Abstract
Human apolipoprotein E has three isoforms: APOE2, APOE3 and APOE41. APOE4 is a major genetic risk factor for Alzheimer’s disease2, 3 and is associated with Down’s syndrome dementia and poor neurological outcome after traumatic brain injury and haemorrhage3. Neurovascular dysfunction is present in normal APOE4 carriers4, 5, 6 and individuals withAPOE4-associated disorders3, 7, 8, 9, 10. In mice, lack of Apoe leads to blood–brain barrier (BBB) breakdown11, 12, whereas APOE4 increases BBB susceptibility to injury13. How APOE genotype affects brain microcirculation remains elusive. Using different APOE transgenic mice, including mice with ablation and/or inhibition of cyclophilin A (CypA), here we show that expression of APOE4 and lack of murine Apoe, but not APOE2 and APOE3, leads to BBB breakdown by activating a proinflammatory CypA–nuclear factor-κB–matrix-metalloproteinase-9 pathway in pericytes. This, in turn, leads to neuronal uptake of multiple blood-derived neurotoxic proteins, and microvascular and cerebral blood flow reductions. We show that the vascular defects in Apoe-deficient and APOE4-expressing mice precede neuronal dysfunction and can initiate neurodegenerative changes. Astrocyte-secreted APOE3, but not APOE4, suppressed the CypA–nuclear factor-κB–matrix-metalloproteinase-9 pathway in pericytes through a lipoprotein receptor. Our data suggest that CypA is a key target for treating APOE4-mediated neurovascular injury and the resulting neuronal dysfunction and degeneration.
Astrocytes are a major source of APOE in the brain2. Importantly, astrocyte-secreted molecules transduce signals to brain microvessels acting on pericytes7, 14, 15. To understand the effects of APOE on brain microcirculation we studied mice with targeted replacement of murine Apoe with each human APOE isoform (TR-APOE)16, mice lacking murine Apoe (Apoe−/−), mice expressing each human APOE isoform under control of the astrocyte-specific glial fibrillary acidic protein (GFAP) promoter on an Apoe-null background, and Apoe−/− and APOE4 transgenic mice with ablation and/or pharmacological inhibition of CypA (see Supplementary Information). In search of molecules that could mediate BBB dysfunction in Apoe−/− and APOE4 mice, we focused on the proinflammatory cytokine CypA, previously demonstrated to have deleterious effects on the vascular system in Apoe−/− mice with aortic aneurysms and atherosclerosis17, 18.
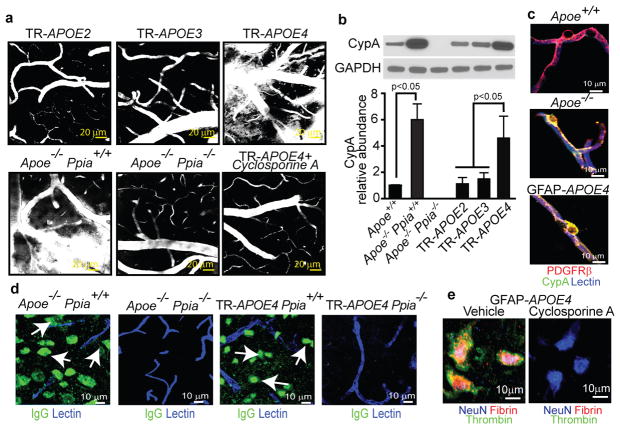
Using multiphoton microscopy of tetramethylrhodamine-conjugated dextran (TMR-dextran)14, we show an intact BBB in TR-APOE2 and TR-APOE3 mice and a leaky BBB in TR-APOE4 and Apoe−/− mice (Fig. 1a and Supplementary Fig. 1a, b), suggesting that APOE2, APOE3 and murine Apoe effectively maintain the BBB, whereas APOE4 promotes BBB disruption. These findings have been replicated in mice expressing each human APOE isoform under control of the GFAP promoter (not shown). Notably, genetic ablation of CypA (encoded by Ppia) eliminated BBB damage in Apoe−/− Ppia−/− mice (Fig. 1a and Supplementary Fig. 1a, b).
Figure 1. CypA deficiency or inhibition reverses BBB breakdown in Apoe−/− and APOE4 mice.
