respond plastically to temperature, e.g. [2], [5], [79], [91]–[95]. Moreover, our findings are in line with the advanced reproductive phenology of the same species grown in a common garden transplant experiment [57]. The advanced spring phenology facilitates setting flowers and seeds before the hot and dry midsummer period. It might therefore enhance plant fitness, but also bears the risk of damage by late frost events, especially in high elevation populations [54], [96], [97]. Furthermore, plant-pollinator-interaction models indicate that pollinating insects might not keep up with shifted flowering periods [98], which might impede insect pollination and thereby reduce reproductive success [99], [100].
In summary, low and high elevation populations exhibited plasticity to temperature in reproductive phenology and several growth-related traits. The advanced flowering and the generally enhanced growth, albeit variable among species, indicate the ability of these species to cope with climate warming at least in the short term.
Genetic Variation in Traits and Differences in Plasticity between Low and High Elevation Populations
We found only little genetic variation among populations within each altitudinal origin (Tables 2 and 3) suggesting small selection differences and high genetic connectivity. Indeed, a previous study on similar populations of the same three species using neutral molecular markers revealed intermediate genetic diversity but only low genetic differentiation among populations and assigned this to extensive historic gene flow [101]. The genetic differentiation between low and high elevation populations in some growth-related and phenological traits (Tables 2 and 3) indicated that selection in the past has acted differently on low and high elevation plants. Alternatively, the observed genetic differences might be due to neutral genetic processes [24], [102]. However, maternal effects were accounted for by including initial plant size as a covariate in the models and genetic drift is unlikely to have acted in the same direction on all sampled populations since seeds were sampled from multiple population pairs distributed over the Swiss Alps.
In contrast to the hypothesis of greater plasticity at more heterogeneous high elevation sites, plasticity of leaf length was reduced in high elevation populations of T. montanum (Table 2; Fig. 1). Similarly, a study with seedlings of European deciduous tree species found that high elevation provenances exhibited less temperature-induced plasticity in growth and leaf phenological traits [103], [104]. The authors argued that low plasticity at high elevations was a result of different directional selection for reduced temperature sensitivity and a stronger influence of photoperiodism, which may reduce the risk of damage by unpredictable late spring frost events [105]. Moreover, the greater plasticity of leaf length in low elevation plants might have been induced by higher levels of competition at these sites [106]. In B. media, plastic responses of leaf length and SLA to elevated temperature were positive in plants from warmer low elevations, but negative in plants from colder high elevations (Table 2; Fig. 1). In low elevation plants, elevated temperature induced longer leaves and greater SLA. Thus, the warming seems to have triggered a greater investment in vegetative growth. High elevation plants invested more in clonal propagation as can be concluded from their shorter and thicker leaves as indicated by the smaller leaf length and SLA in combination with an increase in number of ramets (details not shown). In-situ warming experiments showed similar response patterns, reviewed in [79]. The stronger growth response in low arctic plants was related to higher competition in these communities [107] whereas the greater reproductive response of high arctic plants was attributed to increased colonization efforts in these habitats where competition is less important [108].
In summary, plasticity differed between altitudes of origin in only a few traits and there was little genetic differentiation among populations within each altitudinal origin suggesting uniform responses to climate warming. Moreover, the high connectivity together with the moderate genetic differentiation between altitudes of origin might facilitate future adaptation to climate change.
Selection on Traits and their Plasticities
We found indication for selection on several growth-related and phenological traits (Table 4). In T. montanum, the two phenological traits budding and flowering start were under negative selection with respect to biomass: plants with earlier appearing flower buds and flowers ended up with higher biomass at the end of the growing season. Although we did not record leaf phenology, it is likely that the advanced flowering phenology was related to a similar advance in other spring phenophases [32] allowing these plants more time to accumulate biomass. In B. media, selection for higher growth rates can be explained by the fact that faster growing individuals acquired more above ground biomass [7].
