Abstract
Objective
The prognostic significance of circulating tumor cells (CTCs) is controversial in gastric cancer (GC). We performed a meta-analysis of available studies to assess its prognostic value detected by RT-PCR for patients diagnosed with GC.
Methods
EMBase, PubMed, Ovid, Web of Science, Cochrane library and Google Scholar database search was conducted on all studies reporting the outcomes of interest. The studies were set up according to the inclusion/exclusion criteria. Meta-analysis was performed by using a random-effects model; hazard ratio (HR), risk ratio (RR) and their 95% confidence intervals (95% CIs) were set as effect measures. The information about trial design, results from the data was independently extracted. Heterogeneity of the studies was tested for each pooled analysis.
Results
Nineteen studies published matched the selection criteria and were included in this meta-analysis. CTCs positivity was significantly associated with poor relapse free survival (RFS) (HR 2.42, 95% CI: [1.94–3.02]; P<0.001) and poor overall survival (OS) (HR 2.42, 95% CI: [1.94–3.02]; P<0.001). CTCs positivity were also significantly associated with regional lymph nodes (RLNs) metastasis (RR 1.42, 95% CI: [1.20–1.68]; p<0.0001), depth of infiltration (RR 1.51, 95% CI: [1.27–1.79]; p<0.0001), vascular invasion (RR = 1.43, 95% CI: [1.18–1.74], p = 0.0002) and TNM stage(I,II versus III) (RR 0.63, 95% CI [0.48–0.84]; p = 0.001).
Conclusion
Preoperative CTCs positivity indicates poor prognosis in patients with gastric cancer, and associated with poor clinicopathological prognostic factors.
Introduction
Globally, gastric cancer (GC) is the fourth most common cancer and is the second leading cause of cancer –related death [1]. In China, gastric cancer holds the third place of morbidity among digestive system cancers, due to the difficulties of early diagnosis, quantities of patients were diagnosed with GC until in its advanced stage; unfortunately, even after radical operation and adjuvant therapy, the 5-year overall survival (OS) of GC patient is relatively low (under 50%) [2]; over the past decade, therapy strategy of gastric cancer continuously changes but still fails to improve overall prognosis significantly, most of patients die because of distant metastasis and recurrence. Thus, in order to improve the clinical outcome of GC patients, we need new biomarkers that can help us to identify patients with high-risk of metastasis and pursue specific therapy strategy.
As is known to us, tumor metastasis consists a series of biological procedures, one important step is tumor cells disseminate into blood stream and circulate [3]; thus, to get more insights into metastasis cascade, studies of circulating tumor cells (CTCs) vigorously becomes one of hot academic topics. The concept of CTCs dated back to the study of Ashworth [4] in1869 and was demonstrated by Engell [5] in 1955 who proved the existence of these rare cells. There is a considerable body of evidence indicating that CTCs are shed from the primary tumor mass at a earliest stages of malignant progression [6]; these cells, circulating through the bloodstream, traveling to different tissues of body, are the main cause of overt metastases [7]. Nowadays, numerous studies have investigated the prognostic relevance of CTCs positivity of patients with breast cancer [8], colorectal cancer [9], and proved that CTCs could be a poor prognostic marker.
With regard to gastric cancer, although there are many studies designed to find out the relationship between CTCs and prognosis or other clinicopathologic parameters, the lack of statistical power together with their different study design and results limited the individual clinical value and the prognostic effect of CTCs positivity. Especially, the value of preoperative CTCs positivity in gastric cancer patients has not yet been clearly illustrated. Thus we performed a combined analysis of available studies that will provide a more precise estimate on the prognostic relevance of CTCs in patients with GC.
Methods
Literature Search
PubMed, Embase, Ovid, Web of Science, Google Scholar and Cochrane library data bases were systematically searched without time restrictions. Studies reporting on the molecular detection of CTCs and its effect on prognosis in gastric cancer were identified. The following key words were used: ‘‘Circulating tumor cells’’ or ‘‘CTCs’’, ”gastric cancer’’, “prognosis” and “PCR” were used as the key words. In order to prevent missing relevant publications, “related articles” function of Pubmed and Google Scholar were used to identify other potentially relevant publications. References of the articles were hand-searched for relevant articles, including review articles. Two reviewers (S.Y. Wang and G Zheng) independently screened and retrieved the literature list and, in the case of potentially relevant references, obtained the full articles; Cases of disagreement were resolved by discussing the title and abstract; Full-text articles (n = 39) were examined and 20 were excluded following the criteria below.
