Abstract
Background
Currently, it is well established that cancer arises in chronically inflamed tissue. A number of NOD-like receptors (NLRs) form inflammasomes, intracellular multiprotein complexes critical for generating mature pro-inflammatory cytokines (IL-1β and IL-18). As chronic inflammation of the gastric mucosa is a consequence of Helicobacter pylori infection, we investigated the role of genetic polymorphisms and expression of genes involved in the NLR signalling pathway in H. pylori infection and related gastric cancer (GC).
Materials and Methods
Fifty-one genetic polymorphisms were genotyped in 310 ethnic Chinese (87 non-cardia GC cases and 223 controls with functional dyspepsia). In addition, gene expression of 84 molecules involved in the NLR signalling pathway was assessed in THP-1 cells challenged with two H. pylori strains, GC026 (GC) and 26695 (gastritis).
Results
CARD8-rs11672725, NLRP3-rs10754558, NLRP3-rs4612666, NLRP12-rs199475867 and NLRX1-rs10790286 showed significant associations with GC. On multivariate analysis, CARD8-rs11672725 remained a risk factor (OR: 4.80, 95% CI: 1.39–16.58). Further, NLRP12-rs2866112 increased the risk of H. pylori infection (OR: 2.13, 95% CI: 1.22–3.71). Statistical analyses assessing the joint effect of H. pylori infection and the selected polymorphisms revealed strong associations with GC (CARD8, NLRP3, CASP1 and NLRP12 polymorphisms). In gene expression analyses, five genes encoding NLRs were significantly regulated in H. pylori-challenged cells (NLRC4, NLRC5, NLRP9, NLRP12 and NLRX1). Interestingly, persistent up-regulation of NFKB1 with simultaneous down-regulation of NLRP12 and NLRX1 was observed in H. pylori GC026-challenged cells. Further, NF-κB target genes encoding pro-inflammatory cytokines, chemokines and molecules involved in carcinogenesis were markedly up-regulated in H. pylori GC026-challenged cells.
Conclusions
Novel associations between polymorphisms in the NLR signalling pathway (CARD8, NLRP3, NLRP12, NLRX1, and CASP1) and GC were identified in Chinese individuals. Our genetic polymorphisms and gene expression results highlight the relevance of the NLR signalling pathway in gastric carcinogenesis and its close interaction with NF-κB.
Introduction
The overall incidence of gastric cancer (GC) ranges widely among countries. According to global cancer statistics, a total of 952,000 new GC cases and 723,000 GC-related deaths are estimated to have occurred in 2012, accounting for 6.8% of the total cancer cases and 8.8% of total cancer-related deaths [1]. Over 70% of new cases and deaths occur in developing countries, with the highest incidence rates being reported in East Asia, Eastern Europe, and Central and South America [2].
The bacterial pathogen Helicobacter pylori is an essential aetiological factor for GC (IARC, 1994), however, previous studies suggest that in addition to H. pylori infection and dietary factors, host genetics contribute to GC [3]. Genetic polymorphisms have emerged in recent years as determinants of disease susceptibility and severity, which is particularly true in gastrointestinal malignancy [4]. Therefore, polymorphisms within genes involved in innate and adaptive immunity might play an important role in the pathogenesis of H. pylori infection and development of H. pylori-related complications including GC.
Germ-line encoded receptors known as pattern-recognition receptors (PRRs) are part of the innate immune system and are pivotal for the detection of invariant microbial motifs known as pathogen-associated molecular patterns (PAMPs). PRRs have been divided into five distinct genetic and functional clades: nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), Toll-like receptors, C-type lectin receptors, retinoic acid-inducible gene (RIG)-I-like receptors and absent in melanoma 2 (AIM2)-like receptors [5] [6].
The NLR family not only recognise PAMPs but also damage-associated molecular patterns (DAMPs) in the cytoplasm, which are endogenous ligands produced after tissue injury or cell death [7]. The NLRs characteristic structure includes a central nucleotide-binding and oligomerization (NACHT) domain that is present in all NLR family members, a C-terminal leucine-rich repeats (LRRs) and an N-terminal caspase recruitment (CARD) or pyrin (PYD) domain. Based on phylogenetic analysis of NACHT domains, it was determined that the NLR family comprises three subfamilies: 1) the NOD family which includes NOD1–2, NOD 3/NLRC3, NOD4/NLRC5, NOD5/NLRX1 and CIITA, 2) the NLRPs including NLRP1–14 (also known as NALPs), and 3) the IPAF subfamily which consists of IPAF (NLRC4) and NAIP [7]. The NLRP3 inflammasome, the most fully characterized inflammasome, consists of the NLRP3 scaffold protein, the apoptosis associated speck-like protein (ASC) and caspase-1. NLRP3 interacts and recruits the adaptor ASC via PYD-PYD interaction [8]. This interaction leads to the recruitment of caspase-1, an intracellular aspartate specific cysteine protease, which would subsequently lead to the maturation and release of proinflammatory cytokines such as IL-1β and IL-18.
In H. pylori infection, the first four physical-chemical barriers are the mucus layer, gastric epithelial cells, the TLRs and the NLRs. A limited number of studies have assessed the interaction between the NLR signalling pathway and H. pylori. For example, an initial study by Basak et al. [9] showed that not only H. pylori lipopolysaccharide (LPS) activates caspase-1 but that this caspase-1 activation is involved in LPS-induced IL-1β maturation. Later, Hitzler et al. [10] showed that H. pylori infection activates caspase-1, leading to IL-1β/IL-18 processing and secretion, both in cultured dendritic cells (DCs) and in vivo. Consistently, two recent studies, using human gastric cell lines, confirmed increased expression of caspase-1, IL-1β and IL-18 in H. pylori-infected cells [11], [12]. Further, a recent study by Kim et al. [13] has shown that secretion of IL-1β by DCs infected with H. pylori requires TLR2, NOD2 and the NLRP3 inflammasome.
Therefore, previous studies clearly show that, in response to H. pylori, inflammasome-dependent caspase-1 activation is critical for generating mature pro-inflammatory cytokines that are crucial for Th1 responses associated with gastric immunopathology. Given that little is known about the role of inflammasomes and other molecules involved in the NLR signalling pathway in response to H. pylori infection, and that functionally relevant polymorphisms in genes of this arm of the immune system have the potential to affect the magnitude and direction of the host response against the infection, we investigated the role of the NLR signalling pathway, including inflammasome-related molecules, in H. pylori-related GC development by assessing 51 genetic polymorphisms in Chinese individuals, a known high risk population for GC, and addressing the gene expression of 84 molecules involved in NLR signalling pathways in a monocytic cell line upon exposure to H. pylori.
Materials and Methods
Genotyping of Polymorphisms Involved in the NOD-like Receptor Signalling Pathway
Ethics statement
This study was approved by the Human Ethics Committee (HREC) of the University of New South Wales (UNSW) (HREC 08115 and HREC 02144). Written informed consent was obtained from each individual recruited to the study.
Study subjects
Subjects were ethnic Chinese individuals who underwent upper gastrointestinal endoscopy at the Changi General Hospital (Singapore) and the University Hospital of Malaysia (Kuala Lumpur), between January 2004 and April 2007. Patients known to be infected with the human immunodeficiency virus or who had been prescribed non-steroidal anti-inflammatory drugs, anti-microbial agents or acid suppressants in the two-month period prior to recruitment were excluded.
Eighty-seven patients newly diagnosed with a primary non-cardia GC (International Classification of Diseases, 9th revision, code 151) based on histological confirmation were invited to participate in the study. The control group comprised 223 individuals diagnosed with functional dyspepsia (FD), which was defined as persistent or recurrent symptoms (pain or discomfort centred in the upper abdomen) in the absence of organic disease (including at upper endoscopy), in accordance with Rome II classification system [14].
Helicobacter pylori detection
H. pylori IgG antibodies in these Chinese individuals were determined using an in-house enzyme-linked immunosorbent assay [15] and immunoblot (MPD Helico Blot 2.1, MP Biomedicals, Australia).
Polymorphisms selection
Electronic databases (PUBMED, Scopus, Science Direct, Ovid, Biosis Previews, Scirus databases, CINAHL, IMBIOMED, Scielo and LILACS) were searched up to February 2013 for polymorphisms involved in the NLR signalling pathway that were associated with cancer, infectious disease or were functionally relevant. Fifty one polymorphisms in 6 genes, which were reported to have a minor allele frequency >1% in the National Center for Biotechnology Information (NCBI) dbSNP, were selected for analysis (Table S1 in Tables S1).
Genotyping techniques
For genotyping of the 51 selected polymorphisms in the NLR signalling pathway of each individual included in the study, genomic DNA was extracted from peripheral whole blood samples using the QIAamp Blood DNA Mini Kit as described by the manufacturer (Qiagen; Chadstone, Australia). DNA was rehydrated in sterile water and normalised to 10 ng/µl for customized SNP genotyping through the application of matrix assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry, the Sequenom MassARRAY iPLEX?assay (San Diego, CA, USA) [16], [17] at the Australian Genome Research Facility Ltd, St Lucia, University of Queensland, Australia.
One polymorphism that could not be included in the Sequenom MassARRAY iPLEX assay, known as NLRP3–42 bp-VNTR, was genotyped through standard PCR. Primers were designed with Oligo Primer Analysis Software version 6.71 (Molecular Biology Insights, Inc; Colorado Springs, USA). Experiments were performed using the 2720 Thermal Cycler (Applied Biosystems; Foster City, USA). PCR products were subjected to 1.5% agarose gel electrophoresis and visualised under UV transillumination using the Gel Doc 2000 System (Bio-Rad; Hercules, USA). The primer sequences and thermal cycling conditions for genotyping of this polymorphism are described in Table S2 in in Tables S1.
Four polymorphisms (CARD8-rs2043211, CARD8-rs6509368, CARD8-rs12984929 and NLRP3-rs10754558) genotyped by MALDI-TOF mass spectrometry were randomly selected for confirmation of the results using real-time PCR. The primer sequences and thermal cycling conditions are outlined in Table S2 in Tables S1. As a validation of the methodology implemented for the genotyping of NLRP3–42 bp-VNTR, sequencing analyses were performed in 10% of randomly selected study samples, at the Ramaciotti Centre, UNSW, Australia.
Statistical analysis
The unpaired Student’s t-test was used to analyse the clinical characteristics. The direct count method was used to estimate the genotype and allele frequencies. Deviation from Hardy-Weinberg equilibrium (HWE) was tested using the chi-square goodness-of-fit test (χ2). Determination of linkage disequilibrium (LD) was based on a likelihood ratio test in which the significance of the observed likelihood ratio is found by computing the null distribution of this ratio under the hypothesis of linkage equilibrium, using a permutation procedure [18]. Haplotype inference was performed by means of the Expectation-Maximization (EM) algorithm for multi-locus genotypic data [19]. The odds ratios (OR) and 95% confidence intervals (CI) were calculated by means of the Fisher’s exact probability test (two-tailed p-values). In order to correct for confounding factors, a binary logistic regression (LR) adjusted by H. pylori status and gender was performed. P-values<0.05 were considered as statistically significant. Quality filters for exclusion of polymorphisms from the statistical analysis included call rates below 95% and deviation from HWE. The data was analysed by means of the programs Arlequin version 3.1 [20], GraphPad Prism version 5.02 (GraphPad Software Inc; San Diego, USA) and SPSS version 19.0.0 (SPSS Inc; Chicago, USA).