Although plasticity to temperature was not adaptive in our experiment (Table 5), increased plant size under elevated temperature might eventually lead to higher fitness since flower bud preformation is common in perennials and sexual reproduction is often positively correlated with plant size [109]. Increased growth under warmer temperature might thus be beneficial for plant persistence in the longer term whereas the duration of our experiment might have been too short for observing fitness benefits of plasticity. The magnitude and even the direction of selection on a trait may differ for different components of fitness [74]. This might also have affected the results of our selection analyses and shows the need for more comprehensive fitness measures [110]. Furthermore, it is generally recommended to analyse phenotypic selection on trait means and their plasticities based on genotypic values [111] to avoid environmentally induced covariation [112]. However, our experimental design only allowed us to analyse selection based on individual values or population means, see [113]. This might have resulted in a loss of discriminative power because the observed variation is a combination of phenotypic plasticity and within population genetic variability. Thus, future selection studies at the genotype level are recommended to gain deeper insights into the mechanisms of plasticity in plant populations.
In summary, the absence of selection on phenotypic plasticity indicated that the observed trait plasticity was selectively neutral. Selection on several growth-related and phenological traits suggested that there is potential for future evolution of mean trait values allowing the study species to adapt to elevated temperature provided there is sufficient genetic variation and heritability of the respective traits.
Conclusions
We investigated phenotypic variation in low and high elevation populations of nutrient-poor grassland species in response to elevated temperature. The three species exhibited trait plasticity with respect to temperature whereas genetic differentiation and differences in plasticity between low and high populations were less important. Plasticity in growth and flowering phenology determines the ability of the study species to respond to elevated temperature by buffering against detrimental effects of rapid climate change and allowing time for evolutionary adaptation. However, the capacity of species to respond to environmental changes through phenotypic plasticity is limited and plasticity alone might not be sufficient to cope with climate change. Thus, plants additionally need to adjust through the relatively slow process of evolutionary adaptation. Selection on several traits suggests that the three species have the potential for evolutionary changes, which might allow them to adapt to a future climate.
Supporting Information
Sampling locations in the Swiss Alps of the source population pairs of Trifolium montanum (Tm), Ranunculus bulbosus (Rb) and Briza media (Bm).
(DOCX)
Means and standard errors (SE) grouped by altitude of population origin and temperature treatment for growth-related traits of Trifolium montanum , Ranunculus bulbosus and Briza media .
(DOCX)
Means and standard errors (SE) grouped by altitude of population origin and temperature treatment for reproductive and phenological traits of Trifolium montanum and Ranunculus bulbosus .
(DOCX)
Acknowledgments
The authors thank M. Beck, M. Frei, S. Frischknecht, T. Hahn, M. Heggli, D. Kurth, J. Leuenberger, M. Leuzinger, M. Macsai and P. Matter who helped collecting seeds, cultivating and harvesting plants. They are grateful to F. Grassein, M. Kalisch, M. van Kleunen and I. Till-Bottraud for statistical advice as well as to E.S. Frei, M. Heggli and C.J. Kettle for valuable comments on the manuscript. The authors thank the editor and two anonymous reviewers for helpful comments.
Funding Statement
This study was funded by the Swiss National Science Foundation No. 3100A0-116277 (www.snf.ch) to J.G. and A.R.P., the Competence Center Environment and Sustainability (CCES) BioChange (http://www.cces.ethz.ch/projects/clench/BioChange) to J.G., and the Basler Stiftung für biologische Forschung to E.R.F. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.IPCC (2013) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK et al., editors. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press. 1535 p.
- 2. Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421: 37–42. [DOI] [PubMed] [Google Scholar]
- 3. Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, et al. (2003) Fingerprints of global warming on wild animals and plants. Nature 421: 57–60. [DOI] [PubMed] [Google Scholar]
- 4. Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, et al. (2006) Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences of the United States of America 103: 1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walther GR, Post E, Convey P, Menzel A, Parmesan C, et al. (2002) Ecological responses to recent climate change. Nature 416: 389–395. [DOI] [PubMed] [Google Scholar]
- 6.Hopkins WG, Hüner NPA (2008) Introduction to plant physiology. Hoboken, New Jersey, United States: John Wiley & Sons.
- 7.Lambers H, Chapin FS III, Pons TL (2008) Plant physiological ecology. New York, United States: Springer.