Literature Screening Criteria
To be included in the analysis, studies had to match the following inclusion criteria: (1) any form of reverse transcription PCR (RT-PCR) used for the evaluation of the association between the putative markers of circulating tumor cells and either overall survival (OS), relapse-free survival (RFS), or prognostic factors of gastric cancer; (2) >20 analyzed patients and sufficient data to calculate a hazard ratio (HR) or a risk ratio(RR) with a 95% confidence interval (95% CI) as a comparable effect estimate; (3) samples used in these studies should be peripheral blood and should be collected before surgery; (4) exclusion of letters to the editor, reviews, and articles published in non-English language books or papers.
Data Extraction
Two reviewers (S.Y. Wang and G Zheng) independently extracted the following data from each study: the year of publication, the first author’s surname, the number of cases and controls, the number of different clinical and pathological parameters, and the assessment methods of survival expression. Disagreements were resolved by discussion and were checked by a third investigator.
Statistical Analysis
Statistical analysis were done with Review Manager (RevMan)(Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012). To statistically evaluate the prognostic effect of CTCs, we extracted Hazard Ratio (HR) and their associated standard errors on relapse free survival (RFS) and/or overall survival (OS) from the included studies. If the HRs and their associated standard errors, confidence intervals (CIs), or P values were not directly provided in the original articles, we approximated HRs according to the method described by Parmar et al [10]. By convention, a HR>1 implies a worse prognosis in the CTCs–positive group in comparison to negative group. We pooled the extracted HRs with the use of the generic inverse variance method available in the Review Manager. Because we expected interstudy heterogeneity, we applied a random effect model [11], because it is more conservative by creating a wider CI around the pooled HR than the fixed effect analysis model. When analyzed the association between CTCs and other parameters, Relative Risk (RR) was calculated, a RR>1 implied CTC-positive group was associated with a parameter. All data extractions were performed separately by SY Wang and G Zheng. Disagreements were resolved by discussion. Heterogeneity between studies was tested with the Q test and I2 statistic. We evaluated potential publication bias by a funnel plot, which was further examined by the Egger [12] and Begg’s test [13] using STATA software (Version 11.0, College Station, TX). And pooled analysis of the diagnostic accuracy of CTCs positivity was also calculated by STATA.
Results
Baseline Study Characteristics
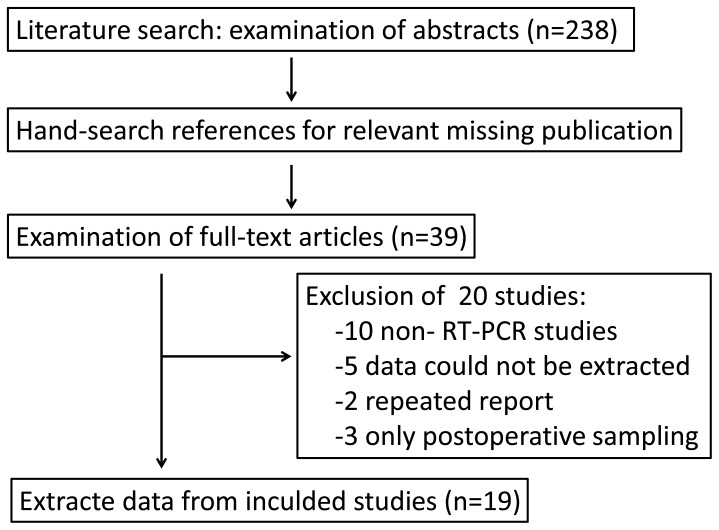
The systematic literature search (Fig. 1) yielded a total of 19 studies [14]–[32] for final analysis. The studies were conducted in 6 countries (China, Germany, Japan, Korea and the United States) and published between 2000 and 2013. All 19 studies analyzing peripheral blood before surgery applied a molecular detection method (PCR, RT-PCR, or RT followed by quantitative PCR) of tumor cells, CEA mRNA was tested in 6 studies, and other genes were tested not more than 3 studies. The baseline characteristics of the included studies are summarized in (Table 1).
