Abstract
Difficulty obtaining reliable transportation to clinic is frequently cited as a barrier to HIV care in sub-Saharan Africa (SSA). Numerous studies have sought to characterize the impact of geographic and transportation-related barriers on HIV outcomes in SSA, but to date there has been no systematic attempt to summarize these findings. In this systematic review, we summarized this body of literature. We searched for studies conducted in SSA examining the following outcomes in the HIV care continuum: (1) voluntary counseling and testing, (2) pre-anti-retroviral therapy (ART) linkage to care, (3) loss to follow-up and mortality, and (4) ART adherence and/or viral suppression. We identified 34 studies containing 52 unique estimates of association between a geographic or transportation-related barrier and an HIV outcome. There was an inverse effect in 23 estimates (44 %), a null association in 26 (50 %), and a paradoxical beneficial impact in 3 (6 %). We conclude that geographic and transportation-related barriers are associated with poor outcomes across the continuum of HIV care.
Keywords: Transportation barriers, Linkage to care, Retention in care, Adherence, Sub-Saharan Africa
Introduction
Early treatment of HIV infection with combination anti-retroviral therapy (ART) improves health outcomes in infected individuals and reduces transmission [1–4]. Many public health experts advocate for expanding treatment provision through a “test and treat” strategy [5, 6], in which HIV-infected individuals are offered ART regardless of clinical status. Such policies have had beneficial impacts on HIV-related outcomes in resource-rich settings [7]. Yet despite the dramatic expansion of ART in sub-Saharan Africa (SSA) during the last decade [8], the mortality rate in this region continues to exceed that in resource-rich settings [9–13]. In SSA, late presentation to care [14], treatment refusal despite eligibility [15], low rates of pre-ART linkage to care [16, 17], high rates of attrition [18, 19], and interrupted treatment [20] all contribute to poor outcomes and hinder scale-up efforts.
The success of a “test and treat” strategy in SSA will depend on thoughtful consideration of the structural barriers that impede the ability of HIV-infected individuals to get tested, link to HIV care, stay in care, and adhere to ART. “Voltage drops” [21] may occur along the continuum of care, leading to diminishing numbers getting tested, successfully linking to and remaining in care, and achieving sustained adherence to ART. Qualitative studies have found that patients in the region frequently cite difficulty obtaining reliable transportation to clinic as a reason for treatment default, poor ART adherence, and other adverse health outcomes [22–24]. We hypothesized that the presence of geographic and transportation-related barriers would be associated with unfavorable outcomes at all points along the continuum of HIV care, and that this effect would be observed across different sub-continental regions, time periods, and study populations. Guided by these hypotheses, we conducted this review to systematically assess the extent to which—and in what manner—geographic and transportation-related barriers affect HIV outcomes in SSA.
Methods
Search Strategy
All procedures were performed according to the PRISMA guidelines [25]. We searched PubMed and Web of Science for manuscripts that were published prior to August 2011, using title and abstract key words to identify studies that examined associations between geographic or transportation-related barriers and HIV outcomes in SSA (for search terms, see Appendix). We used the “Find Duplicates” function in EndNote X4 (Thomson Reuters, New York, NY, USA) to identify and eliminate duplicates. In addition, we manually searched all abstracts from the International Conference on HIV Treatment and Prevention Adherence of the International Association of Physicians in AIDS Care [now International Association of Providers of AIDS Care (IAPAC)] from 2002–2004 and from 2006–2011.
Study Selection and Eligibility Criteria
Two investigators (AJL and MJS), working independently and in duplicate, screened the first 150 abstracts. Agreement on the selection of studies for full-text review was acceptable (k = 0.74), so a single investigator (AJL) completed the remainder of the screening. After the initial screening of abstracts, a single investigator (AJL) obtained full-text journal articles for all records to select studies for inclusion in the final review. We searched PubMed and Google Scholar to identify IAPAC abstracts that had been subsequently published as full-length manuscripts. IAPAC abstracts that had not been subsequently published were also considered for inclusion in the final review. We included all identified manuscripts that were the result of original research that was based at least partially in SSA, described a study population that was either predominantly HIV-infected or prescribed ART for other reasons (e.g. post-exposure prophylaxis), and reported data relating to one of the following four outcomes of interest: (1) voluntary counseling and testing (VCT), (2) pre-ART linkage to care (as defined by Govindasamy et al. [26]), (3) loss to follow-up (LTFU) and/or mortality, and (4) ART adherence and/or viral suppression. Additionally, studies were categorized as eligible during a second round of screening if they described a relationship between at least one of these outcomes and a geographic or transportation-related exposure variable: (1) travel distance, (2) travel time, (3) transportation cost, or (4) rural versus urban setting. In cases where the manuscript or IAPAC abstract contained insufficient information to evaluate estimates of geographic or transportation-related barriers, we contacted the authors to obtain additional information. We also included a limited number of manuscripts and abstracts that were recommended by experts in the field but not identified in our systematic search. There were no language exclusion criteria. In the final compilation of reviewed manuscripts, we only included studies that were conducted in SSA, where the substantial majority of the world’s HIV-infected population resides [8], in order to maintain generalizability of our findings to this region.
Data Extraction
Using a standardized extraction form, data from all eligible studies were extracted by a single investigator (AJL). An initial attempt was made to aggregate study results for meta-analysis; however, substantial heterogeneity in the definition and measurement of study exposures and outcomes precluded this. Therefore, we proceeded with a systematic review. Beyond these quantitative studies, we sorted identified manuscripts into two additional categories of studies—(1) descriptive, and (2) qualitative. These studies were deemed to be important to understanding the geographic and transportation-related barriers to HIV care, but did not report an inferential statistical relationship. Manuscripts in these categories met all other inclusion criteria and were identified during the same systematic search process. We defined as descriptive any study that reported a proportion of respondents indicating a geographic or transportation-related factor to be a barrier to HIV care, but that did not estimate an association between this exposure and one of our outcomes of interest. If the authors estimated an association, the study was defined as quantitative. We defined as qualitative any study that reported general themes regarding geographic or transportation-related barriers to HIV care, but did not report specific proportions.
Data Analysis
In our primary analysis, we examined all eligible quantitative studies. One study [27] did not report data in the form of an odds ratio (OR); therefore, we calculated an OR using the data that were presented. Using author-provided definitions, we considered shorter distance, shorter travel time, lower transportation cost, and urban (versus rural) residence as the referent categories. Each estimate of association was categorized as an inverse effect (i.e. increasing distance, time, cost, or rural location was associated with worsened HIV outcomes such as lower rate of VCT completion, linkage, or adherence; or, greater rate of LTFU or mortality), a null effect, or a positive effect (i.e. increasing distance, time, cost, or rural location was associated with improved HIV outcomes such as higher rate of VCT completion, linkage, or adherence; or, lower rate of LTFU or mortality).
We summarized the percentage of studies demonstrating an inverse, null, or positive effect when categorized by study-level variables such as sub-continental region (Eastern Africa, Southern Africa, or Western Africa) as defined by the United Nations (UN); study population [HIV-infected adults, HIV-infected children, HIV/tuberculosis (TB) co-infected individuals receiving anti-TB therapy, or pregnant women receiving services for the prevention of maternal to child transmission of HIV (PMTCT)]; and study time period (pre–2003, 2003–2006, or post–2006). The study time period date ranges were selected based on 2003 being the initial year of the President’s Emergency Plan for AIDS Relief, and 2006 being the year in which member states at the UN High Level Meeting on AIDS resolved to scale up access to HIV care with a goal of universal access by 2010.
Assessment of Study Quality
For studies reporting a statistical association between a geographic or transportation-related barrier and an HIV outcome, we designed an assessment tool that accounted for seven parameters within the following four domains: (1) study design and population, (2) exposure measurement, (3) outcome measurement, and (4) data analysis.
Results
Study Selection
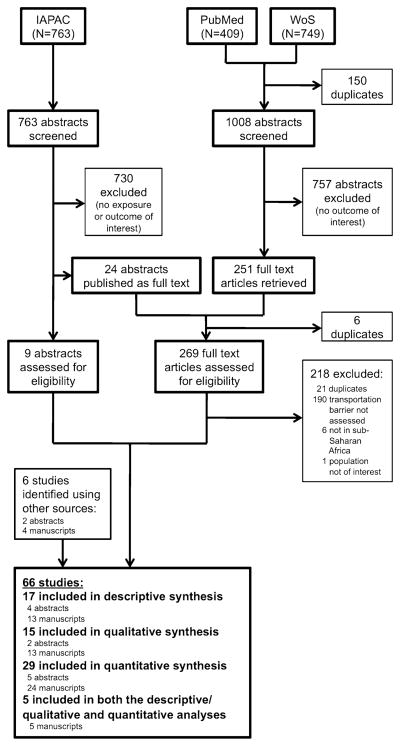
We identified 1,008 full-length manuscripts and 763 conference abstracts during our initial search. After excluding 1,487 records on the basis of the initial screen, we reviewed 273 full-length, published manuscripts and 11 IAPAC abstracts that had not yet been published as manuscripts. We also included six studies identified outside of our systematic screening protocol. A total of 66 studies were included in our review: 29 quantitative studies, 17 descriptive studies, 15 qualitative studies, and five studies that contained both descriptive/ qualitative and quantitative data (Fig. 1). All studies included in the final review were conducted exclusively in SSA. Excluding two qualitative studies that did not report the number of participants, and accounting for studies that included more than one type of data, these studies involved 131,325 participants from 15 different countries in SSA.
Fig. 1.
PRISMA flow diagram of studies identified for review. Studies were identified using a systematic search of PubMed and Web of Science (WoS), as well as manual search of conference abstracts from the International Association of Physicians in AIDS Care (IAPAC) annual meeting from 2002 to 2011
Study Characteristics: Descriptive Studies
In the descriptive studies (Table 1 [28–48]), participants commonly indicated geographic and transportation-related barriers as factors that promoted poor outcomes throughout the continuum of HIV care, including delaying or forgoing HIV testing (percent of study participants ranging from 4.9 to 20.7 %; three studies [28–30]), not successfully linking to HIV care (range 3.8–44 %; two studies [31, 32]), missing clinic visits or dropping out of care (range <5–20.1 %; four studies [33–36]), or failing to adhere to ART (range 5–70 %; 12 studies [37–48]). These studies represented 72,642 subjects in ten countries: Botswana, Cote d’Ivoire, Kenya, Malawi, Nigeria, Tanzania, Togo, Uganda, Zambia, and Zimbabwe.
Table 1.
