TO THE EDITOR
In humans, apolipoprotein E (apoE) has three isoforms: apoE2, apoE3, and apoE4. APOE4 is a major genetic risk factor for Alzheimer’s disease (AD).1 ApoE4 has direct effects on the cerebrovascular system resulting in microvascular lesions and blood-brain barrier (BBB) damage, as recently reviewed.2 Neurovascular dysfunction is also present in cognitively normal APOE4 carriers and individuals with APOE4-associated disorders including AD.1 - 3 Moreover, post-mortem brain tissue analysis has indicated that BBB breakdown in AD patients is more pronounced in APOE4 carriers compared to APOE3 or APOE2.4 - 6 Our recent studies in transgenic mice have demonstrated that apoE4 leads to BBB breakdown by activating the proinflammatory cyclophilin A (CypA)-matrix metalloproteinase-9 (MMP-9) pathway in brain pericytes which in turn results in degradation of the BBB tight junctions and basement membrane proteins.7 It has also been shown that apoE4-mediated BBB breakdown leads to secondary neuronal injury and cognitive decline in transgenic mice.7 ApoE2 and apoE3 maintained normal BBB integrity in transgenic mice by suppressing the CypA-MMP9 pathway.7 Here, we studied the cerebrospinal fluid (CSF)/plasma albumin quotient (QAlb), an established marker of BBB breakdown8, and CypA and active MMP-9 levels in CSF of cognitively normal individuals with different APOE genotypes to determine whether apoE4-dependent changes in BBB permeability and CypA-MMP9 pathway as shown in APOE4, but not APOE3 and APOE2 transgenic mice, also occur in humans.
METHODS
Participants were volunteers who were recruited through advertisements or from the Memory Education and Research Initiative Program at the Nathan S. Kline Institute for Psychiatric Research.9 Participants gave their informed consent to participate in studies approved by the Institutional Review Board of the Nathan S. Kline Institute for Psychiatric Research and New York University School of Medicine. We studied a total of 49 cognitively normal individuals as indicated by the Clinical Dementia Rating (CDR) score of 0 and Mini-Mental State Examination (MMSE) score of approximately 30. This study did not exclude participants meeting criteria for Major Depressive Disorder as there were no differences in the studied markers of BBB damage in this group compared to controls. The studied individuals represented 3 different APOE genotypes: APOE2/E2 (n = 11), APOE3/E3 (n = 28) and APOE3/E4 (n = 10). Within each genotype, individuals were stratified into two age groups, 40-65 and 66-85 years old to control for age-dependent effects (Table 1). CSF and plasma collection and APOE genotyping were performed as described.9 Enzyme-linked immunosorbent assays (ELISA) were used to determine levels of CypA (Cat. No. sE90979Hu, USCN Life Science, Houston, TX), active MMP-9 (Cat. No. 72017, AnaSpec, Fremont, CA) and albumin (Cat. No. E-80AL, Immunology Consultant Laboratories, Portland, OR). Data were analyzed by multifactorial analysis of variance with 2 factors (age and APOE genotype), with Bonferroni post-hoc tests to adjust for multiple comparisons, and Pearson’s correlation analysis using Graphpad Prism 5.0. Analyses were performed by an investigator blinded to the experimental conditions. A P value of less than 0.05 was considered statistically significant.
Table 1. Demographic data.
|
APOE genotype / age (years) |
||||||
|---|---|---|---|---|---|---|
|
APOE2/E3 (40-65) |
APOE2/E3 (66-85) |
APOE3/E3 (40-65) |
APOE3/E3 (66-85) |
APOE3/E4 (40-65) |
APOE3/E4 (66-85) |
|
| n | 5 | 6 | 18 | 10 | 5 | 5 |
| Female (%) | 40.0 | 16.7 | 55.6 | 50.0 | 80.0 | 20.0 |
| Age at LP, years (SD) | 53.0 (10.6) | 73.3 (4.7) | 59.2 (7.3) | 70.8 (4.8) | 56.2 (7.8) | 75.2 (4.4) |
| MMSE (SD) | 29.6 (0.5) | 29.2 (1.2) | 29.7 (0.7) | 29.4 (0.8) | 30.0 (0) | 29.4 (0.5) |
| CDR Score | 0 | 0 | 0 | 0 | 0 | 0 |
| Education, years (SD) | 14.6 (1.7) | 16.2 (3.1) | 15.6 (2.5) | 15.2 (2.4) | 15.6 (0.9) | 19.2 (2.7) |
Abbreviations: APOE, apolipoprotein E; CDR, Clinical Dementia Rating; LP, lumbar puncture; MMSE, Mini-Mental State Examination; SD, standard deviation
RESULTS
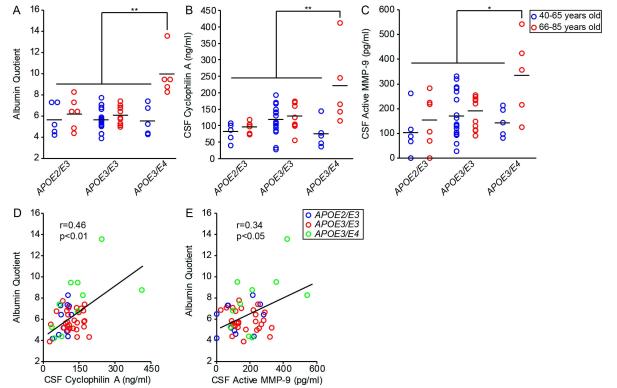
Older cognitively normal individuals carrying one APOE4 allele compared to younger cognitively normal APOE4 carriers or age-matched APOE4 non-carriers had increased QAlb by approximately 77% and 67%, respectively (p < 0.01; Figure 1A). No age-dependent increase in QAlb was associated with APOE2 or APOE3 alleles. Compared to cognitively normal younger APOE4 carriers or age-matched APOE4 non-carriers, older cognitively normal APOE4 carriers had increased CSF levels of CypA by approximately 190% and 95%, respectively (p < 0.01; Figure 1B) and active MMP-9 by 167% and 110%, respectively (p < 0.05; Figure 1C). No age-dependent changes in CypA or MMP-9 CSF levels were associated with APOE2 or APOE3 alleles. Importantly, increases in QAlb values correlated positively with both CypA and active MMP-9 CSF levels in all studied individuals (r = 0.37, p < 0.01; r = 0.45, p < 0.01) (Figure 1D and E) indicating the greater the increase in CypA and active MMP-9 levels the greater the magnitude of BBB breakdown assayed by QAlb.
