Abstract
Background
Adolescent alcohol use is a major public health concern and is strongly correlated with the development of alcohol abuse problems in adulthood. Adolescence is characterized by maturation and remodeling of brain regions implicated in decision making and therefore may be uniquely vulnerable to environmental insults such as alcohol exposure. We have previously demonstrated that voluntary alcohol consumption in adolescence results in maladaptive risk-based decision making in adulthood. However, it is unclear whether this effect on risk-based decision making can be attributed to chronic alcohol use in general or to a selective effect of alcohol use during the adolescent period.
Methods
Ethanol was presented to adolescent (PND 30–49) and adult rats (PND 80–99) for 20 days, either 24h or 1h/day, in a gel matrix consisting of distilled water, gelatin, Polycose (10%), and ethanol (10%). The 24h time course of ethanol intake was measured and compared between adolescent and adult animals. Following 20 days of withdrawal from ethanol, we assessed risk-based decision making with a concurrent instrumental probability-discounting task. Blood ethanol concentrations (BECs) were taken from trunk blood and assessed using the Analox micro-stat GM7 in separate groups of animals at different time points.
Results
Unlike animals exposed to ethanol during adolescence, animals exposed to alcohol during adulthood did not display differences in risk preference compared to controls. Adolescent and adult rats displayed similar ethanol intake levels and patterns when given either 24h or 1h access/day. In addition, while both groups reached significant BEC levels we failed to find a difference between adult and adolescent animals.
Conclusions
Here we show that adolescent, but not adult, ethanol intake leads to a persistent increase in risk preference which cannot be attributed to differences in intake levels or BECs attained. Our findings support previous work implicating adolescence as a time period of heightened susceptibility to the long-term negative effects of alcohol exposure.
Introduction
Adolescence is a critical period of neurobiological and behavioral development and a time when individuals often have their first exposure to alcohol. Indeed, despite efforts to curb its use, alcohol remains the number one used and abused substance by adolescents (Witt, 2010). Data from Monitoring the Future Survey of 2011 indicates that 70% of 12th graders and 33% of 8th graders have had some exposure to alcohol in their lifetime (Johnston et al., 2011). In addition, early life alcohol use is one of the best predictors of problem drinking in adulthood (Bonomo et al., 2004; Jennison, 2004), and more recently has been linked to subsequent deficits in decision-making processes, which may represent a vulnerability to the development of addictive disorders (Bechara and Damasio, 2002; Crews and Boettiger, 2009; de Wit, 2009; Goudriaan et al., 2007; Johnson et al., 2008; Redish et al., 2008; Stout et al., 2005). Thus, a detailed examination of the consequences of chronic alcohol use, including the delineation of those specifically attributable to exposure during the adolescent time period, is critical in understanding the antecedents to life-long alcohol abuse disorders.
Adolescence is characterized by maturation and synaptic remodeling of brain regions implicated in reward and decision-making processes including the limbic system, the prefrontal cortex, and the hippocampus (Chambers et al., 2003; Spear, 2000). The malleable nature of the adolescent brain may render it uniquely vulnerable to environmental insults such as ethanol exposure (Chambers et al., 2003; Crews et al., 2007; Philpot et al., 2009; Spear, 2000). Indeed, prior studies in rodents have demonstrated that chronic ethanol exposure during adolescence results in activation of inflammatory processes, cell death, and structural changes in numerous brain regions (Coleman et al., 2013; Crews et al., 2000; Pascual et al., 2007; Vetreno and Crews, 2012). Moreover, behavioral studies have demonstrated that ethanol exposure during adolescence results in multiple alterations including increased motivation for ethanol, novelty seeking, increased anxiety-like and depressive-like behavior, and motor impairments (Alaux-Cantin et al., 2012; Pascual et al., 2007; Slawecki et al., 2004; Stansfield and Kirstein, 2007; White et al., 2000). Finally, work related to the consequences of alcohol exposure on learning and memory have revealed impaired fear conditioning, behavioral flexibility, conditional discrimination learning, spatial working memory, and memory recall in adult animals receiving ethanol administration as adolescents (Bergstrom et al., 2006; Broadwater and Spear, 2013; Coleman et al., 2013; Pascual et al., 2007; Sircar and Sircar, 2005; Vetreno and Crews, 2012).
We have previously demonstrated that adolescent animals who voluntarily consume ethanol subsequently display impaired decision making as defined by increased risk-taking behavior on a probability-discounting task weeks or even months later when they are adults (Clark et al., 2012; Nasrallah et al., 2011). This decision-making task involves a choice between obtaining a large but uncertain reward (a risky option) and obtaining a smaller but certain reward (a safe option), a behavioral process thought to be dependent on dopaminergic signaling in the ventral striatum (Cardinal et al., 2005; Kuhnen and Jnutson, 2005; St Onge and Floresco, 2009). In our model, adult animals exposed to ethanol during adolescence consistently choose the risky option over the certain option, even when choosing the risky option results in obtaining significantly less reward (Clark et al., 2012; Nasrallah et al., 2009; Nasrallah et al., 2011;). These data, together with the correlation between early life exposure to alcohol and later alcohol abuse problems, suggest an important link between developmental exposure to ethanol, aberrant decision-making processes, and a potentially unique vulnerability to the development of addictive disorders.
