Abstract
The pathway causing CD4 T-cell death in HIV-infected hosts remains poorly understood. Apoptosis has been proposed as the key mechanism for CD4 T-cell loss. We now show that caspase-3-mediated apoptosis accounts for the death of only a small fraction of productively infected cells. The remaining >95% of quiescent lymphoid CD4 T-cells die by caspase-1-mediated pyroptosis triggered by abortive viral infection. Pyroptosis corresponds to an intensely inflammatory form of programmed cell death where cytoplasmic contents and pro-inflammatory cytokines including IL-1β, are released. This death pathway thus links the two signature events in HIV infection––CD4 T-cell depletion and chronic inflammation––and creates a vicious pathogenic cycle where dying CD4 T-cells release inflammatory signals that attract more cells to die. This cycle can be broken by caspase-1 inhibitors shown to be safe in humans, raising the possibility of a new class of “anti-AIDS” therapeutics targeting the host rather than the virus.
The progressive loss of CD4 T cells in HIV-infected individuals lies at the root of AIDS. Despite more than three decades of study, the precise mechanism(s) underlying the demise of CD4 T cells during HIV infection remains poorly understood and has been highlighted as one of the key questions in HIV research1. In almost all cases, loss of CD4 T cells has been linked to apoptosis, both in in vivo2-6 and ex vivo5,7,8 studies. However, various features of apoptotic cell death including maturation of executioner caspase-3, DNA fragmentation, and plasma membrane permeabilization are commonly shared with other programmed cell death pathways9. Importantly, most studies have focused on the death of productively infected cells circulating in peripheral blood10. Very little is known about the death of “bystander” CD4 T cells in tissues that are refractory to productive HIV infection. These resting CD4 T lymphocytes in fact represent the major cellular targets encountered by HIV in lymphoid tissues11-13.
To investigate how CD4 T cells die during HIV infection, we took advantage of an ex vivo human lymphoid aggregate culture (HLAC) system formed with fresh human tonsil or spleen tissues13. HLACs can be infected with a small number of viral particles in the absence of artificial mitogens, allowing analysis of HIV cytopathicity in a natural and preserved lymphoid microenvironment12. Infection of these cultures with HIV-1 produces extensive loss of CD4 T cells, but >95% of the dying cells are abortively infected with HIV reflecting their nonpermissive, quiescent state. The HIV life cycle is attenuated during the chain elongation phase of reverse transcription, giving rise to incomplete cytosolic viral DNA transcripts. Cell death is ultimately caused by a cellular innate immune response elicited by these cytosolic DNA intermediates11. This response is associated with production of type I interferon and activation of both caspase-3 and caspase-1. While caspase-3 activation leads to apoptosis without inflammation14, caspase-1 activation can trigger pyroptosis, a highly inflammatory form of programmed cell death where dying cells release their cytoplasmic contents, including inflammatory cytokines, into the extracellular space9,15. The consequences of apoptosis-versus-pyroptosis may affect HIV pathogenesis by influencing the state of inflammation and immune activation, but their relative contribution to CD4 T-cell death in lymphoid tissues had remained unexplored.
Results
Host permissivity determines the form of cell death
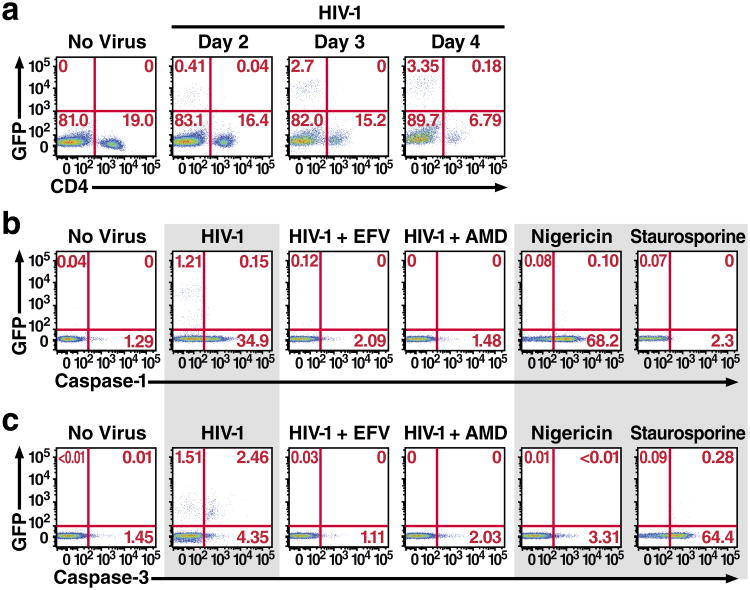
Previous reports have implicated caspase-3 activation and apoptosis in most instances of cell death caused by HIV-13,7,8. To explore the role of caspase-1 in dying HIV-infected CD4 T cells, HLACs formed with freshly dissected human tonsillar tissues were infected with a GFP reporter virus (NLENG1), prepared from the X4-tropic NL4-3 strain of HIV-1. This reporter produces fully replication-competent viruses. An IRES upstream of the nef gene preserves Nef expression and supports LTR-driven GFP expression16, allowing simultaneous quantification of HIV-1 infection and caspase activation in CD4 T cells. NL4-3 was selected because tonsillar tissue contains a high percentage of CD4 T cells that express CXCR4 (90–100%). Consistent with our previous report11, infection with HIV-1 produced extensive depletion of “bystander” non-productively infected CD4 T cells. No more than 4% of the CD4 T cells were productively infected with HIV-1, but most of the remaining CD4 T cells underwent abortive infection and ultimately died after four days in culture (Fig. 1a).