Gene Expression of Molecules Involved in the NOD-like Receptor Signalling Pathway
Mammalian cell culture
The human monocytic leukaemia cell line THP-1 (code: TIB-202) (American Type Culture Collection; Manassas, USA), was cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen; Mulgrave, Australia), 1 mM sodium pyruvate (Invitrogen), 2500 mg/L sodium bicarbonate (Invitrogen) and 100 µg/mL penicillin-streptomycin solution (Invitrogen). Cells were maintained in 25-cm2 tissue culture flasks (Greiner-Bio-On; Frickenhausen, Germany) at 37°C with 5% CO2.
Bacterial culture
The H pylori strain GC026 (cagA+, cagE+, cagL+, cagT+, vacA s1 m1+, babA+, oipA+, dupA+ and sabA+) was isolated from a GC patient at the University Hospital of Malaysia, Kuala Lumpur [21], [22]. The H. pylori strain 26695 (cagA+ and vacA s1m1+) was isolated from a patient with gastritis [23] (ATCC code 700392). Both H. pylori strains were grown for two days on horse blood agar plates (Blood Agar Base No.2 supplemented with 6% sterile defibrinated horse blood (Oxoid, Heidelberg West, Vic., Australia) at 37°C in an anaerobic jar containing a gas generating kit (Oxoid) to provide a microaerobic atmosphere of 6% O2, 10% CO2 and 84% N2.
Infection assay
THP-1 cells were seeded in a 6-well culture plate at a concentration of 5×105 cells/ml and subsequently differentiated into macrophages with phorbol myrastate acetate (Sigma-Aldrich) for 72 hours [24]. Prior to bacterial infection, mammalian cells were incubated in antibiotic-free medium. The H. pylori strains GC026 and 26695 were then added to the medium at a multiplicity of infection of 1, and incubated for 6 hours at 37°C and 5% CO2. The bacterial concentration added was determined based on a standard curve and optical density (OD) readings at 595 nm using a Bio-Rad microplate reader model 550 (Bio-Rad). The actual concentration added was confirmed by counting colony forming units (CFU) grown on horse blood agar plates after serial dilution of the bacterial suspension.
RNA extraction, cDNA preparation and PCR arrays
After infection, non-challenged and H. pylori-challenged THP-1 cells were used for the isolation of mRNA using the Qiagen RNeasy Mini Kit as described by the manufacturer (Qiagen). For the analysis of the gene expression of 84 molecules involved in the NLR signalling pathway, cDNA was synthesised from 0.8 µg total RNA using the RT2 First Strand cDNA Kit (Qiagen) and further analysed using the Human Inflammasome RT2 Profiler PCR array (PAHS-097R) (Qiagen) as recommended by manufacturer. Gene expression profiles were obtained from three independent experiments of H. pylori GC026-infected, H. pylori 26695-infected and corresponding non-infected (control) samples. The experiments were performed employing the Rotor-Gene Q cycler (Corbett Life Sciences; Doncaster, Australia).
For gene expression data analysis, the ΔΔ Ct-based method of relative quantification was implemented using the Web-based RT2 Profiler PCR Array Data Analysis version 3.5 (http://pcrdataanalysis.sabiosciences.com/pcr/arrayanalysis.php). At least two-fold changes (≥2, ≤0.5) and P-values<0.05 were considered as statistically significant.
SDS-PAGE and Western Blotting
THP-1 cells were seeded, differentiated and infected at a MOI of 1, as previously described. Proteins were extracted using RIPA buffer, separated on 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels, and transferred to methanol-treated polyvinylidine difluoride membranes using the Trans-blot cell transfer system (Bio-Rad). Membranes were immunolabeled with rabbit anti-human polyclonal antibodies against NLRX1 (1∶200) or mouse anti-human monoclonal antibodies against β-actin (1∶1000) (Santa Cruz). Goat anti-rabbit and anti-mouse IgG antibodies coupled to HRP (1∶2000; Bio-Rad) were used as secondary antibodies, respectively. Membranes were probed in accordance with the Pierce ECL Western Blotting Substrate protocol (Thermo Scientific; Scoresby, Australia).
Results
Clinical Characteristics
The clinical characteristics of the study subjects including gender, H. pylori infection status and median age are shown in Table S3 in Tables S1. Male gender was found to be more predominant in GC patients than in FD controls, showing a positive association with the development of GC in this ethnic Chinese population (OR: 2.15, 95% CI: 1.29–3.58, P-value: 0.0036). H. pylori infection was found to be present in 68.7% of individuals in this study (73/87 GC patients and 140/223 FD controls). Comparison of the prevalence of H. pylori infection in GC patients and FD controls showed that H. pylori infection was a risk factor for the development of GC (OR: 2.38, 95% CI: 1.41–3.99, P-value:<0.0001). Even though subjects in this study were matched according to 5-years age groups, the median age of GC patients (65.3 yrs) was significantly higher than that of FD controls (54.3 yrs) (P-value:<0.0001).
Polymorphisms in Genes Involved in the NOD-like Receptor Signalling Pathway are Associated with Gastric Cancer in Ethnic Chinese Individuals
Fifty-one polymorphisms in genes involved in the NLR signalling pathway were genotyped in 310 ethnic Chinese individuals (87 GC cases and 223 FD controls). The call rate of all the selected polymorphisms was 97–100% in this study. All polymorphisms in the NLR signalling pathway were found to be in HWE in the control group showing non-significant χ2 values except for CARD8-rs12984929 (χ2: 35.98), CARD8-rs4802445 (χ2: 5.87) and CARD8-rs6509368 (χ2: 5.43). Twenty one polymorphisms in NLRP3 (n = 5), NLRP12 (n = 3), NLRX1 (n = 3), ASC (n = 4) and CASP1 (n = 6) were found to be non-polymorphic in this ethnic Chinese population (Table S1 in in Tables S1).
Allele and genotype frequencies as well as ORs and 95% CI for the remaining 27 polymorphisms are shown in Table 1 and Table S4 in Tables S1. The CARD8-rs11672725 TT genotype showed a strong association with GC in the bivariate statistical analysis (OR: 4.80, 95% CI: 1.39–16.58). In addition to CARD8-rs11672725, another four polymorphisms showed borderline results in the bivariate analysis (NLRP3-rs10754558, NLRP3-rs4612666, NLRP12-rs199475867 and NLRX1-rs10790286) (Table 1). Further multivariate statistical analyses, adjusting for H. pylori infection and male gender, showed that the CARD8-rs11672725 TT genotype remained a risk factor for GC in this ethnic Chinese population (Table 2).
Table 1. Association between polymorphisms involved in the NOD-like receptors signalling pathway and risk of gastric cancer in ethnic Chinese individuals (bivariate statistical analysis).
| Gene | Polymorphism | Nucleotide change | AlleleAnalysis | GenotypeAnalysis | |||||||||||||||||
| GC cases | FD controls | OR | 95% CI | P-value* | GC cases | FD controls | XX vs YY | XX vs XY | |||||||||||||
| X | Y | X | Y | XX | XY | YY | XX | XY | YY | OR | 95% CI | P-value* | OR | 95% CI | P-value* | ||||||
| CARD8 | rs10405717 | C>T | 118 | 54 | 294 | 130 | 1.04 | 0.70–1.52 | 0.9221 | 40 | 38 | 8 | 98 | 98 | 16 | 1.23 | 0.49–3.09 | 0.6374 | 0.95 | 0.56–1.61 | 0.8939 |
| rs11670259 | C>T | 138 | 34 | 340 | 84 | 1.00 | 0.64–1.56 | 1 | 55 | 28 | 3 | 134 | 72 | 6 | 1.22 | 0.29–5.05 | 0.723 | 0.95 | 0.55–1.62 | 0.8918 | |
| rs11672725 | C>T | 130 | 42 | 332 | 84 | 1.28 | 0.84–1.95 | 0.2701 | 53 | 24 | 9 | 129 | 74 | 5 | 4.38 | 1.40–13.69 | 0.0134 | 0.79 | 0.45–1.38 | 0.4833 | |
| rs16981829 | T>C | 79 | 93 | 208 | 210 | 1.17 | 0.82–1.67 | 0.4156 | 19 | 41 | 26 | 57 | 94 | 58 | 1.35 | 0.68–2.70 | 0.482 | 1.31 | 0.69–2.47 | 0.4313 | |
| rs1966625 | G>A | 142 | 30 | 336 | 88 | 0.81 | 0.51–1.28 | 0.4272 | 58 | 26 | 2 | 130 | 76 | 6 | 0.75 | 0.15–3.82 | 1 | 0.77 | 0.45–1.32 | 0.3471 | |
| rs2043211 | A>T | 79 | 93 | 210 | 214 | 1.16 | 0.81–1.65 | 0.4695 | 21 | 37 | 28 | 54 | 102 | 56 | 1.29 | 0.65–2.53 | 0.4956 | 0.93 | 0.50–1.75 | 0.8725 | |
| rs4802449 | G>A | 133 | 39 | 326 | 98 | 0.98 | 0.64–1.49 | 1 | 51 | 31 | 4 | 122 | 82 | 8 | 1.20 | 0.34–4.15 | 0.7522 | 0.90 | 0.53–1.53 | 0.7894 | |
| NLRP3 | rs10754558 | C>G | 112 | 60 | 244 | 178 | 0.73 | 0.51–1.06 | 0.1165 | 38 | 36 | 12 | 74 | 96 | 41 | 0.57 | 0.27–1.21 | 0.1518 | 0.73 | 0.42–1.26 | 0.2674 |
| rs10925026 | A>C | 103 | 69 | 250 | 176 | 0.95 | 0.66–1.37 | 0.8543 | 31 | 41 | 14 | 72 | 106 | 35 | 0.93 | 0.44–1.97 | 1 | 0.90 | 0.52–1.56 | 0.7769 | |
| rs12079994 | G>A | 149 | 23 | 373 | 53 | 1.09 | 0.64–1.84 | 0.7866 | 63 | 23 | 0 | 165 | 43 | 5 | 0.24 | 0.01–4.35 | 0.3273 | 1.40 | 0.78–2.51 | 0.2831 | |