However, whether increased risk-taking behavior following chronic ethanol intake in adolescence is specific to intake during this developmental period, and thus age-dependent, or a general consequence of chronic ethanol intake at any age remains unknown. Further, while previous studies have reported differences in ethanol intake and/or blood ethanol concentration (BEC) levels reached between adolescents and adults (Bell et al., 2006; Doremus et al., 2005; Garcia-Burgos et al., 2009; Little et al., 1996; Silveri and Spear, 2000; Spear, 2013; Vetter et al., 2007; Walker and Ehlers, 2009; Walker et al., 2008; Willey et al., 2012; Wills et al., 2008), this has not been assayed in our model of voluntary ethanol intake using a polycose-gelatin delivery method (Peris et al., 2006; Rowland et al., 2005). Thus, in the current work we exposed both adolescent and adult animals to ethanol or control treatment and examined the intake patterns and BEC levels achieved. Following withdrawal from ethanol, we assayed risk-taking behavior using a probability-discounting task in animals exposed to ethanol in adolescence or adulthood to test the central hypothesis that sub-optimal decision making following chronic ethanol use selectively results from exposure during the adolescent period. Based on data demonstrating that the adolescent brain undergoes active development and may be uniquely susceptible to disruption by ethanol exposure, we hypothesized that adolescent, but not adult, animals exposed to ethanol would demonstrate enduring neurobiological effects that translate into aberrant decision making later in adulthood.
Methods
Animals and housing
Male Sprague Dawley rats (Charles River, Hollister, CA) aged PND 27 or PND 77 at the start of experiments were housed individually in polycarbonate tubs on a 12h light/dark cycle (lights on at 06:00). Water and Teklad (vendor) rodent chow was available ad libitum except as noted. Rats were weighed and handled daily throughout the course of the experiment. Animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Alcohol preparation, administration, and withdrawal
Alcohol was presented to adolescent (PND 30–49) and adult rats (PND 80–99) in a gel matrix consisting of distilled water, Knox gelatin, Polycose (10%), and ethanol (10%). Control-gels had ethanol replaced with distilled water. Preparation was as previously described (Clark et al., 2012; Nasrallah et al., 2009; Nasrallah et al., 2011; Rowland et al., 2005). The ethanol-gels were designed to minimize evaporation of ethanol and have been previously validated to result in accurate ethanol content (Rowland et al., 2005) and to produce alterations in brain chemistry (Li et al., 2008; Peris et al., 2006). The gels were made available 24h/day, unless otherwise noted, in addition to ad libitum water and chow, and fresh gel-containing jars were presented every day. Ethanol-gel intake levels were monitored daily and expressed in g/kg of body weight using individual gel consumption and body weights measured daily. Rats failing to consume gel during the control-gel pre-exposure, exhibiting 3 consecutive days of no consumption, or burying of the gel in bedding once the ethanol-gel exposure began were excluded from the study. Experiments began with 3 days of pre-exposure to control-gel. Subsequently, adolescent and adult rats were each split into ethanol-gel and control-gel groups matched by weight and baseline intake. 20 days of 24h/day gel exposure followed. Upon completion of the 20 day exposure, gel access for all groups was discontinued and rats were monitored daily for withdrawal symptoms for the following 20 days. No overt signs of withdrawal were observed.
Gel-intake and BEC time course
Experiments began with 4–7 days of pre-exposure to control-gel. Subsequently, adolescent and adult rats were exposed to 24h/day ethanol-gels. Once stable intake levels were obtained, a gel-intake time course was conducted during the subsequent 24h period by weighing of the gel containing jars every 2–3h. This intake time course was then used to establish the time points during the 24h gel exposure period where levels of gel intake were the highest. These two time points (3h and 11.45h into the dark-cycle) were then used for subsequent blood collection and BEC analysis. For the two time points, rats were matched by weight and intake levels.
Short access ethanol exposure and BEC
Experiments began with 3–4 days of pre-exposure to control-gel. Subsequently, adolescent and adult rats were exposed to 24h/day ethanol-gels. Following 5 days of 24h/day ethanol-gel access, rats were subsequently allowed only 1h/day ethanol-gel access (1h/day occurring from 13:00–14:00 PST). Once stable ethanol-gel intake levels were obtained, blood was collected 30–40 min into the 1h access period on the following day for BEC analysis.
Blood ethanol content measurements
Adolescent and adult rats were deeply anesthetized with isoflurane prior to decapitation and collection of trunk blood on ice. Blood samples were subsequently centrifuged at 11,000 g for 15 min at 4⍛C. The resulting plasma supernatants were then transferred to fresh micro-centrifuge tubes and BEC levels were determined using the Analox micro-stat GM7 (Analox Inst. Ltd.; Lunenberg, MA). All samples were run in duplicate.
