Abstract
Several mouse models of multiple sclerosis (MS) are now available. We have established a mouse model, in which ocular infection with a recombinant HSV-1 that expresses murine IL-2 constitutively (HSV-IL-2) causes CNS demyelination in different strains of mice. This model differs from most other models in that it represents a mixture of viral and immune triggers. In the present study, we directly compared MOG35–55, MBP35–47, and PLP190–209 models of EAE with our HSV-IL-2-induced MS model. Mice with HSV-IL-2-induced and MOG-induced demyelinating diseases demonstrated a similar pattern and distribution of demyelination in their brain, spinal cord, and optic nerves. In contrast, no demyelination was detected in the optic nerves of MBP- and PLP-injected mice. IFN-β injections significantly reduced demyelination in brains of all groups, in the spinal cords of the MOG and MBP groups, and completely blocked it in the spinal cords of the PLP and HSV-IL-2 groups as well as in optic nerves of MOG and HSV-IL-2 groups. In contrast to IFN-β treatment, IL-12p70 protected the HSV-IL-2 group from demyelination, while IL-4 was not effective at all in preventing demyelination. MOG-injected mice showed clinical signs of paralysis and disease-related mortality whereas mice in the other treatment groups did not. Collectively, the results indicate that the HSV-IL-2 model and the MOG model complement each other and, together, provide unique insights into the heterogeneity of human MS.
Keywords: HSV-IL-2, MOG, PLP, MBP, EAE, multiple sclerosis
INTRODUCTION
Multiple sclerosis (MS) affects approximately 400,000 individuals in the United States. The clinical symptomology ranges from relatively benign to devastating. In severe cases, MS can render a person unable to write, speak, or walk with most MS patients experiencing muscle weakness in their extremities and difficulty with coordination and balance 1. The course of the disease differs among affected individuals with some exhibiting relapsing-remitting disease and some progressive disease. Like other presumed autoimmune diseases, MS is more common in females and clinical symptoms are often first manifested during young adulthood 1, 2. The disease is characterized by the development of lesions in the central nervous system (CNS) that have an inflammatory component and frequently, but not always, by demyelination. The location of the lesions within the CNS differs amongst affected individuals and can occur in several different locations in any one individual. Epidemiologic studies of MS have implicated environmental as well as genetic factors in the disease process. Evidence has accumulated that infectious agents, particularly viral, may be involved 3, 4; however, this remains controversial 5–7. If an infectious agent is involved, it alone may not be sufficient to initiate the observed pathology.
The primary experimental animal model for the study of MS is experimental autoimmune encephalitis (EAE), in which the disease is incited by aggressive immunization with specific peptides in both mice and rats 8–11. This model has been used extensively and proven extremely useful. EAE includes a group of disease models that differ in terms of the species and strain of the animals used, the agent/peptides used to elicit the autoimmune response, and the protocols used for immunization including the type of adjuvant employed 8, 10, 12. The disease process is influenced by these variables and, therefore, care must be taken in extrapolating results from one EAE model to another 10, 13.
Other currently available models are based on viral infection 14–20. We found that infection with HSV-1 did not cause demyelination in mice; however when we combined HSV-1 infection with administration of IL-2, an MS-like pathology (CNS inflammatory demyelination) was observed in the infected mice. This inflammatory demyelination occurred when IL-2 was delivered using a viral vector (HSV-IL-2) 21–25 or when mice were injected with IL-2 and then infected with wild-type (WT) HSV-1 26. Ocular infection of different strains of mice with HSV-IL-2 induces CNS demyelination in the periventricular white matter, brain stem, stem cell (SC) white matter and optic nerves (ON) of infected mice, whereas WT HSV-1 or recombinant viruses expressing IL-4, IFN-γ, IL-12p35, IL-12p40, or IL-12p70 do not induce this demyelinating neuropathy 21, 23–25. Similar results are obtained upon delivery of IL-2 into the mouse brain using osmotic mini-pumps or injection of mice with rIL-2 protein, IL-2 DNA, or IL-2 synthetic peptides prior to infection with WT HSV-1 strains McKrae and KOS 26. Notably, a single mutation at position T27A in the IL-2 open-reading frame completely blocked CNS demyelination in this model 26. Thus, the HSV-IL-2 model of CNS demyelination offers a novel alternative experimental animal model for MS that incorporates both a viral and an immune component 21–25.
The availability of two or more independent animal models could prove extremely useful in elucidating the pathogenic mechanisms underlying human disease and identifying effective therapeutic strategies. Effective use of the model depends, however, on a thorough understanding of the similarities and differences among the models, especially in terms of the parameters that are most likely to relate to the human disease. Therefore, in this study we compared the HSV-IL-2 model of CNS demyelination with the MOG35–55, MBP35–47, and PLP190–209 models of EAE using standardized protocols and female C57BL/6 mice of the same age. We also compared the effects of controlling CNS demyelination by delivery of interferon-β (IFN-β), IL-4 and IL-12p70 in the different models. The results of this simultaneous comparison of different models of CNS demyelination provides insight into their possible relevance to each other and, ultimately, to MS.
RESULTS
Demyelination in the CNS
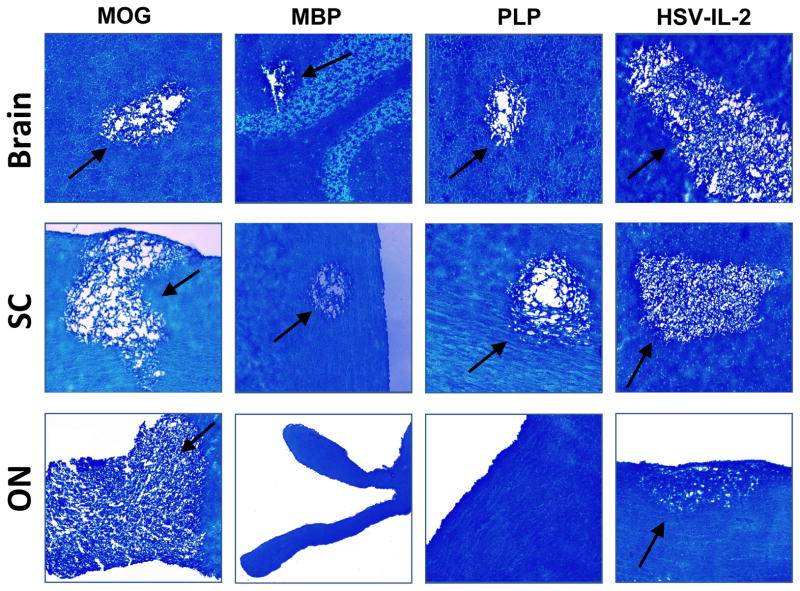
Six-week-old female C57BL/6 mice were administered the MOG, MBP or PLP peptide or infected with HSV-IL-2 recombinant virus as described in Materials and Methods. There were 5–8 mice per group and the treatment groups are referred to as MOG, MBP and PLP and HSV-IL-2 hereafter. At day 29 PI, the mice were sacrificed and the brains, SCs and ONs collected, post-fixed and stained with the myelin stain, LFB. Representative photomicrographs of the LFB-stained brain, SC, and ON sections are shown in Figure 1. Demyelination was observed in the brain and SC of mice in all the treatment groups. Demyelination also was observed in the ON of mice in the MOG or HSV-IL-2 treatment group; however, demyelination was not detected in the ON of mice in the MBP or PLP treatment group.