| rs1539019 | G>T | 103 | 69 | 247 | 177 | 0.93 | 0.65–1.34 | 0.783 | 30 | 43 | 13 | 70 | 107 | 35 | 0.87 | 0.40–1.87 | 0.847 | 0.94 | 0.54–1.63 | 0.8873 | |
| rs3806265 | T>C | 81 | 91 | 223 | 203 | 1.23 | 0.87–1.76 | 0.2783 | 21 | 39 | 26 | 61 | 101 | 51 | 1.48 | 0.75–2.94 | 0.2987 | 1.12 | 0.60–2.08 | 0.7562 | |
| rs4612666 | C>T | 79 | 91 | 227 | 199 | 1.31 | 0.92–1.88 | 0.1467 | 21 | 37 | 27 | 62 | 103 | 48 | 1.66 | 0.84–3.29 | 0.1675 | 1.06 | 0.57–1.97 | 0.876 | |
| rs4925650 | G>A | 100 | 72 | 225 | 191 | 0.85 | 0.59–1.22 | 0.4121 | 33 | 34 | 19 | 67 | 91 | 50 | 0.77 | 0.39–1.51 | 0.5001 | 0.76 | 0.43–1.35 | 0.3802 | |
| NLRP12 | rs104895565 | A>del | 171 | 1 | 433 | 3 | 0.84 | 0.09–8.18 | 1 | 85 | 1 | 0 | 215 | 3 | 0 | 2.52 | 0.05–128.20 | 1 | 0.84 | 0.09–8.22 | 1 |
| rs199475867 | T>G | 172 | 0 | 427 | 9 | 0.13 | 0.01–2.26 | 0.0671 | 86 | 0 | 0 | 209 | 9 | 0 | 2.42 | 0.05–123.20 | 1 | 0.13 | 0.01–2.22 | 0.0651 | |
| rs2866112 | C>G | 132 | 40 | 333 | 101 | 1.00 | 0.66–1.52 | 1 | 51 | 30 | 5 | 128 | 77 | 12 | 1.05 | 0.35–3.12 | 1 | 0.98 | 0.57–1.67 | 1 | |
| rs4419163 | T>A | 125 | 45 | 309 | 113 | 0.98 | 0.66–1.47 | 1 | 46 | 33 | 6 | 115 | 79 | 17 | 0.88 | 0.33–2.38 | 1 | 1.04 | 0.61–1.78 | 0.8927 | |
| rs4539722 | A>G | 90 | 80 | 218 | 214 | 0.91 | 0.63–1.30 | 0.5884 | 24 | 42 | 19 | 58 | 102 | 56 | 0.82 | 0.41–1.66 | 0.5964 | 1.00 | 0.55–1.81 | 1 | |
| NLRX1 | rs10790286 | T>C | 107 | 65 | 297 | 137 | 1.32 | 0.91–1.90 | 0.1523 | 33 | 41 | 12 | 107 | 83 | 27 | 1.44 | 0.66–3.16 | 0.4051 | 1.60 | 0.93–2.75 | 0.1 |
| ASC | rs151056688 | C>T | 162 | 0 | 423 | 1 | 0.87 | 0.04–21.45 | 1 | 81 | 0 | 0 | 211 | 1 | 0 | 2.50 | 0.05–127.30 | 1 | 0.83 | 0.03–20.70 | 1 |
| rs8056505 | T>C | 120 | 52 | 320 | 116 | 1.20 | 0.81–1.76 | 0.3669 | 42 | 36 | 8 | 120 | 80 | 18 | 1.27 | 0.51–3.14 | 0.6351 | 1.29 | 0.76–2.18 | 0.4168 | |
| CASP1 | rs2282659 | A>G | 123 | 49 | 316 | 116 | 1.09 | 0.73–1.61 | 0.6867 | 43 | 37 | 6 | 117 | 82 | 17 | 0.96 | 0.36–2.60 | 1 | 1.23 | 0.73–2.07 | 0.5037 |
| rs501192 | G>A | 172 | 0 | 435 | 1 | 0.84 | 0.03–20.77 | 1 | 86 | 0 | 0 | 217 | 1 | 0 | 2.51 | 0.05–127.90 | 1 | 0.84 | 0.03–20.79 | 1 | |
| rs530537 | A>G | 123 | 49 | 310 | 122 | 1.01 | 0.68–1.50 | 1 | 43 | 37 | 6 | 117 | 76 | 23 | 0.71 | 0.27–1.86 | 0.6459 | 1.33 | 0.78–2.24 | 0.3449 | |
| rs61751523 | T>C | 160 | 10 | 415 | 21 | 1.24 | 0.57–2.68 | 0.6815 | 76 | 8 | 1 | 197 | 21 | 0 | 7.75 | 0.31–192.40 | 0.281 | 0.99 | 0.42–2.33 | 1 | |
GC, gastric cancer; FD, functional dyspepsia; X, wild allele; Y, mutant allele; XX, wild homozygote; XY, heterozygote; YY, mutant homozygote; OR, odds ratio; CI, confidence intervals.
*Fisher’s exact test two-tailed P-value.
Table 2. Association between identified risk factors and gastric cancer in ethnic Chinese individuals (binary logistic regression).
| Variable | Adjusted OR | 95% CI | P-value* |
| H. pylori infection | 3.22 | 1.68–6.19 | 0.0001 |
| Male gender | 1.99 | 1.16–3.41 | 0.013 |
| CARD8 rs11672725 TT | 4.8 | 1.39–16.58 | 0.013 |
OR, odds ratio; CI, confidence intervals.
*P-values were obtained from a binary logistic regression (backward Wald) adjusted by Helicobacter pylori status and gender.
Haplotype-based analyses are a powerful approach to dissect the architecture of complex diseases. In the current study, the EM algorithm identified 25 haplotypes with a frequency >0.01 in GC cases (Table S5 in Tables S1). Using the major haplotype in the control group as the reference haplotype, two CARD8-NLRP12 (Hap1 and Hap8), one NLRP3 (Hap3) and three NLRX1-CASP1 (Hap21, 23 and 24) haplotypes were found to increase the risk of GC in this ethnic Chinese population. Interestingly, Hap1 harbours the CARD8-rs11672725 T allele, and Hap21 and Hap23 harbour the NLRX1-rs10790286 C allele, which shows consistency with the results obtained from the allele and genotype analyses.
Polymorphisms in Genes Involved in the NOD-like Receptor Signalling Pathway are Associated with Helicobacter Pylori Infection in Ethnic Chinese Individuals
Given that H. pylori is known to be a major risk factor associated with GC, further analyses were performed to assess the association between genetic polymorphisms involved in the NLR signalling pathway and H. pylori infection. Interestingly, our ethnic Chinese case-control study showed that NLRX1-rs10790286, NLRP12-rs2866112, NLRP12-rs4419163 and ASC-rs8056505 were associated with a significantly increased risk of H. pylori infection in these individuals (Table S6 in Tables S1). Multivariate statistical analysis confirmed that NLRP12-rs2866112 was associated with an increased risk of H. pylori in Chinese individuals (OR: 2.13, 95% CI: 1.22–3.71).
Joint Effect of Helicobacter Pylori Infection and Genetic Polymorphisms Markedly Increases the Risk of Gastric Cancer in Ethnic Chinese Individuals
Further analyses were performed to assess not only the presence or absence of the selected polymorphisms but also H. pylori status in relation to the risk of GC, in an attempt to determine the existence of biological interaction in the form of synergism or antagonism. Strikingly, individuals harbouring ten of the selected polymorphisms (CARD8-rs10405717, NLRP3-rs12079994, NLRP3-rs3806265, NLRP3-rs4612666, NLRP12-rs2866112, NLRP12-rs4419163, NLRX1-rs10790286, CASP1-rs2282659, CASP1-rs530537 and CASP1-rs61751523) and infected with H. pylori were observed to be at most risk of GC, the majority of these ORs being in the range 4.0–5.0 (Table 3). In contrast, in the absence of H. pylori infection, CARD8-rs2043211 significantly decreased the risk of GC (OR: 0.19, 95% CI: 0.06–0.63).
Table 3. Joint effect of Helicobacter pylori infection and genetic polymorphisms involved in the NOD-like receptors signalling pathway on gastric cancer risk.
| Polymorphism | HP status | Genotype | Cases | Controls | OR | 95% CI | P-value* |
| CARD8 rs10405717 | HP (−) | CC | 8 | 34 | 1 | ||
| HP (−) | T carrier | 6 | 45 | 0.57 | 0.18–1.79 | 0.3897 | |
| HP (+) | CC | 32 | 64 | 2.13 | 0.88–5.12 | 0.1051 | |
| HP (+) | T carrier | 40 | 69 | 2.46 | 1.04–5.84 | 0.0504 | |
| CARD8 rs2043211 | HP (−) | AA | 8 | 16 | 1 | ||
| HP (−) | T carrier | 6 | 63 | 0.19 | 0.058–0.63 | 0.0071 | |
| HP (+) | AA | 13 | 38 | 0.68 | 0.24–1.97 | 0.5833 | |
| HP (+) | T carrier | 59 | 95 | 1.24 | 0.50–3.08 | 0.8213 | |
| NLRP3 rs12079994 | HP (−) | GG | 10 | 59 | 1 | ||
| HP (−) | A carrier | 4 | 21 | 1.12 | 0.32–3.97 | 1 | |
| HP (+) | GG | 53 | 106 | 2.95 | 1.40–6.23 | 0.0036 | |
| HP (+) | A carrier | 19 | 27 | 4.15 | 1.70–10.12 | 0.0019 | |
| NLRP3 rs3806265 | HP (−) | TT | 4 | 23 | 1 | ||
| HP (−) | C carrier | 10 | 57 | 1.01 | 0.29–3.55 | 1 | |
| HP (+) | TT | 17 | 38 | 2.57 | 0.77–8.60 | 0.178 | |
| HP (+) | C carrier | 55 | 95 | 3.33 | 1.09–10.13 | 0.0276 | |
| NLRP3 rs4612666 | HP (−) | CC | 3 | 21 | 1 | ||
| HP (−) | T carrier | 11 | 59 | 1.31 | 0.33–5.14 | 1 | |
| HP (+) | CC | 18 | 41 | 3.07 | 0.81–11.63 | 0.1025 | |
| HP (+) | T carrier | 53 | 92 | 4.03 | 1.15–14.16 | 0.0201 | |
| NLRP12 rs2866112 | HP (−) | CC | 8 | 59 | 1 | ||
| HP (−) | G carrier | 6 | 22 | 2.01 | 0.63–6.46 | 0.34 | |
| HP (+) | CC | 29 | 69 | 3.10 | 1.32–7.30 | 0.008 | |
| HP (+) | G carrier | 43 | 67 | 4.73 | 2.06–10.88 | <0.0001 | |
| NLRP12 rs4419163 | HP (−) | TT | 11 | 48 | 1 | ||
| HP (−) | A carrier | 3 | 31 | 0.42 | 0.11–1.64 | 0.243 | |
| HP (+) | TT | 35 | 67 | 2.28 | 1.05–4.94 | 0.0459 | |
| HP (+) | A carrier | 36 | 65 | 2.42 | 1.12–5.23 | 0.0304 | |
| NLRX1 rs10790286 | HP (−) | TT | 7 | 45 | 1 | ||
| HP (−) | C carrier | 7 | 36 | 1.25 | 0.40–3.89 | 0.7755 | |
| HP (+) | TT | 26 | 62 | 2.70 | 1.08–6.76 | 0.0389 | |
| HP (+) | C carrier | 46 | 74 | 4.00 | 1.66–9.61 | 0.0011 | |
| CASP1 rs2282659 | HP (−) | AA | 5 | 39 | 1 | ||
| HP (−) | G carrier | 9 | 42 | 1.67 | 0.52–5.42 | 0.563 | |
| HP (+) | AA | 38 | 78 | 3.80 | 1.39–10.42 | 0.0087 | |
| HP (+) | G carrier | 34 | 57 | 4.65 | 1.67–12.95 | 0.0021 | |
| CASP1 rs530537 | HP (−) | AA | 5 | 39 | 1 | ||
| HP (−) | G carrier | 9 | 42 | 1.67 | 0.52–5.42 | 0.563 | |
| HP (+) | AA | 38 | 78 | 3.80 | 1.39–10.42 | 0.0087 | |
| HP (+) | G carrier | 34 | 57 | 4.65 | 1.67–12.95 | 0.0021 | |
| CASP1 rs61751523 | HP (−) | TT | 14 | 71 | 1 | ||
| HP (−) | C carrier | 0 | 11 | 0.21 | 0.01–3.85 | 0.358 | |
| HP (+) | TT | 62 | 126 | 2.50 | 1.30–4.78 | 0.0054 | |
| HP (+) | C carrier | 9 | 10 | 4.56 | 1.57–13.28 | 0.0113 | |
HP, Helicobacter pylori; OR, odds ratio; CI, confidence intervals.