Descriptive studies
| Reference | Duration; country | Population (# of participants) | Setting | Findings related to geographic or transportation-related barriers |
|---|---|---|---|---|
| Primary outcome: VCT completion | ||||
| Kaawa-Mafigiri et al. [28] | 2008–2009; Uganda | HIV-infected adults (n = 889) | 2 Public hospital-based clinics | 23/470 (4.9 %) men and 32/466 (6.9 %) women reported that lack of transportation was a reason for delaying HIV testing |
| Morin et al. [29] | 2002–2003; Zimbabwe | Adults participating in a mobile VCT program (n = 350) | Mobile VCT vans in public marketplaces | Among those testing for HIV for the first time, 72/348 (20.7 %) reported that “location is not convenient” was a reason for not seeking prior HIV testing |
| Uganda Ministry of Health and ORC Macro [30] | 2004–2005; Uganda | Adults participating in a national survey (n = 15,497) | National survey | Among individuals who reported never having been tested for HIV, 2.7 % of women and 4.6 % of men reported that this was due to the testing center being too far away |
| Primary outcome: linkage to carea | ||||
| Amuron et al. [31] | 2007; Uganda | HIV-infected, ART-eligible adults undergoing pre-ART counseling and assessment (n = 2,483) | NGO-based clinic | Among subjects who were eligible and alive at study follow-up but did not initiate ART, 70/158 (44 %) reported that inability to afford transportation costs was a reason for not initiating ART; this was the most common reason given for failure to initiate ART |
| Parkes-Ratanshi et al. [32] | 2007; Uganda | HIV-infected adults with CD4 count 200 in a trial of fluconazole prophylaxis for cryptococcal disease, who delayed initiation of ART for >3 months (n = 400) | 2 NGO-based clinics | Among participants who delayed ART for >3 months after enrollment in the study, 15/400 (3.8 %) cited transportation cost as a reason for the delay |
| Primary outcomes: loss to follow-up (LTFU) & mortalityb | ||||
| Braitstein et al. [33] | 2009–2010; Kenya | HIV-infected, HIV-exposed, or HIV status unknown children who were LTFU (n = 97) | 2 public hospital-based clinics | Among children who were LTFU and later interviewed, 7/97 (7 %) reported that transportation cost was the primary reason.; 23 % of children who were LTFU were on ART at the last visit |
| Krebs et al. [34] | 2005; Zambia | HIV-infected adults enrolled in an ART program (n = 430) | 12 public clinics | Among the 271/430 (63 %) of patients who provided reasons for missed visits,<5 % cited inability to pay for transportation as an important reason |
| McGuire et al. [35] | 2004–2007; Malawi | HIV-infected adults who were LTFU (n = 221) | 1 NGO-based district hospital and 10 affiliated health centers | Among respondents, 31/172 (20.1 %) of those who were not on ART and 5/49 (12.8 %) of those on ART reported transportation cost as a reason for defaulting; 49/221 (22 %) of respondents were on ART at the time of LTFU |
| Ochieng-Ooko et al. [36] | 2001–2007; Kenya | HIV-infected adults, including ART-naïve and those on ART (n = 50,275) | 23 public clinics | Among the subset of participants that were interviewed, 124/1037 (12 %) men and 360/2217 (17 %) women cited high transportation cost as a reason for missing scheduled clinic visits |
| Primary outcomes: ART adherence & viral suppression | ||||
| Adeyemo et al. [37] | Time not reported; Nigeria | HIV-infected adults and adolescents defaulting on ART (n = 127) | Not reported | 76/127 (59.8 %) of participants attributed poor adherence to financial challenges due to transportation costs or purchasing food |
| Asiimwe et al. [38] | 2005; Uganda | HIV-infected adults and children on ART for >1 year (n = 84) | Public clinic | 13 % of participants reported transportation problems as a barrier to accessing care |
| Bajunirwe et al. [39] | 2006; Uganda | HIV-infected adults on ART (n = 175) | Public hospital-based clinic | Of patients who were interviewed, 9/47 (19 %) reported an inability to secure money for transportation to the clinic as the reason for running out of ART medicines |
| Eholie et al. [40] | 2002; Cote d’Ivoire | HIV-infected adults on ART (n = 308) | 3 public clinics | Among participants with incomplete adherence, 14 % reported “trips or weariness” as a reason for missing a dose of ART |
| Kip et al. [41] | 2007; Botswana | HIV-infected adults enrolled in an ART clinic (n = 400) | 4 randomly selected ART clinics situated throughout the country | 180/400 (45 %) respondents reported being unable to pay for transportation to ART clinics |
| Kirsten et al. [42] | 2008–2009; Tanzania | HIV-infected women enrolling in a PMTCT program (n = 122) | Public hospital-based antenatal clinic | 3/19 (16 %) women who missed drug collection at least once mentioned transportation difficulties as a reason |
| Munseri et al. [43] | 2001–2005; Tanzania | HIV-infected adults initiating treatment for latent TB (n = 8) | Clinical trial of TB vaccine | 1/8 (13 %) interviewed participants who did not complete latent TB treatment reported travel distance to clinic as a significant factor in the decision to stop therapy |
| Olowookere et al. [44] | 2007; Nigeria | HIV-infected adults on ART (n = 216) | Public hospital-based clinic | Of those reporting at least one missed dose, 70/216 (32.4 %) and 54/216 (25 %) indicated that distance to clinic and transportation cost, respectively, were contributing factors |
| Potchoo et al. [45] | 2005; Togo | HIV-infected adults on ART (n = 99) | NGO-based pharmacies affiliated with a national HIV treatment program | Among patients who missed at least one ART dose intake, 11/43 (25.6 %) reported travel as a factor in their poor adherence |
| Van Dijk et al. [46] | 2007–2008; Zambia | HIV-infected children and caretakers (n = 192) | Public hospital-based clinic | Among the 73 % of caretakers who reported difficulty accessing care, 60 % reported that this was due to lack of money, 54 % reported this was due to lack of transportation, and 32 % reported this was due to poor road conditions |
| Weiser et al. [47] | 2000; Botswana | HIV-infected adults on ART, providers (n = 169) | 3 private clinics | Of participants who were interviewed, 4/75 (5 %) of respondents indicated that distance from clinic caused them to miss doses of ART; of the 32/108 (30 %) of participants who identified frequency of required clinic visits as a barrier to treatment, 16/32 (50 %) indicated that living too far away was a reason for this |
| Yahaya et al. [48] | Year not reported; Nigeria | HIV-infected adults who were poorly adherent on ART (n = 100) | Public hospital | Among the respondents (all of whom were classified as poorly adherent), 70/100 (70 %) lived >10 km from the treatment facility |
NGO non-governmental organization
Including eligibility assessment, enrollment in pre-ART care, ART initiation, and pre-ART retention in care [26]
Including measures of LTFU/retention either while on ART, or a combined measure of pre-ART and on-ART LTFU/retention
Study Characteristics: Qualitative Studies
Among the qualitative studies that we identified (Table 2 [24, 49–63]), participants described geographic and transportation-related barriers as factors that impeded successful navigation of several points along the continuum of HIV care, including pre-ART linkage to care (four studies [49–52]), retention in care once on ART (one study [53]), and maintenance of optimal adherence to ART (11 studies [24, 54–63]). In these studies, prominent themes included lack of money to pay for transportation to clinic [24, 49–54, 57, 59], being forced to decide between paying for transportation to clinic and basic necessities such as feeding one’s family or purchasing medications for opportunistic infection prophylaxis [52, 63], and the need to draw on social supports to overcome transportation barriers [62]. Poor road conditions [46], difficulty accessing reliable transportation [24], and the inability to take time off from work to travel long distances to clinic [51], were also described as factors that contributed to transportation difficulties. These studies represented at least 5,373 participants in ten countries: Botswana, Ethiopia, the Gambia, Kenya, Malawi, Namibia, Nigeria, South Africa, Tanzania, and Uganda.
Table 2.
Qualitative studies
| Reference | Year(s); country | Population (# of participants) | Setting | Findings related to geographic or transportation-related barriers |
|---|---|---|---|---|
| Primary outcome: linkage to carea | ||||
| Assefa et al. [49] | 2005–2008; Ethiopia | HIV/AIDS program managers, care providers, and HIV-infected patients on ART (n = not reported) | Various NGO- and public hospital-based clinics | Semi-structured interviews with program managers, care providers, and patients revealed that distance to treatment sites and high transportation costs were among the main reasons described for poor linkage to care |
| Bowie et al. [50] | 2003–2008; Malawi | HIV-infected adults (n = 1,266) | Home-based care administered by a public hospital | Among 131 WHO Stage III or IV patients not receiving ART, many cited difficulty obtaining transportation to the clinic as a barrier to receiving ART |
| Chan et al. [51] | 2004–2008; Malawi | HIV-infected HCWs, HIV care providers, and hospital administrators (n = 306) | Public hospital-based clinic providing care specifically for HIV- infected HCWs | Several of the respondents who participated in in-depth interviews or focus group discussions indicated that distance from the rural health centers and transportation cost were barriers to uptake of services at the HCW HIV clinic |
| Lubega et al. [52] | 2008; Uganda | HIV-infected, ART-naïve adults, health center staff, and community members (n = 74) | Public pre-ART clinic | Transportation difficulties were often mentioned as a reason for dropping out of pre-ART care |
| Primary outcomes: loss to follow-up (LTFU) & mortalityb | ||||
| Bwirire et al. [53] | 2004; Malawi | HIV-infected pregnant women; nurse midwives (n = 25) | Public hospital | Focus groups identified transportation costs as a barrier to attending PMTCT visits |
| Primary outcomes: ART adherence & viral suppression | ||||
| Abrahams et al. [54] | 2005–2006; South Africa | HIV-uninfected female adult rape victims initiating ART for PEP (n = 29) | 2 hospital-based public health facilities | High transportation costs were identified as a barrier to PEP adherence among some women from the more rural of the 2 sites, which had a larger catchment area and primarily served patients with low socioeconomic status |
| Byakika- Tusiime et al. [55] | 2004–2005; Uganda | HIV-infected adults and children on ART (n = 177) | Public hospital-based clinic | Of patients who were interviewed, one of the reasons given for poor adherence was lack of money for transportation to clinic to refill their prescriptions |
| Hardon et al. [24] | 2005; Uganda, Tanzania, Botswana | Health care workers; HIV-infected adults on ART (n = 272) | 12 public or private clinics across 3 countries | Transportation cost was reported by patients and health care workers as an important reason for failure to follow-up at health facilities to refill medications |
| Nam et al. [56] | Year not reported; Botswana | HIV-infected adults on ART (n = 32) | 1 public and 1 private clinic located in the capital city | Transportation cost was not specifically enumerated by participants as a barrier to adherence; however, transportation cost is referenced in the theoretical framework put forth by the authors |
| Nassali et al. [57] | 2006–2007; Uganda | HIV-infected women discharged from a hospital post-natal ward (n = 289) | Public hospital | Among women who did not return for post-natal PMTCT, transportation cost to the health unit was identified as a hindrance to returning |
| Peterson et al. [58] | Year not reported; The Gambia | HIV-infected adults initiating ART (n = 64) | Public clinic | Among all participants, travel was the most commonly reported barrier to adherence |
| Pyne-Mercier et al. [59] | 2007–2008; Kenya | HIV-infected adults on ART (n = 2,534) | Public clinic | Among participants who were interviewed, transportation was cited as an important barrier to receiving treatment during a period of post-election violence |
| Rowe et al. [60] | 2001; South Africa | HIV-infected adults enrolled in a TB prophylaxis program who did not complete a 6-month course of isoniazid (n = 6) | Public hospital-based clinic | Participants who were unable to complete a 6-month course of TB prophylaxis therapy cited the cost of obtaining transportation to clinic as an obstacle to completing therapy |
| Thobias et al. [61] | Year not reported; Namibia | HIV-infected individuals on ART (n = not reported) | Public hospital | Participants identified long distances to health facilities as an important factor influencing adherence |
| Ware et al. [62] | Year not reported; Nigeria, Tanzania, Uganda | HIV-infected adults on ART, treatment partners, and providers (n = 252) | 3 public clinics | Participants reported that obtaining transportation to clinic was a significant barrier to optimal adherence and that they often had to rely on social supports to overcome these difficulties |
| Weiser et al. [63] | 2007; Uganda | HIV-infected adults (n = 47) | Public hospital-based clinic | Participants reported that often times they were forced to decide between spending money on transportation to clinic and taking care of other essential needs, such as securing food for their families or purchasing medications to treat opportunistic infections |
NGO non-governmental organization, WHO World Health Organization, HCW health care worker, PEP post exposure prophylaxis
Including eligibility assessment, enrollment in pre-ART care, ART initiation, and pre-ART retention in care [26]
Including measures of LTFU/retention either while on ART, or a combined measure of pre-ART and on-ART LTFU/retention
Study Characteristics: Quantitative Studies
In the 34 quantitative studies (Table 3 [27, 36, 42–44, 59, 64–91]), there were 52 estimated associations between geographic or transportation-related barriers and HIV-related outcomes of interest: VCT [two (4 %)], pre-ART linkage to care [eight (15 %)], LTFU or mortality [17 (33 %)], and ART adherence or viral suppression [25 (48 %)]. Geographic or transportation-related barriers had an inverse association with HIV outcomes in 23 estimates (44 %), whereas 26 estimates (50 %) were null and three estimates (6 %) demonstrated a positive effect. When we evaluated these estimates by outcome of interest, we found an inverse association between geographic or transportation-related barriers and HIV outcomes for 2/2 (100 %) VCT estimates, 4/8 (50 %) pre-ART linkage estimates, 8/17 (47 %) LTFU or mortality estimates, and 9/25 (36 %) adherence or viral suppression estimates (Table 4). These studies represented 106,574 participants from nine countries: Ethiopia, Kenya, Malawi, Mozambique, Nigeria, South Africa, Tanzania, Uganda, and Zambia.