Instrumental training
Following 20 days of withdrawal, rats began food restriction to ~90% of their free-feeding body weight. Rats were first exposed to 45 mg sucrose pellets (Bio-Serve, Frenchtown, NJ) in their home cage to reduce neophobia. Next, rats underwent magazine training in a standard operant chamber (Med Associates, St. Albans, WT), where they were given 15 min to consume 10 sucrose pellets in the magazine tray. Following magazine training, rats were next trained on an instrumental FR1 schedule for single sucrose pellets on two separate levers to a criterion of ≥24 level presses out of 30 trials. Finally, once criterion was met, rats were autoshaped over the course of 4 days (day 1 of autoshaping required rats to perform a nose-poke into the food tray for trial initiation; day 2 increased the inter-trial interval from 0 s to 15 s; day 3 reduced the time to perform trial-initiating nose-poke to 10 s; day 4 increased the inter-trial interval from 15 s to 30 s).
Probability-discounting task
As previously described (Clark et al., 2012; Nasrallah et al., 2009; Nasrallah et al., 2011), rats were tested on a concurrent instrumental response task involving the presentation of two levers (lever assignments were counterbalanced across groups). The certain lever was associated with the certain delivery (1.00) of two sucrose pellets and the uncertain lever was associated with the probabilistic delivery (either 1.00, 0.75, 0.50, 0.25, or 0.00) of four sucrose pellets. Daily sessions consisted of 24 forced trials followed by 24 free choice trials with a single probability per session completed in descending order with rats experiencing each probability once. In order to decrease position bias, all trials began with illumination of a light in the food tray cueing the rat to make a nose-poke in the tray within 10 sec, ensuring that each rat was centered in the chamber at the start of each trial. During forced trials, a successful nose-poke by the rat resulted in the extension of a single lever presented pseudorandomly. If the rat responded by lever press within 10 s the tray light was illuminated and reward was delivered based on the associated probability of that lever for that day. A 45 s ITI followed, and in all cases, failure to respond correctly within 10 s resulted in trial termination and return to the chamber ITI state. These forced choice sessions served to expose the rat to each lever's associated expected value and the subsequent free choice trials had the same probability in effect for the uncertain lever. Free choice trials were the same as above but a successful nose-poke resulted in extension of both levers, giving the rat 10 s to choose, and thus assessed the rat's preference between high and low reward options.
Statistical analysis
Differences between groups were determined using repeated or non-repeated measures two-way ANOVA. Student's unpaired, one-sample or two-sample t-tests with the Bonferroni correction for multiple tests were used to determine statistical differences between pair-wise comparisons. The Pearson correlation test was used to assess the relationship between ethanol-gel intake and BEC levels. Statistical analyses were conducted using Graph Pad Prism 4.0 (San Diego, CA).
Results
Alcohol exposure during adolescence, but not adulthood, promotes maladaptive risk-taking behavior
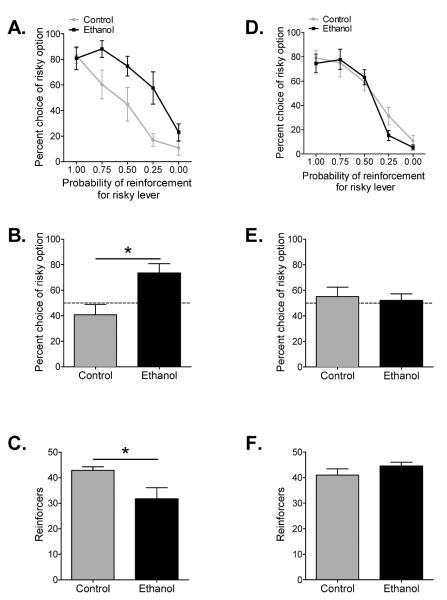
Analysis of choice behavior on the probability-discounting task generated standard discounting curves for choice of the larger but uncertain reward option over all conditions, with decreasing probability of the large reward delivery resulting in decreased choice of the large reward option for each group (F(4,44) = 25.64, p < 0.0001) (Fig. 1A). In line with our previous reports, ethanol and control groups differed significantly in their probability discounting curves (F(1,11) = 6.95, p = 0.023), with animals that received ethanol during adolescence demonstrating a flatter discounting curve and increased preference for large, probabilistic rewards over small, certain rewards (Fig. 1A). Importantly, this separation in choice behavior is selective to conditions where risk is present, as the ethanol treated group did not differ from controls in either their preference for higher rewards at 100% probability of delivery or their ability to shift preference once delivery of the high reward was reduced to 0%. To generate an overall risk preference score, we then collapsed choice behavior for the large risky reward option over the three probability test conditions (0.75, 0.50, and 0.25) as the expected value (probability × magnitude) for the certain and risky options are equal when the conditions are combined. This analysis revealed that animals that ingested ethanol during adolescence had a risk preference score that was significantly elevated above risk neutrality (50% choice for the risky option; Nasrallah et al., 2009) (t(5) = 3.23, p = 0.023) (Fig. 1B). Conversely, control animals did not demonstrate a preference that significantly differed from risk neutral behavior (t(6) = 1.15, p > 0.05). Combined choice behavior between ethanol exposed and control animals was also significantly different (t(11) = 2.97, p = 0.013) (Fig. 1B). Finally, choice behavior that results in the optimal receipt of reward shifts systematically across probabilistic conditions with choice of the small, certain reward being the optimal choice at the 0.25 probability of high reward delivery. Thus, the number of rewards obtained can be used as a definition of whether or not choice behavior is optimal. As depicted in Fig. 1C, animals that ingested ethanol during adolescence obtained significantly fewer pellets than control animals when reward delivery was unlikely (t(11) = 2.57, p = 0.026). Together these data demonstrate that adolescent alcohol intake produces enduring and maladaptive risk preference in adulthood.
