Abstract
Objectives
Bipolar disorder (BD) is a psychiatric disorder with high morbidity and mortality that cannot be distinguished from major depressive disorder (MDD) until the first manic episode. A biomarker able to differentiate BD and MDD could help clinicians avoid risks of treating BD with antidepressants without mood stabilizers.
Methods
Cortical thickness differences were assessed using magnetic resonance imaging in BD depressed patients (n = 18), MDD depressed patients (n = 56), and healthy volunteers (HVs) (n = 54). A general linear model identified clusters of cortical thickness difference between diagnostic groups.
Results
Compared to the HV group, the BD group had decreased cortical thickness in six regions, after controlling for age and sex, located within frontal and parietal lobes, and posterior cingulate cortex. Mean cortical thickness changes in clusters ranged from 7.6–9.6% (cluster wise p-values from 1.0 e−4 to 0.037). When compared to MDD, three clusters of lower cortical thickness in BD were identified that overlapped with clusters that differentiated the BD and HV groups. Mean cortical thickness changes in the clusters ranged from 7.5–8.2% (cluster wise p-values from 1.0 e−4 to 0.023). The difference in cortical thickness was more pronounced when the subgroup of subjects with bipolar I disorder (BD-I) was compared to the MDD group.
Conclusions
Cortical thickness patterns were distinct between BD and MDD. These results are a step toward developing an imaging test to differentiate the two disorders.
Keywords: bipolar disorder, cortical thickness, magnetic resonance imaging, major depressive disorder, neuroimaging
Bipolar disorder (BD) affects 1–2% of the population worldwide; it is a leading cause of disability and carries an elevated risk for suicide (1–3). Although mania or hypomania are the defining features of the disorder, depression is the most common presenting state and accounts for the most lifetime disability in the disorder. BD depressive episodes cannot reliably be distinguished from those of major depressive disorder (MDD) besides by patient history, and if the patient has not had a manic or hypomanic episode, the two disorders remain indistinguishable. Differentiating between MDD and BD depression is important clinically because treatment with antidepressants in the absence of a mood stabilizer carries the risk of precipitating mania and may increase rates of cycling between mood states (4). Developing a reliable test to distinguish the two conditions is therefore an important research goal (5). Disorder specific neuroimaging findings may be useful as a biomarker for BD.
Changes in cortical thickness can reflect differences in neuroplasticity, degeneration or neurotoxicity. Several studies have found less cortical thickness in BD compared to healthy volunteers in a number of regions, including several subregions of the frontal cortex and the anterior cingulate cortex (6–10). However, one study reported no cortical thickness differences in BD (11).
One potential confound in most studies published to date has been inclusion of patients taking medications. Although the effects of medication on cortical thickness have not been fully elucidated, lithium is associated with increased grey matter density (12). Antidepressant medications have also been shown to have neurotrophic effects in cell culture and within the hippocampus of mouse models (13). To limit this potential complication, we included only depressed patients who were off of all psychiatric medications at the time of scanning.
To our knowledge, no previous studies have directly compared cortical thickness differences between MD and BD subtypes of depression, the primary aim of this study. In fact, only a handful of neuroimaging studies have ever been published comparing bipolar and unipolar depression (14). As this is the first study to evaluate this question, whole cortex cluster-wise analyses were performed to examine the entire cortical mantle in an exploratory manner. As more previous studies have shown cortical thickness changes in subjects with BD, we hypothesized that the BD group would demonstrate less cortical thickness relative to the MDD group in a region specific manner.
Methods and materials
Participants
Eighteen patients with DSM-IV BD and 56 patients with MDD were diagnosed based on the Structured Clinical Interview for Axis I disorders (SCID-I/P) (15), psychiatric interview, and chart review, as well as a consensus conference employing all sources of information. Patients with BD and MDD met criteria for a major depressive episode at the time of recruitment, with a 17-item Hamilton Depression Rating Scale (HAM-D) score > 16 (16). Fifty-four healthy volunteers (HVs) were also enrolled after a SCID-NP demonstrated no Axis I pathology, and interview revealed no history of mood disorders, psychotic disorders, or suicide in first-degree relatives. Diagnostic evaluations were performed by masters or doctoral level psychologists and were confirmed by a research psychiatrist. Participant ages ranged between 18–65 years. All patients taking psychotropic medications were tapered off medications before magnetic resonance imaging (MRI) scanning, and none were receiving fluoxetine or depot antipsychotics. Benzodiazepines were permitted in small doses. For all participants, exclusion criteria was (i) active medical conditions, (ii) positive urine toxicology screen, (iii) positive pregnancy test or planned pregnancy, and (iv) lifetime exposure to 3,4-methylenedioxymethamphetamine (MDMA). Subjects with past MDMA use were excluded because these scans had been obtained as part of another experiment that focused on the serotonin signaling system. Depressed patients were excluded if they had a history of alcohol or substance use disorder in the previous six months. HVs were excluded if they had any history of alcohol or substance use disorder. Manic symptoms were assessed with the Young Mania Rating Scale (YMRS) (17). The Institutional Review Board of the New York State Psychiatric Institute approved the study, and the procedures were in accordance with the Helsinki Declaration of 1975. All participants gave written informed consent. Participants were recruited for the study between March 2006 and August 2012. This is a secondary analysis of MRI data that were acquired for ongoing positron emission tomography studies.
MRI acquisition
High-resolution anatomical T1-weighted MRI images were acquired from a 3-T GE Signa HDx scanner using a three-dimensional spoiled gradient echo sequence: 1 mm sagittal slices with repetition time (TR): 5.0–7.5 msec, echo time (TE): 1.3–3.0 msec, flip angle 9–11°, acquisition matrix of 256 ×256, number of axial slices: 180, voxel size 1 mm3. All scans were inspected to check for motion artifacts and to rule out gross neuropathology.