Table 3.
Quantitative Studies
| Study code | Reference | Year(s); country | Population (# of participants) | Setting | Exposure(s) of interest | Outcome(s) of interest | Findings related to geographic or transportation-related barriers | Potential confounding co- variables included |
|---|---|---|---|---|---|---|---|---|
| Primary outcome: VCT completion | ||||||||
| SA1 | Hutchinson et al. [27] | 2002–2003; South Africa | Adults participating in a cross- sectional survey (n = 3,520) | Regional community- based survey | Urban versus rural residence | Use of VCT service | Urban individuals were more likely to use VCT services than rural individuals (OR 3.15; 95 % CI 2.59–3.82) | Univariable analysis only |
| TA1 | Wringe et al. [64] | 2003–2004; Tanzania | Adult women participating in a longitudinal serologic surveillance study (n = 4,990) | Community- based cohort | Urban versus peri- urban versus rural residence | Return to testing site in 1 week to receive result and complete VCT | Peri-urban women were more likely to complete testing than rural women (AOR = 1.50; 95 % CI 1.12–2.02); however, urban women were not significantly more likely to complete testing than rural women | Age, marital status, marital change, education, ethnicity, religion, HIV status, spouse HIV status and VCT use, risky partner in last year, perceived HIV risk, heard of VCT, prior VCT |
| Primary outcome: linkage to carea | ||||||||
| MO1 | Cook et al. [65] | 2007–2008; Mozambique | HIV-infected pregnant women receiving PMTCT who followed up for adult HIV care (n = 227) | Public hospital- based clinic | Self-reported distance to clinic | Linkage from PMTCT to EID services | Mother-infant pairs that were successfully linked to EID care were more likely to live farther from clinic (OR 2.14; 95 % CI 1.01–4.51) | Not reported |
| SA2 | Ingle et al. [66] | 2004–2007; South Africa | HIV-infected individuals eligible for ART (n = 22,083) | Public clinics (28 free- standing and 8 hospital- based) | Distance to treatment site; urban/peri-urban versus rural assessment site | Initiation of ART; pre-ART mortality | Participants with initial assessment site >15 km from treatment site were less likely to initiate ART (HR 0.72; 95 % CI 0.66–0.78) and had higher pre-ART mortality (HR 1.65; 95 % CI 1.45–1.88) than those initially assessed and treated at the same site; participants from rural assessment sites were less likely to initiate ART (HR 0.89; 95 % CI 0.83–0.96) and had higher pre-ART mortality (HR 1.42; 95 % CI 1.23–1.64) than those from urban or peri-urban assessment sites | Age, sex, weight, CD4 count at time of ART eligibility, enrollment year, staffing levels of treatment facility, urban vs rural |
| SA3 | Lessells et al. [67] | 2007; South Africa | HIV-infected adults not yet eligible for ART (CD4 >200) (n = 930) | Public clinic | Distance to clinic (methodology not reported) | Pre-ART retention in care | Distance to clinic not significantly associated with pre-ART retention in care (OR not reported) | Multivariable analysis performed, but variables not reported |
| ZA1 | Sutcliffe et al. [68] | 2005–2008; Zambia | HIV-infected children receiving care (n = 835) | Public clinics (1 urban and 2 rural) | Self-reported distance to clinic; GIS- mapped straight line distance to clinic; attendance at urban versus rural clinic | Default prior to ART initiation; time to ART initiation | Living farther from clinic was significantly associated with a higher rate of default on ART (p = 0.05), but not with initiating ART; compared to children attending an urban clinic, those attending a rural clinic were less likely to initiate ART (AHR = 0.38; 95 % CI 0.29–0.48 among children initially eligible for ART, and AHR 0.59; 95 % CI 0.37–0.95 among children initially ineligible for ART) | For ART default, univariable analysis only; for ART initiation, adjusted for location, time, and any variables either found to be associated with p <0.05 or a priori known to be associated with the outcome |
| Primary outcomes: loss to follow-up (LTFU) & mortalityb | ||||||||
| UG1 | Elbireer et al. [69] | 2006–2008; Uganda | HIV/TB co-infected adults receiving TB therapy (n = 344) | Public clinic | Self-reported distance to clinic | LTFU on TB therapy | Those living>10 km from the TB treatment facility were significantly more like to default on TB therapy (AOR = 2.22; 95 % CI 1.21–4.06) | Any variable found to be significant at the level of p <0.25 on univariable analysis (variables not specified) |
| UG2 | Geng et al. [70] | 2004–2007; Uganda | HIV-infected adults initiating ART who became LTFU from initial clinic (n = 48) | Public hospital- based clinic | Self-reported distance to clinic | Retention in care at a different clinic among patients initially LTFU | Those living farther from the initial clinic were more likely to be connected to care at a different clinic (AOR = 1.45 per every 10 km; 95 % CI 1.11–1.90) | Age, years since last clinic visit |
| KE1 | Karcher et al. [71] | 2004–2005; Kenya | HIV-infected adults who either previously received PMTCT care or whose wife or mother received PMTCT care (n = 153) | Public hospital- based clinic | Self-reported distance to clinic | Initiation of ART; LTFU (excluding mortality); mortality | Distance to clinic was not significantly associated with initiating ART (OR 0.78; 95 % CI 0.34–1.79), LTFU (OR 0.41, 95 % CI 0.14–1.22), or mortality (OR 0.39, 95 % CI 0.11–1.37) | Univariable analysis only |
| ZA2 | Kempf et al. [72] | 1994–1998; Zambia | HIV sero- discordant couples enrolling in a clinical trial of VCT (n = 1,771 couples) | VCT clinic | Patient perception of living “near” or “far” from clinic | LTFU | Among M+F- serodiscordant couples, living “far” from clinic was significantly associated with LTFU (OR 1.7; 95 % CI 1.0–2.8) from the study cohort | Any variable found to be significant at the level of p <0.05 in univariable analysis (variables not specified) |
| MA1 | Massaquoi et al. [73] | 2006–2007; Malawi | HIV-infected adults initiating ART (n = 4,074) | Public district hospital and nine rural clinics | Enrollment at central hospital versus rural health center | ART default; mortality | Patients treated at a rural health center were less likely to default on ART (HR 0.22; 95 % CI 0.13–0.35) but had higher mortality (HR 2.02; 95 % CI 1.63–2.49) than those treated at the district hospital | Univariable analysis only |
| TA2 | Mossdorf et al. [74] | 2005–2008; Tanzania | HIV-infected adults initiating ART (n = 1,463) | Public hospital | Self-reported distance to clinic | 12-month LTFU/ mortality composite | Distance to clinic was not significantly associated with mortality/LTFU composite (p = 0.561) | Univariable analysis only |
| ET1 | Mulissa et al. [75] | 2003–2008; Ethiopia | HIV-infected adults initiating ART (n = 1,428) | Public hospital | Rural versus urban residence | LTFU (analysis only includes those on ART); mortality | Urban patients were less likely than rural patients to be LTFU (AHR = 0.5; 95 % CI 0.3–0.6), but there was no significant difference in mortality; also, authors did not make a distinction of whether before or after decentralization in this analysis, although separate analyses were performed looking at outcomes by pre- or post- decentralization) | Multivariable analysis performed (variables not specified) |
| KE2 | Ochieng- Ooko et al. [36] | 2001–2007; Kenya | HIV-infected adults (n = 50,275) | 2 public clinics | Self-reported travel time to clinic; urban versus rural clinic | LTFU | Among patients on ART, longer travel time to clinic was significantly associated with LTFU (HR 1.11, 95 % CI 1.04–1.19); attending a rural clinic was associated with a lower rate of pre- ART retention (HR 0.82, 95 % CI 0.77–0.88), but there was no significant association between rural clinic site and retention in patients already on ART | Sex, other variables found to be significant at the level of p <0.05 on univariable analysis (variables not specified) |
| Primary outcomes: ART adherence & viral suppression | ||||||||
| NI1 | Adeyemi et al. [76] | 2009; Nigeria | HIV-infected adults on ART (n = 320) | Not reported | Self-reported travel time to clinic | Adherence, as defined by late or missed clinic visit >7 days | Those living >3 h from clinic were more likely to have poor adherence than those <3 h away (OR 1.9; 95 % CI 1.4–2.8) | Not reported |
| UG3 | Bagenda et al. [77] | 2004–2007; Uganda | HIV-infected children initiating ART (n = 129) | Public hospital- based clinic | Self-reported distance to clinic | >95 % adherence by pharmacy refill records | There was no significant association between distance to clinic and adherence (p = 0.74) | Univariable analysis only |
| SA4 | Barth et al. [78] | 2003–2007; South Africa | HIV-infected children who have been on ART for at least one year (n = 81) | Public clinic | Self-reported distance to clinic | Viral suppression | Among children who had a good initial virologic response to ART, there was no significant association between distance to clinic and sustained viral suppression (OR not reported) | Multivariable analysis performed (variables not specified) |
| UG4 | Byakika- Tusiime et al. [79] | 2002; Uganda | HIV-infected adults purchasing ART who have been on ART for at least one month (n = 304) | 2 public and 1 private clinic | Self-reported distance to clinic | Adherence >95 % by 3-day self- report | Travel distance to clinic was not associated with adherence (AOR = 1.01; 95 % CI 0.45–1.25) | Not reported |
| ZA3 | Carlucci et al. [80] | 2006; Zambia | HIV-infected adults on ART (n = 409) | Public hospital- based clinic | Self-reported cost and travel time to clinic; odometer-tracked distance to clinic; GPS-estimated linear distance from nearest health center to clinic | Adherence >95 % by pill count | There was no significant association between linear distance (p = 0.1), actual travel distance (p = 0.1), transportation cost (OR 0.7, 95 % CI 0.35–1.4), or travel time (OR 1.0, 95 % CI 0.91–1.0) and adherence | Only travel time and transportation cost were modeled in multivariable analysis (variables not specified) |
| NI2 | Charurat et al. [81] | 2005–2006; Nigeria | HIV-infected adults initiating ART (n = 5,760) | 5 tertiary hospitals | Self-reported travel time to clinic | Adherence by pharmacy refill records; LTFU | Those traveling >2 h to reach clinic were significantly more likely to be non- adherent than those traveling <1 h (AOR = 1.11; 95 % CI 1.01–1.23); travel time was not significantly associated with LTFU | Adherence analysis adjusted for age, sex, education, disclosure, employment, baseline CD4, time on ART, and ART regimen; LTFU analysis was univariable only |
| UG5 | Haberer et al. [82] | 2008–2010; Uganda | HIV-infected children on ART and living within 20 km of clinic (n = 121) | Public hospital- based clinic | Self-reported travel time to clinic; self- reported transport cost to reach clinic | Adherence >90 %; treatment interruption >48 h (by MEMS) | Travel time (AOR = 1.00; 95 % CI 1.00–1.01) and transportation cost (AOR = 1.00; 95 % CI 1.00–1.01) were not significantly associated with adherence | Any variable found to be significant at the level of p <0.1 (variables not specified) |