Figure 1. Flowchart of studies screening process.
Table 1. Baseline characteristics of included studies for the meta-analyses.
| First author | Year | Number of patients | RT-PCR detection method | Circulating tumor cell incidence | Cancer stages | Follow-up (months) | Outcomes measured |
| Majima | 2000 | 52 | CK19,CK20 | 5(9.6%) | I–IV | 18 | OS |
| Nishida | 2000 | 41 | CEA | 12(29.3%) | I–IV | NR | NR |
| Miyazono | 2001 | 57 | CEA | 21(36.8%) | I–IV | 15(6–30) | RFS |
| Shin | 2002 | 65 | hTert | 30(46.2%) | I–IV | NR | NR |
| 42 | cMET | 9(21.4%) | |||||
| Sumikura | 2003 | 106 | CEA | 43(40.6%) | I–III | 21(12–60) | RFS |
| Seo | 2005 | 46 | CEA | 9(19.6%) | I–III | >6 | RFS |
| Illert | 2005 | 70 | CK20 | 29(41.4%) | I–IV | 20(1–57) | OS |
| Ikeguchi | 2005 | 59 | CEA | 27(45.8%) | I–IV | 20.1(2–31) | RFS |
| Wu | 2006 | 42 | Htert | 26(61.9%) | I–IV | 18(10–26) | RFS |
| CK19 | 29(69%) | ||||||
| CK20 | 26(61.9%) | ||||||
| CEA | 33(78.6%) | ||||||
| Uen | 2006 | 52 | MUC1 | 37(71.2%) | I–IV | 36 | RFS |
| c-MET | 32(61.5%) | ||||||
| Kosaka | 2007 | 90 | VEGFR-1 | 34(37.8%) | I–IV | 9.8(4–24) | RFS |
| Koga | 2008 | 69 | CK19 | 8(11.6%) | I–IV | NR | OS |
| CK20 | 10(14.5%) | ||||||
| Yie | 2008 | 55 | Survivin | 25(45.5%) | I–IV | 36 | RFS |
| Bertazza | 2009 | 70 | Survivin | 69(98.6%) | I–IV | 15(6–119) | OS |
| Kita | 2009 | 846 | uPAR | 404(47.8%) | I–IV | NR | NR |
| Qiu | 2010 | 123 | CEA | 45(36.6%) | I–IV | 37(3–73.6) | RFS |
| Arigami | 2011 | 95 | B7-H3 | 48(50.5%) | I–IV | 24(1–74) | OS |
| Cao | 2011 | 98 | Survivin | 45(45.9%) | I–IV | 47.5(36.5–56) | RFS |
| Agarami | 2013 | 93 | STCs | 43(46.2%) | I–IV | 25(1–74) | OS |
Foot note: NR not reported, OS overall survival, RFS recurrence-free survival.
Diagnostic Accuracy of CTCs Detection
To evaluate the overall test performance of included studies [14]–[17], [19], [21]–[32], we calculated the pooled diagnostic accuracy of CTCs detection. The combined sensitivity and specificity was 0.45 (95% CI: [0.34–0.57]) and 0.99 (95% CI: [0.96–1.00]) respectively (Figure S1), with significant heterogeneity (I2 = 91%, p<0.05 and I2 = 69.83%, p<0.05). Positive Likelihood Ratio (PLR) is 37.1 (95% CI: [11.7–118.1]), Negative Likelihood Ratio (NLR) was 0.55 (95% CI: [0.38–0.89]) (Figure S2). Combined diagnostic odds ratio was 67.08 (95% CI: [19.75–227.86]) (Figure S3) and the area under SROC curve was 0.93 (95% CI: [0.91–0.95] (Figure S4).