(a) Multiphoton microscopy of TMR-Dextran (white) in 6-month-old TR-APOE2, TR-APOE3, TR-APOE4, Apoe−/− Ppia+/+, Apoe−/− Ppia−/− and cyclosporine A-treated TR-APOE4 mice. Bar=20 mm. (b) CypA immunobloting in brain microvessels from apoE transgenic mice. (c) CypA (green) colocalization with PDGFRb-positive pericytes (red; yellow, merged) in hippocampal microvessels from Apoe+/+, Apoe−/− and GFAP-APOE4 mice. Blue, lectin-positive endothelium. Bar=10mm. (d) IgG neuronal uptake (green; lectin-positive vessels, blue) in Apoe−/− Ppia+/+, Apoe−/− Ppia−/−, TR-APOE4 Ppia+/+ and TR-APOE4 Ppia−/− mice. (e) Fibrin (red) and thrombin (green) in NeuN-positive neurons (blue) in the hippocampus of 9-month-old GFAP-APOE4 mice untreated and cyclosporine A-treated. a and c–e, representative results from 4–6 experiments. Scale bar, 10 μm.
Compared to littermate controls, TR- or GFAP-APOE2 and -APOE3 mice, Apoe−/− and APOE4 mice had five- to sixfold higher CypA levels in cerebral microvessels (Fig. 1b and Supplementary Fig. 1c, d), mainly because of an increased CypA expression in pericytes (Fig. 1c and Supplementary Fig. 1e). CypA levels in microvessel-depleted brain were not affected by APOE (Supplementary Fig. 1f). These data suggest that APOE2, APOE3 and murine Apoe, but not APOE4, effectively maintain physiological CypA levels in brain microvessels by controlling CypA expression in pericytes. To determine whether BBB disruption in APOE4 mice can be corrected with cyclosporine A, a drug that binds intracellular CypA and inhibits its effects19, we treated TR-APOE4 and GFAP-APOE4 mice with a low dose of cyclosporine A previously shown not to cause systemic or central nervous system toxicity. Cyclosporine A accumulates in brain microvessels, but does not cross the BBB20. In APOE4 mice cyclosporine A eliminated BBB disruption (Fig. 1a and Supplementary Fig. 1a, b) and neuronal accumulation of systemically administered cadaverine15 (Supplementary Fig. 1g), indicating that BBB changes are reversible and CypA can be therapeutically targeted to correctAPOE4-induced BBB breakdown.
To understand better the pathological implications of BBB breakdown, we studied leakage of endogenous blood-derived proteins in the brain. As shown in the hippocampus, 18-month-old GFAP-APOE3 and control mice had negligible extravascular accumulation of serum IgG in contrast to GFAP-APOE4 and Apoe−/− mice (Supplementary Fig. 2a, b). Ppia genetic deletion eliminated IgG extravascular deposits (Supplementary Fig. 2a, b) and neuronal accumulation in Apoe−/− and APOE4 mice (Fig. 1d). Cyclosporine A diminished IgG leakage by ~80% in TR-APOE4 or GFAP-APOE4 mice (Supplementary Fig. 2c) and inhibited neuronal accumulation of blood-derived thrombin and fibrin (Fig. 1e), consistent with restoration of the BBB. APOE4 mice had numerous brain perivascular fibrin and haemosiderin foci (Supplementary Fig. 2d–f) and elevated thrombin levels that were normalized with cyclosporine A (Supplementary Fig. 2g, h). Thrombin is neurotoxic21, fibrin accelerates neurovascular damage22 and haemosiderin generates reactive oxygen species23, thus implicating multiple potential BBB-derived sources of injury.