Magnetic resonance scan processing and calculations of cortical thickness
Freesurfer package 5.1.0, a freeware set of automated sequences (http://surfer.nmr.mgh.harvard.edu) was used for cortical surface reconstruction and thickness measurement (18, 19). A more detailed explanation of the procedure can be found in (19–22), and at the Freesurfer website listed above. Briefly, the T1 anatomical volume was registered to the Talairach atlas (23) and corrected for intensity variations due to magnetic field homogeneities. The normalized intensity image was then skull-stripped (22) using a deformable template model. The voxels in the brain were classified as white matter or non-white matter based on intensity and neighbor constraints. Cutting planes were computed, which separate the cerebral hemispheres and remove the cerebellum and brain stem. Any interior holes in the components representing white matter were filled. The initial triangular tessellation was formed on the surface of this white matter mass to create a surface mesh representation, and then smoothed using a deformable surface algorithm to form the gray/white surface. The algorithm was further used to expand the surface to obtain the gray matter-CSF surface (18, 20). Since expanding the inner white matter surface generates the outer cortical surface, there is a one-to-one correspondence between mesh nodes. Finally, the images were registered to a spherical atlas and thickness at any given node was calculated by finding the closest point on the opposite surface, performing the same calculation for the corresponding node on the opposite surface, and averaging the two values (19). The cortical thickness surface map was prepared for statistical analysis by performing a smoothing using a Gaussian Kernel with full-width half maximum of 10 mm.
Statistical analysis
Freesurfer’s Qdec (http://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/QdecGroupAnalysis) application was used to obtain cortical thickness difference maps between groups. Qdec uses a general linear model (GLM) to model thickness at each surface vertex as a linear combination of effects related to variables of interests, incorporating the effects of potential confounds. The main effect between each pair of diagnostic groups was evaluated with age and sex as covariates of no interest. Both age and sex have been associated with cortical thickness changes (24–26). Because the rate of past substance use disorder history was different between patients with MDD and BD, and patients with BD had a trend longer course of illness, subsequent analyses included these variables as covariates of no interest. To further characterize cortical thickness in the patients with MDD, a subgroup of patients with a positive family history of mood disorders and a subgroup of patients that were medication naïve were compared to the HV group. Statistical thickness difference maps were constructed using −log10(p value). Monte Carlo permutation cluster analysis with 10,000 iterations was performed to correct for multiple comparisons. Cluster-wise probabilities (CWP) were reported. Clusters were mapped on a Desikan–Killiany atlas based on gyral and sulcal structure (27, 28).
Regions-of-interest were defined based on the clusters that were significantly different between groups. These regions of interest were mapped on each brain in the study in order to obtain a quantitative measure of the average cortical thickness in that region. The region-of-interest analysis was used to evaluate potential confounds in the data and to measure the fold change in cortical thickness between groups.
Statistical differences between categorical groups were compared using a two-tailed Student’s t-test or one-way ANOVA with Bonferroni post-hoc tests. Linear regression analyses were used to evaluate correlations between two sets of data. We used SPSS, 2012, version 20.
Results
Participants
Demographic and clinical characteristics are summarized in Table 1. The three groups were matched for sex and handedness, but differed in age. Post-hoc testing showed no pair-wise differences between the groups in age, however. The BD group had higher HAM-D scores and higher rates of lifetime substance use disorders than patients with MDD, including higher rates of remitted alcohol dependence and alcohol abuse. Twelve of the 18 patients with BD were diagnosed with BD-I, and the rest (n = 6) were diagnosed with bipolar II disorder (BD-II). One patient with BD was in a mixed episode as determined by a YMRS > 25 and clinical determination at the time of screening. All patients were not taking medications for more than 14 days, other than two subjects with BD who had finished their medication taper less than seven days before scanning. Rates of lifetime exposure to psychiatric medications are included in Table 1.
Table 1.
Demographic and clinical information for participants in the study
| BD (n = 18) | MDD (n = 56) | HV (n = 54) | p-value | |
|---|---|---|---|---|
|
General demographics | ||||
| ANOVA; p = 0.033c | ||||
| BD versus HV; p = 0.174 | ||||
| BD versus MDD; p = 1.00 | ||||
| Age, years, mean (SD) | 37.6 (9.4) | 36.9 (12.2) | 31.8 (10.5) | MDD versus HV; p = 0.055 |
| Sex, female, n (%) | 9 (50.0) | 32 (57.1) | 26 (48.1) | 0.65d |
| Handedness, left, n (%) | 0 (0) | 6 (10.7) | 4 (7.4) | 0.44d |
|
Disorder characteristics | ||||
| Weeks of current episode, median (SD) | 14.5 (194.0) | 67.0 (350.8) | N/A | 0.15e |
| Years of illness, mean (SD) | 20.1 (10.9) | 13.6 (12.9) | N/A | 0.06e |
| Age of onset, years, mean (SD) | 17.5 (5.6) | 23.4 (12.3) | N/A | 0.06e |
| HAM-D score, mean (SD) | 29.4 (5.9) | 23.8 (6.0) | N/A | 9.0 e−4e |
| Family history, n (%)a | 10 (55.6) | 24 (42.9) | N/A | 0.42d |
|
Lifetime comorbid psychiatric disorders, n (%) | ||||
| Anxiety disorder | 9 (50.0) | 26 (46.4) | N/A | 1.0d |
| Eating disorder | 1 (5.6) | 1 (1.8) | N/A | 0.43d |
| ADHD | 2 (11.1) | 1 (1.8) | N/A | 0.14d |
| Any substance use disorder | 11 (61.1) | 7 (12.5) | N/A | 1.1 e−4d |
| Alcohol dependence | 6 (33.3) | 4 (7.1) | N/A | 0.01d |
| Alcohol abuse | 4 (22.2) | 2 (3.6) | N/A | 0.03d |
| Marijuana use disorder | 1 (5.6) | 1 (1.8) | N/A | 0.43d |
| Cocaine abuse | 1 (5.6) | 0 (0) | N/A | 0.24d |
| Polysubstance | 1 (5.6) | 0 (0) | N/A | 0.24d |
|
Lifetime medication history, n (%) | ||||
| Lithium | 5 (27.7) | 3 (5.4) | N/A | 0.02d |
| Other mood stabilizer | 8 (44.4) | 8 (14.3) | N/A | 0.02d |
| Antidepressant | 14 (77.8) | 29 (51.8) | N/A | 0.06d |
| Antipsychoticb | 6 (33.3) | 3 (5.4) | N/A | 5.1 e−3d |
BD = bipolar disorder; MDD = major depressive disorder; HV = healthy volunteers; SD = standard deviation; HAM-D = Hamilton Depression Rating Scale; ADHD = attention-deficit hyperactivity disorder; N/A = not applicable.