| SA5 | Hirschhorn et al. [83] | Year not reported; South Africa | HIV-infected adults on ART (n = 407) | Not reported | Self-report of “difficult to get to clinic” | Adherence by visual analog scale, last dose missed, and # doses missed in last week | Self-report of “less difficult to get to clinic” was associated with higher adherence (OR not reported) | Not reported |
| NI3 | Iroha et al. [84] | 2008; Nigeria | HIV-infected children on ART (n = 212) | Public hospital- based clinic | Self-reported distance to clinic | Adherence by 3-day self report of at least 1 missed dose | Travel distance was not significantly associated with adherence (for >20 km, OR 0.69; 95 % CI 0.24–1.99) | Univariable analysis only |
| MA2 | Kirsten et al. [42] | 2008–2009; Tanzania | HIV-infected women enrolling in a PMTCT program (n = 122) | Public hospital- based antenatal clinic | Self-reported travel time to clinic; self- reported transportation cost | Acceptance of PMTCT medications; adherence to PMTCT regimen by medication possession ratio | Travel time was not significantly associated with maternal acceptance of PMTCT (OR 0.74; 95 % CI 0.34–1.64) or adherence to PMTCT (p = 0.788); transportation cost was not significantly associated with maternal acceptance of PMTCT (OR 0.91; 95 % CI 0.40–2.08) or adherence to PMTCT (p = 0.728) | Age, participation in an income-generating activity, gestational age |
| NI4 | Kurlander et al. [85] | 2005–2006; Nigeria | HIV-infected adults on ART (n = 116) | Public hospital | Self-reported travel time to clinic | >95 % adherence by pharmacy refill records | There was no significant association between travel time to clinic and adherence (OR not reported) | Not reported |
| SA6 | Maqutu et al. [86] | 2004–2006; South Africa | HIV-infected adults initiating ART (n = 688) | 2 public clinics (1 urban and 1 rural) | Urban versus rural treatment site | >95 % adherence by pharmacy refill records | Patients receiving care at the urban treatment site were more likely to achieve >95 % adherence than those at the rural treatment site (AOR = 4.35; 95 % CI 2.26–8.37) | Multivariable analysis performed (variables not specified) |
| NI5 | Mbaezue et al. [87] | 2005–2006; Nigeria | HIV-infected adults initiating ART (n = 228) | Public hospital | Self-reported travel time to clinic | Failure to pick up medications within 1 week of scheduled appointment | There was no significant association between travel time to clinic and failure to pick up medications within one week of scheduled appointment (OR not reported) | Not reported |
| TA3 | Munseri et al. [43] | 2001–2005; Tanzania | HIV-infected adults initiating IPT for latent TB (n = 117) | Clinical trial of TB vaccine | Self-reported distance to clinic | Completion of IPT course | Self-reported distance to clinic was not significantly associated with IPT completion (p = 0.055); however, patient subjective assessment of “clinic far from home” was associated with non-completion of IPT (p = 0.04) | Univariable analysis only |
| NI6 | Olowookere et al. [44] | 2007; Nigeria | HIV-infected adults on ART (n = 216) | Public hospital- based clinic | Self-report of “unable to pay for transport” | Adherence >95 % by 1-week self- report | “Unable to pay for transport” was not significantly associated with adherence (OR 1.83; 95 % CI 0.98–3.4) | Multivariable analysis performed (variables not specified) |
| KE3 | Pyne- Mercier et al. [59] | 2007–2008; Kenya | HIV-infected adults on ART (n = 2,534) | Public clinic (during period of post- election violence) | Self-reported travel time to clinic | Treatment interruption of >48 h | Patients who lived >3 h from clinic were significantly more likely to have at least one treatment interruption than those who lived within 1–2 h of clinic (AOR = 1.86; 95 % CI 1.28–2.71) | Sex, time on ART |
| TA4 | Ramadhani et al. [88] | 2005; Tanzania | HIV-infected adults on ART (n = 150) | Public hospital- based clinic | Self-reported walking time to clinic | Adherence by self-report of at least one missed dose in last 6 months | Walking time to clinic was not significantly associated with non-adherence (AOR = 1.2; 95 % CI 0.94–1.6) | Multivariable analysis performed (variables not specified) |
| UG6 | Sethi et al. [89] | Year not reported; Uganda | HIV-infected adults (n = 132) | Public hospital- based clinic | Self-reported distance to clinic | Adherence by self-report of never having missed a dose of ART | Those living >5 km from clinic were significantly more likely to have ever missed a dose of ART than those living <5 km from clinic (OR 2.6; 95 % CI 1.1–6.0) | Sex |
| NI7 | Taiwo et al. [90] | 2006–2007; Nigeria | HIV-infected adults initiating ART (n = 499) | Public clinic | Self-reported distance to clinic | Adherence >95 % by pharmacy refill | Those living <20 km from clinic were significantly more likely to have >95 % adherence at 24 weeks (AOR = 2.31; 95 % CI 1.30–4.10) and at 48 weeks (AOR = 2.35; 95 % CI 1.43–3.87) after initiating ART than those living >100 km from clinic | Age, sex, disclosure status, presence of treatment partner |
| ZA4 | Van Dijk et al. [91] | 2007–2010; Zambia | HIV-infected children initiating ART (n = 267) | Public hospital- based clinic | Self-reported travel time to clinic | Viral suppression; mortality | Patients who lived >5 h from clinic were significantly less likely to achieve viral suppression at 6 months after ART initiation (OR 0.10; 95 % CI 0.01–0.82) compared to those living <1 h from clinic; travel time to clinic was not significantly associated with mortality (for >5 h, HR 2.93; 95 % CI 0.37–23.48) | Any variable found to be significant at p <0.1 in univariable analysis (variables not specified) |
EID early infant diagnosis, GIS geographic information system, GPS global positioning system, MEMS medication event monitoring system, IPT isoniazid prophylactic therapy, OR odds ratio, AOR adjusted odds ratio, HR hazard ratio, AHR adjusted hazard ratio
Including eligibility assessment, enrollment in pre-ART care, ART initiation, and pre-ART retention in care [26]
Including measures of LTFU/retention either while on ART, or a combined measure of pre-ART and on-ART LTFU/retention
Table 4.
Quantitative studies grouped by exposure and outcome of interest
| Transportation variable | Outcome of interest
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VCT
|
Linkage to carea
|
LTFU & mortalityb
|
Adherence & viral suppression
|
|||||||||
| Inverse effect | Null effect | Positive effect | Inverse effect | Null effect | Positive effect | Inverse effect | Null effect | Positive effect | Inverse effect | Null effect | Positive effect | |
| Travel distance | SA2 | SA3, ZA1, KE1 | MO1 | SA2, ZA1, UG1, ZA2 | KE1c, KE1d, TA2 | UG2 | UG6, NI7, TA3f | UG3, SA4, UG4, ZA3, NI3, TA3e | ||||
| Travel time | KE2 | NI2, ZA4 | NI1, NI2, KE3, ZA4 | ZA3, UG5, MA2, NI4, NI5, TA4 | ||||||||
| Transportation Cost | SA5 | ZA3, UG5, MA2, NI6 | ||||||||||
| Rural residence or clinic location | SA1, TA1 | SA2, ZA1, KE2 | SA2, MA1d, ET1c | ET1d, KE2 | MA1c | SA6 | ||||||
| Total estimates | 2 | 0 | 0 | 4 | 3 | 1 | 8 | 7 | 2 | 9 | 16 | 0 |
Study codes (from Table 3) populate individual cells
Including eligibility assessment, enrollment in pre-ART care, ART initiation, and pre-ART retention in care [26]
Including measures of LTFU/retention either while on ART, or a combined measure of pre-ART and on-ART LTFU/retention
LTFU outcome
Mortality outcome
Self-reported distance
Self-report of living “far from clinic”
Exposure and Outcome Heterogeneity
The frequency with which geographic and transportation-related barriers were estimated to have an inverse effect on HIV outcomes varied according to the method of exposure measurement, sub-continental region, time period, study population, number of study participants, and number of study sites (Table 5). Among the three estimates based on an objective measure of distance, two (67 %) demonstrated an inverse effect. Among the 12 estimates where the exposure was residence in a rural area, nine (75 %) demonstrated an inverse effect. In contrast, an inverse effect was shown in only 3/15 (20 %) estimates based on self-reported distance, 5/13 (38 %) based on self-reported travel time, and 0/4 (0 %) based on self-reported transportation cost. When analyzed by sub-continental region, an inverse effect was demonstrated in 13/35 (37 %) studies conducted in Eastern Africa, 3/8 (38 %) in Western Africa, and 7/9 (78 %) in Southern Africa. There was limited variability in the percentage of estimates demonstrating an inverse association when categorized by time period, with 5/8 (63 %) studies conducted prior to 2003, 17/36 (47 %) conducted from 2003 to 2006, and 15/32 (47 %) conducted after 2006 demonstrating an inverse effect. Similarly, there was limited variability in these findings between studies conducted in different patient populations, with the exception of studies of women enrolled in PMTCT care, where 0/3 (0 %) of studies found an inverse effect. An inverse effect was demonstrated with greater frequency in larger studies, with 2/15 (13 %) studies with n <200, 8/19 (42 %) studies with n = 201–1,000, and 12/17 (72 %) studies with n >1,000 demonstrating an inverse effect. Furthermore, multi-site studies [13/18 (72 %)] were more likely than single site studies [7/30 (23 %)] to demonstrate an inverse effect. Additionally, there was significant heterogeneity in the definition and measurement of both exposure and outcomes between studies (Table 6).
Table 5.
Variability in estimates by study parameter
| Study parametera | % (n/N) of estimates demonstrating an inverse association between a geographic/transportation-related barrier and an HIV outcome |
|---|---|
| Geographic/transportation barrier | |
| Rural | 75 % (9/12) |
| Distance (objective) | 67 % (2/3) |
| Distance (self-reported) | 20 % (3/15) |
| ravel time (self-reported) | 38 % (5/13) |
| Transport cost (self-reported) | 0 % (0/4) |
| Region | |
| Eastern Africa | 37 % (13/35) |
| Western Africa | 38 % (3/8) |
| Southern Africa | 78 % (7/9) |
| Time period | |
| Pre–2003 | 63 % (5/8) |
| 2003–2006 | 47 % (17/36) |
| Post–2006 | 47 % (15/32) |
| Study population | |
| HIV-infected adults | 50 % (18/36) |
| HIV-infected children | 30 % (3/10) |
| HIV/TB care | 67 % (2/3) |
| PMTCT care | 0 % (0/3) |
| Number of participants | |
| n <200 | 13 % (2/15) |
| n = 20–1,000 | 42 % (8/19) |
| n >1,000 | 72 % (12/17) |
| Number of study sites | |
| Single site | 23 % (7/30) |
| Multiple sites | 72 % (13/18) |
For some estimates, certain study parameters were not reported and therefore not considered in this calculation. For study time period, a single study may be classified in more than one time period if it was reported to span multiple time period categories
Table 6.
