Abstract
Purpose
Autoimmune diseases are thought to be caused by a loss of self-tolerance of the immune system. One candidate marker of immune dysregulation in autoimmune disease is the presence of increased double negative T cells (DNTs) in the periphery. DNTs are characteristically elevated in autoimmune lymphoproliferative syndrome, a systemic autoimmune disease caused by defective lymphocyte apoptosis due to Fas pathway defects. DNTs have also been found in the peripheral blood of adult patients with systemic lupus erythematosus (SLE), where they may be pathogenic. DNTs in children with autoimmune disease have not been investigated.
Methods
We evaluated DNTs in pediatric patients with SLE, mixed connective tissue disease (MCTD), juvenile idiopathic arthritis (JIA), or elevated antinuclear antibody (ANA) but no systemic disease. DNTs (CD3+CD56−TCRαβ+CD4−CD8−) from peripheral blood mononuclear cells were analyzed by flow cytometry from 54 pediatric patients including: 23 SLE, 15 JIA, 11 ANA and 5 MCTD compared to 28 healthy controls.
Results
Sixteen cases (29.6%) had elevated DNTs (≥ 2.5% of CD3+CD56−TCRαβ+ cells) compared to 1 (3.6%) control. Medication usage including cytotoxic drugs and absolute lymphocyte count were not associated with DNT levels, and percentages of DNTs were stable over time. Analysis of multiple phenotypic and activation markers showed increased CD45RA expression on DNTs from patients with autoimmune disease compared to controls.
Conclusion
DNTs are elevated in a subset of pediatric patients with autoimmune disease and additional investigations are required to determine their precise role in autoimmunity.
Keywords: T cells, double negative T cells, pediatric, autoimmunity
Introduction
Systemic pediatric autoimmune diseases result from failure of immunologic tolerance leading to response against self [1]. While systemic autoimmunity affects many children, immune dysregulation mechanisms remain poorly understood.
A potential marker of disease and immunoregulatory defects in systemic lupus erythematosus (SLE) are circulating double negative T cells (DNTs) [2]. DNTs are mature post-thymic T cells that express CD3/TCRα/β receptor but lack CD4/CD8 [3]. Patients with FAS/FAS ligand defects develop autoimmune lymphoproliferative syndrome (ALPS) with significant DNT accumulation, signifying escaped peripheral tolerance [4–6]. Importantly, in ALPS, somatic FAS mutations in DNTs results in similar clinical symptoms as germline mutations suggesting a pathogenic role [7,8].
Adults with SLE have elevated DNTs, and studies have suggested DNTs may be pathogenic [9–12]. However, while DNTs have been associated with many disease processes, their precise role is uncertain [13,14]. DNTs in pediatric autoimmune disease have not been evaluated. We hypothesize that DNTs may contribute to pediatric autoimmunity and investigated the frequency and phenotype in this population.
Materials and Methods
Subjects
Pediatric patients (≤21 years old) from a single institution diagnosed by a pediatric rheumatologist with SLE [15], mixed connective tissue disease (MCTD), ANA positive oligoarticular or polyarticular juvenile idiopathic arthritis (JIA) [16], or an elevated antinuclear antibody (ANA) ≥1:1280 without systemic autoimmune disease were included in this study. No patients met classification criteria for ALPS [5]. Control subjects (≤25 years old) were healthy without autoimmunity. Washington University School of Medicine Human Research Protection Office approved this study.
Flow cytometry
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood (Ficoll-Paque), pre-incubated with mouse IgG (Sigma, St. Louis, MO), and stained for TCRγ/δ (clone: 11F2), CD4 (RPA-T4), CD8 (HIT8a), and CD19 (SJ25C1) (BD Biosciences, San Jose, CA); TCRα/β (IP26) and CD3 (OKT-3) (e-Biosciences, San Diego, CA); and CD56 (N901; Beckman Coulter, Brea, CA). T, B, and natural killer (NK) cell percentages were calculated with standard gating strategies. DNTs were calculated as the percentage of CD3+/CD56−/TCRα/β+/TCRγ/δ− T lymphocytes that were CD4−/CD8−. DNT percentages greater than or equal to 2.5% were considered elevated based upon previous studies [5,17]. For patients with more than one visit, DNT percentages and absolute lymphocyte count are from the time of study entry.