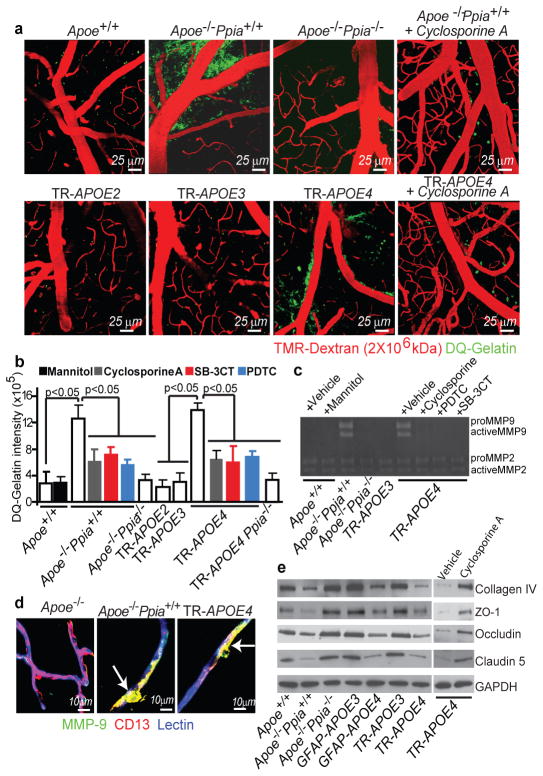
To elucidate the molecular mechanisms underlying CypA-mediated BBB breakdown we studied matrix metalloproteinases (MMP)2 and MMP9 (gelatinases), which are activated by CypA in the vessel wall in a mouse model of aortic aneurism17. Gelatinases disrupt the BBB by degrading the capillary basement membrane and tight-junction proteins7, 24. Multiphoton microscopy of DQ-gelatin25 revealed an increase in cerebrovascular gelatinase activity in Apoe−/− Ppia+/+ and TR-APOE4 mice compared with controls, TR-APOE2 and TR-APOE3 mice (Fig. 2a, b). Gelatin zymography of brain tissue demonstrated an increase in pro-MMP9 and activated MMP9, but not MMP2, in Apoe−/− and TR-APOE4 mice (Fig. 2c), which correlated with the appearance of MMP9-positive pericytes (Fig. 2d and Supplementary Fig. 3a, b). To establish causality and demonstrate that increased MMP9 activity does not only correlate with, but is required for, BBB breakdown in Apoe−/− and TR-APOE4 mice, we studied the effects of pharmacological inhibition of MMP9 in vivo with 2-[[(4-phenoxyphenyl)sulfonyl]methyl]-Thiirane (SB-3CT), an MMP9 inhibitor, and of MMP9 and MMP2 silencing by short interfering (si)RNA administration into the hippocampus, as reported26. SB-3CT eliminated MMP9 gelatinase activity (Fig. 2b, c) and reversed the leaky BBB phenotype (Supplementary Fig. 4a) in both mouse lines. Similarly, MMP9, but not MMP2, silencing reversed the BBB phenotype in TR-APOE4 mice (Supplementary Fig. 4b). Similar results were obtained with siMmp9 treatment in Apoe−/− mice (not shown).
Figure 2. CypA activates NF-kB-MMP-9 pathway causing BBB breakdown in Apoe−/− and APOE4 mice.
(a) Multiphoton microscopy of DQ-gelatin (green) in 8–9-month-old control, Apoe−/− Ppia+/+, Apoe−/− Ppia−/−, TR-APOE2, TR-APOE3, TR-APOE4 and cyclosporine A-treated Apoe−/− Ppia+/+ and TR-APOE4 mice. Red, cortical vessels. (b) Quantification of DQ-gelatin signal in apoE transgenic mice. Effects of cyclosporine A, PDTC, SB-3CT and CypA deletion in Apoe−/− and TR-APOE4 mice. Mean±s.e.m., n=3–6 animals per group.(c) Gelatin zymography of brain tissue in control, Apoe−/− Ppia+/+, Apoe−/− Ppia−/−, TR-APOE3 and TR-APOE4 mice treated with vehicle, cyclosporine A, PDTC or SB-3CT. (d) MMP-9 (green) colocalization with CD13-positive pericytes (red; yellow, merged) in cortical microvessels from 9-month-old Apoe+/+Ppia+/+, Apoe−/− Ppia+/+ and TR-APOE4 mice. Blue, lectin-positive endothelium. Bar=10 mm. (e) Reduced collagen-IV, ZO-1, occludin and claudin-5 levels in 2-week-old Apoe−/− and TR-APOE4 mice and reversal by CypA ablation (Apoe−/− Ppia+/+) and cyclosporine A (TR-APOE4). c and e, representative results from 4–6 experiments.
Consistent with MMP9 activation, several MMP9 substrates including collagen IV and tight-junction proteins ZO-1 (also known as Tjp1), occludin and claudin 5, which are required for normal BBB integrity7, 24 were reduced in brain microvessels in young Apoe−/− and APOE4 mice, indicating BBB breakdown (Fig. 2e and Supplementary Fig. 3c–f). SB-3CT (Supplementary Fig. 4c) and siMMP-9, but not siMMP-2 or control siRNA, normalized the levels of tight-junction and basement-membrane proteins in APOE4 (Supplementary Fig. 4b) and Apoe−/− mice (not shown). These data explain how MMP9 inhibition permits reversal of BBB disruption in Apoe−/− and APOE4 mice at a molecular level. Notably, genetic deletion of Ppia or cyclosporine A substantially inhibited gelatinase/MMP9 activity (Fig. 2a–c) and restored the basement-membrane and tight-junction proteins in Apoe−/− Ppia−/− and TR-APOE4 mice (Fig. 2e and Supplementary Figs 3c–f, 4c).