Number of patients with at least one first-degree relative with a history of mania or depression.
All antipsychotics listed were atypical besides thioridazine that had been taken by one patient with MDD.
One-way ANOVA with Bonferroni post-hoc comparisons.
Fisher’s Exact test.
Two-tailed Student’s t-test.
BD group comparison to HVs
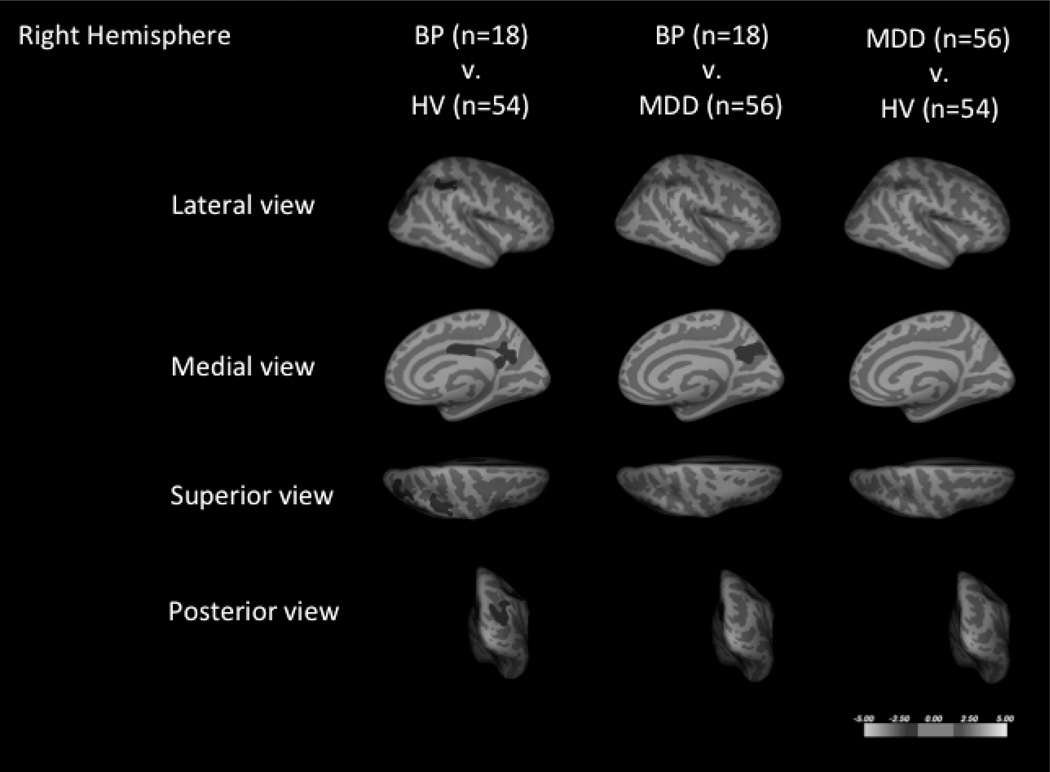
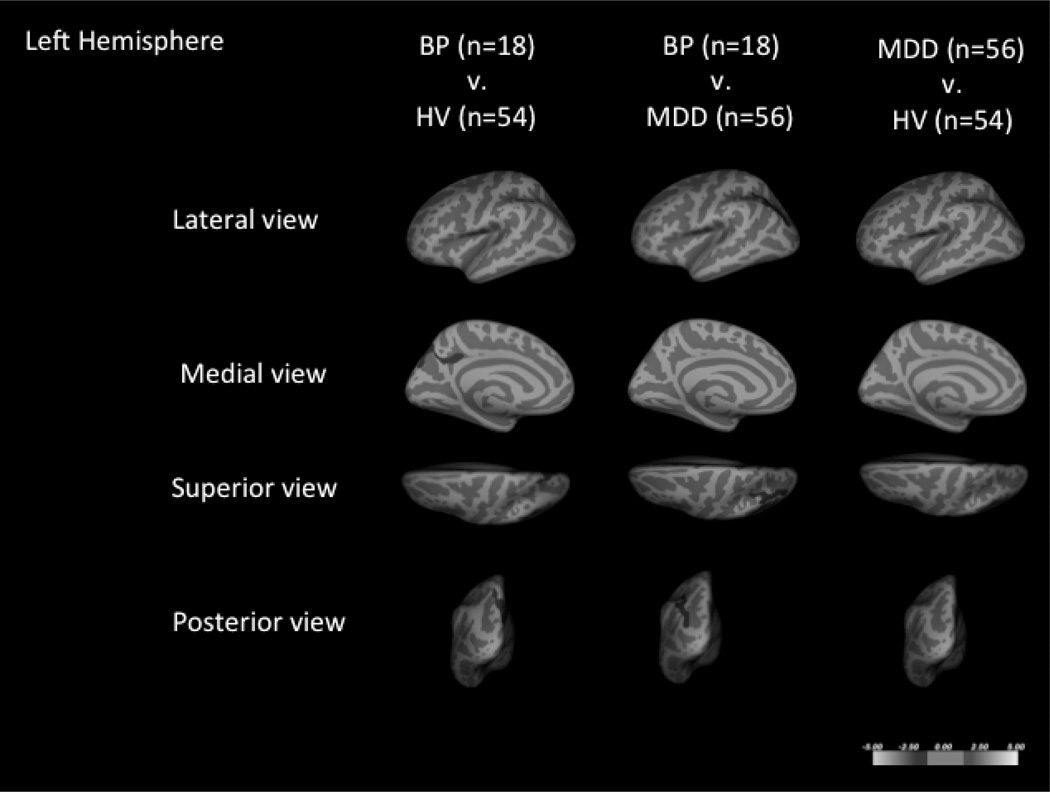
Whole brain cluster-wise analysis of patients with BD and HVs, co-varying for age and sex, demonstrated six clusters that were significantly thinner in the BD group (Fig. 1 and Table 2). On average, these regions were between 7.6–9.6% thinner than that of the HV group.