Fig. 1. Demyelination in CNS of treated mice.
Mice were injected with MOG, MBP, or PLP or infected ocularly with HSV-IL-2 as described in Materials and Methods. After 29 days, brains, SCs, and ONs were removed, sectioned, and stained for LFB. Representative micrographs are shown. 10X direct magnification. Arrows indicate areas of demyelination in the brain, SC, and ON of the treated mice.
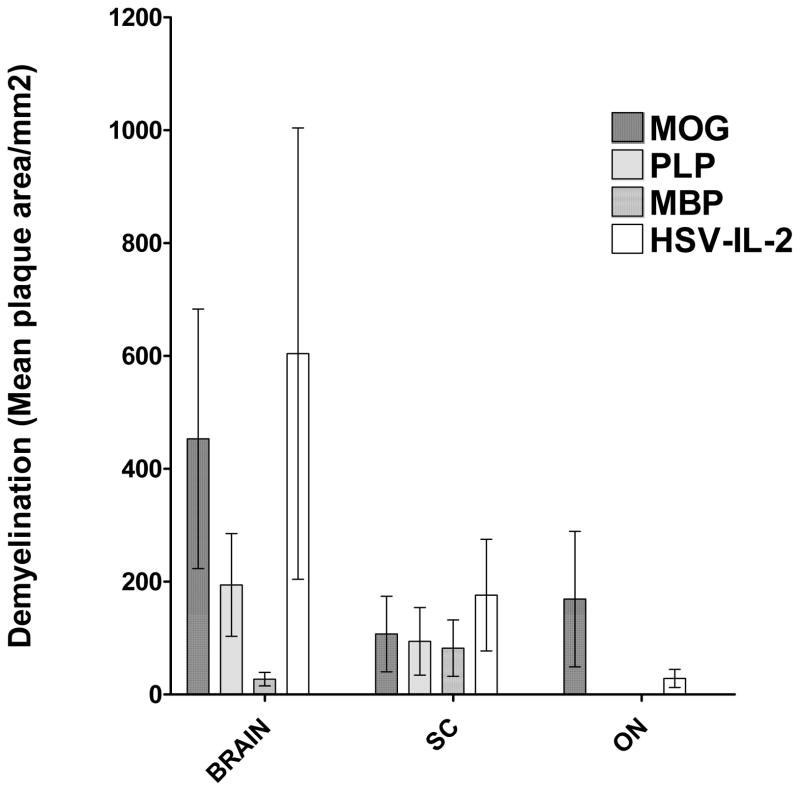
Visual inspection of the slides suggested that the severity of CNS demyelination was greater in the mice in the MOG and HSV-IL-2 treatment groups than in the MBP and PLP treatment groups. To quantify the extent of the demyelination, we counted the number and measured the size of the observed demyelination plaques in the brains, SCs and ONs. The data are shown as the area of demyelination per total stained sections in Figure 2. The area of brain demyelination was significantly greater in the mice in the MOG and HSV-IL-2 treatment groups than in the mice in the PLP or MBP group (Fig. 2, brain). Although the area of brain demyelination was somewhat higher in the HSV-IL-2 group than the MOG group, the differences did not reach statistical significance (Fig. 2, brain, P>0.05). The mice treated in the MBP group showed the lowest degree of brain demyelination out of the four experimental groups (Fig. 2, brain). In contrast to the observed differences in the level of demyelination in the brains of the mice in the different treatment groups, the levels of SC demyelination were similar in all the groups (Fig. 2, SC). The area of demyelination was greater in the ONs of mice in the MOG group than in the ONs of the mice that were ocularly infected with HSV-IL-2 (Fig. 2, ON). No demyelination was detected in the ONs of any of the mice in the PLP or MBP group (Fig. 2, ON).
Fig. 2. Severity of CNS demyelination in treated mice.
The brains, SCs, and ONs of the mice described in Figure 1 above were sectioned, stained with LFB, and the size of the demyelination plaques in the entire sections of brain, SC, and ON determined as described in Materials and Methods. Data are presented as mean demyelination using a total of 150 sections for brain and SC and 30 sections for ON from 5 mice per group.
Relationship between demyelination and inflammation
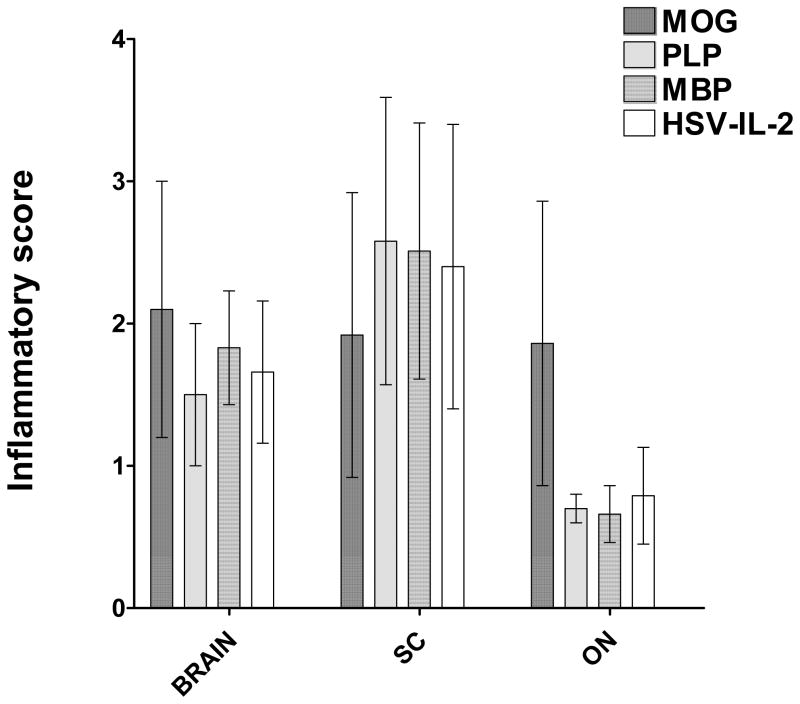
Inflammation at one or more sites in the CNS is associated with the initial event in MS 13, 27, 28. Our results (Figures 1 and 2) suggested that there is greater demyelination in the brains and ONs of the mice in the MOG and HSV-IL-2 treatment groups than those in the PLP and MBP groups. To determine if the extent of demyelination correlated with the level of inflammation, we stained sections from the brains, SCs and ONs with H&E (Figure S1) and estimated the severity of inflammation as described in Materials and Methods. The data are shown as the inflammatory score per total stained sections in Figure 3. The inflammatory scores for the sections of brain and SC from mice in the MOG and HSV-IL-2 treatment groups were similar to those of mice in the PLP and MBP groups (Fig. 3). In contrast, the inflammatory score for ONs of the mice in the MOG treatment group was significantly higher than the scores for mice in the PLP, MBP or HSV-IL-2 groups (Fig. 3). The inflammatory responses in the ONs of the MOG treatment group correlated with the severity of demyelination (Fig. 2, ON). This was an exception, however, and overall, the inflammatory responses did not correlate with the severity of demyelination.