Overall Analysis of Survival for Gastric Cancer Patients
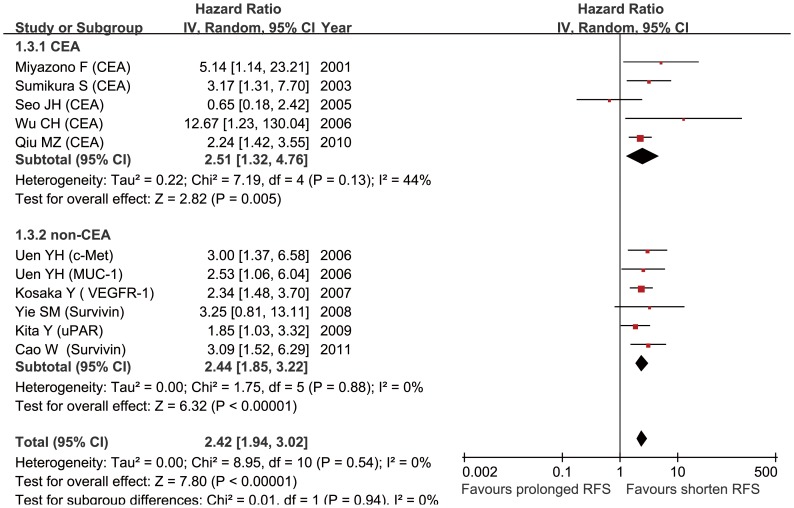
Data on RFS were available in 10 studies [16], [18], [19], [22]–[24], [26], [28], [29], [31], the pooled analysis showed a prognostic effect of CTCs positivity (HR = 2.42, [95% CI: 1.94–3.02]; P<0.001) (Figure 2), with no between-study heterogeneity (I2 = 0%, p = 0.54). We also stratified studies of CEA-mRNA positive CTCs [16], [18], [19], [22], [29] for subgroup, pooled analysis suggested an association between poor RFS and CTCs positivity (HR = 2.51, 95% CI: [1.32–4.76], p<0.001%), and between-study heterogeneity was moderate (I2 = 44%, p = 0.13). Publication bias, tested by Egger’s test (p = 0.578) and funnel plot (Figure S5), was negligible for the pooled analysis of RFS.
Figure 2. Summary estimates of hazard ratio (HR) for RFS.
RFS, relapse-free survival.
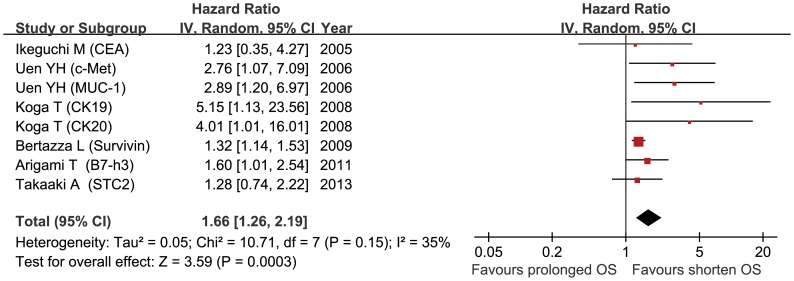
Pooled analysis of studies [21], [23], [25], [27], [30], [32] on OS showed that presence of CTCs was associated with poor OS (HR = 1.66, 95% CI: 1.26–2.19; p<0.001) (Figure 3), and the between-study heterogeneity (I2 = 35%, p = 0.15) was not significance. Egger’s test (p = 0.017) and funnel plot (Fig. S6) showed this combined analysis had publication bias.
Figure 3. Summary estimates of hazard ratio (HR) for OS.
OS, overall survival.