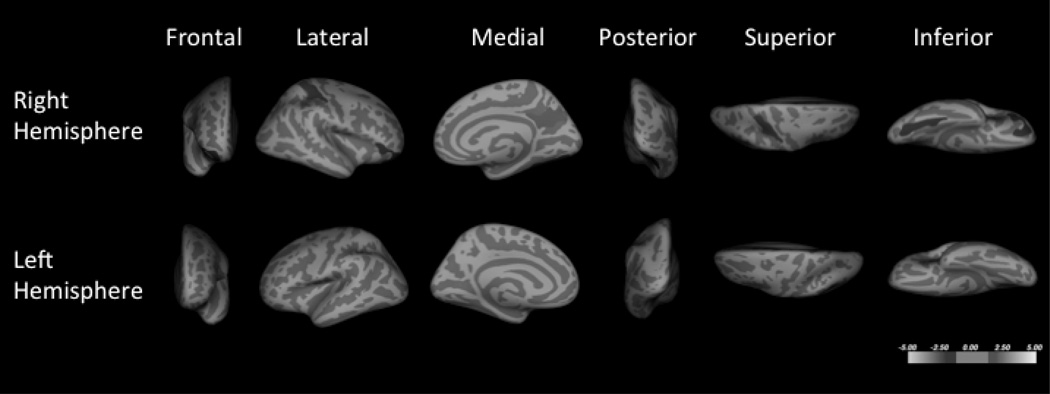
Fig. 1.
Cortical thickness comparisons of subjects with bipolar disorder (BD), major depressive disorder (MDD), and healthy volunteers (HVs) in the right (A) and left hemispheres (B). Six regions were thinner in patients with BD when compared to HVs, including those in the left inferior parietal, the right caudal middle frontal, the left superior parietal, the right posterior cingulate, the right inferior parietal, and the right supramarginal. Three regions with significant spatial overlap with the regions of BD and HV comparison were found when subjects with BD were compared to subjects with MDD, including the right caudal middle frontal region, the left inferior parietal, and the right precuneus regions. No regions were found altered between the MDD and HV groups. Blue coloration indicates significance threshold of p-value < 0.05. Clusters are overlaid on average inflated images with sulci displayed as darker than gyri.
Table 2.
Results of whole brain cluster-wise analysis of cortical thickness comparing subjects with bipolar disorder (BD) (n = 18), healthy volunteers (n = 54), and subjects with major depressive disorder (MDD) (n = 56)
| Size | Talairach coordinatesb |
|||||
|---|---|---|---|---|---|---|
| (mm2) | % changea | x | y | z | CWP | |
| BD versus HVc | ||||||
| L inferiorparietal | 2069.3 | 9.5 | −35.7 | −67.6 | 44.9 | 1.0 e−4 |
| R caudalmiddlefrontal | 1369.9 | 9.1 | 39.7 | 13.8 | 47.5 | 1.2 e−3 |
| L superiorparietal | 1073.5 | 9.3 | −11.3 | −70.8 | 42.7 | 8.0 e−3 |
| R posteriorcingulate | 1011.2 | 9.6 | 4.8 | −11.2 | 27.8 | 0.015 |
| R inferiorparietal | 964.8 | 8.8 | 36.9 | −79.0 | 24.2 | 0.022 |
| R supramarginal | 881.6 | 7.6 | 42.9 | −41.8 | 34.8 | 0.037 |
| BD versus MDDc | ||||||
| R caudalmiddlefrontal | 1750.1 | 7.5 | 38.6 | 14.4 | 48.3 | 1.0 e−4 |
| L inferiorparietal | 924.3 | 8.2 | −35.5 | −65.2 | 45.9 | 0.021 |
| R precuneus | 941.2 | 7.5 | 4.7 | −56.0 | 26.1 | 0.023 |
No significant results were identified when MDD was compared to HV. CWP = cluster-wise p-value; R = right; L = left.
% change: the percent difference in means in cortical thickness; all regions were thinner in BD. This value was derived from the region-of-interest data of each cluster that was found to be statistically different between the two groups.
Coordinates of the maximum voxel for the cluster.
Age and sex as covariates.
BD comparison to MDD
Whole brain cluster-wise analysis of subjects with BD and subjects with MDD, co-varying for age and sex, demonstrated three clusters that were thinner in subjects with BD (Fig. 1 and Table 2). These clusters had significant spatial overlap with clusters that were thinner when the BD group was compared to the HV group. The average cortical thickness differences in these regions ranged from 7.5–8.2%.
MDD group comparison to HVs
Whole brain cluster-wise analysis of subjects with MDD and HVs, co-varying for age and sex, demonstrated no significant clusters that differed between the two groups (Fig. 1).
Analysis of potential confounds
To assess whether the cortical thickness differences in BD were associated with illness characteristics, we studied correlations between clinical characteristics and average cortical thickness measurements of subjects with BD and subjects with MDD (n = 74). Cortical thickness correlated with age within regions that were found to differ between the two groups as expected, but was not correlated with the HAM-D, age at onset of first mood episode, or total brain volume (Table 3).
Table 3.