*Fisher’s exact test two-tailed P-value.
Helicobacter Pylori Influences the Expression of Several Molecules Involved in the NOD-like Receptor Signalling Pathway
To further characterise the effect of H. pylori on molecules involved in the NLR signalling pathway, we assessed the expression of 84 genes encoding NLRs, other inflammasome components, negative regulators, pro-inflammatory cytokines, pro-inflammatory caspases and molecules involved in downstream signalling, in THP-1-derived macrophages following exposure to two different H. pylori strains (GC026 and 26695). In H. pylori GC026-challenged THP1 cells, 49 genes were differentially expressed when compared to the control group (Table S7 in Tables S1 and Figure S1 in Figures S1). Of these, statistically significant up-regulation and down-regulation of at least two-fold was found in 17 and 28 genes, respectively (Tables 4 and 5). Thirty five genes were differentially expressed between the control group and the group exposed to H. pylori 26695 (Table S7 in Tables S1 and Figure S2 in Figures S1). Of these, statistically significant up-regulation and down-regulation of at least two-fold was found in 5 and 23 genes, respectively (Tables 4 and 5).
Table 4. Significant up-regulation of genes involved in the NOD-like receptor signalling pathway in a monocytic cell line upon exposure to Helicobacter pylori.
| Regulation* | Gene symbol | Gene name | Helicobacter pylori GC026 | Helicobacter pylori 26695 | ||||
| Medianfoldchange | 95% CI | P-value | Medianfoldchange | 95% CI | P-value | |||
| Up-regulated | BIRC3 | Baculoviral IAP repeat containing 3 | 12.29 | (8.37, 16.20) | 0.0016 | 0.99 | (0.00001, 2.11) | 0.7263 |
| CASP5 | Caspase 5, apoptosis-relatedcysteine peptidase | 3.09 | (2.63, 3.55) | 0.0004 | 1.00 | (1.00, 1.00) | 0.0000 | |
| CCL5 | Chemokine (C-C motif)ligand 5 | 2.28 | (1.30, 3.26) | 0.0366 | 0.94 | (0.00001, 2.19) | 0.9940 | |
| CXCL1 | Chemokine (C-X-C motif)ligand 1 | 22.45 | (8.83, 36.08) | 0.0006 | 2.95 | (0.75, 5.14) | 0.0188 | |
| CXCL2 | Chemokine (C-X-C motif)ligand 2 | 60.08 | (27.53, 92.64) | 0.0002 | 20.73 | (0.00001, 42.73) | 0.0575 | |
| IFNB1 | Interferon, beta 1,fibroblast | 32.50 | (15.41, 49.58) | 0.0001 | 3.49 | (0.57, 6.41) | 0.0642 | |
| IL12A | Interleukin 12A | 3.59 | (2.49, 4.69) | 0.0003 | 0.60 | (0.00001, 1.32) | 0.4203 | |
| IL12B | Interleukin 12B | 434.20 | (0.00001, 1093.32) | 0.0399 | 5.36 | (0.00001, 17.63) | 0.2003 | |
| IL1B | Interleukin 1, beta | 3.70 | (1.56, 5.83) | 0.0086 | 63.26 | (0.00001, 478.44) | 0.0446 | |
| IL33 | Interleukin 33 | 4.78 | (3.81, 5.74) | 0.0000 | 15.82 | (0.00001, 43.71) | 0.0522 | |
| IL6 | Interleukin 6 | 262.39 | (32.53, 492.24) | 0.0008 | 94.57 | (0.00001, 234.99) | 0.0292 | |
| NFKB1 | Nuclear factor of kappa light polypeptidegene enhancer in B-cells 1 | 2.50 | (1.90, 3.10) | 0.0068 | 0.58 | (0.00001, 1.25) | 0.4679 | |
| NFKBIA | Nuclear factor of kappa light polypeptidegene enhancer in B-cells inhibitor, alpha | 6.60 | (4.38, 8.82) | 0.0001 | 1.46 | (0.43, 2.49) | 0.3920 | |
| P2RX7 | Purinergic receptor P2X,ligand-gated ion channel, 7 | 6.99 | (5.17, 8.81) | 0.0017 | 3.10 | (0.00001, 7.12) | 0.2016 | |
| PTGS2 | Prostaglandin-endoperoxidesynthase 2 | 54.03 | (10.88, 97.17) | 0.0247 | 19.56 | (7.32, 31.81) | 0.0161 | |
| RIPK2 | Receptor-interacting serine-threoninekinase 2 | 3.09 | (2.26, 3.92) | 0.0035 | 0.67 | (0.13, 1.21) | 0.4007 | |
| TNF | Tumor necrosis factor | 32.12 | (19.19, 45.05) | 0.0003 | 14.32 | (7.45, 21.19) | 0.0108 | |
CI, confidence intervals. * Corresponds to genes showing at least two-fold changes (≥2 indicates up-regulation) and P-values<0.05, in THP-1 cells challenged with H. pylori GC026 and/or 26695.
Table 5. Significant down-regulation of genes involved in the NOD-like receptor signalling pathway in a monocytic cell line upon exposure to Helicobacter pylori.
| Regulation* | Genesymbol | Gene name | Helicobacter pylori GC026 | Helicobacter pylori 26695 | ||||
| Medianfold change | 95% CI | P-value | Medianfold change | 95% CI | P-value | |||
| Down-regulated | AIM2 | Absent in melanoma 2 | 0.07 | (0.00001, 0.15) | 0.0016 | 0.06 | (0.01, 0.11) | 0.0110 |
| BCL2L1 | BCL2-like 1 | 0.09 | (0.02, 0.16) | 0.0332 | 0.23 | (0.00001, 0.56) | 0.1923 | |
| CARD6 | Caspase recruitmentdomain family, member 6 | 0.29 | (0.18, 0.41) | 0.0029 | 0.22 | (0.00001, 0.59) | 0.1449 | |
| CASP1 | Caspase 1, apoptosis-relatedcysteine peptidase | 1.04 | (0.85, 1.22) | 0.7008 | 0.32 | (0.12, 0.52) | 0.0182 | |
| CASP4 | Caspase 4, apoptosis-relatedcysteine peptidase | 0.54 | (0.38, 0.71) | 0.0093 | 0.21 | (0.11, 0.32) | 0.0220 | |
| CASP8 | Caspase 8, apoptosis-relatedcysteine peptidase | 0.28 | (0.20, 0.36) | 0.0054 | 0.06 | (0.00001, 0.17) | 0.0060 | |
| CCL2 | Chemokine (C-C motif) ligand 2 | 1.40 | (1.02, 1.79) | 0.0737 | 0.12 | (0.05, 0.19) | 0.0272 | |
| CCL7 | Chemokine (C-C motif) ligand 7 | 0.59 | (0.45, 0.73) | 0.0124 | 0.11 | (0.00001, 0.24) | 0.0417 | |
| CD40LG | CD40 ligand | 0.05 | (0.04, 0.06) | 0.0000 | 2.15 | (0.00001, 7.38) | 0.3977 | |
| CHUK | Conserved helix-loop-helixubiquitous kinase | 0.86 | (0.32, 1.41) | 0.4720 | 0.20 | (0.05, 0.35) | 0.0223 | |
| CTSB | Cathepsin B | 0.44 | (0.42, 0.46) | 0.0000 | 0.15 | (0.08, 0.22) | 0.0013 | |
| FADD | Fas (TNFRSF6)-associatedvia death domain | 0.10 | (0.00001, 0.20) | 0.0255 | 0.04 | (0.00001, 0.15) | 0.1890 | |
| HSP90AA1 | Heat shock protein 90 kDa alpha(cytosolic), class A member 1 | 0.63 | (0.56, 0.71) | 0.0031 | 0.16 | (0.10, 0.22) | 0.0003 | |
| HSP90AB1 | Heat shock protein 90 kDa alpha(cytosolic), class B member 1 | 0.96 | (0.55, 1.38) | 0.7407 | 0.11 | (0.01, 0.22) | 0.0204 | |
| HSP90B1 | Heat shock protein 90 kDa beta(Grp94), member 1 | 0.42 | (0.26, 0.58) | 0.0040 | 0.19 | (0.05, 0.33) | 0.0894 | |
| IKBKB | Inhibitor of kappa lightpolypeptide gene enhancer inB-cells, kinase beta | 0.65 | (0.42, 0.87) | 0.0970 | 0.10 | (0.03, 0.17) | 0.0364 | |
| IL18 | Interleukin 18 | 0.43 | (0.29, 0.57) | 0.0072 | 0.12 | (0.02, 0.22) | 0.0694 | |
| IRF2 | Interferon regulatory factor 2 | 0.21 | (0.13, 0.28) | 0.0108 | 0.09 | (0.01, 0.17) | 0.0796 | |
| MAP3K7 | Mitogen-activated proteinkinase kinase kinase 7 | 0.54 | (0.44, 0.64) | 0.0071 | 0.15 | (0.07, 0.23) | 0.0053 | |
| TAB1 | TGF-beta activated kinase1/MAP3K7 binding protein 1 | 0.09 | (0.05, 0.14) | 0.0021 | 0.09 | (0.00001, 0.19) | 0.1703 | |