Sequencing
Patient DNA was isolated from peripheral blood or saliva and the sequence of promoter regions, exons, and intron/exon junctions of FAS determined by Sanger sequencing using primers previously described [18]. Sequences were compared to the reference FAS sequence (NCBI accession NG_009089) for variation and frequency of single nucleotide polymorphisms (SNPs) in dbSNP (http://www.ncbi.nlm.nih.gov/SNP/).
Phenotyping of DNTs
PBMCs were surface-stained with anti-TCRα/β/TCRγ/δ/CD3//CD4/CD8/CD56 and anti-CD25 (BC96), CD45RA (HI100), CD45RO (UCHL1), CD69 (FN50) or HLA-DR (G46-6), (Biolegend, San Diego, CA). For intracellular staining, cells were fixed and permeabilized (BD Biosciences, San Jose, CA), and stained with granzyme B (GB12; Invitrogen, Grand Island, NY) or perforin (dG9; Biolegend, San Diego, CA). FoxP3 staining (206D; Biolegend, San Diego, CA) was performed as per manufacturer's recommendations (eBioscience, San Diego, CA).
Statistical analysis
Statistical analysis was performed utilizing GraphPad Prism 6.0 (GraphPad Software Inc, La Jolla, CA). Specific tests are noted with results and/or in the figure legend. A p-value <0.05 was considered statistically significant.
Results
DNTs were evaluated in 82 subjects, 54 cases and 28 controls (Supplemental Table 1). The majority were Caucasian (62.2%) and female (79.3%), with a mean age 15±5 years. Controls were slightly older than cases (p=0.001). To ensure there was no significant difference in DNT staining of PBMC versus whole blood, as has been reported for other immune cell types [19], staining of PBMC and whole blood was compared in 4 age-matched normal donors and found to be similar (Supplemental Figure 1).
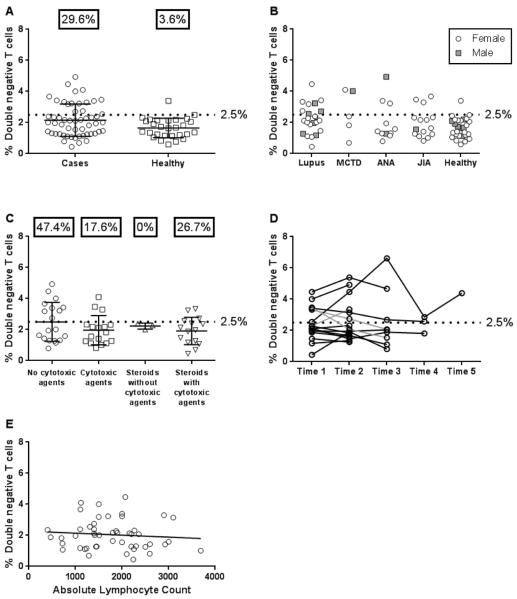
A higher percentage of pediatric patients with autoimmune disease had elevated DNTs>2.5% compared to controls (p=0.008, Figure 1A; Fischer's exact test). Eight of 23 patients (34.8%) with SLE, 20.0% (3/15) with JIA, 27.3% (3/11) with elevated ANA, and 40.0% (2/5) with MCTD had increased DNTs (Figure 1B). While no patients met criteria for ALPS, the coding and regulatory regions of FAS was sequenced in 15 of the subjects (14 cases and one control) with elevated DNTs and available DNA. No insertions/deletions were found, and only previously reported SNPs with allele frequencies >1.5% were identified (data not shown), suggesting that no subjects had pathogenic FAS mutations.
Fig 1. Frequency of DNTs in pediatric patients.
(A) Percentage of DNTs from flow cytometric analysis of PBMC from 54 patients (circles) and 28 healthy controls (squares). Percentages shown represent the percent of subjects with DNTs greater than or equal to 2.5%. (B) DNT percentages by disease category. (C) Association between medication use at study entry and DNTs. Nineteen patients were taking no cytotoxic medications; 17 were treated with cytotoxic agents including 6-mercaptopurine, mycophenolate mofetil, cyclophosphamide, methotrexate, and leflunomide; 3 received steroids without cytotoxic drugs; and 15 were taking steroids plus cytotoxic agent(s). There was no difference among groups by ANOVA or percentage of patients with DNT values greater or less than 2.5% between groups by Chi-square. (D) DNT values were determined at multiple time points for an average of 8 months. Three of 16 patients had DNT percentages cross 2.5% on subsequent visits (gray line). (E) Comparison of DNTs to absolute lymphocyte count (ALC) in patients with autoimmune disease (n=49). There was no association of DNTs to ALC (r=−0.11, p=0.45, Spearman's correlation). Abbreviations: Mixed connective tissue disease (MCTD), anti-nuclear antibody (ANA), juvenile idiopathic arthritis (JIA).