Effect of disease severity and total brain volume on the cortical thickness findings
| Cluster annotation | HAM-D | Age at first episode |
Current age | Total brain volume |
|---|---|---|---|---|
| R2 = 9.4 e−3 | R2 = −9.4 e−3 | R2 = 0.11 | R2 = 5.9 e−3 | |
| R caudalmiddlefrontal | p = 0.20 | p = 0.57 | p = 2.5 e−3 | p = 0.45 |
| R2 = 0.01 | R2 = 4.3 e−4 | R2 = 0.13 | R2 = 0.02 | |
| L inferiorparietal | p = 0.18 | p = 0.31 | p = 8.9 e−4 | p = 0.14 |
| R2 = 5.5 e−3 | R2 = 5.4 e−3 | R2 = 0.13 | R2 = 8.3 e−3 | |
| R precuneus | p = 0.24 | p = 0.24 | p = 9.5 e−4 | p = 0.53 |
To assess the effect of disease severity and total brain volume on the cortical thickness findings, linear regression analyses of Hamilton Depression Rating Scale (HAM-D) score age at first episode and current age of subject were performed on the average cortical thickness measurements from regions that were found to be significantly different between the bipolar disorder (BD) and major depressive disorder (MDD) groups. Correlations were based on cortical thickness measurements from both BD and MDD groups combined (n = 74). Age was correlated with cortical thickness in three brain regions as expected, but HAM-D score, age at first episode, and total brain volume were not. R = right; L = left.
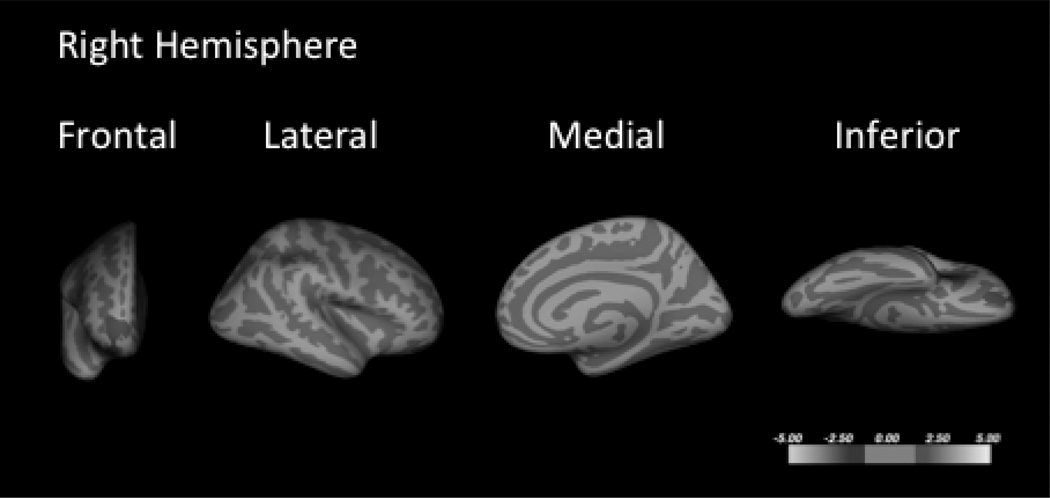
When the subset of subjects with BD-I (n = 12) was compared to the MDD group (n = 56), the regions of less cortical thickness were more expansive than with the combined BD-I and BD-II group (Fig. 2 and Table 4). Previous reports have demonstrated less cortical thickness with alcohol use disorders in HVs (73–75) and patients with BD (11). When past substance use history and current age were included as covariates of no interest, the right dorsal lateral frontal cluster remained thinner in the BD group when compared to the MDD group (Supplementary Fig. 1). To assess the effect of medication history, subjects with MDD that were medication naïve were compared to medication-exposed subjects with MDD; no clusters were found to have significantly different cortical thickness between the groups. Only three subjects with BD were medication naïve, precluding any group wise comparisons.
Fig. 2.
Whole brain cluster-wise comparison of cortical thickness of subgroups of the primary comparisons with age and sex as covariates. Whole brain cluster-wise comparison of cortical thickness of subjects with bipolar I disorder (n = 12) when compared to subjects with major depressive disorder (n = 56). The pattern of cortical thickness decreases was more extensive in this subgroup of patients than the combined bipolar disorder population.
Table 4.
Results of whole brain cluster-wise analysis of cortical thickness comparing the subgroup of subjects with bipolar I disorder (BD-I) (n = 12) and subjects with major depressive disorder (MDD) (n = 56)
| Talairach coordinatesa |
|||||
|---|---|---|---|---|---|
| Size (mm2) | x | y | z | CWP | |
| BD-I versus MDDb | |||||
| L superiorfrontal | 4470.3 | −6.9 | 29.7 | 46.9 | 1.0 e−4 |
| L inferiorparietal | 6008.4 | −34.3 | −65.9 | 45.7 | 1.0 e−4 |
| R superiorfrontal | 5006.7 | 8.5 | −5.5 | 47.7 | 1.0 e−4 |
| R superiorpareital | 1804.7 | 22.5 | −54.2 | 59.2 | 1.0 e−4 |
| R precuneus | 1314.5 | 4.7 | −55.6 | 23.9 | 1.8 e−3 |
| R superiorparietal | 1155.9 | 24.7 | −39.7 | 56.9 | 6.0 e−3 |
| R rostralmiddlefrontal | 951.6 | 26.0 | 49.5 | −12.3 | 0.024 |
| R fusiform | 890.0 | 27.0 | −76.0 | 2.2 | 0.035 |
CWP = cluster-wise p-value; L = left; R = right.
Coordinates of the maximum voxel for the cluster.
Age and sex as covariates.
A previous study demonstrated less cortical thickness in subjects who were at high risk for depression based on a strong family history of major depression (29). We therefore assessed the effect of family history in our cohort of subjects with MDD on cortical thickness. Whole brain cluster-wise analysis was performed in a combined group of subjects with BD and subjects with MDD who had a first-degree relative with MDD (n = 24) compared to HVs (n = 54), co-varying for age and sex. One cluster was identified with greater cortical thickness (Fig. 3 and Table 4).
Fig. 3.
Whole brain cluster-wise comparison of cortical thickness of subjects with major depressive disorder (MDD) with a first-degree relative with a history of mood disorder (n = 24) and healthy volunteers (n = 54). One cluster in the lateral orbital frontal region was found to be thicker in patients in the MDD group.