Nuclear-factor-κB (NF-κB) transcriptionally activates MMP9 in cerebral vessels, causing BBB breakdown24. Consistent with findings that CypA at pathophysiological levels activates NF-κB and the NF-κB–MMP9 pathway18, 24, 27, we found NF-κB nuclear translocation in brain capillary pericytes in Apoe−/− and APOE4 mice (Supplementary Fig. 5a, b). In both Apoe−/− and APOE4 mice, NF-κB nuclear translocation was inhibited by Ppia gene deletion and/or cyclosporine A (Supplementary Fig. 5a, b).
To establish the causality between NF-κB activation, increased gelatinase activity and BBB breakdown, we treated Apoe−/− and APOE4 mice with pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-κB nuclear translocation. In Apoe−/− and APOE4 mice, PDTC markedly reduced MMP9 activation (Fig. 2b, c and Supplementary Fig. 3b) and reversed the leaky BBB phenotype (Supplementary Fig. 4a). Consistent with these data, NF-κB inhibition in the hippocampus by silencing Rela, which encodes the p65 subunit of NF-κB, inhibited MMP9 and reversed the BBB phenotype in APOE4 mice (Supplementary Fig. 4c). Similar results were obtained by silencing Rela in Apoe−/− mice (not shown). Our findings therefore clearly establish that each of the molecules studied (that is, CypA, NF-κB and MMP9) have important and required roles in BBB disruption in Apoe−/− and APOE4 mice.
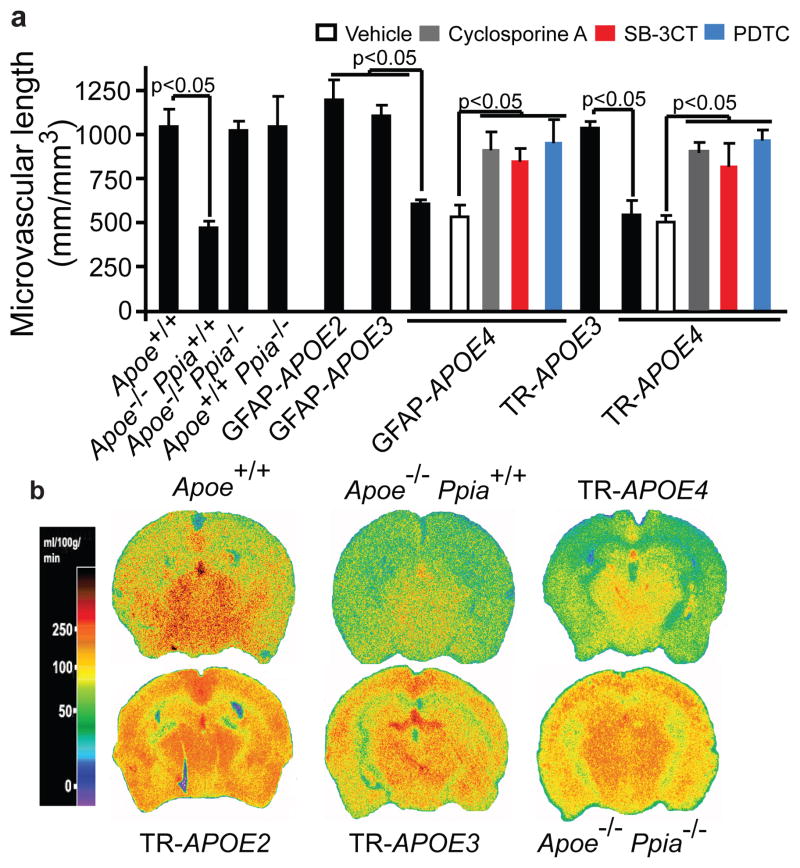
Consistent with reports that chronic BBB breakdown leads to microvascular reductions7, 14, 22, we found microvascular degeneration in APOE4 and Apoe−/− mice, including DNA fragmentation in pericytes and endothelial cells (Supplementary Fig. 6a), diminished pericyte coverage (Supplementary Fig. 5b, c) and reductions in microvascular length (Fig. 3a and Supplementary Fig. 6d), which correlated with the degree of BBB breakdown (Supplementary Fig. 6e) and regional cerebral blood flow (CBF) reductions (Fig. 3b and Supplementary Table 1). Notably, Ppia deletion and cyclosporine A, SB-3CT or PDTC normalized microvascular reductions in Apoe−/− and APOE4 mice (Fig. 3a and Supplementary Fig. 6c). Ppia deletion also normalized CBF reductions in Apoe−/− mice (Fig. 3b).