Figure 1.
Host permissivity determines the CD4 T-cell death pathway employed following HIV infection. a. Kinetics of spreading viral infection versus depletion of CD4 T cells after infection of HLACs with a replication-competent HIV reporter virus encoding GFP. The relative proportion of CD8 T cells was not altered (not shown). Consistent with our previous report, HIV-infected HLACs contain a small number of productively infected cells, while almost all of the dying cells are abortively infected 11. b. Abortively infected CD4 T cells exclusively activate caspase-1. Nigericin induces abundant caspase-1 activation in uninfected cells. c. Productively-infected CD4 T cells activate caspase-3, but not caspase-1. (b) and (c) represent cells from the same infected tonsil culture. Efavirenz and AMD3100 were added to the indicated cultures prior to HIV infection. These data are representative of four independent experiments performed with tonsil cells isolated from four different donors.
To determine the distribution of active caspase-1 and caspase-3 in the dying CD4 T cells, we used fluorescently labeled inhibitor of caspases (FLICA) probes with sequences targeted by specific activated caspases17. Interestingly, the majority of non-productively infected CD4 T cells exhibited activation of caspase-1. Conversely, essentially no caspase-1 activity was detected in the productively infected cells (Fig. 1b). Caspase-3 activity was markedly less abundant, and mainly confined to the productively infected subset of cells (Fig. 1c). Treatment, with efavirenz (a non-nucleoside reverse transcriptase inhibitor, NNRTI) or AMD3100 (an inhibitor of CXCR4-dependent HIV entry) prevented activation of both caspases. Infection with the primary, dual-tropic 89.6 HIV isolate18 produced similar results (Extended Data Fig. 1). The two FLICA probes appeared to bind their respective caspases with reasonable specificity based on exclusive caspase-3 staining in cells treated with staurosporine, a protein kinase inhibitor known to induce apoptosis versus robust caspase-1 staining in cells treated with the cationic ionophore nigericin that promotes NLRP3 inflammasome assembly, caspase-1 activation, and pyroptosis19.
Healthy lymphoid CD4 T cells express pro-IL-1β
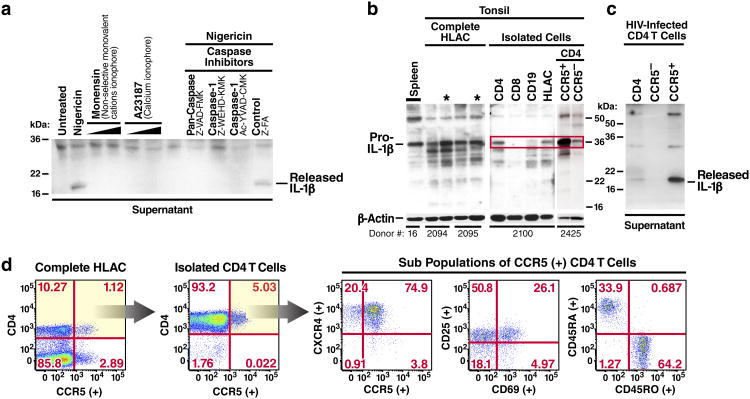
IL-1β activity is controlled at multiple levels including pro-IL-1β expression, processing, and secretion. Proinflammatory stimuli induce expression of pro-IL-1β while processing and release are regulated by caspase-1 activation in inflammasomes20. The signals required for caspase-1 activation and release of IL-1β differ between immune cells. In circulating human blood monocytes, caspase-1 is constitutively active21. Stimulation of these cells with LPS promotes pro-IL-1β expression leading to the rapid release of bioactive IL-1β. In contrast, macrophages and dendritic cells require a second signal to activate caspase-122. Nigericin can function as this second signal activating caspase-1 in LPS-primed macrophages19,23. Surprisingly, nigericin proved sufficient alone to activate caspase-1 in uninfected lymphoid CD4 T cells (Fig. 1b) and to promote the release of the 17-kDa bioactive form of IL-1β (Fig. 2a). Treatment with monensin, a different monovalent cationic ionophore, or A23187, a calcium ionophore, did not promote mature IL-1β release23,24. Maturation and secretion of the bioactive form of IL-1β was inhibited by Z-VAD-FMK (a pan-caspase inhibitor), Z-WEHD-FMK, or Z-YVAD-FMK (two independent caspase-1 inhibitors, which also block other inflammatory caspases i.e. caspase-4 and -5), but not by Z-FA-FMK (negative control for caspase inhibitors) suggesting that caspase-1 activation was required.
Figure 2.
Lymphoid CD4 T cells are primed to mount an inflammatory response and constitutively express high-levels of pro-IL-1β. a. A secondary inflammatory stimulus by nigericin induces lymphoid CD4 T cells to process and release bioactive IL-1β. Supernatants from cell cultures were filtered to remove all remaining cells and subjected to SDS-PAGE immunoblotting analyses for bioactive 17-kDa IL-1β. b. High levels of constitutive pro-IL-1β are selectively expressed in lymphoid CD4 T cells. Levels of intracellular pro-IL-1β were assessed in HLACs from fresh tonsils or spleen tissue from different donors. Asterisks indicate samples in which dead cells were removed. CD4 T cells were positively isolated from HLAC. Cells were lysed and analyzed for pro-IL-1β expression. c. Nearly all bioactive IL-1β produced by HIV-infected lymphoid CD4 T cells is released from CCR5-expressing cells. Indicated CD4 T-cell populations were isolated from HLAC and infected with HIV-1. Supernatants of cultures were filtered and analyzed for bioactive 17-kDa IL-1β. d. HLACs were characterized for expression of memory and activation markers by flow cytometry. The majority of CCR5-expressing CD4 T lymphocytes exhibit a memory phenotype. All CCR5-expressing CD4 T cells co-express the CXCR4 receptor.