Correlation of Circulating Tumor Cells with Clinicopathologic Parameters
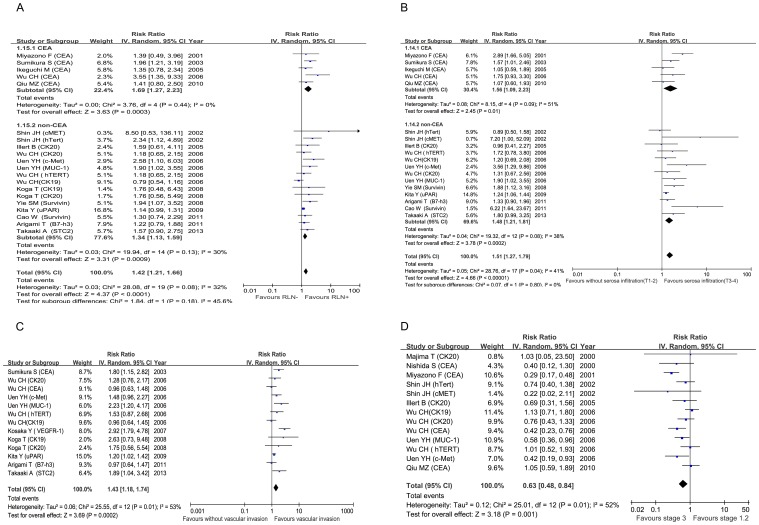
14 studies [16]–[18], [20]–[23], [25], [26], [28]–[32] were assessed the relationship between CTCs positivity and regional lymph nodes (RLNs) metastasis (RR = 1.42, 95% CI: [1.21–1.66]; p<0.0001) (Fig. 4A), with no significant between-study heterogeneity (I2 = 32%, p = 0.08), subgroup analysis showed that CEA-mRNA positive CTCs were associated with RLNs metastasis (RR = 1.69, 95%CI:[1.27–2.23]; p = 0.0003), and the between-study heterogeneity decreased (I2 = 0%, p = 0.44). Studies [16]–[18], [20]–[23], [26], [30]–[32] assessed by pooled analysis showed significant association of CTCs positivity with the depth of tumor infiltration (RR = 1.51, 95% CI: [1.27–1.79]; p<0.0001) (Fig. 4B), between-study heterogeneity was significant (I2 = 41%, p = 0.04), subgroup analysis showed that CEA-mRNA positive CTCs were associated with depth of tumor infiltration (RR = 1.56, 95% CI:[1.09–2.23], p = 0.01), with same between-study heterogeneity (I2 = 51%, p = 0.09). Vascular invasion[18], [22]–[25], [28], [30], [32] (RR = 1.43, 95% CI: [1.18–1.74]; p = 0.0002) was associated with CTCs positivity (Fig. 4C), but the between-study heterogeneity was significant (I2 = 55%, p = 0.01).
Figure 4. Summary estimates of risk ratio (RR) for RLNs metastasis (A), depth of infiltration (B), vascular invasion (C) and TNM stage (D) (Stage I,II vs Stage III) associated with CTCs positivity.
RLNs, regional lymph nodes.
Eight studies [14]–[17], [20], [22], [23], [29] reported the relationship between CTCs positivity and TNM stage, the overall positive rate of CTCs in stage I and II group was 36.7% compared with 56.6% of stage III group. Pooled analysis showed that CTCs positivity in stage III is greater than on stage I and II (RR 0.63, 95% CI 0.48–0.84; p = 0.001), with between-study heterogeneity (I2 = 52%, p = 0.01) as shown in Figure 4D. When pooled analysis [14]–[17], [20], [22], [23], [26], [29] was introduced to compare CTC positivity in stage I with stage II, the CTCs positivity was higher in stage II versus stage I (RR = 0.55; [95% CI 0.36–0.84], p = 0.005). However, when stage II and stage III groups were compared [15]–[17], [20], [22], [23], [29], data showed no statistically significant (RR = 0.87; [95% CI: 0.73–1.04], p = 0.93).
Discussion
From the clinical perspective, the assessment of patients’ prognosis by CTCs detection in the PB can supply important prognostic information. Bizard et al [33] found that even a single CTC detected in 7.5 ml of blood was associated with the subsequent development of metastases, which means CTCs have strong potential of distant metastasis. Besides, CTCs detection, with the advantage of time- and cost- saving, appears comfortable for the patient and may be easily repeated as a monitoring tool. To date, encouraging results concerning an association between CTC positivity and metastatic progression in patients with metastatic breast [34], prostate [35], and colorectal [36] cancer have been recently published. However, there is currently very limited data on the clinical relevance of CTC positivity in GC patient, the results of our collective evaluation suggest that CTCs positivity in PB should indeed be considered as a prognostic marker.