- 8. Walther GR, Beissner S, Burga CA (2005) Trends in the upward shift of alpine plants. Journal of Vegetation Science 16: 541–548. [Google Scholar]
- 9. Frei E, Bodin J, Walther GR (2010) Plant species’ range shifts in mountainous areas – all uphill from here? Botanica Helvetica 120: 117–128. [Google Scholar]
- 10. Gottfried M, Pauli H, Futschik A, Akhalkatsi M, Barancok P, et al. (2012) Continent-wide response of mountain vegetation to climate change. Nature Climate Change 2: 111–115. [Google Scholar]
- 11. Engler R, Randin CF, Thuiller W, Dullinger S, Zimmermann NE, et al. (2011) 21st century climate change threatens mountain flora unequally across Europe. Global Change Biology 17: 2330–2341. [Google Scholar]
- 12. Lenoir J, Gegout JC, Marquet PA, de Ruffray P, Brisse H (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science 320: 1768–1771. [DOI] [PubMed] [Google Scholar]
- 13. Huntley B (1991) How plants respond to climate change: migration rates, individualism and the consequences for plant communities. Annals of Botany 67: 15–22. [Google Scholar]
- 14. Davis MB, Shaw RG (2001) Range shifts and adaptive responses to quaternary climate change. Science 292: 673–679. [DOI] [PubMed] [Google Scholar]
- 15. Collingham YC, Huntley B (2000) Impacts of habitat fragmentation and patch size upon migration rates. Ecological Applications 10: 131–144. [Google Scholar]
- 16. Honnay O, Verheyen K, Butaye J, Jacquemyn H, Bossuyt B, et al. (2002) Possible effects of habitat fragmentation and climate change on the range of forest plant species. Ecology Letters 5: 525–530. [Google Scholar]
- 17. Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, et al. (2004) Extinction risk from climate change. Nature 427: 145–148. [DOI] [PubMed] [Google Scholar]
- 18. Lesica P, McCune B (2004) Decline of arctic-alpine plants at the southern margin of their range following a decade of climatic warming. Journal of Vegetation Science 15: 679–690. [Google Scholar]
- 19. Hoffmann AA, Sgro CM (2011) Climate change and evolutionary adaptation. Nature 470: 479–485. [DOI] [PubMed] [Google Scholar]
- 20. Jump AS, Penuelas J (2005) Running to stand still: adaptation and the response of plants to rapid climate change. Ecology Letters 8: 1010–1020. [DOI] [PubMed] [Google Scholar]
- 21. Nicotra AB, Atkin OK, Bonser SP, Davidson AM, Finnegan EJ, et al. (2010) Plant phenotypic plasticity in a changing climate. Trends in Plant Science 15: 684–692. [DOI] [PubMed] [Google Scholar]
- 22. Gienapp P, Teplitsky C, Alho JS, Mills JA, Merila J (2008) Climate change and evolution: disentangling environmental and genetic responses. Molecular Ecology 17: 167–178. [DOI] [PubMed] [Google Scholar]
- 23. Joshi J, Schmid B, Caldeira M, Dimitrakopoulos P, Good J, et al. (2001) Local adaptation enhances performance of common plant species. Ecology Letters 4: 536–544. [Google Scholar]
- 24. Linhart YB, Grant MC (1996) Evolutionary significance of local genetic differentiation in plants. Annual Review of Ecology and Systematics 27: 237–277. [Google Scholar]
- 25. Macel M, Lawson CS, Mortimer SR, Smilauerova M, Bischoff A, et al. (2007) Climate vs. soil factors in local adaptation of two common plant species. Ecology 88: 424–433. [DOI] [PubMed] [Google Scholar]
- 26. Becker U, Colling G, Dostal P, Jakobsson A, Matthies D (2006) Local adaptation in the monocarpic perennial Carlina vulgaris at different spatial scales across Europe. Oecologia 150: 506–518. [DOI] [PubMed] [Google Scholar]
- 27. Franks SJ, Sim S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences of the United States of America 104: 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Davis MB, Shaw RG, Etterson JR (2005) Evolutionary responses to changing climate. Ecology 86: 1704–1714. [Google Scholar]
- 29. Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13: 115–155. [Google Scholar]
- 30. Valladares F, Sanchez-Gomez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. Journal of Ecology 94: 1103–1116. [Google Scholar]
- 31. Ghalambor CK, McKay JK, Carroll SP, Reznick DN (2007) Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Functional Ecology 21: 394–407. [Google Scholar]
- 32. Menzel A, Sparks TH, Estrella N, Koch E, Aasa A, et al. (2006) European phenological response to climate change matches the warming pattern. Global Change Biology 12: 1969–1976. [Google Scholar]
- 33. van Vliet AJH, de Groot RS, Bellens Y, Braun P, Bruegger R, et al. (2003) The European Phenology Network. International Journal of Biometeorology 47: 202–212. [DOI] [PubMed] [Google Scholar]