Pro-IL-1β expression in human tonsil and spleen HLACs was next examined. Western blotting analysis surprisingly revealed large amounts of intracellular pro-IL-1β in both untreated tonsil and spleen HLACs (Fig. 2b). Removal of dead cells by ficoll-hypaque density centrifugation resulted in an even higher intracellular pro-IL-1β signal, suggesting that these normal lymphoid tissues constitutively express high levels of pro-IL-1β. The presence of pro-IL-1β in spleen argued that expression in tonsil is not solely caused by infection (tonsillitis). Fractionation of the lymphocytes present in these HLACs revealed high levels of intracellular pro-IL-1β in isolated CD4 T cells, but not in CD8 T or B-cell populations.
Most tonsillar CD4 T cells express CXCR4, but only ∼5% of these cells also express CCR512,25. Interestingly, when CCR5-positive and -negative lymphoid CD4 T-cell subsets were isolated and studied, the CCR5-expressing cells displayed much higher levels of intracellular pro-IL-1β (Fig. 2b). The CCR5-expressing CD4 T cells also released significantly more 17 kDa IL-1β into the supernatant after infection with HIV-1 (Fig. 2c). These results suggest that most of the mature form of IL-1β is released by the small population of CCR5-expressing CD4 T cells. The resident CCR5-expressing cells in lymphoid tissues are primarily memory CD4 T cells, which might be more permissive for productive HIV infection26. However, the activation status of these cells varied (Fig. 2d). Two thirds exhibited a memory phenotype as determined by surface expression of CD45RO but only a small fraction of these cells were permissive to productive infection with either X4-tropic or R5-tropic HIV-1 strains (Extended Data Fig. 2). Notably, lymphoid CCR5-expressing CD4 T cells also express CXCR4 and thus can be targeted by either X4 or R5-tropic HIV-1 strains12,27,28. Memory T cells continually recirculate within lymphoid tissues scanning for presentation of their cognate antigen29-31. It seems likely that many of these cells have returned to a sufficient state of quiescence that they are susceptible to abortive HIV infection and thus could contribute importantly to chronic inflammation through the release of bioactive IL-1β.
CD4 T-cell death by HIV-1 is mediated by pyroptosis
Caspase-1 is a proinflammatory caspase whose catalytic activity is tightly regulated by signal-dependent autoactivation within inflammasomes20. Inflammasome-dependent caspase-1 activity results in a highly inflammatory form of cell death known as pyroptosis, primarily described in myeloid cells infected with intracellular bacterial pathogens9,15,32. Pyroptosis is caspase-1-dependent by definition and occurs independently of other proapoptotic caspases9,32. Based on our finding that caspase-1 is activated in lymphoid CD4 T cells following abortive HIV infection, we investigated whether pyroptosis is triggered within these cells.
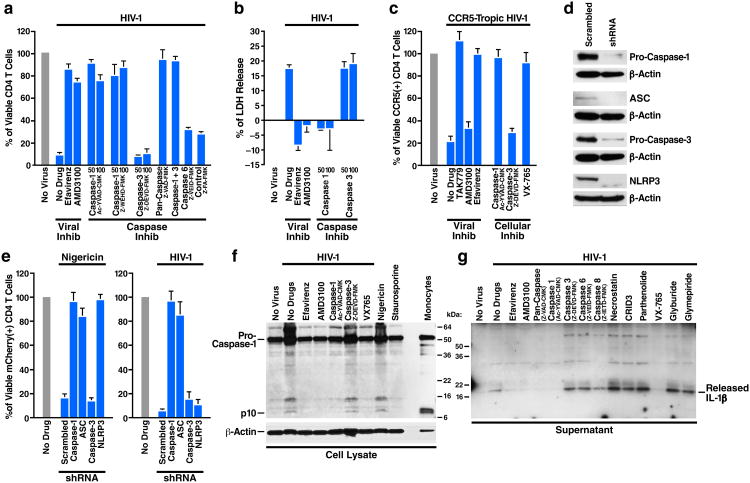
Fresh HLACs were infected with HIV-1 and cultured for 12 hours to initiate viral spread and then treated with various caspase inhibitors or controls. Extensive and selective depletion of CD4 T cells occurred in untreated, HIV-infected cultures after 3 days. However, treatment with either pan-caspase or caspase-1 inhibitors prevented the depletion of CD4 T cells as efficiently as the viral inhibitors efavirenz and AMD3100 (Fig. 3a). Inhibitors of caspase-3 or caspase-6 and the control compound did not prevent CD4 T-cell depletion. Necrostatin-1, a RIP1 inhibitor, did not inhibit CD4 T-cell depletion (Extended Data Fig. 3a, b), suggesting that cell death does not reflect necroptosis. Analysis of spleen cells yielded similar results (Extended Data Fig. 3c). Inhibiting type-I interferon signaling with neutralizing antibodies directed against IFNα/β receptor did not prevent CD4 T-cell death (Extended Data Fig. 4), indicating that this antiviral response is not critical for the innate immune-mediated onset of programmed cell death. Distinct from apoptosis, pyroptosis features cellular swelling, plasma membrane rupture, and release of intracellular content into the extracellular milieu15, including cytosolic enzymes like lactate dehydrogenase (LDH)33. LDH release was readily detected after HIV infection (Fig. 3b), but completely blocked by an inhibitor of caspase-1, efavirenz and AMD3100, not by a caspase-3 inhibitor. Thus, the form of cell death associated with abortive HIV infection appears to involve caspase-1 activation and the release of cytoplasmic contents. Caspase-1 inhibitors also prevented death of CCR5-expressing CD4 T cells in HLACs infected with a CCR5-dependent strain of HIV-1 (Fig. 3c). Inhibition of cell death by the caspase-1 inhibitor was as effective as the CCR5 receptor antagonist TAK779, suggesting that most CCR5-expressing CD4 T cells die by caspase-1-mediated pyroptosis. These findings are consistent with the large amounts of bioactive IL-1β released by these cells after HIV-1 infection.