During the process of our meta-analysis, we restrict sampling time and site for our design in order to minimize heterogeneity, but we still notice a certain degree of heterogeneity. Potential sources of heterogeneity may derive from differences in the detection protocol, types and numbers of target genes selection, standard of CTCs positivity, as well as in demographic or clinicopathologic data of included patients. In theory, postoperative CTCs status may be important and informative, it reflects the combined information of preoperative CTCs and intraoperative tumor cell release by surgical manipulation [37]. But the rapid apoptotic death of freshly shredded CTCs may release mass tumor gene or antigens because of the loss of survival microenvironment in the systemic circulation; this may lead to certain degree of detection bias. Sampling time is another important factor that interfere the prognositic value of CTCs positivity and leads to heterogeneity. Ikeguchi M et al. [21] studied the association between postoperative CTCs positivity and prognosis, they found that, if the blood samples were postoperatively collected within 48 hours, CTCs positive patients had better prognosis than CTCs negative ones. Thus, further studies of CTCs should take sampling time into consideration, evaluate and confirm the best sampling time. A further source for the observed heterogeneity may be the CTCs pool itself, it was consisted of heterogeneous population of cancer cells, within this population only a specific fraction had prognostic effect [38]. Furthermore, characterization of CTCs with breast cancer, gastric cancer, or colorectal cancer showed that only a minority of these cells express proliferation-associated markers, growth factor receptors, immune response antigens, adhesion molecules, and proteases or protease-associated proteins [39].In addition, tumor cells dissemination is an early event during distant metastasis, and random aberrations for metastasis-specific gene may be acquired after CTCs shedding into the blood circulation [40]. This model may explain the genomic and functional heterogeneity of CTCs.
There are some limitations of this meta-analysis. Firstly, limitations caused by the heterogeneity mentioned before and the inability to access primary data of the included studies. We addressed the issue of heterogeneity by a rigorous methodological approach that used the random-effects model for more conservative estimates. Prognostic factors of gastric cancer are complicated, our data for meta-analysis was from the included studies and primary data was hard to get, we were unable to exclude every possible confounding factors; approaches based on RT-PCR have high sensitivity for the detection of CTCs, but they cannot quantify the number of CTCs and lack biologic specificity [38]. Secondly, languages selection brings another bias, we have restricted our analysis to published studies written in English, other language such as Japanese, German were excluded based on language criteria. This may result in language bias leading to an overestimation of effect sizes [41]. Thirdly, we notice that certain degree of publication bias exists, especially in the pooled analysis for OS, one reason may be that studies reported positive results are much easier to be published and accessed; besides, studies introduced to pooled analysis have relatively small sample size. Although we were unable to conduct analyses considering certain potentially relevant factors, CTC positivity representing an indicator of poor prognosis in GC patients was consistently present in the pooled analysis; however, our results should be interpreted with caution and it requires more detailed and accurate data to verify.
In conclusion, our study based on available evidence supports the notion of a strong prognostic value of CTCs in the peripheral blood and relates to poor prognosis of GC. Identification of various methodological flaws and sources of heterogeneity in currently available prognostic factor studies could contribute to improve design and reporting of future prognostic and predictive factor studies. Our results also offer a hint that additional studies should use standardized testing method, optimized sampling time, complete analysis and report of results, or identification of certain cellular subgroup such as circulating stem-like cells [42]; in this way can we derive clearer and more accurate prognostic significance of CTCs in GC patients.
Supporting Information
Forest Plot for pooled analysis of SEN and SPE. SEN, sensitivity; SPE, specificity.
(TIF)
Forest Plot for pooled analysis of PLR and NLR. PLR, positive likelihood ratio; NLR, negative likelihood ratio.
(TIF)
Forest Plot for pooled analysis of DOR. DOR, diagnostic odds ratio.
(TIF)
Summary ROC curve with confidence and prediction regions of sensitivity and specificity.
(TIF)
Funnel plot for summary estimates of RFS. RFS, relapse-free survival.
(TIF)
Funnel plot for summary estimates of OS. OS, overall survival.
(TIF)
PRISMA checklist.