Fig. 3. Severity of CNS inflammation in treated mice.
The brains, SCs, and ONs of the mice described in Figure 1 were sectioned, stained with H&E, and the severity of inflammation in the entire sections of brain, SC, and ON determined as described in Materials and Methods. Data are presented as the mean inflammatory score using a total of 10 random sections of brain, SC, or ON per group.
Cellular composition of leukocytes within the CNS
Many cell types have been implicated in MS and animal models of MS 24, 29–33. To investigate the contribution of T cells, B cells, macrophages and dendritic cells (DCs) to the development of autoimmunity in the CNS of the different models, we assessed the number of immune cells in the brains and SCs by multiparameter flow cytometry analysis of cells stained with anti-CD45, anti-CD3, anti-CD19, anti-CD11b, and anti-CD11c mAbs as described in Materials and Methods. Three main populations of common leukocyte antigen CD45-positive cells were identified in the brains and SCs based on expression of CD3 (Fig. 4A), CD11b (Fig. 4B) and CD11c (Fig. 4C). Mice in the HSV-IL-2 treatment group had the highest number of T cells in the brain and SC as compared to mice in the other groups (Fig. 4A, p<0.05). Among the EAE models, the MOG mice had the highest number of T cells (Fig. 4A, p<0.05). No T cells were detected in SC of control mice (Fig. 4A). The number of CD11b+ cells was high in brains of all all treatment groups, including the mock-treated control group (Fig. 4B, p>0.05). A higher number of CD11b+ cells was also found in the SCs of the mock-treated control group as compared to all the other groups, while the number of CD11b+ cells was lower in the HSV-IL-2 treatment group than in any of the other treatment groups (Fig. 4B, p=0.05). The lower amounts of CD11b+ cells in SC of HSV-IL-2 infected mice suggest that HSV-IL-2 infection suppresses CD11b+ cells in this tissue. There were no significant differences in the number of CD11c+ cells among the groups except that the number of CD11c+ cells were significantly higher in the SCs of the mice in the MOG group (Fig. 4C). Overall, the number of CD11b+ cells was higher (Fig. 4B) as compared to CD3 (Fig. 4A) or CD11c+ (Fig. 4C) in the brains of all treatment groups, including the mock-treated control group. B cell infiltrates were not detected in the brains or SCs of mice in any of the treatment groups (not shown).
Fig. 4. Detection of cellular infiltrates in the brains and spinal cords of treated mice.
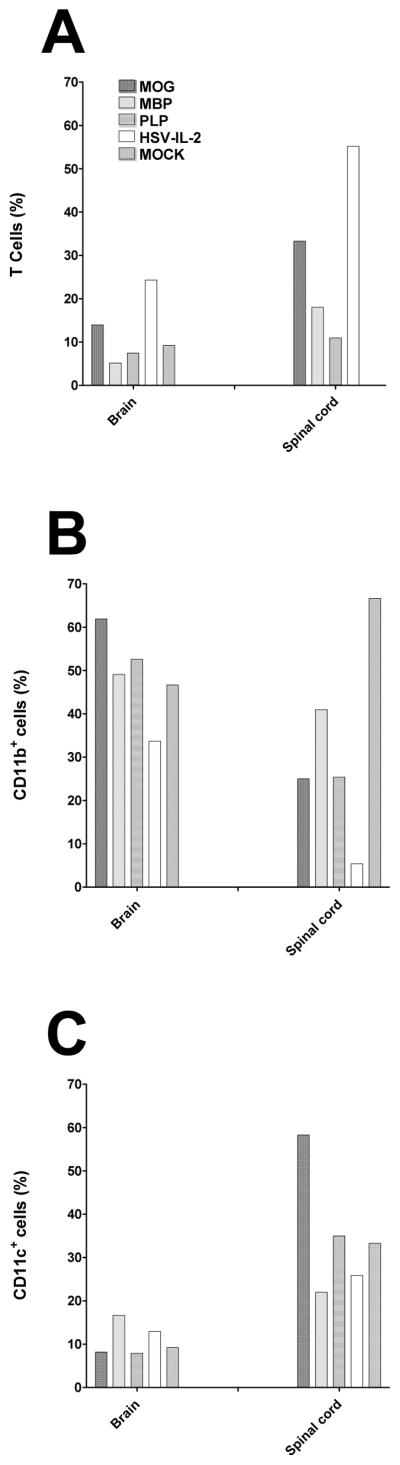
Mice were injected with MOG, PLP, and MBP or infected ocularly with HSV-IL-2. Naive mice were used as a mock control. Fourteen days post-treatment, brain and spinal cord from three mice per group were harvested and digested with collagenase (400UI/TG). The cell suspension was filtered through a 45mm cell strainer and stained with anti-CD45, anti-CD3, anti-CD11b, and anti-CD11c then analyzed by flow cytometry. CD45+ cells were gated for expression of T cells, CD11b, or CD11c. Panels: (A) T cells; (B) CD11b+ cells; and (C) CD11c+ cells.