Figure 3. CypA ablation or inhibition reverses microvascular and CBF reductions in Apoe−/− and APOE4 mice.
(a) Capillary length in the hippocampus of apoE transgenic mice including Apoe−/− Ppia+/+, Apoe−/− Ppia−/− and GFAP-APOE4 and TR-APOE4 mice treated with cyclosporine A, SB-3CT or PDTC (mean±s.e.m., n=5 animals per group). (b) 14C-iodoantipyrine CBF autoradiograms in 9-month-old transgenic apoE mice. b, representative results from 6 experiments.
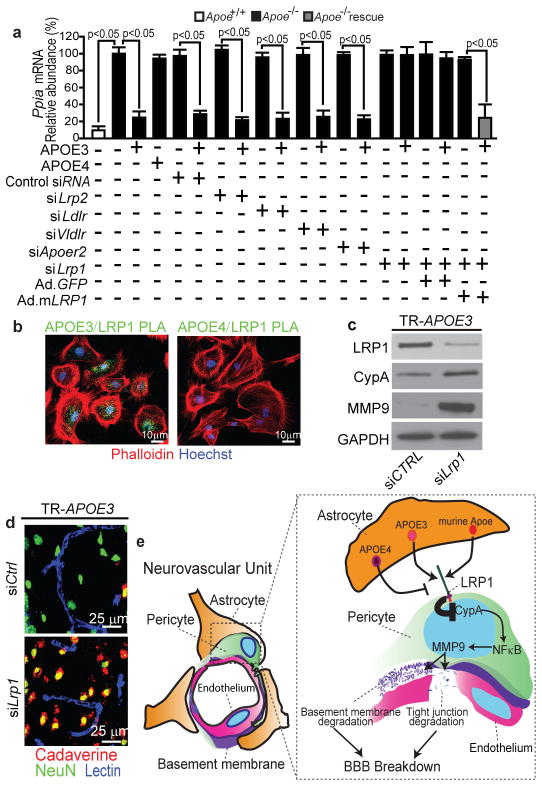
Given that CypA expression and NF-κB and MMP9 activation are increased in pericytes in Apoe−/− and APOE4 mice, we next studied which low-density lipoprotein (LDL)/APOE receptor1 in pericytes regulates CypA in response to astrocyte-derived APOE. After confirming the specificity of our siRNA reagents (Supplementary Fig. 7), we showed by quantifying the effects of siRNA inhibition (Fig. 4a and Supplementary Fig. 8a–d) and by administering antibodies to block the function of specific LDL receptors (Supplementary Fig. 8e–g) that astrocyte-derived APOE3 and murine Apoe require low-density lipoprotein receptor-related protein 1 (LRP1) to maintain CypA synthesis within a physiological range. Adenoviral-mediated re-expression of a human LRP1 minigene rescued the ability of APOE3 to downregulate Ppia mRNA (Fig. 4a) and protein (Supplementary Fig. 8b) in pericytes with siRNA-induced LRP1 knockdown. By imaging APOE/LRP1 proximity ligation in pericytes we demonstrated that APOE3 (Fig. 4b) and murine Apoe (not shown) bind with high affinity to LRP1, whereas the APOE4–LRP1 interaction was barely detectable (Fig. 4b). Together these data explain at the molecular level why APOE4 is unable to properly regulate physiologic CypA levels, which is consistent with previously reported statistically insignificant interactions of APOE4 with LRP1 in cerebral microvessels and at the BBB in vivo28.
Figure 4. ApoE isoform-specific regulation of CypA-NF-kB-MMP-9 pathway in pericytes.
(a) CypA mRNA quantification in Apoe−/− pericytes after treatment with astrocyte-secreted apoE3, apoE4, siRNA silencing of LDL/apoE receptors and adenoviral-mediated re-expression of LRP1 minigene (Ad.m LRP1). Mean±s.e.m., n=3 independent cultures. (b) Proximity ligation imaging of apoE3 and apoE4 interaction with LRP1. (c–d) LRP1, CypA and MMP-9 immunodetection (c) and neuronal uptake (NeuN, green) of cadaverine-Alexa-Fluor-555 (red; yellow, merged) (d) in the hippocampus of 6-month-old GFAP-APOE3 mice after siLRP1 or control siRNA infusion. Blue, lectin-positive capillaries. (e) A schematic showing that astrocyte-secreted apoE3 and murine apoE, but not apoE4, signal to pericytes via LRP1 suppressing the CypA-NF-kB-MMP-9 pathway that causes BBB breakdown. b and c–d, representative results from 6 experiments.