Heterogeneity in measurement and definition of exposures and outcomes
| Exposure measure (# of estimates) | Study codes | Total estimates |
|---|---|---|
| Travel distance (20) | ||
| Self-reported integer | MO1, ZA1, UG1, UG2, KE1, TA2, UG3, SA4, UG4, NI3, TA3, UG6, NI7 | 13 |
| GPS or odometer tracked | ZA3 | 1 |
| Straight line (approximate) | ZA1, ZA3 | 2 |
| Patient perception of difficulty | ZA2, SA5 | 2 |
| Other | SA2, SA3 | 2 |
| Travel time (11) | ||
| Self-reported integer | KE2, NI1, ZA3, NI2, UG5, MA2, NI4, NI5, KE3, TA4, ZA4 | 11 |
| Transportation cost (4) | ||
| Self-reported integer | ZA3, UG5, MA2 | 3 |
| Other | NI6 | 1 |
| Urban vs. rural (8) | ||
| Urban+rural | SA1, ZA1, ET1, KE2, SA6 | 5 |
| Urban+peri-urban+rural | TA1, SA2 | 2 |
| Other | MA1 | 1 |
| Outcome measure (# of estimates) | Study codes | Total estimates |
|---|---|---|
| Linkage to care (6) | ||
| Pre-ART retention in care | SA3, ZA1 | 2 |
| ART initiation | SA2, ZA1, KE1 | 3 |
| Other | MO1 | 1 |
| Retention in care/mortality (12) | ||
| Pre-ART mortality | SA2 | 1 |
| Post-ART | MA1, ET1, ZA4 | 3 |
| Without respect to ART status | SA2, UG1, UG2, KE1, ZA2, TA2, KE2, NI2 | 8 |
| Adherence/viral suppression (20) | ||
| Self-reported | UG4, SA5, NI3, TA4, UG6 | 5 |
| Pharmacy refill or pill count | UG3, ZA3, NI2, MA2, NI4, SA6, NI7 | 7 |
| MEMS device | UG5 | 1 |
| Treatment interruption <1 week | UG5, KE3 | 2 |
| Treatment interruption at least 1 week | NI5 | 1 |
| Viral suppression | SA4, ZA4 | 2 |
| Other | NI1, TA3 | 2 |
GPS global positioning system, MEMS medication event monitoring system
Assessment of Study Quality and Risk for Bias
Of the 34 quantitative studies that we identified, 25 (74 %) involved a longitudinal cohort, four (12 %) were specifically designed to measure an association between a geographic or transportation-related barrier and an HIV outcome, five (15 %) reported an objectively measured exposure variable, 28 (82 %) reported an objectively measured outcome variable, 20 (59 %) performed a multivariable analysis to adjust for potential confounders, and six (18 %) adjusted for a marker of wealth in the multivariable analysis (Table 7). Among the 25 cohort studies in which accounting for LTFU, censoring, or missing data would be relevant to potential bias, 14 (56 %) adequately did so. Of the four studies that were specifically designed to measure an association between a geographic or transportation-related barrier and an HIV outcome, three (75 %) demonstrated an inverse effect. Of the ten studies that included a multivariable analysis, used an objectively measured outcome variable, and adequately accounted for LTFU, censoring, and missing data, seven (70 %) found an inverse effect.
Table 7.
Assessment of study quality and risk of bias
| Reference | Study design and population
|
Exposure measurement | Outcome measurement
|
Analysis
|
|||
|---|---|---|---|---|---|---|---|
| Cross-sectional, case control, or cohort? | Specifically designed to assess geographic or transportation barriers ? | Objective or self-reported? | Accounted for LTFU, censoring, and/or missing data? | Objective or self-reported? | Included multivariable analysis? | Adjusted for marker of wealth? | |
| Hutchinson et al. [27] | Cross-sectional | No | Objective | n/a | Self-reported | No | No |
| Wringe et al. [64] | Cohort | No | Unclear | No | Objective | Yes | No |
| Cook et al. [65] | Cohort | No | Self-reported | No (excluded 226 mothers who did not enroll in adult HIV care after PMTCT) | Objective | Yes | Yes |
| Ingle et al. [66] | Cohort | No | Unclear | Yes | Objective | Yes | No |
| Lessells et al. [67] | Cohort | No | Unclear | Yes | Objective | Yes | No |
| Sutcliffe et al. [68] | Cohort | Yes | Objective | Yes | Objective | Yes | No |
| Elbireer et al. [69] | Case control | No | Self-reported | n/a | Objective | Yes | No |
| Geng et al. [70] | Cohort | No | Self-reported | Yes | Objective | Yes | No |
| Karcher et al. [71] | Cohort | No | Self-reported | Yes | Objective | No | No |
| Kempf et al. [72] | Cohort | No | Self-reported | Yes | Objective | Yes | Yes |
| Massaquoi et al. [73] | Cohort | No | Objective | No | Objective | No | No |
| Mossdorf et al. [74] | Cohort | No | Self-reported | Yes | Objective | No | No |
| Mulissa et al. [75] | Cohort | No | Unclear | No | Objective | Yes | No |
| Ochieng- Ooko et al. [36] | Cohort | No | Unclear | Yes | Objective | Yes | No |
| Adeyemi et al. [76] | Cross-sectional | No | Unclear | n/a | Objective | No | No |
| Bagenda et al. [77] | Cohort | No | Self-reported | Yes | Objective | No | No |
| Barth et al. [78] | Cohort | No | Self-reported | Yes | Objective | Yes | Yes |
| Byakika- Tusiime et al. [79] | Cross-sectional | No | Self-reported | n/a | Self-reported | No | No |
| Carlucci et al. [80] | Cohort | Yes | Objective | No (excluded 75 subjects who were LTFU or died prior to study) | Objective | No | No |
| Charurat et al. [81] | Cohort | No | Self-reported | Yes | Objective | Yes | Yes |
| Haberer et al. [82] | Cohort | No | Self-reported | No (excluded subjects living >20 km from clinic) | Objective | Yes | Unclear |
| Hirschhorn et al. [83] | Cohort | No | Self-reported | Unclear | Self-reported | No | No |
| Iroha et al. [84] | Cross-sectional | No | Self-reported | n/a | Self-reported | No | No |
| Kirsten et al. [42] | Cohort | No | Self-reported | No | Objective | No | No |
| Kurlander et al. [85] | Cohort | No | Self-reported | No (excluded 14 subjects who were LTFU) | Objective | No | No |
| Maqutu et al. [86] | Cohort | No | Objective | No | Objective | Yes | Yes |
| Mbaezue et al. [87] | Case control | No | Self-reported | n/a | Objective | No | No |
| Munseri et al. [43] | Cohort | No | Self-reported | No | Objective | No | No |
| Olowookere et al. [44] | Cross-sectional | No | Self-reported | n/a | Self-reported | Yes | No |
| Pyne-Mercier et al. [59] | Cohort | No | Self-reported | No | Objective | Yes | No |
| Ramadhani et al. [88] | Cross-sectional | No | Self-reported | n/a | Self-reported | Yes | No |
| Sethi et al. [89] | Cross-sectional | Yes | Self-reported | n/a | Self-reported | Yes | No |
| Taiwo et al. [90] | Cohort | No | Self-reported | Yes | Objective | Yes | No |
| Van Dijk et al. [91] | Cohort | Yes | Self-reported | Yes | Objective | Yes | Yes |
Discussion
In this systematic review of 66 studies representing over 130,000 persons receiving HIV care across 15 countries in SSA, we found that geographic and transportation-related barriers were associated with worse outcomes throughout the continuum of HIV care. These inverse associations were observed with variable frequency across different regions, different time periods, and among several sub-populations of HIV-infected individuals. In addition, geographic and transportation-related barriers were characterized as important by a large proportion of participants in descriptive and qualitative studies. This substantial body of evidence supports our hypothesis that geographic and transportation-related barriers contribute to poor outcomes for HIV-infected individuals in SSA at all points along the continuum of HIV care.
Overall, we found that ~50 % of estimates demonstrated an inverse association between a geographic or transportation-related barrier and an HIV-related outcome. This proportion varied across different study-level parameters, including sub-continental region, time period, study population, number of participants, and number of study sites. It is notable that studies with greater numbers of participants were more likely to report an inverse effect of geographic and transportation barriers on HIV outcomes, suggesting that smaller studies may not have had sufficient statistical power to estimate such an association with precision. Furthermore, in our assessment of study quality and risk for bias, we found that the higher quality studies were more likely than those of lesser quality to have reported an inverse association between geographic or transportation barriers and an HIV outcome.
Interpretation of our findings is subject to several important limitations. First, most of the quantitative observational studies that we identified were not designed to evaluate geographic or transportation-related barriers as the primary exposure of interest. In our analysis of study quality, we did find that studies specifically designed for this purpose were more likely to report an inverse association than those for which such an analysis was a secondary aim. Second, authors often neglected to adjust for potential confounding variables (or did not report the variables that were adjusted for). However, studies that did adjust for potential confounding variables were more likely to report an inverse effect than those that failed to do so. Third, our summary measures are crude relative to the pooled relative risks and odds ratios that would be calculated in a meta-analysis. However, as previously noted, the heterogeneity in study designs among the identified studies precluded a formal meta-analysis. A fourth and related limitation was the significant variability in measurement and definition of both exposure and outcome variables (as demonstrated in Table 6). Self-reported measures of geographic and transportation-related barriers, such as travel time, distance, and cost, are intrinsically subjective measures that may either under- or over-estimate true difficulties in health care access [92]. For example, in one recently published study of people on HIV treatment in rural Uganda, distance measures based on global positioning systems were inversely associated with missed clinic visits, while self-reported distance measures were not [93]. Additionally, most quantitative studies identified in this review defined the exposure variable as categorical or binary, rather than continuous. This resulted in a wide range of cut-off values when comparing relatively “shorter” versus “longer” travel distance and time, or relatively “higher” versus “lower” transportation cost. Finally, the null findings of some studies may have resulted from the design of the study itself. For example, Haberer et al. [82] found that, among HIV-infected children in Uganda, neither travel time nor transportation cost had a statistically significant association with ART adherence. It should be noted that participants living>20 km from clinic were excluded from that study, and that this was the most common reason for exclusion. If the effect of travel time on ART adherence were non-linear (e.g. no association below a certain threshold and an inverse effect at distances above the threshold), then the exclusion criteria of this study would preclude valid estimation of an association between distance and adherence.
We were surprised to find a small number of studies that demonstrated a paradoxically beneficial impact of geographic and transportation-related barriers on HIV outcomes [65, 70, 73]. It is notable that none of these three studies was specifically designed to estimate the effect of a geographic or transportation barrier on an HIV outcome. In certain cases, the findings were not fully inconsistent with our hypothesis that geographic and transportation barriers negatively affect HIV outcomes. For example, Geng et al. [70] found that among patients LTFU from clinic, greater distance to clinic was associated with increased likelihood of re-establishing care at different site. The authors hypothesized that this was due to emergence of new clinics as part of treatment decentralization in Uganda. Importantly, the reference clinic was the only ART provider in southwest Uganda in year 2000, while in 2009 (at the time of the study) there were over 60 rural treatment sites in the same region. Although Cook and colleagues found that greater distance to clinic was associated with a higher rate of linkage from PMTCT to early infant diagnosis care, this study excluded mothers who failed to enroll in adult HIV care after their pregnancy, which accounted for nearly half of the total sample [65]. Finally, although Massaquoi and colleagues found that patients treated at a rural health center were significantly less likely to default on ART than those treated at an urban center, the authors also found that rural patients had a significantly higher mortality rate compared to their urban counterparts [73]. Furthermore, this analysis was not adjusted to account for potential confounding variables.