Hind limb weakness
Some, but not all, patients with MS exhibit limb weakness or paralysis. Early pathological changes in the lumbar spinal cord tissue have been described in a mouse MS model and occurred in the presence or absence of overt clinical symptoms 34. The mice were monitored and scored daily for signs of paralysis for 29 days or until death (if earlier than day 29), using the scoring system described in Material and Methods. The mean day of onset, mean and maximum clinical score, incidence of symptoms and mortality were calculated. Greater than 60% of mice in the MOG treatment group showed signs of muscular weakness (Table 1). Among the mice in the MOG group, the first sign of paralysis (usually tail hypotonia or atonia) appeared on day 11 post-MOG injection and different degrees of hind limb paralysis were observed among these mice until day 29-post injection (the final experimental time point). In contrast, no signs of paralysis were observed in the mice in the MBP (Table 2), PLP (Table 3) or HSV-IL-2 (Table 4) treatment groups within the time-frame of this experiment.
Table 1.
Summary of LFB staining in brain, spinal cord, and ON and clinical symptoms in mice injected with MOG. a
| Demyelination | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brain | Spinal cord | Optic Nerve | Clinical symptom | ||||||||
| Treatment | %b | Meanc ± SEM | % | Mean ± SEM | % | Mean ± SEM | % | Score | Range | Onset (days) | Mortality |
| MOG | 87 | 454±234 | 50 | 108±68 | 37 | 169±133 | 68 | 1.96 | 0–5 | 12.4 | 12.5% |
| MOG+IFN-β DNA | 50% | 68±36 | 50 | 24±17 | 0 | 0 | 50% | 0.5 | 0–0.5 | 20 | 0 |
| MOG+HSV-IL-12p70 | 100 | 401±140 | 100% | 100±17 | 67% | 180±39 | 100 | 2.88 | 0–5 | 14 | 33% |
| MOG+HSV-IL-4 | 80 | 501±159 | 60 | 152±71 | 60 | 155±89 | 80 | 1.92 | 0–5 | 14.5 | 40% |
Mice were immunized with MOG peptide as we described in Materials and methods. Some of the MOG immunized mice were injected with IFN-β DNA, while some mice were ocularly infected with 2X107 PFU/eye of HSV-IL-12p70 or HSV-IL-4. The presence of demyelination in ONs, SCs, and brains were assessed on day 29 post treatment. Clinical symptomatology was assessed daily for 29 days or until death.
Percent of mice showing demyelination.
Area of plaque/section ± standard error of mean (SEM) in treated mice.
Table 2.
Summary of LFB staining in brain, spinal cord, and ON and clinical symptoms in mice injected with MBP. a
| Demyelination | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain | Spinal cord | Optic Nerve | Clinical symptom | ||||||
| Treatment | %b | Meanc ± SEM | % b | Meanc ± SEM | % b | Meanc ± SEM | % | Score | Mortality |
| MBP | 71 | 27±13 | 57 | 83±51 | 0 | 0 | 0 | 0 | 0 |
| MBP+IFN-β DNA | 33 | 4±2 | 33 | 3±2 | 0 | 0 | 0 | 0 | 0 |
| MBP+HSV-IL-12p70 | 67 | 35±15 | 67 | 105±10 | 0 | 0 | 0 | 0 | 0 |
Mice were immunized with MBP peptide as we described in Materials and methods. Some of the MOG immunized mice were injected with IFN-β DNA, while some mice were ocularly infected with 2X107 PFU/eye of HSV-IL-12p70. The presence of demyelination in ONs, SCs, and brains was assessed on day 29 post treatment. Clinical symptomatology was assessed daily for 29 days or until death.
Percent of mice showing demyelination.
Area of plaque/section ± standard error of mean (SEM) in treated mice.
Table 3.
Summary of LFB staining in brain, spinal cord, and ON and clinical symptoms in mice injected with PLP. a
| Demyelination | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain | Spinal cord | Optic Nerve | Clinical symptom | ||||||
| Treatment | % b | Meanc ± SEM | % b | Meanc ± SEM | % b | Meanc ± SEM | % | Score | Mortality |
| PLP | 87 | 194±96 | 50 | 82±64 | 0 | 0 | 0 | 0 | 0 |
| PLP+IFN-β DNA | 33 | 5±2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PLP+HSV-IL-12p70 | 100 | 206±18 | 50 | 85±25 | 0 | 0 | 0 | 0 | 0 |
Mice were immunized with PLP peptide as we described in Materials and methods. Some of the MOG immunized mice were injected with IFN-β DNA, while some mice were ocularly infected with 2X107 PFU/eye of HSV-IL-12p70. The presence of demyelination in ONs, SCs, and brains was assessed on day 29 post treatment. Clinical symptomatology was assessed daily for 29 days or until death.
Percent of mice showing demyelination.
Area of plaque/section ± standard error of mean (SEM) in treated mice.
Table 4.
Summary of LFB staining in brain, spinal cord, and ON and clinical symptoms in mice infected with HSV-IL-2. a
| Demyelination | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain | Spinal cord | Optic Nerve | Clinical symptom | ||||||
| Treatment | % b | Meanc ± SEM | % b | Meanc ± SEM | % b | Meanc ± SEM | % | Score | Mortality |
| HSV-IL-2 | 100 | 605±542 | 100 | 176±110 | 100 | 29±18 | 0 | 0 | 0 |
| HSV-IL-2+IFN-β DNA | 67 | 47±9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HSV-IL-2+HSV-IL-12p70 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Mice were ocularly infected with 2X105 PFU/eye of HSV-IL-2 as we described in Materials and methods. Some of the infected mice were injected with IFN-β DNA, while some mice were ocularly infected with 2X107 PFU/eye of HSV-IL-12p70. The presence of demyelination in ONs, SCs, and brains was assessed on day 29 post treatment. Clinical symptomatology was assessed daily for 29 days or until death.
Percent of mice showing demyelination.
Area of plaque/section ± standard error of mean (SEM) in treated mice.