- 34. Wolfe DW, Schwartz MD, Lakso AN, Otsuki Y, Pool RM, et al. (2005) Climate change and shifts in spring phenology of three horticultural woody perennials in northeastern USA. International Journal of Biometeorology 49: 303–309. [DOI] [PubMed] [Google Scholar]
- 35. Atkin OK, Loveys BR, Atkinson LJ, Pons TL (2006) Phenotypic plasticity and growth temperature: understanding interspecific variability. Journal of Experimental Botany 57: 267–281. [DOI] [PubMed] [Google Scholar]
- 36. Steadman KJ, Ellery AJ, Chapman R, Moore A, Turner NC (2004) Maturation temperature and rainfall influence seed dormancy characteristics of annual ryegrass (Lolium rigidum). Australian Journal of Agricultural Research 55: 1047–1057. [Google Scholar]
- 37. Qaderi MM, Cavers PB, Hamill AS, Downs MP, Bernards MA (2006) Maturation temperature regulates germinability and chemical constituents of Scotch thistle (Onopordum acanthium) cypselas. Canadian Journal of Botany-Revue Canadienne De Botanique 84: 28–38. [Google Scholar]
- 38. Kochanek J, Buckley YM, Probert RJ, Adkins SW, Steadman KJ (2010) Pre-zygotic parental environment modulates seed longevity. Austral Ecology 35: 837–848. [Google Scholar]
- 39. van Kleunen M, Fischer M (2005) Constraints on the evolution of adaptive phenotypic plasticity in plants. Research review. New Phytologist 166: 49–60. [DOI] [PubMed] [Google Scholar]
- 40. Lande R (2009) Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. Journal of Evolutionary Biology 22: 1435–1446. [DOI] [PubMed] [Google Scholar]
- 41. Chevin LM, Lande R, Mace GM (2010) Adaptation, plasticity, and extinction in a changing environment: towards a predictive theory. PLOS Biology 8: e1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Draghi JA, Whitlock MC (2012) Phenotypic plasticity facilitates mutational variance, genetic variance, and evolvability along the major axis of environmental variation. Evolution 66: 2891–2902. [DOI] [PubMed] [Google Scholar]
- 43. Price TD, Qvarnström A, Irwin DE (2003) The role of phenotypic plasticity in driving genetic evolution. Proceedings of the Royal Society of London Series B: Biological Sciences 270: 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matesanz S, Gianoli E, Valladares F (2010) Global change and the evolution of phenotypic plasticity in plants. Annals of the New York Academy of Sciences 1206: 35–55. [DOI] [PubMed] [Google Scholar]
- 45. Dudley SA, Schmitt J (1996) Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis . American Naturalist 147: 445–465. [Google Scholar]
- 46. Gianoli E, Gonzalez-Teuber M (2005) Environmental heterogeneity and population differentiation in plasticity to drought in Convolvulus chilensis (Convolvulaceae). Evolutionary Ecology 19: 603–613. [Google Scholar]
- 47. Heschel MS, Sultan SE, Glover S, Sloan D (2004) Population differentiation and plastic responses to drought stress in the generalist annual Polygonum persicaria . International Journal of Plant Sciences 165: 817–824. [Google Scholar]
- 48. van Kleunen M, Lenssen JPM, Fischer M, de Kroon H (2007) Selection on phenotypic plasticity of morphological traits in response to flooding and competition in the clonal shore plant Ranunculus reptans . Journal of Evolutionary Biology 20: 2126–2137. [DOI] [PubMed] [Google Scholar]
- 49. Springate DA, Kover PX (2014) Plant responses to elevated temperatures: a field study on phenological sensitivity and fitness responses to simulated climate warming. Global Change Biology 20: 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pratt JD, Mooney KA (2013) Clinal adaptation and adaptive plasticity in Artemisia californica: implications for the response of a foundation species to predicted climate change. Global Change Biology 19: 2454–2466. [DOI] [PubMed] [Google Scholar]
- 51. Etterson JR (2004) Evolutionary potential of Chamaecrista fasciculata in relation to climate change. 1. Clinal patterns of selection along an environmental gradient in the great plains. Evolution 58: 1446–1458. [DOI] [PubMed] [Google Scholar]
- 52. Via S, Lande R (1985) Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39: 505–522. [DOI] [PubMed] [Google Scholar]
- 53. Sultan SE, Spencer HG (2002) Metapopulation structure favors plasticity over local adaptation. American Naturalist 160: 271–283. [DOI] [PubMed] [Google Scholar]
- 54.Körner C (2003) Alpine plant life: functional plant ecology of high mountain ecosystems. Berlin, Germany: Springer.