Discussion
Significance of right caudal middle frontal result
Less cortical thickness in the right dorsal lateral frontal region in BD is the most robust finding of this study. The region was thinner in subjects with BD when compared to HV and MDD groups and could not be explained by age, sex, or substance use disorder history. A recent meta-analysis of past voxel-based morphometry studies identified the right prefrontal cortex and temporal lobe grey matter as the most robust differences between BD and HV groups (30). Our results indicate this finding to be a BD-specific phenomenon, as we did not find it in MDD. Using magnetic resonance spectroscopy, one study identified a lower N-acetyl aspartate/total creatinine ratio in the dorsolateral prefrontal cortex of patients with BD when compared to controls, implying a deficit in neuronal viability in the region (31). Postmortem brain studies also have identified lower neuronal density and glial abnormalities in individuals with BD in the region, and have provided evidence for a functional shift from excitatory to inhibitory neurotransmission (32, 33). Several functional MRI studies have shown alterations during behavioral tasks in the right dorsolateral prefrontal cortex of patients with BD, such as processing affect in images of faces (34, 35) and performing tasks of executive function (36, 37). Also, there is evidence of benefit with transcranial magnetic stimulation applied to the right dorsolateral prefrontal cortex in BD (38, 39), adding further convergent data implicating the region in the pathophysiology and treatment of the disorder.
The right dorsal lateral prefrontal cortex is implicated in a number of executive functions including working memory (40, 41), decision making (42, 43) and attentional control (44, 45). Indeed, deficits in executive function in BD are an important prognostic factor (46, 47). This region is part of the cortico-thalamic-insular circuit thought to be involved in the pathophysiology of mood disorders. In this neural circuitry model, a deficit in dorsolateral prefrontal cortex would result in decreased cortical regulation of emotional processing driven by insular cortex. This dysregulation is hypothesized to manifest as symptoms of mania and depression (48, 49).
Structural changes beyond the right dorsolateral prefrontal region in BD
Other regions we identified as having less cortical thickness in BD have been reported to have lower cortical thickness in BD in previous studies. For example, the precuneus, a region found thinner here in BD, has been found to have less activation using functional MRI when euthymic patients with BD were exposed to negatively valenced stimuli (50), less deactivation when euthymic patients with BD performed a verbal fluency task (51), and less activation when manic patients performed a continuous performance task (52). Using fluorodeoxyglucose positron emission tomography (FDG PET), the region had less metabolism during mania (53). This region has been implicated in a number of cognitive functions including self- and external-agency attribution and theory of mind (54, 55). It is also activated during the resting state of the brain (56).
We found less cortical thickness bilaterally in inferior parietal lobe of subjects with BD compared to HVs which is consistent with decreased activation in the inferior parietal lobe reported in subjects with BD compared to HVs during tasks of selective attention using functional MRI (57, 58). Also, a resting state FDG PET study and a voxel-based morphometry study found alterations in the inferior parietal lobe in patients with BD (59, 60). This region, along with the caudal middle frontal region, has been associated with working memory tasks (40, 41) and linked with attention, particularly spatial attention (61, 62).
We found lower cortical thickness in the left posterior cingulate of subjects with BD compared to HVs. Work from our group using voxel-based morphometry analyses comport with this, showing lower volume in the posterior cingulate cortex (PCC) when compared to HVs (63–65). The PCC has also been differentially activated in individuals with BD during tasks with functional MRI tasks where subjects process positive- or negative-valenced cues (50, 66, 67) or perform cognitive tasks (51, 52, 68). The PCC has been found to be part of the default mode network (69) and is involved with consciousness (70). The pathophysiological relevance of this observation requires further work.
Previous MRI studies have reported other structural changes in BD including increased rates of white matter hyperintensities, primarily in the periventricular region, and enlarged lateral ventricles (71, 72). How our findings of less cortical thickness in BD relate to widespread changes to the structure of the brain implicated in the pathophysiology of BD requires further investigation. More extensive differences in cortical thickness were found in BD-I than the combined BD-I and BD-II population, indicating that severity of manic symptomatology is related to this biological process.
Family history in MDD
Subjects with MDD with a family history of a mood disorder showed greater cortical thickness in the orbitofrontal cortex when compared to the HV group. Greater cortical thickness in the medial aspect of the right orbitofrontal lobe has also been reported in subjects at high familial risk for major depression (29). This cortical difference may therefore be a familial marker of MDD or the risk to develop MDD.
Study limitations
Changes to cortical thickness by medications cannot be ruled out as a confounder in this study. The patients with BD had more lifetime exposure to antidepressant, antipsychotic, and mood-stabilizer medications, including lithium, compared with patients with MDD. The groups were also not matched for the number of subjects with a history of substance use disorders. Further studies are needed to evaluate the individual contributions of substance use disorders and BD to cortical thickness changes.
Future directions
Cortical thickness may prove to be a useful tool to distinguish BD from MDD, and may contribute to the pathophysiology of manic episodes. A prospective, hypothesis-driven study in a larger sample could determine predictive properties of this index of cortical thickness. Determining whether patients show differences in cortical thickness at their first symptoms of BD could determine the use of this finding for diagnostic purposes. Such an experiment would enable a calculation of the predictive capacity of this measure. Future studies may combine measures of cortical thickness with other imaging modalities for the purpose of differentiating BD from MDD. Postmortem brain studies focusing on molecular and cellular differences between the two disorders in the cortical regions found altered here are also warranted. Localization of deficits in cortical thickness associated with both alcohol use disorders and BD may lead to a better understanding of the high rates of comorbidity between these conditions.
Supplementary Material
Supplementary Figure 1. Whole brain cluster-wise comparison of cortical thickness of bipolar disorder (n = 18) and major depressive disorder (n = 56) groups when age and a history of substance use disorder were included in the model as covariates. Only the right caudal middle frontal lobe remained significant between the two disorders with a cluster size of 1223.3 mm2; Talairach coordinates of x = 32.1, y = 14.2, z = 46.1; and cluster-wise p-value of 4.2 e−3.