Effect of IFN-β on CNS demyelination
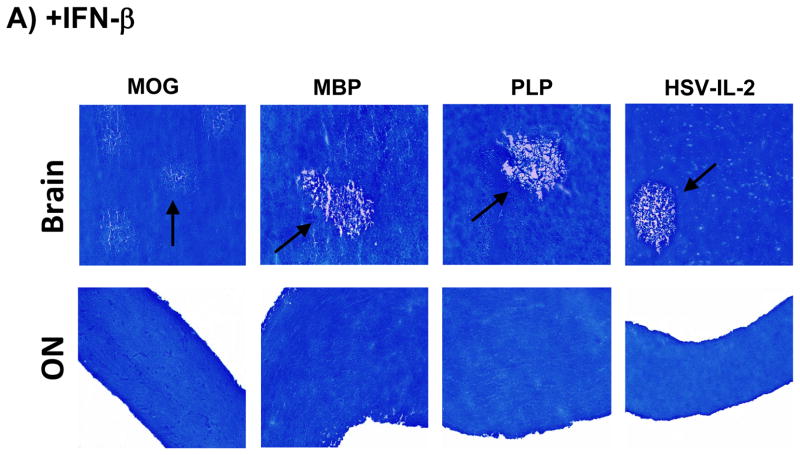
IFN-β is used for the treatment of relapsing-remitting type MS (RRMS). We therefore explored the ability of IFN-β to block or reduce CNS demyelination in the different mouse models. The mice were injected 3X with IFN-β DNA or vector DNA as described in Materials and Methods and demyelination in the brains, SCs and ONs was measured on day 29 post-treatment. Representative photomicrographs of the LFB-stained sections are shown in Figure 5A and the data are summarized in Tables 1–4. Injection with IFN-β DNA reduced demyelination in both the brains and SCs of mice in the MOG group by 50% and completely blocked the demyelination in the ONs (Figure 5A, Table 1). Moreover, the intramuscular injections of IFN-β DNA delayed the onset of clinical disease, reduced the severity of clinical symptoms and eliminated mortality in this group of mice (Table 1). The injection of IFN-β DNA also reduced the levels of demyelination in both the brains and SCs of mice in the MBP group (Fig. 5A, Table 2). The injection with IFN-β DNA resulted in a reduction in demyelination in the brains of mice in the PLP group and demyelination of the SCs was significantly lower than in the vector-treated controls (Fig. 5A, Table 3). Finally, in the HSV-IL-2 group injected with IFN-β, brain demyelination was reduced by more than 90% and SCs and ONs demyelination was blocked completely as compared with the group injected with vector DNA alone (Fig. 5A, Table 4). Overall, IFN-β was effective in reducing or blocking CNS demyelination in all groups but it was most effective in the HSV-IL-2 group followed by the PLP, MBP, and MOG groups. In addition, IFN-β injection of all groups was less efficacious in reducing demyelination in brains of treated mice than in their SCs or ONs. This reduced efficacy is possibly associated with the brain being an immunological privileged site, while SC and ON are not. Thus, the blood-brain barrier may reduce the entrance of IFN-β antigen into the brain but not SC and ON and this may contribute to lower protection from demyelination in all groups.
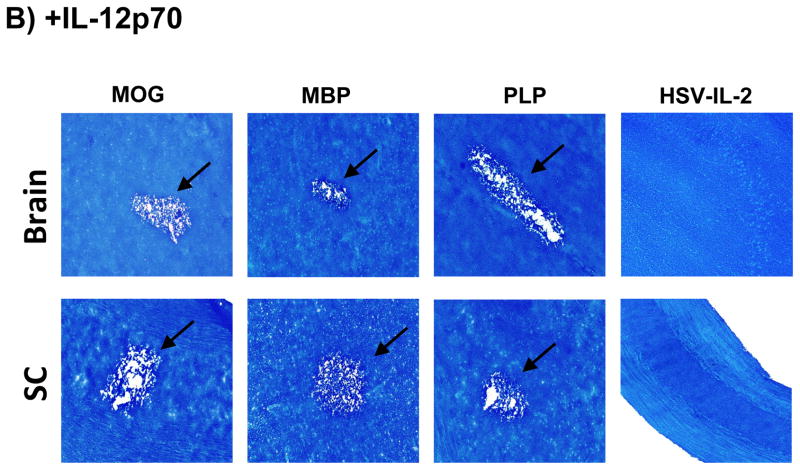
Fig. 5. Effects of IFN-β and IL-12p70 on blocking CNS demyelination.
Mice were treated with MOG, PLP, MBP, or HSV-IL-2 as described in Figure 1 above. Some of the treated mice were injected with IFN-β DNA or infected ocularly with 2X107 PFU/eye of HSV-IL-12p70 as described in Materials and Methods. On day 29 post-treatment with MOG, PLP, MBP or HSV-IL-2, the brains, ONs and SCs were collected, fixed, sectioned, and stained with LFB. Representative photomicrographs are shown. 10X direct magnification. Arrows indicate areas of demyelination. Panels: (A) Effects of IFN-β on blocking CNS demyelination; and (B) Effects of HSV-IL-12p70 on blocking CNS demyelination.
Effects of infection with HSV-IL-12p70 recombinant virus on demyelination in the different models
Previously, we reported that ocular infection of mice with a recombinant HSV-1 expressing IL-12p70 (HSV-IL-12p70) or injection of mice with IL-12p70 DNA completely blocks CNS demyelination in HSV-IL-2-infected mice 25. It has been reported that IL-12p70 is a critical cytokine in the pathogenesis of EAE 35 and subsequently it was shown that the IL-12p40 component of IL-12p70 is involved in EAE-induced CNS pathology 36, 37. Agents that block the common IL-12p70 and IL-23 p40 chain have now been entered into clinical trials 38. Therefore, we evaluated the effects of HSV-IL-12p70 on CNS demyelination in the various models. Mice were injected with MOG, MBP, or PLP as described in figure 1 above. Injected mice were infected ocularly with 2X107 PFU/eye of HSV-IL12p70 and control mice were co-infected with HSV-IL-2 plus HSV-IL12p70 viruses. The clinical signs of disease were monitored from day 1 to day 29 post treatment. At day 29 post ocular infection, the mice were sacrificed and the brains, SCs and ONs were stained with LFB. The results are summarized in Tables 1 to 4 and representative photomicrographs of the LFB-stained sections are shown in Figure 5B. As expected, infection with HSV-IL-12p70 resulted in the inhibition of CNS demyelination in the HSV-IL-2 model (Fig. 5B, Table 4). Infection with HSV-IL-12p70 did not result in inhibition of CNS demyelination in the MOG (Fig. 5B, Table 1), MBP (Fig. 5B, Table 2), or PLP (Fig. 5B, Table 3) models. Indeed, in marked contrast to the protective effect of HSV-IL-12p70 in blocking CNS demyelination in the HSV-IL-2 model, infection with the HSV-IL-12p70 virus exacerbated the level of demyelination and the severity of paralysis and increased mortality in the MOG model (Table 1). However, infection with HSV-IL-12p70 virus did not increase severity of demyelination in the MBP (Table 2) and PLP (Table 3) models. Thus, our results suggest that while IL-12p70 is protective in the HSV-IL-2 model, it promotes demyelination and exacerbates the clinical symptoms in the MOG model.