Ppia silencing, cyclosporine A and astrocyte-derived APOE3, but not APOE4, inhibited NF-κB nuclear translocation in Apoe−/− pericytes (Supplementary Fig. 9a), as in vivo. By using siLrp1 silencing, cyclosporine A or PDTC, we showed that LRP1 is required for APOE3-mediated inhibition of NF-κB-dependent MMP9 activation and transcriptional suppression24 (Supplementary Fig. 9). APOE4 did not have an effect on MMP9 in pericytes, consistent with its barely detectable binding to LRP1 (Fig. 4b). In vivo, LRP1 inhibition in APOE3 mice through siRNA administration in the hippocampus26 reproduced vascular phenotypes seen in APOE4 mice, including elevated CypA and MMP9 levels and increased CypA and MMP9 expression in pericytes (Fig. 4c and Supplementary Fig. 10a, b), and BBB breakdown (Fig. 4d). As expected, LRP1 inhibition in mice with Ppia genetic deletion did not influence MMP9 expression in pericytes (Supplementary Fig. 10d) or BBB integrity (Supplementary Fig. 10d). Together, these data clearly implicate APOE3/LRP1-mediated CypA regulation in pericytes, conferring APOE3 isoform-specific protection of the BBB (Fig. 4e).
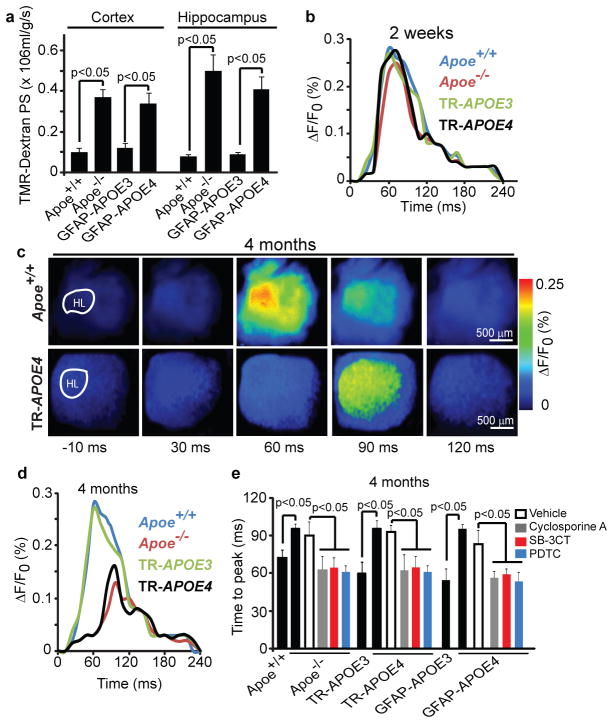
Vascular defects in Apoe−/− and APOE4 mice were detectable at 2 weeks of age, including leakage of dextran (Fig. 5a) and serum IgG (Supplementary Fig. 11a), and reductions in tight-junction and basement-membrane proteins, pericyte coverage, capillary length and regional CBF, which progressively increased with age (Fig. 2e and Supplementary Figs 11b–e, 12 and Supplementary Table 1). We next asked whether vascular damage precedes neuronal changes in Apoe−/− mice29, and neuronal and synaptic dysfunction in APOE4 mice2. Cortical activity determined in vivo by voltage-sensitive dye (VSD) imaging indicated normal time-lapse imaging profiles in 2-week-oldApoe−/− and APOE4 mice (Fig. 5b and Supplementary Fig. 13a, b) and normal neuritic density and levels of pre-synaptic and post-synaptic proteins (Supplementary Fig. 14a–d). At 4 months of age, however, Apoe−/− and APOE4 mice showed a lower amplitude of the VSD signal, longer time-to-peak and a slower duration of the spreading of depolarization (Fig. 5c, d), which was accompanied by age-dependent reductions in neuritic density and pre-synaptic and post-synaptic proteins (Supplementary Fig. 14a–f). These data indicate that Apoe−/− and APOE4 mice develop vascular defects before neuronal and synaptic changes occur. Cyclosporine A, PDTC and SB-3CT improved functional and structural neuronal changes in Apoe−/− and APOE4 mice (Fig. 5e and Supplementary Fig. 15), indicating that normalization of BBB integrity through inhibition of the CypA–NF-κB–MMP9 pathway is required for neuronal and synaptic repair.