(DOC)
Funding Statement
The work was supported by National High Technology Research and Development Program of China (Grant No. 2012AA02A502, 2012AA02A506). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, et al. (2009) Recent patterns in gastric cancer: a global overview. Int J Cancer 125: 666–673. [DOI] [PubMed] [Google Scholar]
- 2. Yang L (2006) Incidence and mortality of gastric cancer in China. World J Gastroenterol 12: 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12: 895–904. [DOI] [PubMed] [Google Scholar]
- 4. Ashworth TR (1869) A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 14: 146149. [Google Scholar]
- 5.ENGELL HC (1955) Cancer cells in the circulating blood; a clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumour area at operation. Acta Chir Scand Suppl 201: 1–70. [PubMed]
- 6. Bernards R, Weinberg RA (2002) A progression puzzle. Nature 418: 823. [DOI] [PubMed] [Google Scholar]
- 7. Pantel K, Alix-Panabieres C (2010) Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 16: 398–406. [DOI] [PubMed] [Google Scholar]
- 8. Braun S, Vogl FD, Naume B, Janni W, Osborne MP, et al. (2005) A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 353: 793–802. [DOI] [PubMed] [Google Scholar]
- 9. Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, et al. (2010) Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 138: 1714–1726. [DOI] [PubMed] [Google Scholar]
- 10. Parmar MK, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815–2834. [DOI] [PubMed] [Google Scholar]
- 11. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 12. Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 14. Majima T, Ichikura T, Takayama E, Chochi K, Mochizuki H (2000) Detecting circulating cancer cells using reverse transcriptase-polymerase chain reaction for cytokeratin mRNA in peripheral blood from patients with gastric cancer. Jpn J Clin Oncol 30: 499–503. [DOI] [PubMed] [Google Scholar]
- 15. Nishida S, Kitamura K, Ichikawa D, Koike H, Tani N, Yamagishi H (2000) Molecular detection of disseminated cancer cells in the peripheral blood of patients with gastric cancer. Anticancer Res 20: 2155–2159. [PubMed] [Google Scholar]
- 16. Miyazono F, Natsugoe S, Takao S, Tokuda K, Kijima F, et al. (2001) Surgical maneuvers enhance molecular detection of circulating tumor cells during gastric cancer surgery. Ann Surg 233: 189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shin JH, Chung J, Kim HO, Kim YH, Hur YM, et al. (2002) Detection of cancer cells in peripheral blood of stomach cancer patients using RT-PCR amplification of tumour-specific mRNAs. Aliment Pharmacol Ther 16 Suppl 2137–144. [DOI] [PubMed] [Google Scholar]
- 18. Sumikura S, Ishigami S, Natsugoe S, Miyazono F, Tokuda K, et al. (2003) Disseminated cancer cells in the blood and expression of sialylated antigen in gastric cancer. Cancer Lett 200: 77–83. [DOI] [PubMed] [Google Scholar]
- 19. Seo JH, Choi CW, Kim BS, Shin SW, Kim YH, et al. (2005) Follow-up study of peripheral blood carcinoembryonic antigen mRNA using reverse transcription-polymerase chain reaction as an early marker of clinical recurrence in patients with curatively resected gastric cancer. Am J Clin Oncol 28: 24–29. [DOI] [PubMed] [Google Scholar]
- 20. Illert B, Fein M, Otto C, Cording F, Stehle D, et al. (2005) Disseminated tumor cells in the blood of patients with gastric cancer are an independent predictive marker of poor prognosis. Scand J Gastroenterol 40: 843–849. [DOI] [PubMed] [Google Scholar]
- 21. Ikeguchi M, Kaibara N (2005) Detection of circulating cancer cells after a gastrectomy for gastric cancer. Surg Today 35: 436–441. [DOI] [PubMed] [Google Scholar]
- 22. Wu CH, Lin SR, Hsieh JS, Chen FM, Lu CY, et al. (2006) Molecular detection of disseminated tumor cells in the peripheral blood of patients with gastric cancer: evaluation of their prognostic significance. Dis Markers 22: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uen YH, Lin SR, Wu CH, Hsieh JS, Lu CY, et al. (2006) Clinical significance of MUC1 and c-Met RT-PCR detection of circulating tumor cells in patients with gastric carcinoma. Clin Chim Acta 367: 55–61. [DOI] [PubMed] [Google Scholar]