Absolute percentages of DNTs and most other lymphocytes were not different across patient populations (Supplemental Table 2) with the exception of differences in the mean percentages of B cells (p=0.02) and CD56+CD3+ cells (p=0.002) between groups by ANOVA.
DNT percentages did not appear to change with medication as there was no statistically significant difference in the percentage of patients with elevated DNTs when compared to medication usage (Figure 1C). We collected multiple samples from sixteen patients over an average of 8 months and most had relatively stable DNTs above/below 2.5% (Figure 1D). We also investigated whether increased DNTs were associated with lymphopenia, as previously suggested [20] and found no association (r=−0.11, p=0.45, Figure 1E).
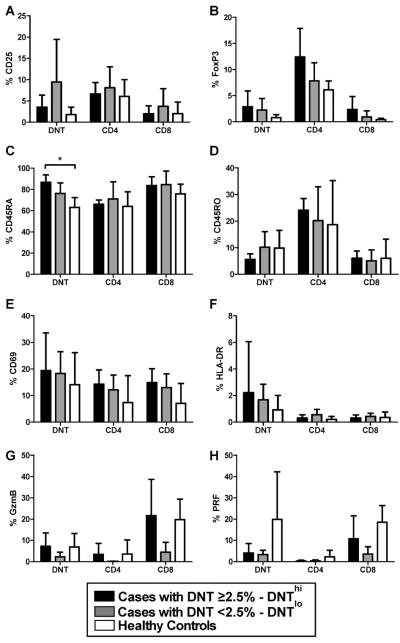
Additional cell surface markers and intracellular proteins in DNTs and single positive CD4/CD8 T cells were evaluated in five autoimmune patients with DNTs>2.5% (DNThi), five with autoimmunity and DNTs<2.5% (DNTlo), and five healthy controls (Figure 2A–H). DNTs from DNThi cases showed increased CD45RA expression compared to healthy control DNTs (p=0.008, Figure 2C), but not DNTlo patients. DNT CD45RA expression from DNThi cases was similar to that seen in CD8 cells, and higher than CD4 cells (p=0.008). Whereas, DNT CD45RO expression from DNThi cases was also similar to CD8 cells (Figure 2D), but lower than CD4 cells (p=0.008).
Fig 2. Expression of phenotypic and activation markers by DNTs, CD4 and CD8 cells in patients with autoimmune disease and healthy controls.
Five cases with DNTs ≥2.5% (DNThi, black), 5 cases with DNTs <2.5% (DNTlo, gray) and 5 healthy controls (white) were evaluated for phenotypic and activation markers. Each case group included 3 SLE, one MCTD, and one patient with an elevated ANA. The percentages of various extracellular and intracellular markers were determined in the following cell types: DNTs, CD3+TCRα/β+CD4+, and CD3+TCRα/β+CD8+ T cells. The following markers were evaluated: A) CD25, B) FoxP3, C) CD45RA, D) CD45RO, E) CD69, F) HLA-DR, G) intracellular granzyme B and H) intracellular perforin. In Figure 6C, DNTs from DNThi cases showed significantly increased CD45RA percentages compared to healthy controls (*, p=0.008, Mann-Whitney test). Abbreviations: Granzyme B (GzmB), perforin (PRF).
Discussion
This is the first study to evaluate DNTs in pediatric autoimmunity. Significantly more patients had DNTs elevated ≥2.5% compared to controls. Additionally, DNTs were stable over ~8 months, suggesting that single observations are significant. Medications did not correlate with DNT%, similar to previous adult lupus studies [2,11]. The presence of elevated DNTs in cases not on cytotoxic treatment also suggests that elevated DNTs are due to autoimmunity and not secondary to medication.