Figure 5. Vascular defects in Apoe−/− and APOE4 mice precede neuronal dysfunction.
(a) The blood-brain barrier permeability surface (PS) product for tetramethylrhodamine (TMR)-dextran (40,000 Da) in the cortex and hippocampus of 2-week-old Apoe+/+, Apoe−/−, GFAP-APOE3 and GFAP-APOE4 mice measured by non-invasive fluorescence spectroscopy. (b) Representative time-lapse imaging profile analysis of fluorescent voltage sensitive dye (VSD) signal response in the hind-limb somatosensory cortex after stimulation in 2-week-old Apoe+/+, Apoe−/−, TR-APOE3 and TR-APOE4 mice. (c) VSD imaging of cortical responses to hind-limb stimulation in 4-month-old Apoe+/+ and TR-APOE4 mice. (d) Representative VSD signal responses in the hind-limb somatosensory cortex region after stimulation in 4-month-old Apoe+/+, Apoe−/−, TR-APOE3 and TR-APOE4 mice. (e) Time to peak in fluorescent VSD signal after hind-limb stimulation in 4-month-old Apoe+/+, Apoe−/−, TR-APOE3, TR-APOE4, GFAP-APOE3 and GFAP-APOE4 mice and in Apoe−/−, TR-APOE4 and GFAP-APOE4 mice treated with cyclosporine A, SB-3CT, PDTC or vehicle. a and e, mean±s.e.m., n= 5 animals per group.
Understanding the contribution of APOE4 to the pathogenesis of Alzheimer’s disease may be one of the most important avenues to a new therapy. Neurovascular dysfunction and BBB defects have been shown in Alzheimer’s disease 7, 30. The findings from this study indicating that abnormal vessels and pericytes can be involved provide an alternative way of thinking about Alzheimer’s disease and neurological disorders affected by APOE4. Our findings demonstrate that APOE maintains cerebrovascular integrity necessary for normal neuronal function by regulating the CypA–NF-κB–MMP9 pathway in pericytes in an isoform-specific manner (Fig. 4e). We also show that CypA is a key target for treating APOE4-mediated neurovascular defects and the resulting neuronal dysfunction.
METHODS SUMMARY
Animals
Apoe−/−, GFAP-APOE mice on murine apoE null background and Ppia−/− mice were acquired from Jackson Laboratories. TR-APOE mice were generated as previously described16. The Ppia−/− mice were crossed to the Apoe−/− and TR-APOE4 mice to generate the Apoe−/− Ppia−/− and TR-APOE4 Ppia−/− mice used in the present study. Mice were housed in plastic cages on a 12 h light cycle with ad libitum access to water and a standard laboratory diet. All studies were performed in accordance with the University of Rochester Institutional Animal Care and Use Committee using National Institute of Health guidelines. All lines were maintained on a C57Bl6 background. No significant phenotypic differences were found between littermate control animals.
Pharmacological inhibition
In some studies, APOE4 or Apoe−/− mice were treated for 7 consecutive days with a low intraperitoneal non-toxic dose of cyclosporine A (Sigma, 30024-25; 10 mg/kg/day for 3 days followed by 5 mg/kg/day for 4 days), or pyrrolidine dithiocarbamate (PDTC, 100 mg/kg/day) or SB-3CT (25 mg/kg/day).
In vivo siRNA infusion
siRNA-mediated LRP1, MMP-9, MMP-2 and RELA knockdown was performed as previously described26.
Blood-brain barrier permeability assays
In vivo multiphoton imaging of TMR-conjugated dextran and detection of endogenous IgG, fibrin, thrombin and Prussian blue deposits in brain tissue was performed as previously described14. Detection of neuronal uptake of systemically administered Alexa fluor 555-conjugated cadaverine was performed as described15.
Stastical analysis
Data were analyzed by multifactorial analysis of variance (ANOVA) followed by Tukey posthoc tests and Pearson’s correlation analysis using GraphPad Prism 3.0 software. A p value less than 0.05 was considered statistically significant in all studies.
A complete description of all experiments performed and associated references are available in the Supplemental Materials and Methods section.