Acknowledgements
We are grateful to all of the subjects who volunteered for this project. We would like to acknowledge the staff at the Brain Imaging Division and Clinical Evaluation Core of the Conte Translational Center for the Neurobiology of Suicidal Behavior for their help with the project. We are also thankful to R. Todd Ogden for his consultation on the statistical analyses and Mike Strupp-Levitsky for coordinating the data and suggesting statistical approaches.
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health grants MH62185, K08-MH67015, K08-MH079033-03, R01-MH074813, R01-MH090276-03, R01 MH040695 21 as well as funding from the Brain and Behavior Research Foundation and American Foundation for Suicide Prevention. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
MAO has received past unrelated grants from Eli Lilly & Co., AstraZeneca, Bristol-Myers Squibb, Janssen, Otsuko, Pfizer, Sanofi-Aventis, and Shire; has received royalties for the use of the Columbia Suicide Severity Rating Scale; and her family owns stock in Bristol-Myers Squibb. MES has received a grant of nutritional supplements from Unicity, International. GS serves as a member of the scientific advisory board of TONIX Pharmaceuticals, Inc. and has received compensation from them; has served as a consultant for Ono Pharma USA, Inc.; and has a US patent application for use of tianeptine. JJM has received past unrelated grants from Novartis and GlaxoSmithKline.
Footnotes
Disclosures
The authors of this paper declare no conflicts of interest in connection with the present manuscript.
References
- 1.Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. 1996;276:293–299. [PubMed] [Google Scholar]
- 2.Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br J Psychiatry. 1997;170:205–228. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- 3.The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization; 2002. [DOI] [PubMed] [Google Scholar]
- 4.Baldessarini RJ, Vieta E, Calabrese JR, et al. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18:143–157. doi: 10.3109/10673221003747955. [DOI] [PubMed] [Google Scholar]
- 5.Belmaker RH. Bipolar disorder. N Engl J Med. 2004;351:476–486. doi: 10.1056/NEJMra035354. [DOI] [PubMed] [Google Scholar]
- 6.Lyoo IK, Sung YH, Dager SR, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- 7.Fornito A, Malhi GS, Lagopoulos J, et al. Anatomical abnormalities of the anterior cingulate and paracingulate cortex in patients with bipolar I disorder. Psychiatry Res. 2008;162:123–132. doi: 10.1016/j.pscychresns.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Rimol LM, Hartberg CB, Nesvag R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 9.Foland-Ross LC, Thompson PM, Sugar CA, et al. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am J Psychiatry. 2011;168:530–539. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartberg CB, Sundet K, Rimol LM, et al. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J Int Neuropsychol Soc. 2011;17:1080–1093. doi: 10.1017/S1355617711001081. [DOI] [PubMed] [Google Scholar]
- 11.Nery FG, Matsuo K, Nicoletti MA, et al. Association between prior alcohol use disorders and decreased prefrontal gray matter volumes in bipolar I disorder patients. Neurosci Lett. 2011;503:136–140. doi: 10.1016/j.neulet.2011.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore GJ, Cortese BM, Glitz DA, et al. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. J Clin Psychiatry. 2009;70:699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- 13.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 14.de Almeida JRC, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol Psychiatry. 2013;73:111–118. doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JBW. SCID-I/P, Version 2.0. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 1994. [Google Scholar]
- 16.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 18.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 21.Fischl B, Sereno MI, Tootell RB, et al. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segonne F, Dale AM, Busa EG, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 23.Talairach JTP. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 24.Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- 25.Preul C, Hund-Georgiadis M, Forstmann BU, et al. Characterization of cortical thickness and ventricular width in normal aging: a morphometric study at 3 Tesla. Journal of magnetic resonance imaging : JMRI. 2006;24:513–519. doi: 10.1002/jmri.20665. [DOI] [PubMed] [Google Scholar]
- 26.Luders E, Narr KL, Thompson PM, et al. Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp. 2006;27:314–324. doi: 10.1002/hbm.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 28.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;3:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Peterson BS, Warner V, Bansal R, et al. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci U S A. 2009;106:6273–6278. doi: 10.1073/pnas.0805311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvaraj S, Arnone D, Job D, et al. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14:135–145. doi: 10.1111/j.1399-5618.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- 31.Winsberg ME, Sachs N, Tate D, et al. Decreased dorsolateral prefrontal Nacetyl aspartate in bipolar disorder. Biol Psychiatry. 2000;47:475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- 32.Lan MJ, McLoughlin GA, Griffin JL, et al. Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol Psychiatry. 2009;14:269–279. doi: 10.1038/sj.mp.4002130. [DOI] [PubMed] [Google Scholar]
- 33.Gigante AD, Young LT, Yatham LN, et al. Morphometric post-mortem studies in bipolar disorder: possible association with oxidative stress and apoptosis. Int J Neuropsychopharmacology. 2011;14:1075–1089. doi: 10.1017/S146114571000146X. [DOI] [PubMed] [Google Scholar]
- 34.Altshuler L, Bookheimer S, Townsend J, et al. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disord. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vizueta N, Rudie JD, Townsend JD, et al. Regional fMRI hypoactivation and altered functional connectivity during emotion processing in nonmedicated depressed patients with bipolar II disorder. Am J Psychiatry. 2012;169:831–840. doi: 10.1176/appi.ajp.2012.11030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Townsend J, Bookheimer SY, Foland-Ross LC, et al. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Res. 2010;182:22–29. doi: 10.1016/j.pscychresns.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strakowski SM, Adler CM, Holland SK, et al. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 38.Tamas RL, Menkes D, El-Mallakh RS. Stimulating research: a prospective, randomized, double-blind, sham-controlled study of slow transcranial magnetic stimulation in depressed bipolar patients. J Neuropsychiatry Clin Neurosci. 2007;19:198–199. doi: 10.1176/jnp.2007.19.2.198. [DOI] [PubMed] [Google Scholar]