Figure 3.
Death of HIV-infected lymphoid CD4 T cells and release of bioactive IL-1β are controlled by caspase-1. a, b. Caspase-1 inhibitors are sufficient to prevent CD4 T-cell death in HIV-infected HLACs. Viable CD4 T cells were counted by flow cytometry, and supernatants were analyzed for levels of cytoplasmic LDH enzyme release33. c, Infection with CCR5-dependent HIV-1 induces pyroptosis of lymphoid CD4 T cells. Death of CCR5-expressing CD4 T cells is prevented by caspase-1 inhibitors and TAK779, but not buy the CXCR4 antagonist, AMD3100. Due to the small number of target CCR5-expressing cells, this experiment was performed by overlaying tonsil cells on a monolayer of 293T cells that had been transfected with an R5-tropic proviral HIV-1 clone, as previously described11. The co-culture conditions for the R5 virus experiment induced no activation of the overlaid cells. d. Efficient repression of target genes by shRNA-coding lentiviral vectors. e. shRNA LV designed to silence either caspase-1 or ASC, key components of the pyroptotic pathway, protect lymphoid CD4 T cells from death by nigericin or HIV-1 infection. To specifically assess non-productively infected cells, cultures were treated with AZT before infections with HIV-1. f. Caspase-1 cleavage in HIV-infected CD4 T cells is blocked by specific caspase-1 inhibitors. g. Inhibitors of caspase-1, but not NLRP3, prevent release of bioactive IL-1β from HIV-infected lymphoid CD4 T cells. Error bars represent SD/√n of at least three independent experiments utilizing tonsil cells from at least three different donors. Protein analyses represent results from three independent experiments utilizing tonsillar CD4 T cells from three different donors.
Because caspase inhibitors are not exquisitely specific. we designed shRNA vectors to silence the expression of caspase-1, the ASC (PYCARD) adaptor, which recruits pro-caspase-1 to inflammasome complexes20, caspase-3, and NLRP3 (Extended Data Fig. 5). For these experiments, a third generation shRNA-encoding lentiviral vector (shRNA LV) pSico34, bearing an EF1α:mCherry reporter expression cassette was used. To relieve the resistance of lymphoid CD4 T cells to shRNA LV infection, target cells were initially challenged with Vpx-harboring lentiviral particles (Vpx-VLPs), which induce proteasomal degradation of SAMHD1 in nonpermissive human resting CD4 T cells35. Infections with Vpx-VLPs did not lead to activation of resting CD4 T cells, as measured by surface expression of the CD69 and CD25 activation markers (not shown). The shRNA LV particles and Vpx-VLPs were pseudotyped with a CXCR4-tropic Env of HIV-1, which supports efficient fusion to quiescent CD4 T lymphocytes36. Under these conditions, infection with shRNA LVs markedly suppressed expression of a variety of targeted genes while the scrambled shRNA LV control did not (Fig. 3d). We next investigated whether any of these shRNA LVs inhibited pyroptosis induced by nigericin. Nigericin induced massive pyroptosis in mCherry positive CD4 T cells infected with scramble and caspase-3 shRNA LV particles, but this response was blocked by the caspase-1, ASC, or NLRP3 shRNA LV particles (Fig. 3e). Next, the effect of these shRNAs on CD4 T-cell death elicited by HIV-1 was examined. HIV-1 infection caused extensive death of mCherry-positive CD4 T cells expressing shRNAs against scramble, caspase-3 and NLRP3, but not caspase-1 and ASC. Thus, cell death occurring during abortive HIV infection appears to be mediated through caspase-1 dependent pyroptosis involving an inflammasome that contains ASC but lacks NLRP3.