There is limited knowledge about the relationship between DNT percentages and absolute lymphocyte count. Some prior reports suggest that a false negative elevation of DNTs is possible in a lymphopenic state due to inaccurate flow cytometry quantification [20]. By evaluating a large number of pediatric patients with autoimmune disease, our study demonstrates that DNT percentage do not correlate with the absolute lymphocyte count in children.
We analyzed various phenotypic and activation markers in DNTs. CD25, FoxP3, CD69, HLA-DR, perforin and granzyme B were not significantly elevated in DNTs. While DNTs appear to lack common markers found in T regulatory cells (e.g., CD25, FoxP3), a previous in vitro study showed that DNTs might have suppressive activity by cell contact [12]. We observed that DNTs from children with autoimmunity are predominantly CD45RA+, consistent with a previous study of DNTs in ALPS [21]. This could indicate lack of prior antigen exposure, upregulation of CD45RA similar to effector memory T cells [22], or physiology similar to end-stage T cells which are effective in cytokine secretion and cytoxicity [23]. Increased DNT CD45RA expression was also seen in adult SLE patients [11]. Interestingly, the C45RA/RO DNT expression pattern in DNThi patients was similar to CD8 cells, consistent with a proposed common lineage [24].
The role of DNTs in autoimmunity remains incompletely understood. DNTs in patients with ALPS are likely pathogenic, or the end result of pathogenic cells, based on somatic FAS mutations selectively in DNTs [8]. In vitro, CD8 cell derived DNTs, are pro-inflammatory and produce IL-1β, IL-17, and IFN-γ [25]. Studies of in situ kidney DNTs from patients with SLE demonstrated IL-17 production that might contribute to lupus nephritis and induce Th17 cell differentiation [9,26]. By contrast, other reports of regulatory DNTs suggest some cells may play beneficial roles by suppressing auto-reactive CD8 cells [27]. Our findings demonstrate that DNTs are elevated in pediatric patients with several types of autoimmune disease, and in patients with high ANAs but no overt autoimmunity, and it remains to be determined whether DNTs contribute to underlying disease. Future investigations are important in elucidating DNT function, understanding their role in autoimmunity, and potentially providing novel therapeutic targets.
Supplementary Material
Acknowledgements
We would like to thank Drs. George Van Hare, David Balzer, and Katie Plax for their help in patient recruitment and sample acquisition. Funding for this project was provided by the Children's Discovery Institute of Washington University and St. Louis Children's Hospital (to M.A.C) and by NIH training grant 5T32AR007279-34 (J.A.T.).
Research reported in this publication was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
The authors declare that they have no conflict of interest.
References
- 1.Thomas JW. Antigen-specific responses in autoimmunity and tolerance. Immunol Res. 2001;23:235–44. doi: 10.1385/IR:23:2-3:235. [DOI] [PubMed] [Google Scholar]
- 2.Sieling PA, Porcelli SA, Duong BT, Spada F, Bloom BR, Diamond B, et al. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J Immunol. 2000;165:5338–44. doi: 10.4049/jimmunol.165.9.5338. [DOI] [PubMed] [Google Scholar]
- 3.Fleisher TA, Oliveira JB. Monogenic defects in lymphocyte apoptosis. Curr Opin Allergy Clin Immunol. 2012;12:609–15. doi: 10.1097/ACI.0b013e3283588da0. [DOI] [PubMed] [Google Scholar]
- 4.Vaishnaw AK, Toubi E, Ohsako S, Drappa J, Buys S, Estrada J, et al. The spectrum of apoptotic defects and clinical manifestations, including systemic lupus erythematosus, in humans with CD95 (Fas/APO-1) mutations. Arthritis Rheum. 1999;42:1833–42. doi: 10.1002/1529-0131(199909)42:9<1833::AID-ANR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turbyville JC, Rao VK. The autoimmune lymphoproliferative syndrome: A rare disorder providing clues about normal tolerance. Autoimmun Rev. 2010;9:488–93. doi: 10.1016/j.autrev.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Dowdell KC, Niemela JE, Price S, Davis J, Hornung RL, Oliveira JB, et al. Somatic FAS mutations are common in patients with genetically undefined autoimmune lymphoproliferative syndrome. Blood. 2010;115:5164–9. doi: 10.1182/blood-2010-01-263145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzelova E, Vonarbourg C, Stolzenberg M-C, Arkwright PD, Selz F, Prieur A-M, et al. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. N Engl J Med. 2004;351:1409–18. doi: 10.1056/NEJMoa040036. [DOI] [PubMed] [Google Scholar]