Effects of co-infection with HSV-IL-4 recombinant virus on demyelination in the MOG model
Previously, it was reported that a recombinant HSV-1 expressing IL-4 protected rhesus monkeys 39 and mice 40, 41 from autoimmune encephalomyelitis. Thus, to determine if MOG-induced CNS demyelination can be blocked by IL-4, mice were injected with MOG and ocularly infected with HSV-IL-4 as described in Materials and Methods. At day 29 post-treatment, mice were sacrificed and the brains, SCs and ONs were collected, postfixed and stained with LFB. The LFB data and the clinical signs of disease are summarized in Table 1. The results indicated that demyelination or paralysis was not reduced in mice that were infected with HSV-IL-4 (Table 1). These results were similar to our previous report showing that HSV-IL-4 did not block HSV-IL-2-induced CNS demyelination 25.
DISCUSSION
The availability of animal models is very helpful, if not critical, for understanding the pathogenesis of MS and ultimately developing methods to control the disease. We recently have described a mixed model of MS that uses both a viral and an immune component to induce CNS demyelination 21–25. In this model, constitutive expression of murine IL-2 by a recombinant HSV-1 (HSV-IL-2) causes CNS demyelination in different strains of mice. EAE is the primary experimental animal model for the study of MS with significant variation on the type of disease models 8, 42. Thus, we initiated this study to answer the following questions: (1) what are the similarities/differences between HSV-IL-2 model and the EAE models? (2) What are the similarities/differences among the entire group of models? And (3) do these models represent different types of MS? The results obtained through this comprehensive, comparative study revealed differences in clinically relevant parameters between the models. Demyelination was observed in the brains and SCs of all four models. Demyelination of the ON in the HSV-IL-2 model, as reported previously 21–25 was also detected in the MOG model. In contrast to the HSV-IL-2 and MOG models, demyelination was not observed in the ON of MBP or PLP models. Inflammatory demyelination of ON, also called optic neuritis, is a common cause of visual and neurologic dysfunction in young adults diagnosed with MS 43–47. Early manifestations of MS often involve degradation of visual acuity and color vision or visual field deficits that result in impaired vision or even blindness 43–47. The results of the Optic Neuritis Treatment Trial, a critical longitudinal prospective cohort study, established a close relationship between optic neuritis and MS 48. About 50–70% of patients with monosymptomatic optic neuritis have clinically silent MS-like lesions on MRI of the brain 43. On longer follow-up, at least 50% of the monosymptomatic optic neuritis patients will be diagnosed as having MS 43–47, and the 15-year risk of developing MS after one episode of optic neuritis is 50% based on clinical criteria alone 48. Measurement of visual-evoked cortical potentials (VECPs) is widely used to assess patients suspected of having MS because of the high sensitivity of this technique and its ability to detect even clinically silent lesions of the visual pathway 49. We have reported previously that mice ocularly infected with HSV-IL-2 showed a significant delay in terms of VECP responses as compared with mice infected with control viruses 23. The results of this comparative study suggest that the HSV-IL-2 model and MOG model of CNS demyelination may be better models of this aspect of MS than the MBP or PLP model.
Using knockout mice, depletion studies and transfer studies, we have established that both CD4+ and CD8+ T cells are involved in the HSV-IL-2-induced demyelination 24. B cells, DCs, and NK cells do not play a role in the HSV-IL-2-induced demyelination 24. Similarly, in the present study, we found elevation in the number of T cells but not B-cells in the brain and SC of HSV-IL-2 infected mice. These findings are consistent with the published data concerning histologic analyses of specimens obtained from patients with MS at autopsy, which have shown a possible correlation between the presence of T cells and the development of demyelinating lesions 50–52. The results are also consistent with the reports that demyelination induced by mouse hepatitis virus (MHV) is associated with T cells 53. In the EAE model of MS, T cells have been associated with CNS demyelination 50, 54 but macrophages also have been implicated in CNS pathology and MS 55, 56 although they may play only an indirect role. In the current study, we observed a suppression of CD11b+ cells in the SC of the HSV-IL-2 group. Previously, we have shown that HSV-IL-2 infection of macrophages alters the balance of IL-12p35 and IL12p40 transcripts 24 and we also have reported that depletion of macrophages induces CNS demyelination following ocular infection of depleted mice with WT HSV-1 22. The suppressive effect of HSV-IL-2 on the balance of IL-12p35 and IL12p40 transcripts were reversed by IL-12p70 22, 24, 25. Similarly, in this study we showed that co-infection of mice with HSV-IL-2 and HSV-IL12p70 completely blocked CNS demyelination in infected mice. In contrast to the results obtained using the HSV-IL-2 model, infection of mice in the MOG treatment group with HSV-IL-12p70 exacerbated the disease outcome. Macrophages are the main source of IL-12 production 57, 58 and blocking the CNS demyelination in HSV-IL-2 infected mice by IL-12p70 is probably due to compensation for the suppressive effect of IL-2 on IL-12p70 formation. IL-12p70 was considered to be a critical cytokine in the pathogenesis of EAE 35. However, later studies showed that although the IL-12p40 component of IL-12p70 is involved in EAE-induced CNS pathology, this effect is mediated by the binding of the IL-12p40 to IL-23p19 rather than its binding to IL-12p35 36, 37. In the current study, in contrast to the suppression of macrophages observed in the HSV-IL-2-treated mice, we did not find an alteration in the number of macrophages in the MOG model. Thus, the exacerbation of disease in the MOG model following infection with HSV-IL-12p70 may be due to the imbalance of IL-12p40 and IL-12p35 and this could contribute to the development of the autoreactive T cells. Previously, we found that the CNS demyelination observed in HSV-IL-2-infected mice was exacerbated on co-infection with HSV-IFN-γ recombinant virus even though mice infected with HSV-IFN-γ recombinant virus alone do not develop CNS demyelination 25.
IL-4 is a pleiotropic lymphokine synthesized primarily by activated TH2 lymphocytes 59, 60. The levels of IL-4, have been reported to be lower than normal in the sera of patients with MS 61, 62. Recombinant IL-4 (rIL-4) has been shown to diminish demyelination and improve the clinical course of mice with EAE, apparently by influencing developing T cells to assume a protective role 40. When fed to mice, retinoids, a group of chemicals that increase IL-4 and TGF-α, also improve the course of EAE 63. In addition, it has been shown that CNS gene therapy with HSV-1 vectors expressing IL-4 protected rhesus monkeys 39 and mice 40, 41 from autoimmune encephalomyelitis. However, in this study a recombinant HSV-1 expressing murine IL-4 (HSV-IL-4) did not block CNS demyelination in the MOG model. Similarly, we reported previously that CNS demyelination was not blocked in mice co-infected with HSV-IL-2 and HSV-IL-4 25. The discrepancy between the results of this study and previous studies could be due to the type of antigen, strain of mice, and dose of infection.