| TAB2 | TGF-beta activated kinase1/MAP3K7 binding protein 2 | 0.39 | (0.29, 0.50) | 0.0093 | 0.12 | (0.00001, 0.27) | 0.2276 | |
| MAPK1 | Mitogen-activated protein kinase 1 | 0.18 | (0.09, 0.26) | 0.0047 | 0.12 | (0.03, 0.21) | 0.0546 | |
| MAPK11 | Mitogen-activated protein kinase 11 | 0.91 | (0.39, 1.43) | 0.7202 | 0.08 | (0.00001, 0.17) | 0.0459 | |
| MAPK12 | Mitogen-activated protein kinase 12 | 0.16 | (0.09, 0.23) | 0.0050 | 0.12 | (0.00001, 0.52) | 0.2075 | |
| MAPK13 | Mitogen-activated protein kinase 13 | 0.34 | (0.18, 0.51) | 0.0560 | 0.11 | (0.01, 0.22) | 0.0191 | |
| MAPK3 | Mitogen-activated protein kinase 3 | 0.22 | (0.05, 0.39) | 0.0560 | 0.05 | (0.00001, 0.14) | 0.0325 | |
| MAPK8 | Mitogen-activated protein kinase 8 | 0.40 | (0.10, 0.70) | 0.0318 | 0.00 | (0.00001, 0.03) | 0.3739 | |
| MAPK9 | Mitogen-activated protein kinase 9 | 0.30 | (0.25, 0.35) | 0.0002 | 0.14 | (0.07, 0.21) | 0.0141 | |
| MEFV | Mediterranean fever | 0.25 | (0.15, 0.36) | 0.0090 | 0.17 | (0.00001, 0.55) | 0.1632 | |
| MYD88 | Myeloid differentiationprimary response gene (88) | 0.11 | (0.05, 0.17) | 0.0096 | 0.13 | (0.00001, 0.29) | 0.1700 | |
| NLRC4 | NLR family, CARDdomain containing 4 | 0.12 | (0.07, 0.17) | 0.0003 | 0.06 | (0.01, 0.10) | 0.0357 | |
| NLRC5 | NLR family, CARDdomain containing 5 | 0.14 | (0.10, 0.18) | 0.0003 | 0.04 | (0.02, 0.06) | 0.0131 | |
| NLRP12 | NLR family, pyrindomain containing 12 | 0.03 | (0.01, 0.04) | 0.0163 | 0.11 | (0.00, 0.21) | 0.1137 | |
| NLRP9 | NLR family, pyrindomain containing 9 | 0.77 | (0.30, 1.24) | 0.4548 | 0.12 | (0.00001, 0.26) | 0.0005 | |
| NLRX1 | NLR family member X1 | 0.02 | (0.00001, 0.05) | 0.0176 | 0.22 | (0.00001, 0.85) | 0.4216 | |
| PEA15 | Phosphoprotein enrichedin astrocytes 15 | 0.49 | (0.00001, 1.07) | 0.2752 | 0.27 | (0.11, 0.42) | 0.0112 | |
| PSTPIP1 | Proline-serine-threoninephosphatase interacting protein 1 | 0.13 | (0.07, 0.19) | 0.0066 | 0.03 | (0.00001, 0.06) | 0.0541 | |
| PYCARD | PYD and CARD domaincontaining | 0.05 | (0.03, 0.07) | 0.0001 | 0.03 | (0.01, 0.05) | 0.0068 | |
| RAGE | Renal tumor antigen | 0.48 | (0.00001, 0.99) | 0.2113 | 0.04 | (0.00, 0.08) | 0.0450 | |
| RELA | V-rel reticuloendotheliosis viraloncogene homolog A (avian) | 0.49 | (0.32, 0.67) | 0.0447 | 0.26 | (0.00001, 0.52) | 0.1310 | |
| SUGT1 | SGT1, suppressor of G2allele of SKP1 (S. cerevisiae) | 0.52 | (0.43, 0.60) | 0.0006 | 0.21 | (0.01, 0.42) | 0.0470 | |
| TNFSF11 | Tumor necrosis factor (ligand)superfamily, member 11 | 0.13 | (0.00001, 0.26) | 0.0092 | 0.48 | (0.00001, 1.69) | 0.3440 | |
| TXNIP | Thioredoxin interacting protein | 0.06 | (0.02, 0.10) | 0.0025 | 0.06 | (0.00001, 0.13) | 0.1814 | |
| XIAP | X-linked inhibitor of apoptosis | 0.42 | (0.22, 0.62) | 0.0429 | 0.13 | (0.00001, 0.27) | 0.1044 | |
CI, confidence intervals.
*Corresponds to genes showing at least two-fold changes (≤0.5 indicates down-regulation) and P-values<0.05, in THP-1 cells challenged with H. pylori GC026 and/or 26695.
Helicobacter Pylori Strains Associated with Gastric Cancer and Gastritis Lead to Different Expression Levels of Cytokines and Chemokines
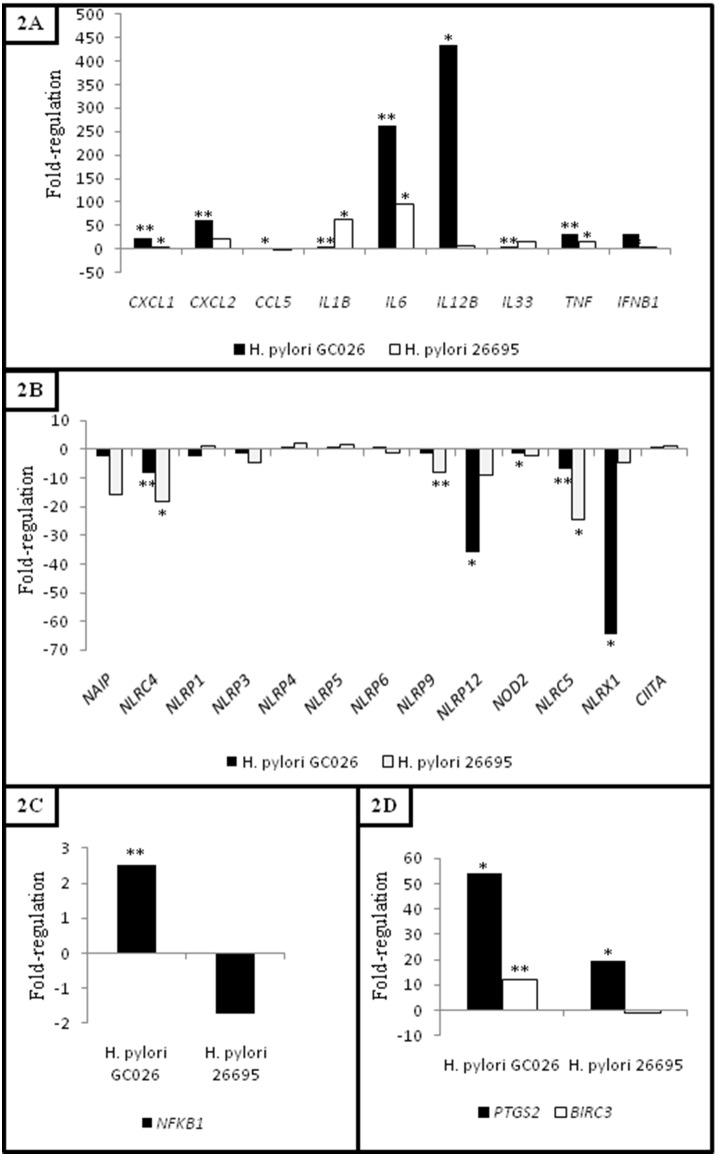
Given that inflammation is a hallmark of gastric carcinogenesis, we further analysed the expression of pro-inflammatory cytokines and chemokines upon exposure to two H. pylori strains. The expression of nine genes encoding pro-inflammatory cytokines (IFNB1, IL1B, IL12B, IL6, IL33 and TNF) and chemokines (CXCL1, CXCL2, CCL5) were significantly up-regulated in THP-1 cells challenged with both H. pylori GC026 and 26695 strains (Table 4). Further, an intense immune response (CXCL1, CXCL2, CCL5, IL6, IL12B, TNF and IFNB1) was initiated against H. pylori GC026 (Figure 1A). Interestingly, a number of genes encoding chemokines (CCL2 and CCL7) and cytokines (IL18, TNFSF11 and CD40LG) were down-regulated in H. pylori-challenged THP-1 cells (Table 5).
Figure 1. Helicobacter pylori up-regulates NFKB1 and NF-κB target genes, and down-regulates NF-κB-negative regulators.
A) Gene expression of cytokines and chemokines in H. pylori-challenged THP-1 cells, B) Gene expression of NOD-like receptors in H. pylori-challenged THP-1 cells, C) NFKB1 expression in H. pylori-challenged THP-1 cells and D) PTGS2 and BIRC3 expression in H. pylori-challenged THP-1 cells. Fold-change (2∧(−Delta Delta Ct)) is the normalized gene expression (2∧(−Delta Ct)) in THP-1 cells challenged with H. pylori (GC026 and 26695) divided the normalized gene expression (2∧(−Delta Ct)) in their respective control group. Fold-regulation represents fold-change results in a biologically meaningful way. Fold-change values greater than one indicate up-regulation, and the fold-regulation is equal to the fold-change. Fold-change values less than one indicate down-regulation, and the fold-regulation is the negative inverse of the fold-change. Fold-difference compared to the control group showing a *P-value<0.05 and a **P-value<0.01. P-values were obtained with a Student’s t-test.
Helicobacter Pylori Infection Results in Up-regulation of NFKB1 with Simultaneous Marked down-Regulation of NLRP12 and NLRX1
Thirteen genes encoding NLRs were assessed in this study including members of the IPAF subfamily (NAIP, NLRC4), NLRPs (NLRP1, NLRP3-6, NLRP9, NLRP12) and NODs (NOD2, NLRC5, NLRX1 and CIITA) (Figure 1B). The expression of five genes encoding NLRs was significantly regulated in H. pylori-challenged cells (NLRC4, NLRC5, NLRP9, NLRP12 and NLRX1) (Table 5). Of these, NLRP12 (fold regulation: 0.03, p-value: 0.016298) and NLRX1 (fold regulation: 0.02, p-value: 0.01762) were markedly down-regulated in H. pylori GC026-challenged cells. As a validation technique, further Western blot assays investigating NLRX1 expression, were conducted. Consistently, decreased NLRX1 levels were found in THP-1 cells challenged with both H. pylori GC026 and 26695 (Supporting Information Figure S3).
Given that NF-κB is negatively regulated by NLRP12 and NLRX1, we further compared the expression of NFKB1 and RELA between H. pylori GC026- and 26695-challenged cells. Remarkably, although RELA showed decreased levels in THP-1 cells exposed to both H. pylori strains (fold regulation: 0.49, p-value: 0.044673 and fold regulation: 0.26, p-value: 0.131017 for H. pylori GC026 and 26695, respectively), statistically significant up-regulation of NFKB1 was only observed in H. pylori GC026-challenged cells (fold regulation: 2.50, p-value: 0.006825) (Figure 1C).