- 55. Gonzalo-Turpin H, Hazard L (2009) Local adaptation occurs along altitudinal gradient despite the existence of gene flow in the alpine plant species Festuca eskia . Journal of Ecology 97: 742–751. [Google Scholar]
- 56. Byars SG, Papst W, Hoffmann AA (2007) Local adaptation and cogradient selection in the alpine plant, Poa hiemata, along a narrow altitudinal gradient. Evolution 61: 2925–2941. [DOI] [PubMed] [Google Scholar]
- 57. Frei ER, Ghazoul J, Matter P, Heggli M, Pluess AR (2014) Plant population differentiation and climate change: responses of grassland species along an elevational gradient. Global Change Biology 20: 441–455. [DOI] [PubMed] [Google Scholar]
- 58. Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37: 1210–1226. [DOI] [PubMed] [Google Scholar]
- 59.Aeschimann D, Lauber K, Moser DM, Theurillat JP (2004) Flora Alpina. Berne, Switzerland: Haupt.
- 60. Schleuning M, Niggemann M, Becker U, Matthies D (2009) Negative effects of habitat degradation and fragmentation on the declining grassland plant Trifolium montanum . Basic and Applied Ecology 10: 61–69. [Google Scholar]
- 61. Barling DM (1955) Some population studies in Ranunculus bulbosus L. Journal of Ecology. 43: 207–218. [Google Scholar]
- 62. Steinbach K, Gottsberger G (1994) Phenology and pollination biology of 5 Ranunculus species in Giessen, Central Germany. Phyton 34: 203–218. [Google Scholar]
- 63. Coles SM (1973) Ranunculus bulbosus L. in Europe. Watsonia 9: 207–228. [Google Scholar]
- 64. Sarukhan J, Harper JL (1973) Studies on plant demography: Ranunculus repens L., R. bulbosus L. and R. acris L.: I. population flux and survivorship. Journal of Ecology 61: 675–716. [Google Scholar]
- 65. Dixon JM (2002) Briza media L. Journal of Ecology. 90: 737–752. [Google Scholar]
- 66. Fischer SF, Poschlod P, Beinlich B (1996) Experimental studies on the dispersal of plants and animals on sheep in calcareous grasslands. Journal of Applied Ecology 33: 1206–1222. [Google Scholar]
- 67.Begert M, Seiz G, Schlegel T, Musa M, Baudraz G, et al.. (2003) Homogenisierung von Klimamessreihen der Schweiz und Bestimmung der Normwerte 1961–1990: Schlussbericht des Projekts NORM90. Zürich, Switzerland: MeteoSchweiz. 170 p.
- 68. Bylesjoe M, Segura V, Soolanayakanahally RY, Rae AM, Trygg J, et al. (2008) LAMINA: a tool for rapid quantification of leaf size and shape parameters. BMC Plant Biology 8: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cornelissen JHC, Lavorel S, Garnier E, Díaz S, Buchmann N, et al. (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. [Google Scholar]
- 70.R Development Core Team (2012) R: a language and environment for statistical computing. 2.15.0 ed. Vienna, Austria: R Foundation for Statistical Computing.
- 71.Faraway JJ (2005) Linear models with R. Boca Raton, United States: Chapman & Hall/CRC.