Supplementary Material
Acknowledgments
We would like to thank the National Institutes of Health for grants R37NS34467(BVZ); R37AG23084(BVZ); RO1AG039452(BVZ); and R37AG13956(DMH) used to support this study.
Footnotes
Author Contributions RDB designed and performed experiments, analyzed data and contributed to writing the paper; EAW designed and performed experiments; IS performed experiments; APS performed CBF experiments; RD designed experiments; ZW gathered pilot data; DMH provided guidance and edited the paper; CB designed experiments and edited the paper, AA performed pilot cadaverine studies; JS generated pilot data; BCB provided guidance and edited the paper; BVZ designed experiments, analyzed data and wrote the paper.
References
- 1.Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E: structure determines function, from atherosclerosis to Alzheimer’s disease to AIDS. J Lipid Res. 2009;50 (Suppl):S183–188. doi: 10.1194/jlr.R800069-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thambisetty M, Beason-Held L, An Y, Kraut MA, Resnick SM. APOE epsilon4 genotype and longitudinal changes in cerebral blood flow in normal aging. Arch Neurol-Chicago. 2010;67:93–98. doi: 10.1001/archneurol.2009.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sheline YI, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30:17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiman EM, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature Rev Nsci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snowdon DA, et al. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 9.Vermeer SE, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 10.Ruitenberg A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789–794. doi: 10.1002/ana.20493. [DOI] [PubMed] [Google Scholar]
- 11.Methia N, et al. ApoE deficiency compromises the blood brain barrier especially after injury. Mol Med. 2001;7:810–815. [PMC free article] [PubMed] [Google Scholar]
- 12.Hafezi-Moghadam A, Thomas KL, Wagner DD. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am J Physiol Cell Physiol. 2007;292:C1256–1262. doi: 10.1152/ajpcell.00563.2005. [DOI] [PubMed] [Google Scholar]
- 13.Nishitsuji K, Hosono T, Nakamura T, Bu G, Michikawa M. Apolipoprotein E regulates the integrity of tight junctions in an isoform-dependent manner in an in vitro blood-brain barrier model. J Bio Chem. 2011;286:17536–17542. doi: 10.1074/jbc.M111.225532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bell RD, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Armulik A, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PM, et al. Reduced levels of human apoE4 protein in an animal model of cognitive impairment. Neurobio Aging. 2011;32:791–801. doi: 10.1016/j.neurobiolaging.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Satoh K, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin ZG, et al. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186–1191. doi: 10.1161/01.ATV.0000130664.51010.28. [DOI] [PubMed] [Google Scholar]
- 19.Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 20.Begley DJ, et al. Permeability of the blood-brain barrier to the immunosuppressive cyclic peptide cyclosporin A. J Neurochem. 1990;55:1222–1230. doi: 10.1111/j.1471-4159.1990.tb03128.x. [DOI] [PubMed] [Google Scholar]
- 21.Grammas P. Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation. 2011;8:26. doi: 10.1186/1742-2094-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul J, Strickland S, Melchor JP. Fibrin deposition accelerates neurovascular damage and neuroinflammation in mouse models of Alzheimer’s disease. J Exp Med. 2007;204:1999–2008. doi: 10.1084/jem.20070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong Z, et al. Activated protein C therapy slows ALS-like disease in mice by transcriptionally inhibiting SOD1 in motor neurons and microglia cells. J Clin Invest. 2009;119:3437–3449. doi: 10.1172/JCI38476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Candelario-Jalil E, et al. Matrix metalloproteinases are associated with increased blood-brain barrier opening in vascular cognitive impairment. Stroke. 2011;42:1345–1350. doi: 10.1161/STROKEAHA.110.600825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Alloza M, et al. Matrix metalloproteinase inhibition reduces oxidative stress associated with cerebral amyloid angiopathy in vivo in transgenic mice. J Neurochem. 2009;109:1636–1647. doi: 10.1111/j.1471-4159.2009.06096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaeger LB, et al. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alz Dis. 2009;17:553–570. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Lu N, Zhou J, Chen ZN, Zhu P. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology. 2008;47:1299–1310. doi: 10.1093/rheumatology/ken225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deane R, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–4013. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masliah E, et al. Neurodegeneration and cognitive impairment in apoE-deficient mice is ameliorated by infusion of recombinant apoE. Brain Res. 1997;751:307–314. doi: 10.1016/s0006-8993(96)01420-5. [DOI] [PubMed] [Google Scholar]
- 30.Zipser BD, et al. Microvascular injury and blood–brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–986. doi: 10.1016/j.neurobiolaging.2006.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.