HIV-1 stimulates caspase-1 to secrete IL-1β
To independently confirm that abortive HIV-1 infection leads to the activation of caspase-1, we investigated appearance of the active p10 subunit of caspase-1. As controls for pyroptosis and apoptosis, uninfected cells were treated with nigericin or staurosporine, respectively. An active 10kDa subunit of caspase-1 (p10) was detected in the lysates of HIV-infected cultures as well as in nigericin-treated cells, and in blood monocytes where caspase-1 is constitutively active21. Treatments with viral or caspase-1, but not caspase-3, inhibitors prevented caspase-1 cleavage (Fig. 3f). These findings confirm the induction of caspase-1 in quiescent CD4 T following abortive infection with HIV-1. Caspase-3 activation in these infected cultures was markedly less abundant (Extended Data Fig. 6). To test whether caspase-1 activation leads to proteolytic maturation of pro-IL-1β, we used various caspase inhibitors and analyzed the culture media for the mature 17-kDa form of IL-1β. Interestingly, release of mature IL-1β was completely inhibited by a pan-caspase inhibitor, and by two different caspase-1 inhibitors (Fig. 3g). Inhibitors of apoptotic caspases, caspase-3, -6, or -8, or necrostatin did not interrupt this release. Similar findings were observed using a quantitative IL-1β enzyme-linked immunosorbent assay (ELISA) (Extended Data Fig. 7a). Thus, caspase-1 activation is specifically required for the release of bioactive IL-1β in lymphoid CD4 T cells infected with HIV-1. In accord with shRNA analyses, treatment with four separate NLRP3 inhibitors did not prevent release of bioactive IL-1β by HIV-1 (Fig. 3g), nor CD4 T-cell death by HIV-1 (Extended Data Fig. 7b, c).
In vivo evidence for HIV-mediated pyroptosis
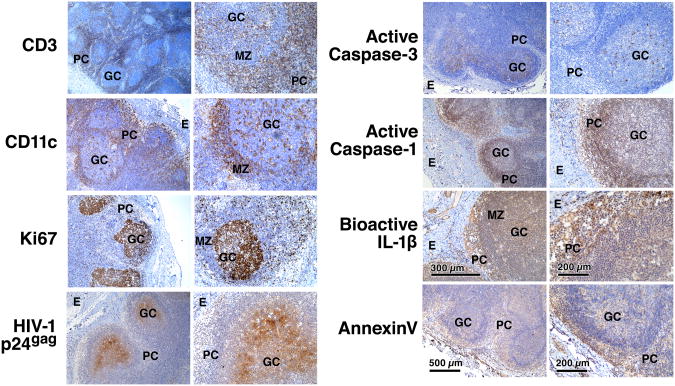
To extend our ex vivo HLAC findings, we next examined fresh lymph node tissue obtained from a consenting untreated subject infected with R5-tropic HIV and displaying a high viral load and a low CD4 T cell count. In-situ immunostaining revealed a distinct zone of HIV p24gag expression between the mantle zone and germinal centers, where activated CD4 T and B cells proliferate (Ki67) and interact in the follicles (Fig. 4). Conversely, staining for caspase-1 revealed abundant activity in the surrounding paracortical zone (CD3) comprised primarily of resting CD4 T cells. Staining of uninfected tonsil or spleen (not shown) tissues revealed no such positive signals (Extended Data Fig. 8). Because this antibody reacts with both the active p20 component of caspase-1 and pro-caspase-1, we cannot completely exclude the possibility that abortive HIV-1 infection produced localized increase in pro-caspase-1 expression. However, large amounts of IL-1β were also detected in the paracortical zone, particularly in the extracellular space between the T cells, as well as the cell death marker annexin V. In sharp contrast, active caspase-3 staining was limited to the areas in the germinal center where HIV-1 p24gag expression was detected. These findings strongly agree with the HLAC results (Fig. 1b) suggesting that caspase-3 activity occurs in a set of productively infected cells, anatomically separated from the majority of resting CD4 T cells undergoing abortive infection, caspase-1 activation, IL-1β processing, and pyroptosis.
Figure 4.
Distinct regions of caspase-1 and caspase-3 activity in lymph node of a patient chronically infected with R5-tropic HIV. Inguinal lymph node was collected from a 50-year-old immunosuppressed HIV-1 infected subject during the chronic phase of disease. The patient was first identified with HIV in 1985, has not been on anti-retroviral therapy and displayed CD4 count of 156 cells/μl and viral load of 85,756 copies/ml at the time of lymph node resection. (See also Extended Data Figure 8). GC, germinal center; MZ, mantle zone; PC, paracortical zone; E, epithelium.
Clinically safe drug blocks pyroptosis by HIV-1
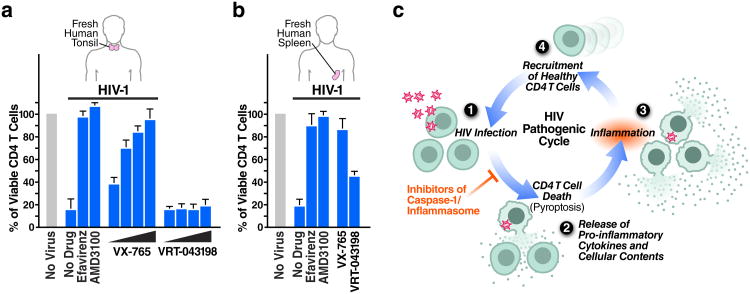
Identifying pyroptosis as the predominant mechanism mediating CD4 T-cell depletion during HIV infection provides novel targets, such as caspase-1, for potential therapeutic intervention. The role of caspase-1 in the chronic inflammatory response has attracted therapeutic interest37. VX-765 is caspase-1 inhibitor that has been tested in chronic epilepsy and psoriasis (Extended Data Fig. 9a)38-41, and found in a phase IIa trial to be safe and well tolerated42. In our studies, VX-765 inhibited IL-1β secretion by nigericin-induced lymphoid CD4 T cells (Extended Data Fig. 7b), indicating it efficiently blocks caspase-1 activity in these cells. VX-765 also blocked caspase-1 cleavage (Fig. 3f), IL-1β secretion (Fig. 3g), and CD4 T-cell death in HIV-infected tonsillar and splenic HLACs (Fig. 3c, 5a, 5b, Extended Data Fig. 9b). Cell death was not markedly inhibited by VRT-043198 (the active form of the VX765 prodrug), likely because of reduced cellular permeability38. HIV-1 infection was not restored to productive infection when caspase-1 was blocked (Extended Data Fig. 10). These findings demonstrate that a small-molecule inhibitor of caspase-1 shown to be safe in humans suppresses CD4 T-cell death and inflammation elicited in lymphoid tissues by HIV-1.