Figure 1.
Adolescent, but not adult, ethanol intake produces maladaptive decision making. (A) Average percent choice of the uncertain lever for each probabilistic test condition on the probability discounting task. Adult rats exposed to ethanol during adolescence exhibited a preference for the large uncertain reward lever when compared to controls (repeated measures two-way ANOVA; n = 6–7). (B) Average percent choice of the uncertain lever averaged across the three uncertain probability test conditions (0.75, 0.50, and 0.25 probability of reinforcement). Dotted line denotes the risk neutral choice. Adult rats exposed to ethanol during adolescence exhibited an averaged preference for risk when compared to controls (Student's unpaired, two-sample t-test; n = 6–7). (C) Average number of reinforcers obtained under the 0.25 probability test condition. Adult rats exposed to ethanol during adolescence received less rewards than controls when choice on the uncertain reward lever was unlikely (Student's unpaired, two-sample t-test; n = 6–7). (D) Average percent choice of the uncertain lever for each probabilistic test condition on the probability discounting task. Adult rats exposed to ethanol during adulthood did not differ in their preference for the large uncertain reward lever when compared to controls (repeated measures two-way ANOVA; n = 8–10). (B) Average percent choice of the uncertain lever averaged across the three uncertain probability test conditions (0.75, 0.50, and 0.25 probability of reinforcement). Dotted line denotes the risk neutral choice. Adult rats exposed to ethanol during adulthood did not differ in averaged preference for risk when compared to controls (Student's unpaired, two-sample t-test; n = 8–10). (C) Averaged number of reinforcers obtained under the 0.25 probability test condition. Adult rats exposed to ethanol during adulthood received similar number of rewards as controls when choice on the uncertain reward lever was unlikely (Student's unpaired, two-sample t-test; n = 8–10). Mean ± SEM. *p < 0.05.
Analysis of choice behavior in animals exposed to ethanol or control-gels during adulthood generated standard probability discounting curves for choice of the larger but uncertain reward option over all conditions, with decreasing probability of the large reward delivery resulting in decreased choice of the large reward option for each group (F(4,64) = 67.67, p < 0.0001) (Fig. 1D). In contrast to animals that ingested ethanol during adolescence, animals that ingested ethanol during adulthood did not significantly differ from controls in their probability discounting curves (F(1,16) = 0.26, p > 0.05) (Fig. 1D). In addition, neither of the experimental groups demonstrated a risk preference score that was significantly different from the risk neutral behavior of 50% choice on the large risky reward option (ethanol: t(9) = 0.71, p > 0.05; control: t(7) = 0.39, p > 0.05) nor did they significantly different from each other (t(16) = 0.33, p > 0.05) (Fig. 1E), again demonstrating the lack of effect on risk-taking behavior of ethanol exposure during adulthood. Likewise, the number of pellets obtained when reward was unlikely (0.25 probability of large risky reward) by animals that ingested ethanol during adulthood did not differ from control animals (t(16) = 1.15, p > 0.05) (Fig. 1F), suggesting that ethanol intake during adulthood did not result in suboptimal choice behavior.
In combination, these findings suggest that the effect of ethanol exposure on decision-making and risk preference is limited to the adolescent period. An alternative explanation is that adolescent ethanol intake accelerates a natural progression to greater risk-taking behavior in adulthood. However, direct comparison of adolescent and adult risk-taking behavior shows that animals that ingested ethanol during adolescence significantly differ from animals that ingested ethanol during adulthood in their probability discounting curves (main effect of age: F(4,48) = 5.003, p < 0.05; interaction: F(4,48) = 3.82, p < 0.01), while adolescence and adult controls did not significantly differ in their probability discounting curves (F(4,60) = 1.002, p > 0.05). Combined with the collapsed analysis in Figures 1B and 1E, this demonstrates that the effect of ethanol exposure on decision-making and risk preference is limited to access during the adolescent period.