- 39.Dell'Osso B, Mundo E, D'Urso N, et al. Augmentative repetitive navigated transcranial magnetic stimulation (rTMS) in drug-resistant bipolar depression. Bipolar Disord. 2009;11:76–81. doi: 10.1111/j.1399-5618.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- 40.Belger A, Puce A, Krystal JH, et al. Dissociation of mnemonic and perceptual processes during spatial and nonspatial working memory using fMRI. Hum Brain Mapp. 1998;6:14–32. doi: 10.1002/(SICI)1097-0193(1998)6:1<14::AID-HBM2>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtis CE. Prefrontal and parietal contributions to spatial working memory. Neuroscience. 2006;139:173–180. doi: 10.1016/j.neuroscience.2005.04.070. [DOI] [PubMed] [Google Scholar]
- 42.Rosenbloom MH, Schmahmann JD, Price BH. The functional neuroanatomy of decision-making. J Neuropsychiatry Clin Neurosci. 2012;24:266–277. doi: 10.1176/appi.neuropsych.11060139. [DOI] [PubMed] [Google Scholar]
- 43.Rorie AE, Newsome WT. A general mechanism for decision-making in the human brain? Trends Cogn Sci. 2005;9:41–43. doi: 10.1016/j.tics.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 45.Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of topdown attentional control. Nature Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg JF, Chengappa KNR. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord. 2009;11(Suppl. 2):123–137. doi: 10.1111/j.1399-5618.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 47.Harvey PD, Wingo AP, Burdick KE, Baldessarini RJ. Cognition and disability in bipolar disorder: lessons from schizophrenia research. Bipolar Disord. 2010;12:364–375. doi: 10.1111/j.1399-5618.2010.00831.x. [DOI] [PubMed] [Google Scholar]
- 48.Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14:326–339. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 49.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Malhi GS, Lagopoulos J, Owen AM, et al. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. J Affect Disord. 2007;97:109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Costafreda SG, Fu CH, Picchioni M, et al. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry. 2011;11:18. doi: 10.1186/1471-244X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strakowski SM, Adler CM, Cerullo M, et al. Magnetic resonance imaging brain activation in first-episode bipolar mania during a response inhibition task. Early Interven Psychiatry. 2008;2:225–233. doi: 10.1111/j.1751-7893.2008.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brooks JO, 3rd, Bonner JC, Rosen AC, et al. Dorsolateral and dorsomedial prefrontal gray matter density changes associated with bipolar depression. Psychiatry Res. 2009;172:200–204. doi: 10.1016/j.pscychresns.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperduti M, Delaveau P, Fossati P, et al. Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Struct Funct. 2011;216:151–157. doi: 10.1007/s00429-010-0298-1. [DOI] [PubMed] [Google Scholar]
- 55.Mar RA. The neural bases of social cognition and story comprehension. Annual Rev Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- 56.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 57.Pompei F, Jogia J, Tatarelli R, et al. Familial and disease specific abnormalities in the neural correlates of the Stroop Task in Bipolar Disorder. Neuroimage. 2011;56:1677–1684. doi: 10.1016/j.neuroimage.2011.02.052. [DOI] [PubMed] [Google Scholar]
- 58.Mullin BC, Perlman SB, Versace A, et al. An fMRI study of attentional control in the context of emotional distracters in euthymic adults with bipolar disorder. Psychiatry Res. 2012;201:196–205. doi: 10.1016/j.pscychresns.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hosokawa T, Momose T, Kasai K. Brain glucose metabolism difference between bipolar and unipolar mood disorders in depressed and euthymic states. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:243–250. doi: 10.1016/j.pnpbp.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 60.Li M, Cui L, Deng W, Ma X, et al. Voxel-based morphometric analysis on the volume of gray matter in bipolar I disorder. Psychiatry Res. 2011;191:92–97. doi: 10.1016/j.pscychresns.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist. 2012;18:502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- 62.Gottlieb J, Snyder LH. Spatial and non-spatial functions of the parietal cortex. Curr Opin Neurobiol. 2010;20:731–740. doi: 10.1016/j.conb.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yatham LN, Lyoo IK, Liddle P, et al. A magnetic resonance imaging study of mood stabilizer- and neuroleptic-naïve first-episode mania. Bipolar Disord. 2007;9:693–697. doi: 10.1111/j.1399-5618.2007.00414.x. [DOI] [PubMed] [Google Scholar]
- 64.Lochhead RA, Parsey RV, Oquendo MA, et al. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxelbased morphometry. Biol Psychiatry. 2004;55:1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 65.Nugent AC, Milham MP, Bain EE, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 66.Kruger S, Seminowicz D, Goldapple K, et al. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 67.Townsend JD, Torrisi SJ, Lieberman MD, et al. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73:127–135. doi: 10.1016/j.biopsych.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sepede G, De Berardis D, Campanella D, et al. Impaired sustained attention in euthymic bipolar disorder patients and non-affected relatives: an fMRI study. Bipolar Disord. 2012;14:764–779. doi: 10.1111/bdi.12007. [DOI] [PubMed] [Google Scholar]
- 69.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 70.Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kempton MJ, Salvador Z, Munafo MR, et al. Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry. 2011;68:675–690. doi: 10.1001/archgenpsychiatry.2011.60. [DOI] [PubMed] [Google Scholar]
- 72.Kempton MJ, Geddes JR, Ettinger U, et al. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 73.Durazzo TC, Tosun D, Buckley S, et al. Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res. 2011;35:1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Momenan R, Steckler LE, Saad ZS, et al. Effects of alcohol dependence on cortical thickness as determined by magnetic resonance imaging. Psychiatry Res. 2012;204:101–111. doi: 10.1016/j.pscychresns.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Fortier CB, Leritz EC, Salat DH, et al. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcohol Clin Exp Res. 2011;35:2193–2201. doi: 10.1111/j.1530-0277.2011.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Whole brain cluster-wise comparison of cortical thickness of bipolar disorder (n = 18) and major depressive disorder (n = 56) groups when age and a history of substance use disorder were included in the model as covariates. Only the right caudal middle frontal lobe remained significant between the two disorders with a cluster size of 1223.3 mm2; Talairach coordinates of x = 32.1, y = 14.2, z = 46.1; and cluster-wise p-value of 4.2 e−3.