Helicobacter Pylori Increases the Expression of PTGS2 and BIRC3
Expression of other molecules involved not only in the NLR signalling pathway but also in carcinogenesis was also analysed by comparing THP-1 cells responses to both H. pylori strains. Interestingly, PTGS2 was significantly up-regulated in THP-1 cells upon exposure to both H. pylori strains, H. pylori GC026-challenged cells (fold regulation: 54.03, p-value: 0.0247) showing significantly higher expression levels when compared to H. pylori 26695-challenged cells (fold regulation: 19.56, p-value: 0.016089) (Figure 1d). Further, a second gene involved in carcinogenesis, BIRC3, was exclusively up-regulated in H. pylori GC026-challenged cells (fold regulation: 12.28, p-value: 0.001638) (Figure 1D).
Discussion
GC is now considered a multifactorial process, in which bacterial, environmental and host genetics factors are involved at different stages in cancer pathophysiology. Currently, it is well established that cancer arises in chronically inflamed tissue, and this is particularly notable in the gastrointestinal tract [4]. As chronic inflammation of the gastric mucosa is a consequence of H. pylori infection and this bacterium is initially targeted by PRRs, we investigated the role of molecules involved in the NLR signalling pathway in H. pylori infection and related GC.
To our knowledge, this is the first reported evidence that polymorphisms involved in the NLR signalling pathway are associated with an increased risk of GC in a human population. Multivariate statistical analysis showed that the CARD8-rs11672725 TT genotype is a consistent risk factor for GC in Chinese individuals. CARD8, located at 19q13, encodes the caspase recruitment domain protein 8 (CARD8), also known as TUCAN, which has been reported to interact with NLRP3, suppress NF-κB activating signals and be overexpressed in human cancer tissues including ovarian, lung and breast cancer tissues [25]–[27]. To date, only one study, conducted in a German population, has assessed the role of this polymorphism in disease [28]. These authors addressed the association between CARD8-rs11672725 and colorectal cancer but failed to show any significant results (OR: 1.02, 95% CI: 0.86–1.21) [28]. Differences in cancer type and study population might account for these conflicting outcomes.
As H. pylori is known to be a major risk factor for GC, we also examined the effect of genetic polymorphisms involved in the NLR signalling pathway on H. pylori infection. On the multivariate analysis, NLRP12-rs2866112 consistently increased the risk of H. pylori infection in this ethnic Chinese population. Like NLRP3, NLRP12 has been shown to interact with ASC to generate an IL-1β-processing inflammasome [29]. To date few studies have addressed the role of NLRP12 in host resistance or susceptibility to infectious agents. Recently, however, a study by Vladimer et al. [30], demonstrated that NLRP12-deficient mice had higher mortality and increased bacterial loads after infection with Yersinia pestis, which correlated with decreased levels of IL-1β, IL18 and IFN-γ suggesting that NLRP12 might form an inflammasome in response to this bacteria. In contrast, Allen et al. [31] showed that NLRP12 did not significantly contribute to the in vivo host innate immune response to Klebsiella pneumonia or Mycobacterium tuberculosis. Further, Zaki et al. [32] have recently showed that NLRP12-deficient mice were highly resistant to Salmonella enterica serovar Typhimurium infection which was associated with the NLRP12-mediated inhibition of NF-κB and ERK activation. Thus, current evidence suggests that NLRP12 may play a dual pathogen-specific role, one in which a NLRP12 inflammasome-dependent response facilitates the eradication of the pathogen (e.g. Y. pestis infection), and a further one in which negative regulation of NF-κB signalling leads to survival and persistence of the pathogen (e.g. S. Typhimurium and possibly, H. pylori infection).
Given that GC is a complex disease, further multivariate statistical analyses were performed to investigate the potential interaction between H. pylori and the selected polymorphisms in the development of GC. Among H. pylori-infected individuals, those ones harbouring CARD8-rs10405717, NLRP3-rs12079994, NLRP3-rs3806265, NLRP3-rs4612666, NLRP12-rs2866112, NLRP12-rs4419163, NLRX1-rs10790286, CASP1-rs2282659, CASP1-rs530537 and CASP1-rs61751523, were at most risk of developing GC. Interestingly, a number of these genetic variants have previously been associated with diverse inflammation-related pathologies including cancer and autoimmune disorders [33], [34].
Surprisingly, in the absence of H. pylori infection, CARD8-rs2043211 significantly decreased the risk of GC in these Chinese individuals. Consistent with this finding, a recent publication by Roberts et al. [35], found that the presence of the CARD8-rs2043211 T allele in combination with the NLRP3-rs35829419 C allele conferred a protective effect against Crohn’s disease (CD) in Caucasian individuals (OR: 0.35, 95% CI: 0.15–0.82 and OR: 0.66, 95% CI: 0.48–0.90 for CARD8 1,1/NLRP3 1,2 and CARD8 1,2/NLRP3 1,1, respectively). In contrast, a study conducted in a Korean population revealed an association between CARD8-rs2043211 and ulcerative colitis (UC) (OR: 1.50, 95% CI: 1.12–2.00, P-value: 0.011) [36]. Further, CARD8-rs2043211 was also associated with elevated levels of IL-1β in female UC patients in this study [36]. Thus, further studies are required to elucidate the association between CARD8-rs2043211 and gastrointestinal inflammatory disorders among different ethnic groups.
Recently, inflammation has been considered the seventh hallmark of cancer and an enabling characteristic that facilitates the acquisition of the other hallmarks (tissue invasion/metastasis, limitless replicative potential, sustained angiogenesis, evasion of programmed-cell death (apoptosis), self-sufficiency in growth signals and insensitivity to growth-inhibitory signals) [37]. Inflammation initiated by innate immune cells, mainly macrophage subtypes, neutrophils, myeloid progenitors and mast cells [38]–[41], designed to fight infections and heal lesions, can instead result in unintentional support of multiple cancer hallmark functions, thereby manifesting the widely accepted tumour-promoting consequences of inflammatory responses [37]. Therefore, to assess the direct effect of H. pylori on the NLR signalling pathway, an important component of innate immunity that has been involved in gastric immunopathology, the expression of 84 genes was investigated in THP-1 cells challenged with two unrelated H. pylori strains (GC026 and 26695). Because pro-inflammatory cytokines play an essential role promoting inflammation in the context of gastrointestinal carcinogenesis [42], we first compared the expression of these genes between non-infected and H. pylori-infected THP-1 cells. Nine genes encoding pro-inflammatory cytokines (IFNB1, IL1B, IL12B, IL6, IL33 and TNF) and chemokines (CXCL1, CXCL2, CCL5) were found to be significantly up-regulated in THP-1 cells challenged with both H. pylori strains. These results are consistent with extensive evidence showing up-regulation of pro-inflammatory cytokines during H. pylori infection [43], [44]. Interestingly, CXCL1, CXCL2, CCL5, IL6, IL12B, TNF and IFNB1 were markedly up-regulated in THP-1 cells challenged with H. pylori GC026, a strain isolated from a GC patient and known to be positive for multiple virulence factors (cagA+, cagE+, cagL+, cagT+, vacA s1m1+, babA+, oipA+, dupA+ and sabA+), when compared to the reference strain H. pylori 26695. Further studies analysing the potential role of specific H. pylori virulence factors in the regulation of the NLR signalling pathway are required.
On the other hand, genes encoding a number of cytokines (IL18, TNFSF11 and CD40LG) and chemokines (CCL2 and CCL7) were found to be down-regulated in H. pylori-challenged THP-1 cells. Of these, only CCL7 showed decreased expression in THP-1 cells challenged with both H. pylori GC026 and 26695 strains. Interestingly, H. pylori-induced down-regulation of chemokines and their receptors has been previously suggested as a novel mechanism of modulating neutrophil migration and activation in the gastric mucosa [45]. As H. pylori might exert a similar effect on CCL7, a chemokine that attracts monocytes and eosinophils and regulates macrophage function, this potential mechanism requires further investigation. Notably, we found expression of IL18 to be significantly down-regulated only in the H. pylori GC026-challenged cells. Given that it has been reported that IL-18 counteracts the pro-inflammatory activities of IL-1β, thereby balancing control of H. pylori infection and prevention of excessive gastric immunopathology [10], it could be postulated that down-regulation of IL18 contributes to the increased inflammation associated with GC.
The expression of thirteen genes encoding NLRs was analysed in the current study. Of these, five genes were significantly down-regulated at least two-fold in THP-1 cells challenged with H. pylori GC026 (NLRC4, NLRC5, NLRP12 and NLRX1) and 26695 (NLRC4, NLRC5 and NLRP9). Interestingly, NLRP3, a gene encoding a NLR that has previously been shown by Kim et al. [13] to cooperate with TLR2 and NOD2 for the secretion of IL-1β in H. pylori-infected DCs, was not differentially expressed in H. pylori infected-cells in our study. However, as described by others, there is considerable variation in phenotype, cytokine secretion, phagocytosis and T cell stimulating capabilities between monocytes, DCs and macrophages, in response to H. pylori infection [46]. Indeed, our results imply that other NLRs expressed in macrophages might be involved in H. pylori recognition and subsequent chronic inflammation including NLRC4 (previously shown to be involved in the recognition of gram-negative bacteria type III and IV secretion systems [47], [48]), NLRC5 (a specific and master regulator of major histocompatibility complex (MHC) class I genes as well as related genes involved in MHC class I antigen presentation [49]) and NLRP9 (recently associated with other inflammation-related disorders [50]).
In addition, our gene expression analyses showed NLRP12 and NLRX1, two genes encoding molecules that have recently been suggested to negatively regulate canonical and non-canonical NF-κB signalling [32], [51]–[54], to be markedly down-regulated in H. pylori GC026-challenged cells. Further, statistically significant up-regulation of NFKB1 was only observed in H. pylori GC026-challenged cells. These results imply that, in THP-1 cells infected with highly virulent H. pylori strains, a dual mechanism may exacerbate the activity of the NF-κB signalling pathway, one of which increases host response to bacterial components leading to NF-κB activation through other PRRs, most likely TLRs, and secondly, down-regulation of the NF-κB-negative regulators NLRP12 and NLRX1.
NLRP12-mediated attenuation of the non-canonical NF-κB pathway has been suggested to be due to NLRP12’s ability to associate with the NF-κB inducing kinase (NIK), which subsequently induces proteasome-dependent degradation of this protein [52]. In addition, evidence suggests that NLRP12 regulates the canonical NF-κB signalling pathway by targeting the interleukin-1 receptor-associated kinase 1 (IRAK1), which results in blockage of IRAK-1 hyperphosphorylation [53]. Similarly, NLRX1 was found to target TNF receptor-associated factor 6 (TRAF6) inhibiting its capacity to signal to NF-κB [51]. Further, upon LPS stimulation, NLRX1 is rapidly ubiquitinated, disassociates from TRAF6, and then binds to the I kappa B kinase (IKK) complex, resulting in inhibition of IKKα and IKKβ phosphorylation and NF-κB activation [54]. In the context of gastrointestinal carcinogenesis, Zaki et al. [55] have demonstrated that NLRP12 plays an essential role in the suppression of pro-inflammatory cytokines and chemokines by controlling the activation of NF-κB and extracellular-signal-regulated kinase (ERK) pathways in response to microbial components, colon inflammation and colorectal tumorigenesis. Further, Allen et al. [56] have found that NLRP12-deficient mice were highly susceptible to colitis and colitis-associated colon cancer showing elevated non-canonical NF-κB activation and increased expression of target genes associated with cancer, including CXCL13 and CXCL12.