- 9.Crispín JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–6. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shivakumar S, Tsokos GC, Datta SK. T cell receptor alpha/beta expressing double-negative (CD4−/CD8−) and CD4+ T helper cells in humans augment the production of pathogenic anti-DNA autoantibodies associated with lupus nephritis. J Immunol. 1989;143:103–12. [PubMed] [Google Scholar]
- 11.Anand A, Dean G, Quereshi K, Isenberg D, Lydyard P. Characterization of CD3+CD4-CD8− (double negative) T cells in patients with systemic lupus erythematosus: activation markers. Lupus. 2002;11:493–500. doi: 10.1191/0961203302lu235oa. [DOI] [PubMed] [Google Scholar]
- 12.Voelkl S, Gary R, Mackensen A. Characterization of the immunoregulatory function of human TCR-αβ+ CD4− CD8− double-negative T cells. Eur J Immunol. 2011;41:739–48. doi: 10.1002/eji.201040982. [DOI] [PubMed] [Google Scholar]
- 13.Hillhouse EE, Lesage S. A comprehensive review of the phenotype and function of antigen-specific immunoregulatory double negative T cells. J Autoimmun. 2012:1–8. doi: 10.1016/j.jaut.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Juvet SC, Zhang L. Double negative regulatory T cells in transplantation and autoimmunity: recent progress and future directions. J Mol Cell Biol. 2012 Feb;4:48–58. doi: 10.1093/jmcb/mjr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochberg M. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 16.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J. Rheumatol. 2004 Feb;31:390–2. [PubMed] [Google Scholar]
- 17.Seif AE, Manno CS, Sheen C, Grupp SA, Teachey DT. Identifying autoimmune lymphoproliferative syndrome in children with Evans syndrome: a multi-institutional study. Blood. 2010;115:2142–5. doi: 10.1182/blood-2009-08-239525. [DOI] [PubMed] [Google Scholar]
- 18.Niemela JE, Hsu AP, Fleisher TA, Puck JM. Single nucleotide polymorphisms in the apoptosis receptor gene TNFRSF6. Mol Cell Probes. 2006 Feb;20:21–6. doi: 10.1016/j.mcp.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Appay V, Reynard S, Voelter V, Romero P, Speiser DE, Leyvraz S. Immuno-monitoring of CD8+ T cells in whole blood versus PBMC samples. J Clin Immunol. 2006 Feb 20;309:192–9. doi: 10.1016/j.jim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Teachey DT, Seif AE, Grupp SA. Advances in the management and understanding of autoimmune lymphoproliferative syndrome (ALPS) Brit J Haematol. 2009;148:205–16. doi: 10.1111/j.1365-2141.2009.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleesing JJ, Brown MR, Novicio C, Guarraia D, Dale JK, Straus SE, et al. A Composite Picture of TcRα/β+ CD4−CD8− T Cells (α/β-DNTCs) in Humans with Autoimmune Lymphoproliferative Syndrome. Clin Immunol. 2002 Jul;104:21–30. doi: 10.1006/clim.2002.5225. [DOI] [PubMed] [Google Scholar]
- 22.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011 Oct;17:1290–7. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henson SM, Riddell NE, Akbar AN. Properties of end-stage human T cells defined by CD45RA re-expression. Curr Opin Immunol. 2012 Aug;24:476–81. doi: 10.1016/j.coi.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Mehal WZ, Crispe IN. TCR ligation on CD8+ T cells creates double-negative cells in vivo. J Immunol. 1998;161:1686–93. [PubMed] [Google Scholar]
- 25.Crispín JC, Tsokos GC. Human TCR-alpha beta+ CD4− CD8− T cells can derive from CD8+ T cells and display an inflammatory effector phenotype. J Immunol. 2009;183:4675–81. doi: 10.4049/jimmunol.0901533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisitkun P, Ha H-L, Wang H, Claudio E, Tivy CC, Zhou H, et al. Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity. 2012;37:1104–15. doi: 10.1016/j.immuni.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer K, Voelkl S, Heymann J, Przybylski GK, Mondal K, Laumer M, et al. Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8− double-negative regulatory T cells. Blood. 2005;105:2828–35. doi: 10.1182/blood-2004-07-2583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.