Adolescent and adult ethanol intake
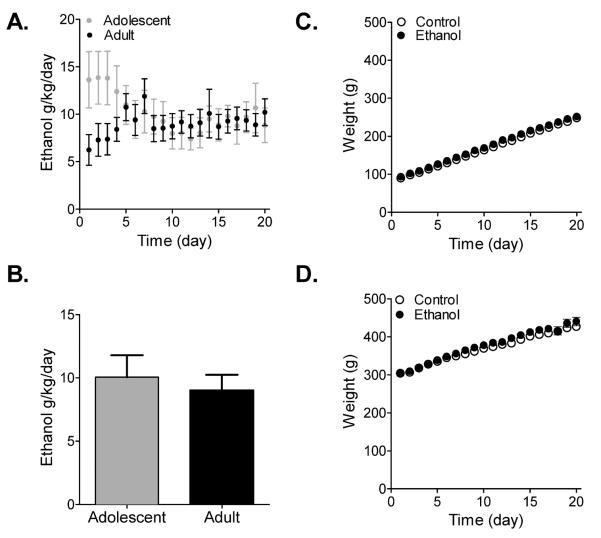
Overall ethanol intake levels across the 20 days of exposure did not differ between adolescent and adult animals (F(1,12) = 0.25, p > 0.05) (Fig. 2A) although a significant interaction effect was detected (age during ethanol intake vs. time: F(19,228) = 4.12, p < 0.0001), which may be due to the apparent separation of intake levels between adolescent and adult animals during the initial 3 days of access (Fig. 2A). When averaged across the 20 days of exposure, adolescent animals consumed 10.05 g/kg/day ± 1.73 and adult animals consumed 9.01 g/kg/day ± 1.23, and these levels did not differ significantly (t(12) = 0.50, p > 0.05) (Fig. 2B). Body weight of both ethanol and control animals increased as a function of time (adolescent: F(19,209) = 2046, p = 0.0001; adult: F(19,304) = 181, p = 0.0001), but did not differ between ethanol and control animals for either adolescents or adults (adolescent: F(1,11) = 2.72, p > 0.05; adult: F(1,16) = 0.29, p > 0.05) (Fig. 2C,D).
Figure 2.
Overall ethanol intake did not differ between adolescent and adult animals. (A,B) Average daily intake for adolescent and adult rats over the 20 days of ethanol exposure. While adolescent rats initially displayed higher intake levels than adult rats (A), intake levels across the 20 days were not different (B; Student's unpaired, two-sample t-test; n = 6–8). (C,D) Average daily body weight for adolescent and adult rats over the 20 days of ethanol exposure. Body weight did not differ between rats exposed to ethanol and controls during adolescence (C) or adulthood (D). Mean ± SEM.
Time course of adolescent and adult ethanol intake during continuous access
In order to fully evaluate the continuous gel access protocol, we assessed the 24-hr pattern of intake in adolescent and adult animals. Previous studies have demonstrated that animals primarily consume ethanol in the dark cycle (Bell et al. 2006; Garcia-Burgos et al. 2009; but see also Walker et al., 2008). Consistent with these findings, both adolescent and adult animals in this study consumed significantly more ethanol during their dark-phase (F(1,18) = 34.89, p = 0.0001), but importantly, the intake patterns did not differ between the two age groups (F(1,18) = 0.09, p > 0.05) (Fig. 3A). Based on the animals' increased intake levels during the dark-phase, we next measured intake every 2–3h during this time frame (Fig. 3B). During the dark-phase, there was a significant effect of time (F(5,90) = 7.02, p = 0.0001) with no difference in intake levels between the adolescent and adult groups (F(1,18) = 0.79, p > 0.05), suggesting that while intake levels for both adolescent and adult animals change during the course of the 12h period, these changes were consistent across both age groups.
Figure 3.
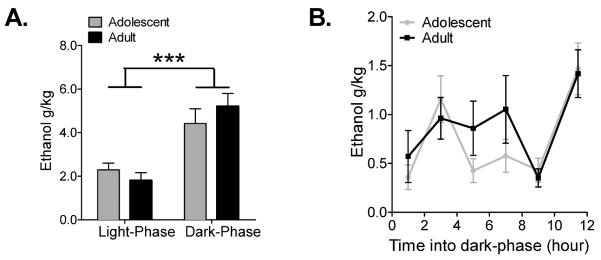
Adolescent and adult rats displayed similar patterns of ethanol intake. (A) Average ethanol intake for adolescent and adult rats during the light-phase and dark-phase of the light/dark cycle. Both adolescent and adult rats consumed higher amounts of ethanol during the dark-phase and intake levels did not differ between the two groups (repeated measures two-way ANOVA; n = 11–13). (B) Averaged ethanol intake for adolescent and adult rats during the dark-phase of the light/dark cycle. Intake levels were measured every 2–3h. Intake patterns and levels between adolescent and adult rats did not differ (repeated measures two-way ANOVA; n = 11–13). Mean ± SEM. *** p < 0.0001
Time course of adolescent and adult BEC during continuous access
Previous work has demonstrated that ethanol intake with the polycose gel delivery method results in significant elevations of blood ethanol concentrations (BEC) that are comparable to other delivery methods (Peris et al., 2006; Rowland et al., 2005). However, these reports investigated BEC levels in adult animals with 1h of ethanol-gel access, but did not assay the BECs attained during 24h access or whether differences exist for adolescent and adult animals exposed to the ethanol-gels. While overall intake levels between our adolescent and adult animals did not differ, previous studies have suggested that adolescent animals reach different BEC levels than adult animals when administered equal amounts of ethanol as a result of differences in metabolism (Little et al., 1996; Silveri and Spear, 2000; Walker and Ehlers, 2009; Walker et al., 2008; Willey et al., 2012; Wills et al., 2008). In order to investigate these processes further, we analyzed BECs attained at 3h and 11.45h into the dark-phase, as these are the two time points of highest intake (1h: 0.46 g/kg ± 0.14; 3h: 1.06 g/kg ± 0.06; 5h: 0.64 g/kg ± 0.16; 7h: 0.82 g/kg ± 0.20; 9h: 0.39 g/kg ± 0.07; 11.45h: 1.44 g/kg ± 0.18) (Fig. 4A). At each time point trunk blood was collected and assayed for BEC. While some animals reached relatively high BEC levels at these two time points, we did not find a significant difference between BEC levels for adolescent and adult animals (F(1,17) = 0.22, p > 0.05) (Fig. 4B).