PTGS2 and BIRC3, two molecules known to be involved in carcinogenesis, were significantly up-regulated in H. pylori-GC026-challenged cells. Prostaglandin-endoperoxide synthase 2 (PTGS2), which is also termed cyclooxygenase 2 (COX2), is the key enzyme that catalyses the conversion of arachidonic acid to prostaglandins. Extensive evidence supports a pivotal role of PTGS2 in gastric inflammation and carcinogenesis [57]. In addition, Zaki et al. [58] showed that elevated PTGS2 expression was evident in colonic tumours of NLRP12-deficient mice leading to the possibility that, in the current study, decreased expression of NLRP12 is related to increased PTGS2 levels in H. pylori GC026-challenged cells.
Further, because baculoviral IAP repeat containing 3 (BIRC3), also known as cellular inhibitor of apoptosis protein 2 (cIAP2), is a member of the inhibitor of apoptosis protein (IAP) family, and is over-expressed in most cancer tissues [59], it can be postulated that stimulus with H. pylori GC026 elevates the already high basal levels of BIRC3 in THP-1 cells, a cell line derived from an acute monocytic leukemia patient. Consistent with this view, Maeda et al. [60] have shown that H. pylori infection increases BIRC3 expression in human GC and cervical cancer cell lines. Further, H. pylori has been shown to increase the expression of BIRC3 in infected-mice after 2 weeks of exposure [61]. In addition, as knocking down of BIRC3 resulted in a 30% decrease in cell proliferation, a 20% increase in apoptosis and delayed migration of SGC-7901 cells, it has been postulated that BIRC3 is a potential target for GC therapy [61].
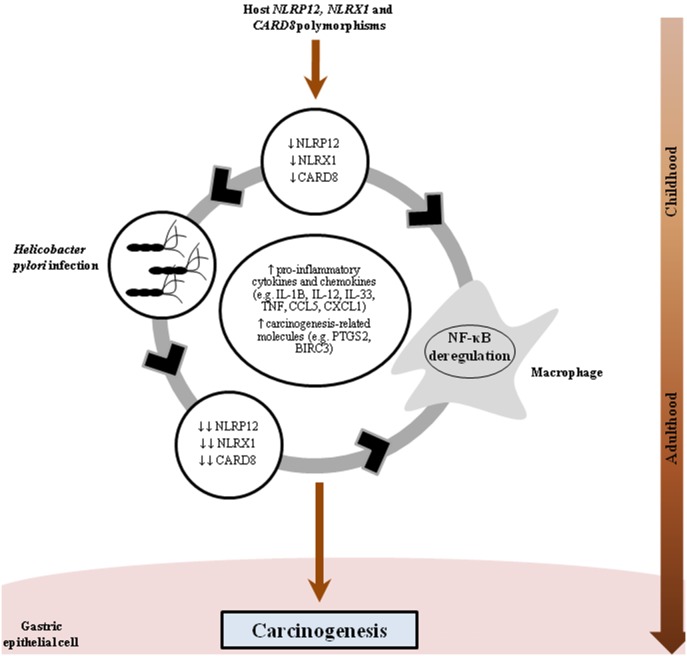
In conclusion, the association between CARD8-rs11672725 and GC was a consistent and novel finding in this study. Further, NLRP12-rs2866112 was found to increase the risk of H. pylori infection in this population, the main risk factor for GC. Additional analyses in our ethnic Chinese population showed that the concomitant presence of polymorphisms involved in the NLR signalling pathway and H. pylori infection dramatically increased the risk of GC in Chinese. Our gene expression analyses showed five genes encoding NLRs to be significantly regulated in H. pylori-challenged cells (NLRC4, NLRC5, NLRP9, NLRP12 and NLRX1). Interestingly, NLRP12 and NLRX1, two known NF-κB negative regulators, were markedly down-regulated, and NFKB1 as well as several NF-κB target genes encoding pro-inflammatory cytokines (IFNB1, IL12B, IL6 and TNF), chemokines (CXCL1, CXCL2, CCL5) and molecules involved in carcinogenesis (PTGS2 and BIRC3) were markedly up-regulated, in THP-1 cells infected with a highly virulent H. pylori strain isolated from a GC patient. Based on our findings, we propose a synergistic interaction between NLRs and H. pylori in GC pathogenesis, which over time, could facilitate the sequence of events that characterises GC development including inflammation, atrophy, metaplasia, dysplasia, carcinoma in situ, and finally invasive GC (Figure 2).
Figure 2. Polymorphisms in the NOD-like receptor signalling pathway increase the risk of Helicobacter pylori-related gastric cancer.
Individuals harbouring NLRP12, NLRX1 and CARD8 polymorphisms would have decreased levels or defective NLRP12, NLRX1 and CARD8, and thus, would be more susceptible to acquisition of H. pylori in childhood and present deregulation of NF-κB. Once established, H. pylori infection appears to intensify the decreased expression of these NF-κB-negative regulators. These factors would lead to the production of pro-inflammatory cytokines (IFNB1, IL1, IL-12B, IL6, IL33 and TNF), chemokines (CXCL1, CXCL2, CCL5) and carcinogenesis-related molecules (PTGS2 and BIRC3), among others, which would facilitate the sequence of events that characterises GC development including inflammation, atrophy, intestinal metaplasia, dysplasia, carcinoma in situ, and finally invasive gastric cancer.
Supporting Information
This file contains Figures S1 and S2. Helicobacter pylori influences the expression of several molecules involved in the NOD-like receptor signalling pathway. THP-1 cells were challenged with two H. pylori strains (GC026 and 26695). Total RNA was extracted from cells after 6 hours infection. Gene expression was detected by quantitative RT-PCR in triplicates. S1) Gene expression of 84 molecules involved in the NOD-like receptor (NLR) signalling pathway in H. pylori GC026-challenged THP-1 cells. S2) Gene expression of 84 molecules involved in the NLR signalling pathway in H. pylori 26695-challenged THP-1 cells. The x-axis plots the log2 of the fold-differences, while the y-axis plots their p-values based on a student’s t-test of the replicate raw Ct data. The red and green circles outside the two vertical lines indicate fold-differences >2. Circles in the volcano plot above the blue line identify fold-differences showing P-values<0.05.
(TIF)
Helicobacter pylori down-regulates NLRX1 levels in THP-1 cells. The levels of NLRX1 were significantly decreased in THP-1 cells challenged with either H. pylori strain GC26 or 26695. A representative experiment of the triplicates performed is shown.
(TIF)
This contains files Tables S1–S7. Table S1. Genetic polymorphisms in genes involved in the NOD-like receptors signalling pathway included in the current study. Table S2. PCR primer sequences and thermal conditions used for genotyping of NLRP3 and CARD8 polymorphisms in gastric cancer patients and functional dyspepsia controls. Table S3. Clinical characteristics of gastric cancer patients and functional dyspepsia controls. Table S4. Association between the NLRP3 42 bp-VNTR polymorphism and risk of gastric cancer in ethnic Chinese individuals (bivariate statistical analysis). Table S5. Association between CARD8-NLRP12, NLRP3, NLRX1-CASP1 and ASC haplotypes and gastric cancer in an ethnic Chinese population. Table S6. Association between polymorphisms involved in the NOD-like receptors signalling pathway and risk of Helicobacter pylori infection in ethnic Chinese individuals (bivariate statistical analysis). Table S7. Effect of Helicobacter pylori infection on the expression of genes involved in the NOD-like receptors signalling pathway.
(DOC)
Acknowledgments
The authors would like to thank all individuals with GC and FD who participated in the ethnic Chinese case-control study.
Funding Statement
This work was supported in part by The Cancer Council of New South Wales, Australia (Grant no.66/04). N.O. Kaakoush is supported by an Early Career fellowship from the National Health and Medical Research Council, Australia. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, et al. (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide. IARC CancerBase No 11 [Internet]. Lyon, France: International Agency for Research on Cancer. [Google Scholar]
- 2. Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez CA, Sala N, Capella G (2002) Genetic susceptibility and gastric cancer risk. Int J Cancer 100: 249–260. [DOI] [PubMed] [Google Scholar]
- 4. Macarthur M, Hold GL, El-Omar EM (2004) Inflammation and Cancer II. Role of chronic inflammation and cytokine gene polymorphisms in the pathogenesis of gastrointestinal malignancy. Am J Physiol Gastrointest Liver Physiol 286: G515–520. [DOI] [PubMed] [Google Scholar]
- 5. Davis BK, Wen H, Ting JP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29: 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, et al. (2010) IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11: 997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schroder K, Tschopp J (2010) The inflammasomes. Cell 140: 821–832. [DOI] [PubMed] [Google Scholar]
- 8. Martinon F, Mayor A, Tschopp J (2009) The inflammasomes: guardians of the body. Annu Rev Immunol 27: 229–265. [DOI] [PubMed] [Google Scholar]
- 9. Basak C, Pathak SK, Bhattacharyya A, Mandal D, Pathak S, et al. (2005) NF-kappaB- and C/EBPbeta-driven interleukin-1beta gene expression and PAK1-mediated caspase-1 activation play essential roles in interleukin-1beta release from Helicobacter pylori lipopolysaccharide-stimulated macrophages. J Biol Chem 280: 4279–4288. [DOI] [PubMed] [Google Scholar]
- 10. Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, et al. (2012) Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1beta and IL-18. J Immunol 188: 3594–3602. [DOI] [PubMed] [Google Scholar]
- 11. Shimada M, Ando T, Peek RM, Watanabe O, Ishiguro K, et al. (2008) Helicobacter pylori infection upregulates interleukin-18 production from gastric epithelial cells. Eur J Gastroenterol Hepatol 20: 1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang JC, Yang HC, Shun CT, Wang TH, Chien CT, et al. (2013) Catechins and Sialic Acid Attenuate Helicobacter pylori-Triggered Epithelial Caspase-1 Activity and Eradicate Helicobacter pylori Infection. Evid Based Complement Alternat Med 2013: 248585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim DJ, Park JH, Franchi L, Backert S, Nunez G (2013) The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1beta production in Helicobacter pylori infected dendritic cells. Eur J Immunol 43: 2650–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Talley NJ, Stanghellini V, Heading RC, Koch KL, Malagelada JR, et al. (1999) Functional gastroduodenal disorders. Gut 45 Suppl 2: II37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitchell H, English DR, Elliott F, Gengos M, Barrett JH, et al. (2008) Immunoblotting using multiple antigens is essential to demonstrate the true risk of Helicobacter pylori infection for gastric cancer. Aliment Pharmacol Ther 28: 903–910. [DOI] [PubMed] [Google Scholar]
- 16. Jurinke C, van den Boom D, Cantor CR, Koster H (2002) The use of MassARRAY technology for high throughput genotyping. Adv Biochem Eng Biotechnol 77: 57–74. [DOI] [PubMed] [Google Scholar]
- 17.Oeth P BM, Park C, Kosman D, del Mistro G, van den Boom D, Jurinke C (2005) iPLEX Assay: Increased Plexing Efficiency and Flexibility for MassARRAY System Through Single Base Primer Extension with Mass-Modified Terminators. Sequenom. pp. 12.