Alternatively, these paradoxical findings may be explained in part by HIV-related stigma. HIV is highly stigmatized throughout SSA [94], with increasing evidence suggesting that the stigma of HIV is an important determinant of health-related behaviors, such as HIV testing [95], disclosure of seropositivity to sexual partners or other social supports [96], retention in care [97], and HIV treatment adherence [98]. It is also possible that in certain settings some people may prefer to travel longer distances for their HIV care in order to maintain their anonymity, a phenomenon that has been discussed in HIV-related studies conducted in various African settings [99–101]. Thus a highly motivated population of patients who intentionally travel long distances to minimize stigma could account for a paradoxically positive association between distance and HIV-related outcomes.
Conclusions
We found that geographic and transportation-related barriers impede access to care at all points in the HIV care continuum. This systematic review has important implications for HIV policy and programming in SSA. The provision of HIV care across large rural catchment areas in many parts of SSA presents a significant challenge to scaling up services for HIV-infected individuals and retaining patients in care with optimal and durable adherence. Although the distance one lives from their HIV clinic is not a readily modifiable risk factor, geographic and transportation-related barriers could be attenuated by public health interventions that seek to improve the accessibility of HIV care facilities. Recommended measures to mitigate these effects have included strengthening investment in rural health care infrastructure [102], decentralization of HIV treatment services [103–105], adoption of simplified management protocols that can be administered at the level of the primary health clinic [106], decreasing visit frequency [107], implementation of mobile clinics [108], point-of-care testing [109], immediate referral [110], improving patient-provider communication of test results and other information [111], and provision of transportation stipends [112, 113].
Additionally, identification of standardized and validated measures of geographic and transportation-related barriers might improve risk-stratification and resource allocation to patients in the region. To optimize and expand HIV care delivery in SSA, it will be important to standardize the measurement of geographic and transportation-related barriers and to develop, evaluate, and scale up interventions to mitigate them. Different interventions will likely need to be designed to address different points in the continuum of HIV care. For example, point-of-care CD4 testing and immediate referral for treatment initiation are likely to be effective interventions for improving pre-ART linkage to care, whereas decreasing visit frequency may be a more effective intervention to help patients overcome geographic and transportation-related barriers to maintaining optimal ART adherence. Decentralization of treatment facilities will diminish the transportation time and cost for patients seeking regular and sustained HIV care. In areas where HIV care decentralization is underway, these efforts should be continued. We also urge policy-makers to aggressively pursue service decentralization, and to prioritize investment in the necessary rural health care infrastructure. Finally, interventions that seek to better monitor ART adherence in real time may help to overcome geographic and transportation-related barriers by identifying and allowing for targeting of patients who are at high risk for viral rebound and other poor outcomes. Such interventions are actively being explored [114–117].
Acknowledgments
This work was supported by the Doris Duke Charitable Foundation International Clinical Research Fellowship at Harvard Medical School; the American Medical Association Foundation Seed Grant Research Program; and the U.S. National Institutes of Health R24TW007988, K23MH099916, K24MH087227, and K23MH096620. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix
Search Terms Used in PubMed and Web of Science
-
PubMed search performed 8/14/11.
(“mortality”[MH] or “survival”[TIAB] or “patient dropouts”[MH] or “lost to follow up”[MH] or retention[TIAB] or default[TIAB] or interruption[TIAB] or “linkage”[TIAB] or “medication adherence”[MH] OR “patient compliance”[MH] or “adherence”[TIAB] or “non-adherence”[TIAB] or “compliance”[TIAB] OR “non-compliance”[TIAB]) AND “africa south of the sahara”[MH] AND (“acquired immunodeficiency syndrome”[MH] OR “HIV”[TIAB] OR “AID-S”[TIAB]) AND (distance[TIAB] or “health services accessibility”[MH] or travel[TIAB] or barriers[TIAB] or structural[TIAB] or rural[TIAB] or “rural health services”[MH] or “rural population”[MH] or “hospitals, rural”[MH]).
-
Web of Science search performed 8/14/11.
TS = (“SSA” OR Africa) AND TS = (“loss to follow up” OR mortality OR survival OR default OR interruption OR linkage OR “non-compliance” OR compliance OR adherence OR “non-adherence”) AND TS = (“human immunodeficiency virus” OR HIV OR “acquired immunodeficiency syndrome” OR “acquired immune deficiency syndrome” OR AIDS) AND TS = (distance or “health services accessibility” or travel or barriers or structural or rural or “rural health services” or “rural population” or “hospitals, rural”).
Footnotes
We declare no conflicts of interest.
Contributor Information
Alexander J. Lankowski, Email: alexlankowski@gmail.com, Departments of Internal Medicine and Pediatrics, University of Pennsylvania, Philadelphia, PA, USA. Center for Global Health, Massachusetts General Hospital, 100 Cambridge Street, 15th Floor, Boston, MA 02114, USA
Mark J. Siedner, Center for Global Health, Massachusetts General Hospital, 100 Cambridge Street, 15th Floor, Boston, MA 02114, USA
David R. Bangsberg, Center for Global Health, Massachusetts General Hospital, 100 Cambridge Street, 15th Floor, Boston, MA 02114, USA. Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, Harvard Medical School, Cambridge, MA, USA. Mbarara University of Science and Technology, Mbarara, Uganda
Alexander C. Tsai, Center for Global Health, Massachusetts General Hospital, 100 Cambridge Street, 15th Floor, Boston, MA 02114, USA. Mbarara University of Science and Technology, Mbarara, Uganda. Chester M. Pierce Division of Global Psychiatry, Department of Psychiatry, Massachusetts General Hospital, Boston, MA, USA
References
- 1.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cain LE, Logan R, Robins JM, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDS-defining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011;154(8):509–15. doi: 10.1059/0003-4819-154-8-201104190-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363(3):257–65. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maman D, Pujades-Rodriguez M, Nicholas S, et al. Response to antiretroviral therapy: improved survival associated with CD4 above 500 cells/ml. AIDS. 2012;26(11):1393–8. doi: 10.1097/QAD.0b013e328352d054. [DOI] [PubMed] [Google Scholar]
- 5.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 6.Hammer SM. Antiretroviral treatment as prevention. N Engl J Med. 2011;365(6):561–2. doi: 10.1056/NEJMe1107487. [DOI] [PubMed] [Google Scholar]
- 7.Geng EH, Hare CB, Kahn JO, et al. The effect of a “universal antiretroviral therapy” recommendation on HIV RNA levels among HIV-infected patients entering care with a CD4 count greater than 500/mL in a public health setting. Clin Infect Dis. 2012;55(12):1690–7. doi: 10.1093/cid/cis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization, UNAIDS, UNICEF. Universal Access. 2011. Global HIV/ AIDS response: epidemic update and health sector progress towards. [Google Scholar]
- 9.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 10.Brinkhof MW, Spycher BD, Yiannoutsos C, et al. Adjusting mortality for loss to follow-up: analysis of five ART programmes in sub-Saharan Africa. PLoS ONE. 2010;5(11):e14149. doi: 10.1371/journal.pone.0014149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta A, Nadkarni G, Yang WT, et al. Early mortality in adults initiating antiretroviral therapy (ART) in low- and middle-income countries (LMIC): a systematic review and meta-analysis. PLoS ONE. 2011;6(12):e28691. doi: 10.1371/journal.pone.0028691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marazzi MC, Liotta G, Germano P, et al. Excessive early mortality in the first year of treatment in HIV type 1-infected patients initiating antiretroviral therapy in resource-limited settings. AIDS Res Hum Retrovir. 2008;24(4):555–60. doi: 10.1089/aid.2007.0217. [DOI] [PubMed] [Google Scholar]
- 14.Kigozi IM, Dobkin LM, Martin JN, et al. Late-disease stage at presentation to an HIV clinic in the era of free antiretroviral therapy in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009;52(2):280–9. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz IT, Essien T, Marinda ET, et al. Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS. 2011;25(17):2177–81. doi: 10.1097/QAD.0b013e32834b6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Losina E, Bassett IV, Giddy J, et al. The “ART” of linkage: pre-treatment loss to care after HIV diagnosis at two PEPFAR sites in Durban, South Africa. PLoS ONE. 2010;5(3):e9538. doi: 10.1371/journal.pone.0009538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassett IV, Regan S, Chetty S, et al. Who starts antiretroviral therapy in Durban, South Africa?… not everyone who should. AIDS. 2010;24(Suppl 1):S37–44. doi: 10.1097/01.aids.0000366081.91192.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4(10):e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geng EH, Nash D, Kambugu A, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7(4):234–44. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oyugi JH, Byakika-Tusiime J, Ragland K, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21(8):965–71. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- 21.Eisenberg JM, Power EJ. Transforming insurance coverage into quality health care: voltage drops from potential to delivered quality. JAMA. 2000;284(16):2100–7. doi: 10.1001/jama.284.16.2100. [DOI] [PubMed] [Google Scholar]
- 22.Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav. 2010;14(4):778–84. doi: 10.1007/s10461-009-9533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biadgilign S, Deribew A, Amberbir A, Deribe K. Barriers and facilitators to antiretroviral medication adherence among HIV-infected paediatric patients in Ethiopia: a qualitative study. SAHARA J. 2009;6(4):148–54. doi: 10.1080/17290376.2009.9724943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardon AP, Akurut D, Comoro C, et al. Hunger, waiting time and transport costs: time to confront challenges to ART adherence in Africa. AIDS Care. 2007;19(5):658–65. doi: 10.1080/09540120701244943. [DOI] [PubMed] [Google Scholar]
- 25.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govindasamy D, Ford N, Kranzer K. Risk factors, barriers and facilitators for linkage to antiretroviral therapy care: a systematic review. AIDS. 2012;26(16):2059–67. doi: 10.1097/QAD.0b013e3283578b9b. [DOI] [PubMed] [Google Scholar]
- 27.Hutchinson PL, Mahlalela X. Utilization of voluntary counseling and testing services in the Eastern Cape, South Africa. AIDS Care. 2006;18(5):446–55. doi: 10.1080/09540120500213511. [DOI] [PubMed] [Google Scholar]
- 28.Kaawa-Mafigiri D, Mcgrath J, Namutiibwa F, et al. Delays in seeking HIV/AIDS testing and treatment among patients on ARVs in urban (Kampala) and rural southwestern Uganda. XIX International AIDS Conference; Washington, DC. 2012. [Google Scholar]