Figure 4.
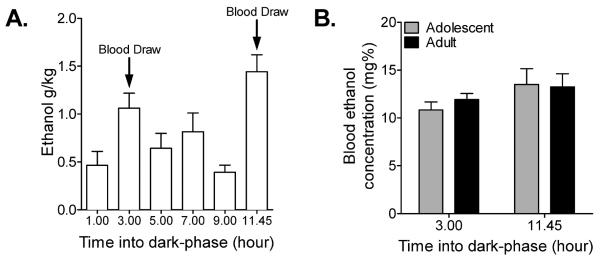
Adolescent and adult rats attained similar BECs during 24h ethanol access. (A) When adolescent and adult rat intake levels were combined, the highest intake levels were 3h and 11.45h into the dark-phase. (B) BECs attained at 3h and 11.45h into the dark-phase did not differ between adolescent and adult rats (repeated measures two-way ANOVA; n = 3–7). Mean ± SEM.
Adolescent and adult intake and BEC during limited access
During the time course study, animals were allowed 24h access to the gels, and while gel intake levels were assessed every 2–3h, individual variation in intake patterns within each 2–3h time window varied across subjects, making a correlation between intake level and BEC difficult to assess. To address this, a separate group of adolescent and adult animals were limited to 1h/day ethanol-gel access and BECs were assayed 30–40 min into the 1h exposure. No difference between adolescent and adult BECs attained was found (t(14) = 0.70, p > 0.05) (Fig. 5A). BEC levels reached by both adolescent and adult animals were significantly correlated with intake levels (adolescent: r = 0.83, p = 0.01; adult: r = 0.94, p = 0.0005). Importantly, no difference in regression slopes was found (F(1,12) = 2.57, p > 0.05) (Fig. 5B), indicating that the relationship between intake and BEC levels does not differ between adolescent and adult animals. Taken together, these data suggest that differences in BEC levels attained between adolescent and adult animals cannot account for behavioral differences seen on the probability-discounting task.
Figure 5.
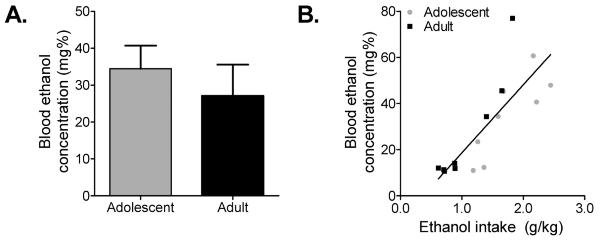
Adolescent and adult rats attained similar BECs during 1h ethanol access. (A) When limited to 1h ethanol access, adolescent and adult rats did not differ in BECs attained (Student's unpaired, two-sample t-test; n = 8). (B) Regression analysis of ethanol vs. BEC revealed significant correlations for both adolescent and adult rats (Pearson correlation test; n = 8) that did not differ between groups (Linear regression to determine if slopes are equal; n = 8). Mean ± SEM.
Discussion
Here we demonstrate that adolescent, but not adult, intake of ethanol results in enduring, maladaptive decision making in adulthood. Furthermore, we demonstrate that this age-selective increase in risk preference cannot be accounted for by differences in intake levels or BECs attained between adolescent and adult animals. These results support the suggestion that adolescence is a period of development uniquely vulnerable to the long-term consequences of alcohol exposure. Indeed, these findings are consistent with previous reports showing age dependent effects of alcohol on subsequent neurobiological and behavior measures (Bergstrom et al., 2006; Broadwater and Spear, 2013; Sircar and Sircar, 2006;).
During the probability-discounting task, animals choose between a high but uncertain reward (risky option) and a low but certain reward (safe option). Such decision-making behavior requires an individual to weigh the associated costs and benefits for each option. The mesolimbic dopamine system, which undergoes substantial development during the adolescent time period (Chambers et al., 2003; Spear, 2000), has been implicated in these processes (Phillips et al., 2007), and is modulated by alcohol (Chambers et al., 2003; Guerri and Pascual, 2010; Spear, 2000). Due to the malleable and vulnerable nature of the adolescent brain, and specifically the mesolimbic dopamine system, alcohol may cause substantial disruption of normal development, as well as neurological damage, which may then translate into long-term negative behavioral consequences such as the increased risk-taking behavior seen in the current study. Conversely, during adulthood many of the brain regions and neurotransmitter systems involved in complex decision-making tasks are fully developed and thus may be more resistant to alcohol's neurobiological effects, which may translate into a lack of long-term negative behavioral consequences, such as that found in the current study. We have previously reported differences in phasic dopamine release to risk-associated cues within the nucleus accumbens (NAc) core in animals exposed to ethanol during adolescence (Nasrallah et al., 2011). Likewise, repeated ethanol exposure during adolescence results in subsequent increased basal levels of DA within the NAc (Badanich et al., 2007; Philpot et al., 2009). Additional studies are required to more fully understand the long-term consequences of adolescent alcohol exposure on the mesolimbic dopamine system and the role such disruption of its normal developmental trajectory might play in subsequent maladaptive behavioral outcomes.