- 18. Slatkin M, Excoffier L (1996) Testing for linkage disequilibrium in genotypic data using the Expectation-Maximization algorithm. Heredity 76 (Pt 4): 377–383. [DOI] [PubMed] [Google Scholar]
- 19. Excoffier L, Slatkin M (1995) Maximum-likelihood estimation of molecular haplotype frequencies in a diploid population. Mol Biol Evol 12: 921–927. [DOI] [PubMed] [Google Scholar]
- 20. Excoffier L, Laval G, Schneider S (2005) Arlequin (version 3.0): an integrated software package for population genetics data analysis. Evol Bioinform Online 1: 47–50. [PMC free article] [PubMed] [Google Scholar]
- 21. Gunaletchumy SP, Teh X, Khosravi Y, Ramli NS, Chua EG, et al. (2012) Draft genome sequences of Helicobacter pylori isolates from Malaysia, cultured from patients with functional dyspepsia and gastric cancer. J Bacteriol 194: 5695–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmidt HM, Andres S, Nilsson C, Kovach Z, Kaakoush NO, et al. (2010) The cag PAI is intact and functional but HP0521 varies significantly in Helicobacter pylori isolates from Malaysia and Singapore. Eur J Clin Microbiol Infect Dis 29: 439–451. [DOI] [PubMed] [Google Scholar]
- 23. Akopyants NS, Eaton KA, Berg DE (1995) Adaptive mutation and cocolonization during Helicobacter pylori infection of gnotobiotic piglets. Infect Immun 63: 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, et al. (1982) Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res 42: 1530–1536. [PubMed] [Google Scholar]
- 25. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, et al. (2004) NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20: 319–325. [DOI] [PubMed] [Google Scholar]
- 26. Bouchier-Hayes L, Conroy H, Egan H, Adrain C, Creagh EM, et al. (2001) CARDINAL, a novel caspase recruitment domain protein, is an inhibitor of multiple NF-kappa B activation pathways. J Biol Chem 276: 44069–44077. [DOI] [PubMed] [Google Scholar]
- 27. Pathan N, Marusawa H, Krajewska M, Matsuzawa S, Kim H, et al. (2001) TUCAN, an antiapoptotic caspase-associated recruitment domain family protein overexpressed in cancer. J Biol Chem 276: 32220–32229. [DOI] [PubMed] [Google Scholar]
- 28. Mockelmann N, von Schonfels W, Buch S, von Kampen O, Sipos B, et al. (2009) Investigation of innate immunity genes CARD4, CARD8 and CARD15 as germline susceptibility factors for colorectal cancer. BMC Gastroenterol 9: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Manji GA, Grenier JM, Al-Garawi A, Merriam S, et al. (2002) PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-kappa B and caspase-1-dependent cytokine processing. J Biol Chem 277: 29874–29880. [DOI] [PubMed] [Google Scholar]
- 30. Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, et al. (2012) The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allen IC, McElvania-TeKippe E, Wilson JE, Lich JD, Arthur JC, et al. (2013) Characterization of NLRP12 during the in vivo host immune response to Klebsiella pneumoniae and Mycobacterium tuberculosis. PLoS One 8: e60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zaki MH, Man SM, Vogel P, Lamkanfi M, Kanneganti TD (2014) Salmonella exploits NLRP12-dependent innate immune signaling to suppress host defenses during infection. Proc Natl Acad Sci U S A 111: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lavender NA, Rogers EN, Yeyeodu S, Rudd J, Hu T, et al. (2012) Interaction among apoptosis-associated sequence variants and joint effects on aggressive prostate cancer. BMC Med Genomics 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Day TG, Ramanan AV, Hinks A, Lamb R, Packham J, et al. (2008) Autoinflammatory genes and susceptibility to psoriatic juvenile idiopathic arthritis. Arthritis Rheum 58: 2142–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roberts RL, Topless RK, Phipps-Green AJ, Gearry RB, Barclay ML, et al. (2010) Evidence of interaction of CARD8 rs2043211 with NALP3 rs35829419 in Crohn’s disease. Genes Immun 11: 351–356. [DOI] [PubMed] [Google Scholar]
- 36. Yang SK, Kim H, Hong M, Lim J, Choi E, et al. (2011) Association of CARD8 with inflammatory bowel disease in Koreans. J Hum Genet 56: 217–223. [DOI] [PubMed] [Google Scholar]
- 37. Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 38. Coffelt SB, Lewis CE, Naldini L, Brown JM, Ferrara N, et al. (2010) Elusive identities and overlapping phenotypes of proangiogenic myeloid cells in tumors. Am J Pathol 176: 1564–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE (2007) Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol 28: 519–524. [DOI] [PubMed] [Google Scholar]
- 40. DeNardo DG, Andreu P, Coussens LM (2010) Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 29: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johansson M, Denardo DG, Coussens LM (2008) Polarized immune responses differentially regulate cancer development. Immunol Rev 222: 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Karin M, Greten FR (2005) NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5: 749–759. [DOI] [PubMed] [Google Scholar]
- 43. Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, et al. (1997) Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut 41: 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wen S, Felley CP, Bouzourene H, Reimers M, Michetti P, et al. (2004) Inflammatory gene profiles in gastric mucosa during Helicobacter pylori infection in humans. J Immunol 172: 2595–2606. [DOI] [PubMed] [Google Scholar]
- 45. Schmausser B, Josenhans C, Endrich S, Suerbaum S, Sitaru C, et al. (2004) Downregulation of CXCR1 and CXCR2 expression on human neutrophils by Helicobacter pylori: a new pathomechanism in H. pylori infection? Infect Immun 72: 6773–6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fehlings M, Drobbe L, Moos V, Renner Viveros P, Hagen J, et al. (2012) Comparative analysis of the interaction of Helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect Immun 80: 2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Faure E, Mear JB, Faure K, Normand S, Couturier-Maillard A, et al. (2014) Pseudomonas aeruginosa Type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am J Respir Crit Care Med. [DOI] [PubMed] [Google Scholar]
- 48. Silveira TN, Zamboni DS (2010) Pore formation triggered by Legionella spp. is an Nlrc4 inflammasome-dependent host cell response that precedes pyroptosis. Infect Immun 78: 1403–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yao Y, Wang Y, Chen F, Huang Y, Zhu S, et al. (2012) NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res 22: 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tadaki H, Saitsu H, Nishimura-Tadaki A, Imagawa T, Kikuchi M, et al. (2011) De novo 19q13.42 duplications involving NLRP gene cluster in a patient with systemic-onset juvenile idiopathic arthritis. J Hum Genet 56: 343–347. [DOI] [PubMed] [Google Scholar]
- 51. Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, et al. (2011) NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity 34: 854–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lich JD, Williams KL, Moore CB, Arthur JC, Davis BK, et al. (2007) Monarch-1 suppresses non-canonical NF-kappaB activation and p52-dependent chemokine expression in monocytes. J Immunol 178: 1256–1260. [DOI] [PubMed] [Google Scholar]
- 53. Williams KL, Lich JD, Duncan JA, Reed W, Rallabhandi P, et al. (2005) The CATERPILLER protein monarch-1 is an antagonist of toll-like receptor-, tumor necrosis factor alpha-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J Biol Chem 280: 39914–39924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xia X, Cui J, Wang HY, Zhu L, Matsueda S, et al. (2011) NLRX1 negatively regulates TLR-induced NF-kappaB signaling by targeting TRAF6 and IKK. Immunity 34: 843–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, et al. (2010) The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32: 379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Allen IC, Wilson JE, Schneider M, Lich JD, Roberts RA, et al. (2012) NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity 36: 742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wu WK, Sung JJ, Lee CW, Yu J, Cho CH (2010) Cyclooxygenase-2 in tumorigenesis of gastrointestinal cancers: an update on the molecular mechanisms. Cancer Lett 295: 7–16. [DOI] [PubMed] [Google Scholar]
- 58. Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, et al. (2011) The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 20: 649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, et al. (2000) Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res 6: 1796–1803. [PubMed] [Google Scholar]
- 60. Maeda S, Yoshida H, Mitsuno Y, Hirata Y, Ogura K, et al. (2002) Analysis of apoptotic and antiapoptotic signalling pathways induced by Helicobacter pylori. Gut 50: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Z, Chen J, Chan KW, Qiao L, Wong BC (2011) A possible role of cIAP2 in Helicobacter pylori-associated gastric cancer. Cancer Lett 313: 192–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Figures S1 and S2. Helicobacter pylori influences the expression of several molecules involved in the NOD-like receptor signalling pathway. THP-1 cells were challenged with two H. pylori strains (GC026 and 26695). Total RNA was extracted from cells after 6 hours infection. Gene expression was detected by quantitative RT-PCR in triplicates. S1) Gene expression of 84 molecules involved in the NOD-like receptor (NLR) signalling pathway in H. pylori GC026-challenged THP-1 cells. S2) Gene expression of 84 molecules involved in the NLR signalling pathway in H. pylori 26695-challenged THP-1 cells. The x-axis plots the log2 of the fold-differences, while the y-axis plots their p-values based on a student’s t-test of the replicate raw Ct data. The red and green circles outside the two vertical lines indicate fold-differences >2. Circles in the volcano plot above the blue line identify fold-differences showing P-values<0.05.
(TIF)
Helicobacter pylori down-regulates NLRX1 levels in THP-1 cells. The levels of NLRX1 were significantly decreased in THP-1 cells challenged with either H. pylori strain GC26 or 26695. A representative experiment of the triplicates performed is shown.
(TIF)
This contains files Tables S1–S7. Table S1. Genetic polymorphisms in genes involved in the NOD-like receptors signalling pathway included in the current study. Table S2. PCR primer sequences and thermal conditions used for genotyping of NLRP3 and CARD8 polymorphisms in gastric cancer patients and functional dyspepsia controls. Table S3. Clinical characteristics of gastric cancer patients and functional dyspepsia controls. Table S4. Association between the NLRP3 42 bp-VNTR polymorphism and risk of gastric cancer in ethnic Chinese individuals (bivariate statistical analysis). Table S5. Association between CARD8-NLRP12, NLRP3, NLRX1-CASP1 and ASC haplotypes and gastric cancer in an ethnic Chinese population. Table S6. Association between polymorphisms involved in the NOD-like receptors signalling pathway and risk of Helicobacter pylori infection in ethnic Chinese individuals (bivariate statistical analysis). Table S7. Effect of Helicobacter pylori infection on the expression of genes involved in the NOD-like receptors signalling pathway.
(DOC)