Figure 5.
Targeting caspase-1 via an orally bioavailable and safe drug prevents lymphoid CD4 T-cell death by HIV-1. (a, b). VX-765 efficiently blocks CD4 T-cell death in HIV-infected tonsillar and splenic lymphoid tissues. No toxicity was observed at any of these drug concentrations. Error bars represent SD/√n of three independent experiments utilizing tonsil or spleen cells from three different donors. c. Pyroptosis in HIV-infected lymphoid tissues may ensue a chronic cycle of CD4 T-cell death and inflammation that ultimately contributes to disease progression and tissue damage. Inhibitors of caspase-1 such as VX-765 may inhibit pyroptosis in a manner that both preserves CD4 T cells and reduces inflammation.
Discussion
HIV's lethal attack on its principal cellular target, the CD4 T cell, has been generally attributed to apoptosis43. We now demonstrate that the permissivity status of the host cell dictates the pathway through which lymphoid CD4 T cells die following HIV infection. Specifically, when HIV infects permissive, activated CD4 T cells, cell death occurs silently through caspase-3-dependent apoptosis. Conversely, when either R5- or X4-tropic HIV abortively infects nonpermissive, quiescent CD4 T cells from lymphoid tissue, death occurs through caspase-1 dependent pyroptosis, an intensely inflammatory form of programmed cell death. In most human lymphoid tissues including tonsil, lymph node, and spleen, the activated and permissive subset of cells represents 5% or less of the total CD4 T cells, while non-permissive quiescent cells represent 95% or more of the targets encountered by HIV12,25. Thus, in sharp contrast to previous studies2,3,7,8,10, caspase-1-mediated pyroptosis, not caspase-3-mediated apoptosis, appears predominantly responsible for driving CD4 T-cell death following HIV infection of these lymphoid tissues. These findings are further supported by analysis of fresh lymph nodes from subjects infected with R5-tropic HIV, where caspase-1 and IL-1β are detected in the paracortical zone rich in resting CD4 T cells while caspase-3 activity is detected in the anatomically distinct germinal centers where productively infected cells are found.
Our studies also highlight how lymphoid CD4 T cells are selectively primed to mount inflammatory responses as evidenced by constitutive expression of cytoplasmic pro-IL-1β. This is particularly prominent within the CCR5-expressing subset of lymphoid CD4 T cells. The pyroptotic death of these cells would lead to high level release of IL-1β potentially further fueling chronic inflammation.
Pyroptosis likely promotes the rapid clearance of various bacterial infections by removing intracellular replication niches and enhancing the host's defensive responses through the release of proinflammatory cytokines and endogenous danger signals. However, in pathogenic chronic inflammation, such as in HIV infection, pyroptosis is not a protective response and does not lead to clearance of the primary infection. In fact, pyroptosis appears to create a vicious pathogenic cycle, where dying CD4 T cells release inflammatory signals that attract more cells into the infected lymphoid tissue to die and to produce more inflammation44 (Fig. 5c). These events establish a chronic state of inflammation that likely fuels disease progression and tissue injury45. Chronic inflammation might also promote maintenance of the latent HIV reservoir through the dysregulated action of the IL-7 or IL-15 cytokines stimulating homeostatic proliferation of memory CD4 T cells. In this regard, it will be interesting to assess to what extent pyroptosis persists in lymphoid tissues of HIV-infected subjects on effective anti-retroviral therapy.
The depletion of CD4 T cells and the development of chronic inflammation are signature processes in HIV pathogenesis that propel disease progression46. Our studies now reveal how pyroptosis provides an unexpected link between these two disease-promoting processes. In non-pathogenic infections where simian immunodeficiency virus (SIV) infects its natural nonhuman primate hosts, caspase-3-apoptosis in productively infected cells may signal for most of the cell death rather than caspase-1, thus avoiding local inflammation. The pathogenic cycle of cell death and inflammation created by pyroptosis critically depends on the activation of caspase-1. As such, it may be possible to break this pathogenic cycle with safe and effective caspase-1 inhibitors. These agents could form a new and exciting “anti-AIDS” therapy for HIV-infected subjects where the treatment targets the host instead of the virus.
Methods Summary
Human tonsil or splenic tissues were obtained from the National Disease Research Interchange and the Cooperative Human Tissue Network and processed as previously described11. Dead cells within the complete HLACs were first removed by Ficoll-Hypaque gradient centrifugation. CD4 T cells (CD3+) were isolated from HLACs by positive selection using CD4 microbeads (Miltenyi) as described11. CCR5-expressing CD4 T cells were positively separated from CD4 T-cell cultures (PlusCellect R&D Systems Cat # PLS180), negatively isolated from complete HLACs (STEMCELL Technologies, EasySep™ Human CD4+ T-cell Enrichment Kit). In shRNA experiments, infections with R5-tropic HIV-1, and when splenic cells that are extremely refractory to HIV-1 infection were used, we modified the infection system by overlaying HLAC cells on a monolayer of 293T cells that had been transfected with HIV-1 proviral clones, as previously described11. Flow cytometry data were collected on a FACS Calibur (BD Biosciences) and analyzed with FlowJo software (Treestar). HIV-1 viruses were generated by transfection of proviral DNA into 293T cells using calcium phosphate.