Figure 1. Age-dependent blood-brain barrier breakdown and elevated cyclophilin A and active MMP-9 levels in cerebrospinal fluid (CSF) of cognitively normal APOE4 carriers.
CSF/plasma albumin quotient (A), CSF levels of cyclophilin A (B), and CSF levels of active MMP-9 (C) in a population of cognitively normal individuals on three different APOE genotypes: APOE2/E3 (n = 11), APOE3/E3 (n = 28), and APOE3/E4 (n = 10). Blue and red points represent individuals ranging from 40-65 and 66-85 years of age, respectively. Pearson correlation coefficients (r) were calculated to study the relationship between CSF cyclophilin A (D) and CSF active MMP-9 (E) levels with albumin quotient in individual subjects. In (D) and (E), blue, red and green points represent APOE2/E3, APOE3/E3 and APOE3/E4 genotypes, respectively. Points represent individual values from n = 49 subjects. Black bars represent mean values. * = P < 0.05 and ** = P < 0.01
DISCUSSION
This study shows that APOE4 carriers may be susceptible to an age-dependent BBB breakdown prior to onset of clinical decline as determined by CDR and MMSE scores. Furthermore, these findings are consistent with experimental studies suggesting that apoE4 leads to BBB damage in transgenic mice via activation of CypA-MMP-9 pathway.7 These findings warrant future longitudinal studies to investigate QAlb and CSF levels of CypA and active MMP-9 in cognitively normal APOE4 carriers as they progress to mild cognitive impairment and eventually AD. With current diagnostic markers, by the time the earliest detectable clinical signs of disease appear, significant brain injury has likely already occurred. Therefore, studying markers of BBB damage along with commonly used β-amyloid-42 and tau CSF levels may contribute to early detection of vascular dysfunction of those at risk for cognitive decline and AD.
ACKNOWLEDGMENTS
Funding/Support: This work was supported by grants R37NS34467 and R37AG23084 to B.V.Z. from the National Institutes of Health (NIH) and by grant R01MH-080405 to N.P. from the NIH.
Additional Contributions: The authors would like to thank Leslie Saint-Louis, M.D., Antero S. Sarreal, M.D. and Raymundo T. Hernando, M.D. for their assistance with patient recruitment.
Footnotes
Conflict of Interest Disclosures: None reported.
Author Contributions: Zlokovic had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Zlokovic, Pomara and Frangione. Acquisition of data: Halliday, Pomara and Sagare. Analysis and interpretation of data: Halliday and Zlokovic. Drafting of the manuscript: Halliday and Zlokovic. Critical revision of the manuscript for important intellectual content: Pomara, Sagare, Frangione and Zlokovic. Statistical analysis: Mack and Halliday. Study supervision: Zlokovic.
REFERENCES
- 1.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zlokovic BV. Cerebrovascular effects of apolipoprotein E: implications for Alzheimer’s disease. JAMA Neurol. 2013;70(4):440–444. doi: 10.1001/jamaneurol.2013.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zipser BD, Johanson CD, Gonzalez L, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28(7):977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 5.Cortes-Canteli M, Zamolodchikov D, Ahn HJ, Strickland S, Norris EH. Fibrinogen and altered hemostasis in Alzheimer’s disease. J Alzheimer’s Dis. 2012;32(3):599–608. doi: 10.3233/JAD-2012-120820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hultman K, Strickland S, Norris EH. The APOE ε4/ε4 genotype potentiates fibrin(ogen) deposition in amyloid-laden vessels in the brains of Alzheimer’s disease patients. J Cereb Blood Flow Metab. 2013 doi: 10.1038/jcbfm.2013.76. doi: 10.1038/jcbfm.2013.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blennow K, Wallin A, Fredman P, et al. Blood-brain barrier disturbance in patients with Alzheimer’s disease is related to vascular factors. Acta Neurol Scand. 1990;81(4):323–326. doi: 10.1111/j.1600-0404.1990.tb01563.x. [DOI] [PubMed] [Google Scholar]
- 9.Pomara N, Bruno D, Sarreal AS, et al. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. Am J Psychiatry. 2012;169(5):523–530. doi: 10.1176/appi.ajp.2011.11081153. [DOI] [PMC free article] [PubMed] [Google Scholar]