The results of previous studies comparing levels of ethanol intake between adolescent and adult animals have varied (Bell et al., 2006; Doremus et al., 2005; Garcia-Burgos et al., 2009; Spear, 2013; Vetter et al., 2007; Walker et al., 2008). These varying reports may be attributable to differences in strain, sex, or route of alcohol self-administration. Here we report no overall difference in ethanol intake levels between adolescent and adult animals. Interestingly, we did find a significant interaction effect between age of ethanol exposure and time. Our adolescent animals initially display elevated ethanol intake levels during the first 3 days of exposure, with intake levels then decreasing to adult levels by day 5. Similar findings have been previously reported (Garcia-Burgos et al., 2009; Vetter et al., 2007) and have been attributed to adolescents' propensity for novelty seeking and insensitivity to the sedative and aversive effects of alcohol (Spear, 2000). However, averaging across days revealed no overall difference in intake levels. Similarly, the pattern of ethanol intake in adolescent and adult animals over the 24h time course did not differ. For both groups the largest intake of ethanol occurred during the dark-phase of the animals' light/dark cycle. Bell et al. (2006) and Garcia-Burgos et al. (2009) reported similar results using 2–4 bottle choice in Wistar animals and alcohol-preferring animals respectively, while Walker et al. (2008) reported increased intake during the light-phase for adolescent animals and no difference in adult animals. Walker et al. (2008) allowed only 1h access of ethanol solution which could account for the differences in intake patterns reported as compared to the current study and other previously published works. Upon closer examination of the 12h dark-phase of the light/dark cycle, we found the highest intake rates to occur immediately prior to the start of the light-phase, which is in accordance with Bell et al. (2006). Finally, the results of work comparing BEC levels reached between adolescent and adult animals given the identical ethanol exposure have varied, with increased, decreased, or no change in the BEC of adolescents as compared to adult animals (Little et al., 1996; Silveri and Spear, 2000; Walker and Ehlers, 2009; Walker et al., 2008; Willey et al., 2012; Wills et al., 2008). Again, these varying reports may be attributable to differences in strain, sex, or route of alcohol self-administration. Here we report no difference in BEC levels between adolescent and adult animals either during 24h access or when access was limited to 1h. Importantly, there was a significant correlation between intake level and BEC, with BECs reaching between 10–80 mg%, supporting the general utility of the gel delivery method for studying drinking behavior and its long-term neurobiological effects in rodents.
In summary, consistent with our hypothesis we have demonstrated that persistent risk preference following chronic alcohol use is restricted to exposure during the adolescent period. Further, these differences in decision making and risk preference between adult animals exposed to ethanol during adolescence or adulthood could not be attributable to differences in total intake levels or BECs attained. The adolescent brain is still undergoing substantial maturational changes that render it uniquely vulnerable to environmental insults such as alcohol exposure, which may explain the increased risk-taking behavior seen specifically in animals exposed to ethanol during adolescence. Future studies will determine which molecular, biochemical, and psychological processes are altered following adolescent alcohol exposure that may subsequently promote increased risk-taking behavior.
Acknowledgements
The authors wish to thank Brendan Walker for assistance with blood alcohol analysis and Scott Ng-Evans for technical support.
Support: This work was supported by NIH grants; R01AA021121 (JJC) and T32AA07455 (AGS)
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2012;67:521–31. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res. 2007;31(5):895–900. doi: 10.1111/j.1530-0277.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–89. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, Murphy JM, McBride WJ. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bergstrom HC, McDonald CG, Smith RF. Alcohol exposure during adolescence impairs auditory fear conditioning in adult Long–Evans rats. Physiol Behav. 2006;88(4–5):466–72. doi: 10.1016/j.physbeh.2006.04.021. [DOI] [PubMed] [Google Scholar]
- Bonomo YA, Bowes G, Coffey C, Carlin JB, Patton GC. Teenage drinking and the onset of alcohol dependence: A cohort study over seven years. Addiction. 2004;99(12):1520–8. doi: 10.1111/j.1360-0443.2004.00846.x. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Spear LP. Consequences of ethanol exposure on cued and contextual fear conditioning and extinction in adulthood differ depending on timing of exposure. Behav Brain Res. 2013;256:10–19. doi: 10.1016/j.bbr.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Howes NJ. Effects of lesions of the nucleus accumbens core on choice between small certain rewards and large uncertain rewards in rats. BMC Neuroscience. 2005;6:37. doi: 10.1186/1471-2202-6-37. PMCID: PMC1177958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Ame J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JJ, Nasrallah NA, Hart AS, Collins AL, Bernstein IL, Phillips PE. Altered risk-based decision making following adolescent alcohol use results from an imbalance in reinforcement learning in rats. PLoS One. 2012;7(5):e37357. doi: 10.1371/journal.pone.0037357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr, Liu W, Oguz I, Styner M, Crews FT. Adolescent binge ethanol treatment alters adult brain regional volumes, cortical extracellular matrix protein and behavioral flexibility. Pharmacol Biochem Behav. 2013;116C:142–151. doi: 10.1016/j.pbb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93(3):237–47. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24(11):1712–23. [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharm Biochem Behav. 2007;86:189–99. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin. Exp. Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- García-Burgos D, González F, Manrique T, Gallo M. Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res. 2009;33(4):722–8. doi: 10.1111/j.1530-0277.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: a longitudinal study. Alcoholism: Clin & Exp Res. 2007;31:928–38. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44(1):15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Jennison KM. The short-term effects and unintended long-term consequences of binge drinking in college: A 10-year follow-up study. Am J Drug Alcohol Abuse. 2004;30(3):659–84. doi: 10.1081/ada-200032331. [DOI] [PubMed] [Google Scholar]