- 29.Morin SF, Khumalo-Sakutukwa G, Charlebois ED, et al. Removing barriers to knowing HIV status: same-day mobile HIV testing in Zimbabwe. J Acquir Immune Defic Syndr. 2006;41(2):218–24. doi: 10.1097/01.qai.0000179455.01068.ab. [DOI] [PubMed] [Google Scholar]
- 30.Minstry of Health Uganda, ORC Macro. Uganda HIV/AIDS sero-behavioural survey 2004–2005. Calverton: Ministry of Health and ORC Macro; 2006. [Google Scholar]
- 31.Amuron B, Namara G, Birungi J, et al. Mortality and loss-to-follow-up during the pre-treatment period in an antiretroviral therapy programme under normal health service conditions in Uganda. BMC Public Health. 2009;9:290. doi: 10.1186/1471-2458-9-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parkes-Ratanshi R, Bufumbo L, Nyanzi-Wakholi B, et al. Barriers to starting ART and how they can be overcome: individual and operational factors associated with early and late start of treatment. Trop Med Int Health. 2010;15(11):1347–56. doi: 10.1111/j.1365-3156.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 33.Braitstein P, Songok J, Vreeman RC, et al. “Wamepotea” (they have become lost): outcomes of HIV-positive and HIV-exposed children lost to follow-up from a large HIV treatment program in western Kenya. J Acquir Immune Defic Syndr. 2011;57(3):e40–6. doi: 10.1097/QAI.0b013e3182167f0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs DW, Chi BH, Mulenga Y, et al. Community-based follow-up for late patients enrolled in a district-wide programme for antiretroviral therapy in Lusaka, Zambia. AIDS Care. 2008;20(3):311–7. doi: 10.1080/09540120701594776. [DOI] [PubMed] [Google Scholar]
- 35.McGuire M, Munyenyembe T, Szumilin E, et al. Vital status of pre-ART and ART patients defaulting from care in rural Malawi. Trop Med Int Health. 2010;15(Suppl 1):55–62. doi: 10.1111/j.1365-3156.2010.02504.x. [DOI] [PubMed] [Google Scholar]
- 36.Ochieng-Ooko V, Ochieng D, Sidle JE, et al. Influence of gender on loss to follow-up in a large HIV treatment programme in western Kenya. Bull World Health Organ. 2010;88(9):681–8. doi: 10.2471/BLT.09.064329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adeyemo A, Tamen F, Ayebe T, Oyigebe G. Socio-gender factors as explanation for poor drug adherence among antiretroviral users. 4th International Conference on HIV Treatment Adherence; Miami. 2009. [Google Scholar]
- 38.Asiimwe B, Tarinyeba E, Kyomuhendo J. Adherence results for 84 patients on anti-retroviral therapy for 1 year at Bushenyi Medical Centre, Katungu in rural south western Uganda. 2nd International Conference on HIV Treatment Adherence; Miami. 2007. [Google Scholar]
- 39.Bajunirwe F, Tisch DJ, King CH, Arts EJ, Debanne SM, Sethi AK. Quality of life and social support among patients receiving anti-retroviral therapy in Western Uganda. AIDS Care. 2009;21(3):271–9. doi: 10.1080/09540120802241863. [DOI] [PubMed] [Google Scholar]
- 40.Eholie SP, Tanon KA, Folquet-Amorissani M, et al. Impact of access to antiretroviral therapy in Cote d’Ivoire. Med Trop (Mars) 2009;69(5):520–4. [PubMed] [Google Scholar]
- 41.Kip E, Ehlers VJ, van der Wal DM. Patients’ adherence to anti-retroviral therapy in Botswana. J Nurs Scholarsh. 2009;41(2):149–57. doi: 10.1111/j.1547-5069.2009.01266.x. [DOI] [PubMed] [Google Scholar]
- 42.Kirsten I, Sewangi J, Kunz A, et al. Adherence to combination prophylaxis for prevention of mother-to-child-transmission of HIV in Tanzania. PLoS ONE. 2011;6(6):e21020. doi: 10.1371/journal.pone.0021020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munseri PJ, Talbot EA, Mtei L, von Reyn CF. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis. 2008;12(9):1037–41. [PubMed] [Google Scholar]
- 44.Olowookere SA, Fatiregun AA, Akinyemi JO, Bamgboye AE, Osagbemi GK. Prevalence and determinants of nonadherence to highly active antiretroviral therapy among people living with HIV/AIDS in Ibadan, Nigeria. J Infect Dev Ctries. 2008;2(5):369–72. doi: 10.3855/jidc.199. [DOI] [PubMed] [Google Scholar]
- 45.Potchoo Y, Tchamdja K, Balogou A, Pitche VP, Guissou IP, Kassang EK. Knowledge and adherence to antiretroviral therapy among adult people living with HIV/AIDS treated in the health care centers of the association “Espoir Vie Togo” in Togo, West Africa. BMC Clin Pharmacol. 2010;10:11. doi: 10.1186/1472-6904-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Dijk JH, Sutcliffe CG, Munsanje B, Hamangaba F, Thuma PE, Moss WJ. Barriers to the care of HIV-infected children in rural Zambia: a cross-sectional analysis. BMC Infect Dis. 2009;9:169. doi: 10.1186/1471-2334-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiser S, Wolfe W, Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003;34(3):281–8. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 48.Yahaya H, Gwarzo U, Liman MS. Forecasting long-term adherence to HAART in GHAIN-supported facility using client’s socioeconomic status. 1st International Conference on HIV Treatment Adherence; Miami. 2006. [Google Scholar]
- 49.Assefa Y, Van Damme W, Mariam DH, Kloos H. Toward universal access to HIV counseling and testing and antiretroviral treatment in Ethiopia: looking beyond HIV testing and ART initiation. AIDS Patient Care STDS. 2010;24(8):521–5. doi: 10.1089/apc.2009.0286. [DOI] [PubMed] [Google Scholar]
- 50.Bowie C, Gondwe N. Changing clinical needs of people living with AIDS and receiving home based care in Malawi: the Bangwe Home Based Care Project 2003–2008—a descriptive study. BMC Public Health. 2010;10:370–1. doi: 10.1186/1471-2458-10-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan AK, van Lettow M, Tenthani L, et al. Outcome assessment of a dedicated HIV positive health care worker clinic at a central hospital in Malawi: a retrospective observational study. PLoS One. 2011;6(5):e19789. doi: 10.1371/journal.pone.0019789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lubega M, Nsabagasani X, Tumwesigye NM, et al. Policy and practice, lost in transition: reasons for high drop-out from pre-antiretroviral care in a resource-poor setting of eastern Uganda. Health Policy. 2010;95(2–3):153–8. doi: 10.1016/j.healthpol.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Bwirire LD, Fitzgerald M, Zachariah R, et al. Reasons for loss to follow-up among mothers registered in a prevention-of-mother-to-child transmission program in rural Malawi. Trans R Soc Trop Med Hyg. 2008;102(12):1195–200. doi: 10.1016/j.trstmh.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Abrahams N, Jewkes R. Barriers to post exposure prophylaxis (PEP) completion after rape: a South African qualitative study. Cult Health Sex. 2010;12(5):471–84. doi: 10.1080/13691050903556316. [DOI] [PubMed] [Google Scholar]
- 55.Byakika-Tusiime J, Crane J, Oyugi JH, et al. Longitudinal antiretroviral adherence in HIV+Ugandan parents and their children initiating HAART in the MTCT-Plus family treatment model: role of depression in declining adherence over time. AIDS Behav. 2009;13(Suppl 1):82–91. doi: 10.1007/s10461-009-9546-x. [DOI] [PubMed] [Google Scholar]
- 56.Nam SL, Fielding K, Avalos A, Dickinson D, Gaolathe T, Geissler PW. The relationship of acceptance or denial of HIV-status to antiretroviral adherence among adult HIV patients in urban Botswana. Soc Sci Med. 2008;67(2):301–10. doi: 10.1016/j.socscimed.2008.03.042. [DOI] [PubMed] [Google Scholar]
- 57.Nassali M, Nakanjako D, Kyabayinze D, Beyeza J, Okoth A, Mutyaba T. Access to HIV/AIDS care for mothers and children in sub-Saharan Africa: adherence to the postnatal PMTCT program. AIDS Care. 2009;21(9):1124–31. doi: 10.1080/09540120802707467. [DOI] [PubMed] [Google Scholar]
- 58.Peterson K, Laly R. Patterns in the use of adherence supports. 1st International Conference on HIV Treatment Adherence; Miami. 2006. [Google Scholar]
- 59.Pyne-Mercier LD, John-Stewart GC, Richardson BA, et al. The consequences of post-election violence on antiretroviral HIV therapy in Kenya. Aids Care. 2011;23(5):562–8. doi: 10.1080/09540121.2010.525615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowe KA, Makhubele B, Hargreaves JR, Porter JD, Hausler HP, Pronyk PM. Adherence to TB preventive therapy for HIV-positive patients in rural South Africa: implications for antiretroviral delivery in resource-poor settings? Int J Tuberc Lung Dis. 2005;9(3):263–9. [PubMed] [Google Scholar]
- 61.Thobias A, Van Wyk B. Issues in adherence to antiretroviral treatment in an urban setting in Namibia. 4th International Conference on HIV Treatment Adherence; Miami. 2009. [Google Scholar]
- 62.Ware NC, Idoko J, Kaaya S, et al. Explaining adherence success in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009;6(1):e11. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiser SD, Tuller DM, Frongillo EA, Senkungu J, Mukiibi N, Bangsberg DR. Food insecurity as a barrier to sustained anti-retroviral therapy adherence in Uganda. PLoS One. 2010;5(4):e10340. doi: 10.1371/journal.pone.0010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wringe A, Isingo R, Urassa M, et al. Uptake of HIV voluntary counselling and testing services in rural Tanzania: implications for effective HIV prevention and equitable access to treatment. Trop Med Int Health. 2008;13(3):319–27. doi: 10.1111/j.1365-3156.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- 65.Cook RE, Ciampa PJ, Sidat M, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambezia, Mozambique. J Acquir Immune Defic Syndr. 2011;56(4):e104–9. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ingle SM, May M, Uebel K, et al. Outcomes in patients waiting for antiretroviral treatment in the Free State Province, South Africa: prospective linkage study. AIDS. 2010;24(17):2717–25. doi: 10.1097/QAD.0b013e32833fb71f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lessells RJ, Mutevedzi PC, Cooke GS, Newell ML. Retention in HIV care for individuals not yet eligible for antiretroviral therapy: rural KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2011;56(3):e79–86. doi: 10.1097/QAI.0b013e3182075ae2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sutcliffe CG, van Dijk JH, Bolton-Moore C, Cotham M, Tambatamba B, Moss WJ. Differences in presentation, treatment initiation, and response among children infected with human immunodeficiency virus in urban and rural Zambia. Pediatr Infect Dis J. 2010;29(9):849–54. doi: 10.1097/INF.0b013e3181e753a8. [DOI] [PubMed] [Google Scholar]
- 69.Elbireer S, Guwatudde D, Mudiope P, Nabbuye-Sekandi J, Manabe YC. Tuberculosis treatment default among HIV-TB co-infected patients in urban Uganda. Trop Med Int Health. 2011;16(8):981–7. doi: 10.1111/j.1365-3156.2011.02800.x. [DOI] [PubMed] [Google Scholar]
- 70.Geng EH, Glidden DV, Bwana MB, et al. Retention in care and connection to care among HIV-infected patients on antiretroviral therapy in Africa: estimation via a sampling-based approach. PLoS One. 2011;6(7):e21797. doi: 10.1371/journal.pone.0021797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karcher H, Omondi A, Odera J, Kunz A, Harms G. Risk factors for treatment denial and loss to follow-up in an antiretroviral treatment cohort in Kenya. Trop Med Int Health. 2007;12(5):687–94. doi: 10.1111/j.1365-3156.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 72.Kempf MC, Allen S, Zulu I, et al. Enrollment and retention of HIV discordant couples in Lusaka, Zambia. J Acquir Immune Defic Syndr. 2008;47(1):116–25. doi: 10.1097/QAI.0b013e31815d2f3f. [DOI] [PubMed] [Google Scholar]