IFN-β is a first-line therapy for patients with relapsing–remitting MS due to its suppression of inflammation in the CNS 64. In our current study, we found that injection of mice with IFN-β DNA significantly reduced or blocked CNS demyelination in all the disease models used in this study. In addition, it delayed the clinical sign of paralysis and the severity of paralysis in the MOG mice. In this study, we only detected paralysis in the MOG mice. We were not able to induce paralysis in the MBP or PLP mice even when a higher dose of peptides was used in the protocol. Similarly, the HSV-IL-2-treated mice showed no signs of paralysis as late as 29 days of follow-up even though they exhibited extensive CNS demyelination and inflammation. However, occasionally we have detected some paralysis in female BALB/c mice infected ocularly with HSV-IL-2 virus.
The results of our study are summarized in Tables 1–5 and indicate that: (1) The pattern of demyelination is the same in the MOG- and HSV-IL-2-treated mice, in which demyelination occurred in the brain, SC and ON. In contrast, MBP and PLP did not induce ON demyelination; (2) The development of the demyelinated lesions after IFN-β DNA intramuscular injection was reduced in the MOG- and MBP-treated mice, while it was significantly reduced in the PLP- and HSV-IL-2-treated mice; (3) In marked contrast to its protective effect in HSV-IL-2-treated mice, infection of mice with HSV-IL-12p70 exacerbated disease in the MOG-treated mice and had no effect on PLP- or MBP-treated mice; (4) IL-4 did not affect demyelination in the MOG-treated mice; and (5) MOG-treated mice were the only group that developed paralysis. In summary, our results suggest that the HSV-IL-2 and MOG models share features that are characteristic of human MS and are complementary in that each has unique attributes that can provide insights into development of the disease.
MATERIALS AND METHODS
Mice, viruses, and cells
Six-week-old Female C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Plaque-purified HSV-IL-2, HSV-IL-4, and HSV-IL-12p70 were grown in rabbit skin (RS) cell monolayers in minimal essential medium (MEM) containing 5% fetal calf serum (FCS) as we described previously 21–25, 65. We have previously described the construction and characterization of HSV-IL-2 and HSV-IL-4 65, 66. Briefly, the complete IL-2 or IL-4 gene, including its polyA site, was inserted into pLAT1.6 just downstream of the LAT promoter. pLAT-IL-2 or pLAT-IL-4 was co-transfected with infectious dLAT2903 DNA by the calcium phosphate method, and a single plaque containing the IL-2 or gene was plaque-purified as described 65, 66. HSV-IL-2 or HSV-IL-4 therefore transcribes mouse IL-2 or IL-4 driven by the LAT promoter, respectively. We chose the LAT promoter since it is the only HSV-1 promoter that expresses high levels of transcripts both in response to primary infection and during periods of latency 67, 68. Construction and characterization of HSV-IL-12p70 (also call M002), a γ134.5-deleted HSV that expresses both murine IL-12p35 and IL-12p40 in-frame as a heterodimer under the transcriptional control of the murine early-growth response-1 promoter (Egr-1), has been described previously 69. In contrast to HSV-IL-2, ocular infection of mice with HSV-IL-4 or HSV-IL-12p70 does not cause CNS demyelination 24, 25.
Ocular infection
Ocular infection was carried out using the standard protocol, we have used routinely 21, 22, 65. Mice were infected or co-infected ocularly with 2 × 105 PFU/eye of HSV-IL-2 or 2 × 107 PFU/eye of HSV-IL-4 or HSV-IL-12p70. Each virus was suspended in 2 μl of tissue culture media and administered as an eye drop.
Peptide synthesis
Mouse myelin basic protein (MBP35–47) (TGILDSIGRFFSG), mouse myelin proteolipid protein (PLP190–209) (SKTSASIGSLCADARMYGVL), and mouse myelin oligodendrocyte glycoprotein (MOG35–55) (MEVGWYRSPFSRVVHLYRNGK) were synthesized by the Brain Research Center, University of British Columbia as described previously 70. The purity of each synthesized peptide was at least 95% as determined by fast protein liquid chromatography (FLPC) analyses.
Peptide administration
A standard protocol was used for EAE induction using synthetic peptides described above 70, 71. Mice were injected subcutaneously with 200 μg of each peptide and 100 μl of Complete Freund’s adjuvant (CFA) in a total volume of 200 μl. Four hours before peptide injection, each mouse received 200 ng of purified Bordetella pertussis toxin (CalBiochem, San Diego, CA) in 100 μl of sterilized water intraperitoneally (i.p.) and this was repeated 48 h post-myelin peptide injection.
Clinical evaluation
All mice were examined daily for 29 days for neurologic clinical signs according to the following criteria: 0, normal/no overt sign of disease; 0.5, tail hypotonia; 1, tail atonia or hind limb weakness; 2, limb tail and hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; and 5, moribund state; death.
Isolation of CNS cells and fluorescence-activated cell sorting (FACS) analysis
The brains and spinal cords (SCs) of mice that were treated with MBP, PLP, MOG, and HSV-IL-2 or mock treated were harvested after perfusion on day 14 post-treatment. The tissues from three mice per each treatment group were combined, cut into small pieces and then treated with 3 mg/ml of collagenase type I (Sigma Chemical Co.) for 2.5 h at 37°C, with gentle passage 3–4X through an eighteen-gauge syringe after the first hour. The single cell suspensions of total CNS cells including mononuclear cells (MNCs) were isolated using Percoll gradients. Single-cell suspensions of brains and SCs from 3 mice per treatment group were stained with anti-CD45, anti-CD3, anti-CD19, anti-CD11b and anti-CD11c mAbs (BD PharMingen, San Diego, CA) and analyzed using a multicolor five laser FACScan instrumentation (Becton Dickinson).
Preparation of optic nerve, spinal cord, and brain for pathologic analysis
The brains, spinal cords (SCs) and optic nerves (ONs) were removed at necropsy on day 29 post treatment. Tissues were snap-frozen in an isopentane-liquid nitrogen bath and stored at −80°C. Transverse sections of each tissue, 8–10 μm thick, were cut, air-dried overnight, and fixed in acetone for 3 min at 25°C 23–25, 72.