- 24. Kosaka Y, Mimori K, Fukagawa T, Ishikawa K, Etoh T, et al. (2007) Identification of the high-risk group for metastasis of gastric cancer cases by vascular endothelial growth factor receptor-1 overexpression in peripheral blood. Br J Cancer 96: 1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koga T, Tokunaga E, Sumiyoshi Y, Oki E, Oda S, et al. (2008) Detection of circulating gastric cancer cells in peripheral blood using real time quantitative RT-PCR. Hepatogastroenterology 55: 1131–1135. [PubMed] [Google Scholar]
- 26. Yie SM, Lou B, Ye SR, Cao M, He X, et al. (2008) Detection of survivin-expressing circulating cancer cells (CCCs) in peripheral blood of patients with gastric and colorectal cancer reveals high risks of relapse. Ann Surg Oncol 15: 3073–3082. [DOI] [PubMed] [Google Scholar]
- 27. Bertazza L, Mocellin S, Marchet A, Pilati P, Gabrieli J, et al. (2009) Survivin gene levels in the peripheral blood of patients with gastric cancer independently predict survival. J Transl Med 7: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kita Y, Fukagawa T, Mimori K, Kosaka Y, Ishikawa K, et al. (2009) Expression of uPAR mRNA in peripheral blood is a favourite marker for metastasis in gastric cancer cases. Br J Cancer 100: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qiu MZ, Li ZH, Zhou ZW, Li YH, Wang ZQ, et al. (2010) Detection of carcinoembryonic antigen messenger RNA in blood using quantitative real-time reverse transcriptase-polymerase chain reaction to predict recurrence of gastric adenocarcinoma. J Transl Med 8: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arigami T, Uenosono Y, Hirata M, Yanagita S, Ishigami S, Natsugoe S (2011) B7-H3 expression in gastric cancer: a novel molecular blood marker for detecting circulating tumor cells. Cancer Sci 102: 1019–1024. [DOI] [PubMed] [Google Scholar]
- 31. Cao W, Yang W, Li H, Lou G, Jiang J, et al. (2011) Using detection of survivin-expressing circulating tumor cells in peripheral blood to predict tumor recurrence following curative resection of gastric cancer. J Surg Oncol 103: 110–115. [DOI] [PubMed] [Google Scholar]
- 32. Arigami T, Uenosono Y, Ishigami S, Yanagita S, Hagihara T, et al. (2013) Clinical significance of stanniocalcin 2 expression as a predictor of tumor progression in gastric cancer. Oncol Rep 30: 2838–2844. [DOI] [PubMed] [Google Scholar]
- 33. Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, et al. (2010) Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol 21: 729–733. [DOI] [PubMed] [Google Scholar]
- 34. Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, et al. (2009) Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol 27: 5153–5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaffer DR, Leversha MA, Danila DC, Lin O, Gonzalez-Espinoza R, et al. (2007) Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res 13: 2023–2029. [DOI] [PubMed] [Google Scholar]
- 36. Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, et al. (2008) Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 26: 3213–3221. [DOI] [PubMed] [Google Scholar]
- 37. Vincent-Salomon A, Bidard FC, Pierga JY (2008) Bone marrow micrometastasis in breast cancer: review of detection methods, prognostic impact and biological issues. J Clin Pathol 61: 570–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wicha MS, Hayes DF (2011) Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol 29: 1508–1511. [DOI] [PubMed] [Google Scholar]
- 39. Pantel K, Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4: 448–456. [DOI] [PubMed] [Google Scholar]
- 40. Klein CA (2009) Parallel progression of primary tumours and metastases. Nat Rev Cancer 9: 302–312. [DOI] [PubMed] [Google Scholar]
- 41. Pham B, Klassen TP, Lawson ML, Moher D (2005) Language of publication restrictions in systematic reviews gave different results depending on whether the intervention was conventional or complementary. J Clin Epidemiol 58: 769–776. [DOI] [PubMed] [Google Scholar]
- 42. Alix-Panabieres C, Schwarzenbach H, Pantel K (2012) Circulating tumor cells and circulating tumor DNA. Annu Rev Med 63: 199–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest Plot for pooled analysis of SEN and SPE. SEN, sensitivity; SPE, specificity.
(TIF)
Forest Plot for pooled analysis of PLR and NLR. PLR, positive likelihood ratio; NLR, negative likelihood ratio.
(TIF)
Forest Plot for pooled analysis of DOR. DOR, diagnostic odds ratio.
(TIF)
Summary ROC curve with confidence and prediction regions of sensitivity and specificity.
(TIF)
Funnel plot for summary estimates of RFS. RFS, relapse-free survival.
(TIF)
Funnel plot for summary estimates of OS. OS, overall survival.
(TIF)
PRISMA checklist.
(DOC)