- 72. Coleman JS, McConnaughay KDM, Ackerly DD (1994) Interpreting phenotypic variation in plants. Trends in Ecology & Evolution 9: 187–191. [DOI] [PubMed] [Google Scholar]
- 73. Roach DA, Wulff RD (1987) Maternal effects in plants. Annual Review of Ecology and Systematics 18: 209–235. [Google Scholar]
- 74. Kingsolver JG, Pfennig DW (2007) Patterns and power of phenotypic selection in nature. Bioscience 57: 561–572. [Google Scholar]
- 75. van Kleunen M, Fischer M (2001) Adaptive evolution of plastic foraging responses in a clonal plant. Ecology 82: 3309–3319. [Google Scholar]
- 76. Funk JL (2008) Differences in plasticity between invasive and native plants from a low resource environment. Journal of Ecology 96: 1162–1173. [Google Scholar]
- 77. Hahn MA, van Kleunen M, Muller-Scharer H (2012) Increased phenotypic plasticity to climate may have boosted the invasion success of polyploid Centaurea stoebe . PLOS ONE 7: e50284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pollock CJ (1990) The response of plants to temperature change. Journal of Agricultural Science 115: 1–5. [Google Scholar]
- 79. Arft AM, Walker MD, Gurevitch J, Alatalo JM, Bret-Harte MS, et al. (1999) Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecological Monographs 69: 491–511. [Google Scholar]
- 80. Elmendorf SC, Henry GHR, Hollister RD, Björk RG, Bjorkman AD, et al. (2012) Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecology Letters 15: 164–175. [DOI] [PubMed] [Google Scholar]
- 81. Roughley RJ, Dart PJ (1970) Growth of Trifolium subterraneum L. selected for sparse and abundant nodulation as affected by root temperature and Rhizobium strain. Journal of Experimental Botany 21: 776–786. [Google Scholar]
- 82. Warwick KR, Taylor G, Blum H (1998) Biomass and compositional changes occur in chalk grassland turves exposed to elevated CO2 for two seasons in FACE. Global Change Biology 4: 375–385. [Google Scholar]
- 83. Molau U (1997) Responses to natural climatic variation and experimental warming in two tundra plant species with contrasting life forms: Cassiope tetragona and Ranunculus glacialis . Global Change Biology 3: 97–107. [Google Scholar]
- 84. Diggle PK (1997) Extreme preformation in alpine Polygonum viviparum: an architectural and developmental analysis. American Journal of Botany 84: 154–169. [PubMed] [Google Scholar]
- 85.Pulido F, Berthold P (2004) Microevolutionary response to climatic change. In: Moller AP, Fielder W, Berthold P, editors. Birds and Climate Change. Oxford, United Kingdom: Elsevier. 151–183.
- 86. Etterson JR, Shaw RG (2001) Constraint to adaptive evolution in response to global warming. Science 294: 151–154. [DOI] [PubMed] [Google Scholar]
- 87. Visser ME (2008) Keeping up with a warming world; assessing the rate of adaptation to climate change. Proceedings of the Royal Society B-Biological Sciences 275: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bürger R, Lynch M (1995) Evolution and extinction in a changing environment - a quantitative-genetic analysis. Evolution 49: 151–163. [DOI] [PubMed] [Google Scholar]
- 89. Whitney KD, Gabler CA (2008) Rapid evolution in introduced species, ‘invasive traits’ and recipient communities: challenges for predicting invasive potential. Diversity and Distributions 14: 569–580. [Google Scholar]
- 90. Didiano TJ, Turley NE, Everwand G, Schaefer H, Crawley MJ, et al. (2014) Experimental test of plant defense evolution in four species using long-term rabbit exclosures. Journal of Ecology 102: 584–594. [Google Scholar]
- 91. Menzel A (2003) Plant phenological anomalies in Germany and their relation to air temperature and NAO. Climatic Change 57: 243–263. [Google Scholar]
- 92. Parmesan C (2007) Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Global Change Biology 13: 1860–1872. [Google Scholar]
- 93. Wilczek AM, Roe JL, Knapp MC, Cooper MD, Lopez-Gallego C, et al. (2009) Effects of genetic perturbation on seasonal life history plasticity. Science 323: 930–934. [DOI] [PubMed] [Google Scholar]
- 94. De Frenne P, Brunet J, Shevtsova A, Kolb A, Graae BJ, et al. (2011) Temperature effects on forest herbs assessed by warming and transplant experiments along a latitudinal gradient. Global Change Biology 17: 3240–3253. [Google Scholar]