Supplementary Material
Acknowledgments
We thank David N. Levy for the NLENG1 plasmid, Luke A. J. O'Neill for CRID3 and parthenolide, Dr. Ronald Collman for the HIV-1 89.6 clone, and Vertex Pharmaceuticals for the VX-765 and VRT-043198 compounds. HIV-infected lymph node tissue was obtained from the SCOPE cohort at HIV/AIDS clinic of the San Francisco General Hospital (SFGH) Positive Health Program, with the help of Rebecca Hoh, and Marian Kerbelski. We thank Dr. Laura Napolitano and Yoyanda Lie from Monogram Biosciences for performing Trofile assays to determine HIV co-receptor tropism in samples of HIV-infected volunteers. The following reagents were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: AMD3100, Efavirenz, and Raltegravir. We thank Caroline Miller, director of the Gladstone Histology Core, for performing the immunostaining assays, and Dr. Marielle Cavrois, Marianne Gesner, and Jaime Tawney for assistance with flow cytometry. We also thank Gary Howard and Anna Lisa Lucido for editorial assistance, John C.W. Carroll, Giovanni Maki, and Teresa Roberts for graphics arts, and Robin Givens and Sue Cammack for administrative assistance. Special thanks to Dr. Nadia Roan for comments on the manuscript, and to Jason Neidleman for stimulating discussions and technical advice. We thank the NIH/NIAID for funding (R21AI102782, 1DP1036502, U19 AI0961133). Funding was also provided by the UCSF/Robert John Sabo Trust Award (Doitsh) and A.P. Giannini Foundation Postdoctoral Research Fellowship (Monroe). We also acknowledge support from NIH P30 AI027763 (UCSF-GIVI Center for AIDS Research) for support to Dr. Sowinski, Dr. Yang, and for Immunology Core services.
Footnotes
Author Contributions: G.D. identified the involvement of caspase-1 and pyroptosis in lymphoid CD4 T-cell death by HIV-1, developed and designed most of the studies, collected the data, and wrote the manuscript; N.LK.G. performed IL-1β protein assays, and examined VX-765 in HIV-infected tonsils,; X.G. performed FLICA and shRNA analyses in HLACs; Z.Y. analyzed caspase cleavage in HIV-infected cultures; K.M.M. examined caspase inhibitors and LDH release assays; O.Z. tested caspase inhibitors, type-I IFN, and pro-IL-1β expression; T.P.H. and H.H. provided HIV-infected lymphoid node from surgeries of SCOPE cohort patients at HIV/AIDS clinic of the San Francisco General Hospital (SFGH); I.M-A. provided reagents and tissues; S.S. coordinated lymph node biopsies; W.C.G supervised all of these studies and participated in the preparation of the final manuscript.
Author Information: Reprints and permissions information are available at www.nature.com/reprints.
The authors have no conflicting financial interests.
Readers are welcome to comment on the online version of the paper.
Online Content Any additional Methods, Extended Data display items and Source Data are available in the online version of the paper; references unique to these sections appear only in the online paper.
References
- 1.Thomas C. Roadblocks in HIV research: five questions. Nat Med. 2009;15:855–859. doi: 10.1038/nm0809-855. [DOI] [PubMed] [Google Scholar]
- 2.Muro-Cacho CA, Pantaleo G, Fauci AS. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 3.Finkel TH, et al. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 4.Huang MB, James CO, Powell MD, Bond VC. Apoptotic peptides derived from HIV-1 Nef induce lymphocyte depletion in mice. Ethnicity & disease. 2008;18:S2-30–37. [PMC free article] [PubMed] [Google Scholar]
- 5.Rosok BI, et al. Correlates of apoptosis of CD4+ and CD8+ T cells in tonsillar tissue in HIV type 1 infection. AIDS research and human retroviruses. 1998;14:1635–1643. doi: 10.1089/aid.1998.14.1635. [DOI] [PubMed] [Google Scholar]
- 6.Gougeon ML, et al. Programmed cell death in peripheral lymphocytes from HIV-infected persons: increased susceptibility to apoptosis of CD4 and CD8 T cells correlates with lymphocyte activation and with disease progression. J Immunol. 1996;156:3509–3520. [PubMed] [Google Scholar]
- 7.Jekle A, et al. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol. 2003;77:5846–5854. doi: 10.1128/JVI.77.10.5846-5854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grivel JC, Malkevitch N, Margolis L. Human immunodeficiency virus type 1 induces apoptosis in CD4(+) but not in CD8(+) T cells in ex vivo-infected human lymphoid tissue. J Virol. 2000;74:8077–8084. doi: 10.1128/jvi.74.17.8077-8084.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamkanfi M, Dixit VM. Manipulation of host cell death pathways during microbial infections. Cell. 2010;8:44–54. doi: 10.1016/j.chom.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Cooper A, et al. HIV-1 causes CD4 cell death through DNA-dependent protein kinase during viral integration. Nature. 2013 doi: 10.1038/nature12274. [DOI] [PubMed] [Google Scholar]
- 11.Doitsh G, et al. Abortive HIV Infection Mediates CD4 T Cell Depletion and Inflammation in Human Lymphoid Tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckstein DA, et al. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity. 2001;15:671–682. doi: 10.1016/s1074-7613(01)00217-5. [DOI] [PubMed] [Google Scholar]
- 13.Glushakova S, Baibakov B, Margolis LB, Zimmerberg J. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat Med. 1995;1:1320–1322. doi: 10.1038/nm1295-1320. [DOI] [PubMed] [Google Scholar]