- Johnson CA, Xiao L, Palmer P, Sun P, Wang Q, Wei Y, Jia Y, Grenard JL, Stacy AW, Bechara A. Affective decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in 10th grade Chinese adolescent binge drinkers. Neuropsychologia. 2008;46:714–26. doi: 10.1016/j.neuropsychologia.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings, 2010. Institute for Social Research, The University of Michigan; Ann Arbor: 2011. p. 77. [Google Scholar]
- Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–70. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Li Z, Zharikova A, Bastian J, Esperon L, Hebert N, Mathes C, Rowland NE, Peris J. High temporal resolution of amino acid levels in rat nucleus accumbens during operant ethanol self-administration: involvement of elevated glycine in anticipation. J Neurochem. 2008;106(1):170–81. doi: 10.1111/j.1471-4159.2008.05346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin. Exp. Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Nasrallah NA, Clark JJ, Collins AL, Akers CA, Phillips PE, Bernstein IL. Risk preference following adolescent alcohol use is associated with corrupted encoding of costs but not rewards by mesolimbic dopamine. Proc Natl Acad Sci. 2011;108(13):5466–71. doi: 10.1073/pnas.1017732108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah NA, Yang TWH, Bernstein IL. Long-term risk preference and suboptimal decision making following adolescent alcohol use. Proc Natl Acad Sci U S A. 2009;106(41):17600–4. doi: 10.1073/pnas.0906629106. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Miñarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci. 2007;25(2):541–50. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Peris J, Zharikova A, Li Z, Lingis M, MacNeill M, Wu MT, Rowland NE. Brain ethanol levels in rats after voluntary ethanol consumption using a sweetened gelatin vehicle. Pharmacol Biochem Behav. 2006;85(3):562–8. doi: 10.1016/j.pbb.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology (Berl) 2007;191(3):483–95. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci. 2009;27(8):805–15. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Redish D, Jensen S, Johnson A. Addiction as vulnerabilities in the decision process. Behav Brain Sci. 2008;31:461–48. doi: 10.1017/S0140525X0800472X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland NE, Nasrallah N, Robertson KL. Accurate caloric compensation in rats for electively consumed ethanol-beer or ethanol-polycose mixtures. Pharm Biochem Behav. 2005;80:109–14. doi: 10.1016/j.pbb.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Ontogeny of ethanol elimination and ethanol-induced hypothermia. Alcohol. 2000;20:45–53. doi: 10.1016/s0741-8329(99)00055-5. [DOI] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29(8):1402–10. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann N Y Acad Sci. 2004;1021:448–52. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobeh. Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. Adolescents and alcohol: Acute sensitivities, enhanced intake, and later consequences. Neurotox and Teratology. 2013 doi: 10.1016/j.ntt.2013.11.006. http://dx.doi.org/10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed]
- St Onge JR, Floresco SB. Dopaminergic modulation of risk-based decision making. Neuropsychopharmacology. 2009;34(3):681–97. doi: 10.1038/npp.2008.121. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Chronic cocaine or ethanol exposure during adolescence alters novelty-related behaviors in adulthood. Pharmacol Biochem Behav. 2007;86(4):637–42. doi: 10.1016/j.pbb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, Finn PR. Psychological processes underlying risky decisions in drug abusers. Psychol Addictive Behav. 2005;19:148–57. doi: 10.1037/0893-164X.19.2.148. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Adolescent binge drinking increases expression of the danger signal receptor agonist HMGB1 and Toll-like receptors in the adult prefrontal cortex. Neuroscience. 2012;226:475–88. doi: 10.1016/j.neuroscience.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31(7):1159–68. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Age-related differences in the blood alcohol levels of Wistar rats. Pharmacol Biochem Behav. 2009;91(4):560–565. doi: 10.1016/j.pbb.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Walker JL, Ehlers CL. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol. 2008;42:83–89. doi: 10.1016/j.alcohol.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 2000;24(8):1251–6. [PubMed] [Google Scholar]
- Willey AR, Anderson RI, Morales M, Ramirez RL, Spear LP. Effects of ethanol administration on corticosterone levels in adolescent and adult rats. Alcohol. 2012;46(1):29–36. doi: 10.1016/j.alcohol.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, Knapp DJ, Overstreet DH, Breese GR. Differential Dietary Ethanol Intake and Blood Ethanol Levels in Adolescent and Adult Rats: Effects on Anxiety-Like Behavior and Seizure Thresholds. Alcohol Clin Exp Res. 2008;32(8):1350–60. doi: 10.1111/j.1530-0277.2008.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44(1):119–24. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]