- 95. Donnelly A, Caffarra A, Kelleher CT, O’Neill BF, Diskin E, et al. (2012) Surviving in a warmer world: environmental and genetic responses. Climate Research 53: 245–262. [Google Scholar]
- 96. Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89: 353–362. [DOI] [PubMed] [Google Scholar]
- 97. De Valpine P, Harte J (2001) Plant responses to experimental warming in a montane meadow. Ecology 82: 637–648. [Google Scholar]
- 98. Thomson JD (2010) Flowering phenology, fruiting success and progressive deterioration of pollination in an early-flowering geophyte. Philosophical Transactions of the Royal Society B-Biological Sciences 365: 3187–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant-pollinator interactions. Ecology Letters 10: 710–717. [DOI] [PubMed] [Google Scholar]
- 100. Hegland SJ, Nielsen A, Lazaro A, Bjerknes AL, Totland O (2009) How does climate warming affect plant-pollinator interactions? Ecology Letters 12: 184–195. [DOI] [PubMed] [Google Scholar]
- 101. Hahn T, Kettle CJ, Ghazoul J, Frei ER, Matter P, et al. (2012) Patterns of genetic variation across altitude in three plant species of semi-dry grasslands. PLOS ONE 7: e41608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Weiner J, Martinez S, Müller-Schärer H, Stoll P, Schmid B (1997) How important are environmental maternal effects in plants? A study with Centaurea maculosa . Journal of Ecology 85: 133–142. [Google Scholar]
- 103. Vitasse Y, Hoch G, Randin CF, Lenz A, Kollas C, et al. (2013) Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 171: 663–678. [DOI] [PubMed] [Google Scholar]
- 104. Vitasse Y, Lenz A, Kollas C, Randin CF, Hoch G, et al. (2014) Genetic vs. non-genetic responses of leaf morphology and growth to elevation in temperate tree species. Functional Ecology 28: 243–252. [Google Scholar]
- 105. Goldstein G, Rada F, Azocar A (1985) Cold hardiness and supercooling along in altitudinal gradient in Andean giant rosette species. Oecologia 68: 147–152. [DOI] [PubMed] [Google Scholar]
- 106.Grime JP, Crick JC, Rincon JE (1986) The ecological significance of plasticity. In: Jennings DH, Trewavas AJ, editors. Plasticity in plants. Cambridge, United Kingdom: Company of Biologists. 5–29.
- 107. Parsons AN, Press MC, Wookey PA, Welker JM, Robinson CH, et al. (1995) Growth responses of Calamagrostis lapponica to simulated environmental change in the Sub Arctic. Oikos 72: 61–66. [Google Scholar]
- 108. Welker JM, Molau U, Parsons AN, Robinson CH, Wookey PA (1997) Responses of Dryas octopetala to ITEX environmental manipulations: a synthesis with circumpolar comparisons. Global Change Biology 3: 61–73. [Google Scholar]
- 109.Weiner J (1988) The influence of competition on plant reproduction. In: Lovett Doust J, Lovett Doust L, editors. Plant reproductive ecology: patterns and strategies. New York, United States: Oxford University Press. 228–245.
- 110. Kingsolver JG, Hoekstra HE, Hoekstra JM, Berrigan D, Vignieri SN, et al. (2001) The strength of phenotypic selection in natural populations. American Naturalist 157: 245–261. [DOI] [PubMed] [Google Scholar]
- 111. Sultan SE (2000) Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5: 537–542. [DOI] [PubMed] [Google Scholar]
- 112. Rausher MD (1992) The measurement of selection on quantitative traits - biases due to environmental covariances between traits and fitness. Evolution 46: 616–626. [DOI] [PubMed] [Google Scholar]
- 113. Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecology Letters 9: 981–993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sampling locations in the Swiss Alps of the source population pairs of Trifolium montanum (Tm), Ranunculus bulbosus (Rb) and Briza media (Bm).
(DOCX)
Means and standard errors (SE) grouped by altitude of population origin and temperature treatment for growth-related traits of Trifolium montanum , Ranunculus bulbosus and Briza media .
(DOCX)
Means and standard errors (SE) grouped by altitude of population origin and temperature treatment for reproductive and phenological traits of Trifolium montanum and Ranunculus bulbosus .
(DOCX)