- 14.Ren Y, Savill J. Apoptosis: the importance of being eaten. Cell Death Differ. 1998;5:563–568. doi: 10.1038/sj.cdd.4400407. [DOI] [PubMed] [Google Scholar]
- 15.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–1916. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy DN, Aldrovandi GM, Kutsch O, Shaw GM. Dynamics of HIV-1 recombination in its natural target cells. Proc Natl Acad Sci U S A. 2004;101:4204–4209. doi: 10.1073/pnas.0306764101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Experimental cell research. 2000;259:308–313. doi: 10.1006/excr.2000.4955. [DOI] [PubMed] [Google Scholar]
- 18.Collman R, et al. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 20.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 21.Netea MG, et al. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood. 2009;113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laliberte RE, Eggler J, Gabel CA. ATP treatment of human monocytes promotes caspase-1 maturation and externalization. J Biol Chem. 1999;274:36944–36951. doi: 10.1074/jbc.274.52.36944. [DOI] [PubMed] [Google Scholar]
- 23.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 24.Perregaux D, et al. IL-1 beta maturation: evidence that mature cytokine formation can be induced specifically by nigericin. J Immunol. 1992;149:1294–1303. [PubMed] [Google Scholar]
- 25.Moore JP, Kitchen SG, Pugach P, Zack JA. The CCR5 and CXCR4 coreceptors--central to understanding the transmission and pathogenesis of human immunodeficiency virus type 1 infection. AIDS research and human retroviruses. 2004;20:111–126. doi: 10.1089/088922204322749567. [DOI] [PubMed] [Google Scholar]
- 26.Schweighardt B, et al. R5 human immunodeficiency virus type 1 (HIV-1) replicates more efficiently in primary CD4+ T-cell cultures than X4 HIV-1. J Virol. 2004;78:9164–9173. doi: 10.1128/JVI.78.17.9164-9173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grivel JC, Margolis LB. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat Med. 1999;5:344–346. doi: 10.1038/6565. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Shen L, Yang HC, Siliciano RF. Preferential cytolysis of peripheral memory CD4+ T cells by in vitro X4-tropic human immunodeficiency virus type 1 infection before the completion of reverse transcription. J Virol. 2008;82:9154–9163. doi: 10.1128/JVI.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 30.Mackay CR. Immunological memory. Advances in immunology. 1993;53:217–265. doi: 10.1016/s0065-2776(08)60501-5. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. Journal of immunological methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- 34.Ventura A, et al. Cre-lox-regulated conditional RNA interference from transgenes. Proc Natl Acad Sci U S A. 2004;101:10380–10385. doi: 10.1073/pnas.0403954101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baldauf HM, et al. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat Med. 2012;18:1682–1689. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agosto LM, et al. The CXCR4-tropic human immunodeficiency virus envelope promotes more-efficient gene delivery to resting CD4+ T cells than the vesicular stomatitis virus glycoprotein G envelope. J Virol. 2009;83:8153–8162. doi: 10.1128/JVI.00220-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boxer MB, Shen M, Auld DS, Wells JA, Thomas CJ. Probe Reports from the NIH Molecular Libraries Program. Bethesda MD: 2010. A small molecule inhibitor of Caspase 1. [Google Scholar]
- 38.Boxer MB, et al. A highly potent and selective caspase 1 inhibitor that utilizes a key 3-cyanopropanoic acid moiety. ChemMedChem. 2010;5:730–738. doi: 10.1002/cmdc.200900531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Randle JC, Harding MW, Ku G, Schonharting M, Kurrle R. ICE/Caspase-1 inhibitors as novel anti-inflammatory drugs. Expert opinion on investigational drugs. 2001;10:1207–1209. doi: 10.1517/13543784.10.7.1207. [DOI] [PubMed] [Google Scholar]
- 40.Stack JH, et al. IL-converting enzyme/caspase-1 inhibitor VX-765 blocks the hypersensitive response to an inflammatory stimulus in monocytes from familial cold autoinflammatory syndrome patients. J Immunol. 2005;175:2630–2634. doi: 10.4049/jimmunol.175.4.2630. [DOI] [PubMed] [Google Scholar]
- 41.Maroso M, et al. Interleukin-1beta biosynthesis inhibition reduces acute seizures and drug resistant chronic epileptic activity in mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2011;8:304–315. doi: 10.1007/s13311-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vezzani A, et al. Current opinion in investigational drugs. Vol. 11. London, England: 2010. ICE/caspase 1 inhibitors and IL-1beta receptor antagonists as potential therapeutics in epilepsy; pp. 43–50. 2000. [PubMed] [Google Scholar]
- 43.Fevrier M, Dorgham K, Rebollo A. CD4+ T cell depletion in human immunodeficiency virus (HIV) infection: role of apoptosis. Viruses. 2011;3:586–612. doi: 10.3390/v3050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biancotto A, et al. HIV-1 induced activation of CD4+ T cells creates new targets for HIV-1 infection in human lymphoid tissue ex vivo. Blood. 2008;111:699–704. doi: 10.1182/blood-2007-05-088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeng M, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. The Journal of clinical investigation. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annual review of medicine. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.