- 73.Massaquoi M, Zachariah R, Manzi M, et al. Patient retention and attrition on antiretroviral treatment at district level in rural Malawi. Trans R Soc Trop Med Hyg. 2009;103(6):594–600. doi: 10.1016/j.trstmh.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 74.Mossdorf E, Stoeckle M, Mwaigomole EG, et al. Improved antiretroviral treatment outcome in a rural African setting is associated with cART initiation at higher CD4 cell counts and better general health condition. BMC Infect Dis. 2011;11:98. doi: 10.1186/1471-2334-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mulissa Z, Jerene D, Lindtjorn B. Patients present earlier and survival has improved, but pre-ART attrition is high in a six-year HIV cohort data from Ethiopia. PLoS ONE. 2010;5(10):e13268. doi: 10.1371/journal.pone.0013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adeyemi A, Olubunmi F, Oluseyi A. Predictors of adherence for patients on highly active antiretroviral therapy in HIV treatment program. 12th Annual International Meeting of the Institute of Human Virology; Tropea. 2010. [Google Scholar]
- 77.Bagenda A, Barlow-Mosha L, Bagenda D, Sakwa R, Fowler MG, Musoke PM. Adherence to tablet and liquid formulations of antiretroviral medication for paediatric HIV treatment at an urban clinic in Uganda. Ann Trop Paediatr. 2011;31(3):235–45. doi: 10.1179/1465328111Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 78.Barth RE, Tempelman HA, Smelt E, Wensing AM, Hoepelman AI, Geelen SP. Long-term outcome of children receiving anti-retroviral treatment in rural South Africa: substantial virologic failure on first-line treatment. Pediatr Infect Dis J. 2011;30(1):52–6. doi: 10.1097/INF.0b013e3181ed2af3. [DOI] [PubMed] [Google Scholar]
- 79.Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, Katabira ET, Mugyenyi PN, Bangsberg DR. Adherence to HIV antiretroviral therapy in HIV plus Ugandan patients purchasing therapy. Int J STD AIDS. 2005;16(1):38–41. doi: 10.1258/0956462052932548. [DOI] [PubMed] [Google Scholar]
- 80.Carlucci JG, Kamanga A, Sheneberger R, et al. Predictors of adherence to antiretroviral therapy in rural Zambia. J Acquir Immune Defic Syndr. 2008;47(5):615–22. doi: 10.1097/QAI.0b013e318165dc25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Charurat M, Oyegunle M, Benjamin R, et al. Patient retention and adherence to antiretrovirals in a large antiretroviral therapy program in Nigeria: a longitudinal analysis for risk factors. PLoS ONE. 2010;5(5):e10584. doi: 10.1371/journal.pone.0010584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haberer JE, Kiwanuka J, Nansera D, Ragland K, Mellins C, Bangsberg DR. Multiple measures reveal antiretroviral adherence successes and challenges in HIV-infected Ugandan children. PLoS ONE. 2012;7(5):e36737. doi: 10.1371/journal.pone.0036737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hirschhorn L, Singal R, Navario P, Mokhele I, Patel A, Maartens G. Adherence to ART in two innovative treatment models in South Africa. 4th International Conference on HIV Treatment Adherence; Miami. 2009. [Google Scholar]
- 84.Iroha E, Esezobor CI, Ezeaka C, Temiye EO, Akinsulie A. Adherence to antiretroviral therapy among HIV-infected children attending a donor-funded clinic at a tertiary hospital in Nigeria. AJAR-Afr J Aids Res. 2010;9(1):25–30. doi: 10.2989/16085906.2010.484543. [DOI] [PubMed] [Google Scholar]
- 85.Kurlander C, Eng ML, Charurat M, Farley JJ, Blattner WA. Evaluation of pharmacy refill rates and self-reported adherence for antiretroviral therapy at a PEPFAR clinic in northern Nigeria. 2nd International Conference on HIV Treatment Adherence; Miami. 2007. [Google Scholar]
- 86.Maqutu D, Zewotir T, North D, Naidoo K, Grobler A. Factors affecting first-month adherence to antiretroviral therapy among HIV-positive adults in South Africa. AJAR-Afr J Aids Res. 2010;9(2):117–24. doi: 10.2989/16085906.2010.517478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mbaezue N, Farley J, Charurat M, Blattner WA. A case control study of nonadherence among Nigerian patients initiating anti-retroviral (ARV) therapy. 2nd International Conference on HIV Treatment Adherence; Miami. 2007. [Google Scholar]
- 88.Ramadhani HO, Thielman NM, Landman KZ, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45(11):1492–8. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 89.Sethi A, Bajunirwe F, Mafigiri D, et al. Distance as a barrier to adherence to antiretroviral therapy among HIV-infected patients in rural southwestern Uganda. 4th International Conference on HIV Treatment Adherence; Miami. 2009. [Google Scholar]
- 90.Taiwo BO, Idoko JA, Welty LJ, et al. Assessing the viorologic and adherence benefits of patient-selected HIV treatment partners in a resource-limited setting. J Acquir Immune Defic Syndr. 2010;54(1):85–92. doi: 10.1097/01.qai.0000371678.25873.1c. [DOI] [PubMed] [Google Scholar]
- 91.van Dijk JH, Sutcliffe CG, Munsanje B, et al. HIV-infected children in rural Zambia achieve good immunologic and virologic outcomes two years after initiating antiretroviral therapy. PLoS One. 2011;6(4):e19006. doi: 10.1371/journal.pone.0019006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bateman J, Garrod GD, Brainard JS, Lovett AS. Measurement issues in the travel cost method: a geographical information systems approach. J Agric Econ. 1996;47(2):191–205. [Google Scholar]
- 93.Siedner MJ, Lankowski A, Tsai AC, et al. GPS-measured distance to clinic, but not self-reported transportation factors, are associated with missed HIV clinic visits in rural Uganda. AIDS. 2013;27(9):1503–8. doi: 10.1097/QAD.0b013e32835fd873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsai AC, Bangsberg DR, Weiser SD. Harnessing poverty alleviation to reduce the stigma of HIV in Sub-Saharan Africa. PLoS Med. 2013;10(11):e1001557. doi: 10.1371/journal.pmed.1001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Turan JM, Bukusi EA, Onono M, Holzemer WL, Miller S, Cohen CR. HIV/AIDS stigma and refusal of HIV testing among pregnant women in rural Kenya: results from the MAMAS Study. AIDS Behav. 2011;15(6):1111–20. doi: 10.1007/s10461-010-9798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsai AC, Bangsberg DR, Kegeles SM, et al. Internalized stigma, social distance, and disclosure of HIV seropositivity in rural Uganda. Ann Behav Med. 2013;46(3):285–94. doi: 10.1007/s12160-013-9514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Naar-King S, Bradford J, Coleman S, Green-Jones M, Cabral H, Tobias C. Retention in care of persons newly diagnosed with HIV: outcomes of the Outreach Initiative. AIDS Patient Care STDS. 2007;21(Suppl 1):S40–8. doi: 10.1089/apc.2007.9988. [DOI] [PubMed] [Google Scholar]
- 98.Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. doi: 10.7448/IAS.16.3.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mekonnen Y, Sanders R, Tibebu S, Emmart P. Equity and access to ART in Ethiopia. Washington, DC: Futures Group, Health Policy Initiative; 2010. [Google Scholar]
- 100.Mahler HR, Kileo B, Curran K, et al. Voluntary medical male circumcision: matching demand and supply with quality and efficiency in a high-volume campaign in Iringa Region, Tanzania. PLoS Med. 2011;8(11):e1001131. doi: 10.1371/journal.pmed.1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gilbert L, Walker L. ‘My biggest fear was that people would reject me once they knew my status…’: stigma as experienced by patients in an HIV/AIDS clinic in Johannesburg, South Africa. Health Soc Care Community. 2010;18(2):139–46. doi: 10.1111/j.1365-2524.2009.00881.x. [DOI] [PubMed] [Google Scholar]
- 102.Wilson DP, Blower S. How far will we need to go to reach HIV-infected people in rural South Africa? BMC Med. 2007;5:16. doi: 10.1186/1741-7015-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boyer S, Eboko F, Camara M, et al. Scaling up access to antiretro-viral treatment for HIV infection: the impact of decentralization of healthcare delivery in Cameroon. AIDS. 2010;24(Suppl 1):S5–15. doi: 10.1097/01.aids.0000366078.45451.46. [DOI] [PubMed] [Google Scholar]
- 104.Bemelmans M, van den Akker T, Ford N, et al. Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health. 2010;15(12):1413–20. doi: 10.1111/j.1365-3156.2010.02649.x. [DOI] [PubMed] [Google Scholar]
- 105.Pfeiffer J, Montoya P, Baptista AJ, et al. Integration of HIV/ AIDS services into African primary health care: lessons learned for health system strengthening in Mozambique: a case study. J Int AIDS Soc. 2010;13:3. doi: 10.1186/1758-2652-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsai AC, Chopra M, Pronyk PM, Martinson NA. Socioeconomic disparities in access to HIV/AIDS treatment programs in resource-limited settings. AIDS Care. 2009;21(1):59–63. doi: 10.1080/09540120802068811. [DOI] [PubMed] [Google Scholar]
- 107.Siedner MJ, Lankowski A, Haberer JE, et al. Rethinking the “pre” in pre-therapy counseling: no benefit of additional visits prior to therapy on adherence or viremia in Ugandans initiating ARVs. PLoS ONE. 2012;7(6):e39894. doi: 10.1371/journal.pone.0039894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wilson DP, Blower SM. Designing equitable antiretroviral allocation strategies in resource-constrained countries. PLoS Med. 2005;2(2):e50. doi: 10.1371/journal.pmed.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jani IV, Sitoe NE, Alfai ER, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378(9802):1572–9. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
- 110.Wanyenze RK, Hahn JA, Liechty CA, et al. Linkage to HIV care and survival following inpatient HIV counseling and testing. AIDS Behav. 2011;15(4):751–60. doi: 10.1007/s10461-010-9704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Siedner MJ, Haberer JE, Bwana MB, Ware NC, Bangsberg DR. High acceptability for cell phone text messages to improve communication of laboratory results with HIV-infected patients in rural Uganda: a cross-sectional survey study. BMC Med Inform Decis Mak. 2012;12:56. doi: 10.1186/1472-6947-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Emenyonu N, Thirumurthy H, Muyindike W, et al. Cash transfers to cover clinic transportation costs improve adherence and retention in care in a HIV treatment program in Rural Uganda. 17th Conference on Retroviruses and Opportunistic Infections; San Francisco. 2010. [Google Scholar]
- 113.Mukherjee JS, Ivers L, Leandre F, Farmer P, Behforouz H. Antiretroviral therapy in resource-poor settings. Decreasing barriers to access and promoting adherence. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S123–6. doi: 10.1097/01.qai.0000248348.25630.74. [DOI] [PubMed] [Google Scholar]
- 114.Haberer JE, Kiwanuka J, Nansera D, et al. Real-time adherence monitoring of antiretroviral therapy among HIV-infected adults and children in rural Uganda. AIDS. 2013;27(13):2166–8. doi: 10.1097/QAD.0b013e328363b53f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bachman Desilva M, Gifford AL, Keyi X, et al. Feasibility and acceptability of a real-time adherence device among HIV-positive IDU patients in China. AIDS Res Treat. 2013;2013:957862. doi: 10.1155/2013/957862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haberer JE, Robbins GK, Ybarra M, et al. Real-time electronic adherence monitoring is feasible, comparable to unannounced pill counts, and acceptable. AIDS Behav. 2012;16(2):375–82. doi: 10.1007/s10461-011-9933-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Haberer JE, Kahane J, Kigozi I, et al. Real-time adherence monitoring for HIV antiretroviral therapy. AIDS Behav. 2010;14(6):1340–6. doi: 10.1007/s10461-010-9799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]