Analysis of demyelination using Luxol Fast Blue (LFB) staining
The presence or absence of demyelination in the brains, SCs and ONs was evaluated using LFB staining of formalin-fixed sections as we described previously 21–25. The number of plaques, size of plaques, and shape of plaques on multiple fields were evaluated by investigators who were blinded to the treatment groups using serial sections of CNS tissues. The amount of myelin loss in the stained sections of brains, SCs and ONs was measured using the NIH Image J software analysis system. The areas of demyelination (clear-white) to normal tissue (blue) were quantified using 150 random sections from the brain and SCs or 30 sections from ONs of each animal. Demyelination in each section was confirmed by monitoring adjacent sections. The percentage of myelin loss was calculated by dividing the lesion size into the total area for each section.
Analysis of inflammation using Hematoxylin and Eosin (HE) staining
The inflammatory responses were quantified using 10 random sections of each brain, SC and ON of each animal. The H & E staining protocol consisted of the following steps: slides were rewarmed to RT (for 5–10 min), deparaffinized and dehydrated in Xylene 3X for 3 min, then 95% ethanol 3X for 1 min, followed by 100% ethanol 3X for 1 min. Slides were washed in tap water for 1 min and then distilled water for 1 min. Hematoxylin solution was applied for 2 min, Clarifier solution for 1 minute and running tap water for 1 min, followed by Bluing Solution for 1 min and running tap water for 1 min. Slides were dipped in in Eosin 8X, washed and cleared with 95% ethanol for 1 min, 2 times, then 100% ethanol 3X for 1 min and Xylene 3X for 1 min. Coverslips were mounted and microscopic analysis of inflammation was performed the following day by investigators who were blinded to the treatment groups, based on the following scale: 0, no infiltration; 1, perivascular infiltration; 2, perivascular inflammatory cuff; and 3, inflammation of CNS substance.
DNA injection
The complete open-reading frame (ORF) for IFN-β (pORF-mIFNβ) was purchased from InvivoGen (San Diego, CA). Plasmid DNA was purified using a cesium chloride gradient. In each experiment, mice were injected 3X intramuscularly (in the quadriceps) using a 27 gauge needle with 100 μg of cesium chloride-purified DNA in a total volume of 50 μl of PBS. As a negative control, we used mock-treated mice that were similarly injected with vector DNA alone. The mice were injected on days 0, 7, and 14 relative to peptide injection or HSV-IL-2 infection.
Supplementary Material
Table 5.
Summary of presence or absence of demyelination, clinical symptoms, and mortality in experimental groups.
| Demyelination | |||||
|---|---|---|---|---|---|
| Treatment | Brain | SC | ON | Clinical symptom | Mortality |
| MOG | Yes | Yes | Yes | Yes | Yes |
| MBP | Yes | Yes | No | No | No |
| PLP | Yes | Yes | No | No | No |
| HSV-IL-2 | Yes | Yes | Yes | No | No |
Acknowledgments
This work was supported by Public Health Service grants EY15557, EY13615, and AI093941. We thank Dr. James M. Markert of the Division of Neurosurgery, Department of Surgery, University of Alabama at Birmingham for providing the M002 (HSV-IL-12p70) virus.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary information is available at Gene Therapy’s website.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–52. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Hunter SF, Weinshenker BG, Carter JL, Noseworthy JH. Rational clinical immunotherapy for multiple sclerosis. Mayo Clin Proc. 1997;72(8):765–80. doi: 10.1016/S0025-6196(11)63598-2. [DOI] [PubMed] [Google Scholar]
- 3.Challoner PB, Smith KT, Parker JD, MacLeod DL, Coulter SN, Rose TM, et al. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc Natl Acad Sci U S A. 1995;92(16):7440–4. doi: 10.1073/pnas.92.16.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman JE, Lyons MJ, Cu G, Ablashl DV, Whitman JE, Edgar M, et al. The association of the human herpesvirus-6 and MS. Mult Scler. 1999;5(5):355–62. doi: 10.1177/135245859900500509. [DOI] [PubMed] [Google Scholar]
- 5.Boman J, Roblin PM, Sundstrom P, Sandstrom M, Hammerschlag MR. Failure to detect Chlamydia pneumoniae in the central nervous system of patients with MS. Neurology. 2000;54(1):265. doi: 10.1212/wnl.54.1.265. [DOI] [PubMed] [Google Scholar]
- 6.Martin C, Enbom M, Soderstrom M, Fredrikson S, Dahl H, Lycke J, et al. Absence of seven human herpesviruses, including HHV-6, by polymerase chain reaction in CSF and blood from patients with multiple sclerosis and optic neuritis. Acta Neurol Scand. 1997;95(5):280–3. doi: 10.1111/j.1600-0404.1997.tb00210.x. [DOI] [PubMed] [Google Scholar]
- 7.Mirandola P, Stefan A, Brambilla E, Campadelli-Fiume G, Grimaldi LM. Absence of human herpesvirus 6 and 7 from spinal fluid and serum of multiple sclerosis patients. Neurology. 1999;53(6):1367–8. doi: 10.1212/wnl.53.6.1367-a. [DOI] [PubMed] [Google Scholar]
- 8.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain: a journal of neurology. 2006;129(Pt 8):1953–71. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 9.t Hart BA, Hintzen RQ, Laman JD. Multiple sclerosis - a response-to-damage model. Trends in molecular medicine. 2009;15(6):235–44. doi: 10.1016/j.molmed.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Ransohoff RM. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat Neurosci. 2012;15(8):1074–7. doi: 10.1038/nn.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.t Hart BA, Gran B, Weissert R. EAE: imperfect but useful models of multiple sclerosis. Trends in molecular medicine. 2011;17(3):119–25. doi: 10.1016/j.molmed.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 13.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–87. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 14.Ferrante P, Omodeo-Zorini E, Caldarelli-Stefano R, Mediati M, Fainardi E, Granieri E, et al. Detection of JC virus DNA in cerebrospinal fluid from multiple sclerosis patients. Mult Scler. 1998;4(2):49–54. doi: 10.1177/135245859800400202. [DOI] [PubMed] [Google Scholar]
- 15.Morris MM, Dyson H, Baker D, Harbige LS, Fazakerley JK, Amor S. Characterization of the cellular and cytokine response in the central nervous system following Semliki Forest virus infection. J Neuroimmunol. 1997;74(1–2):185–97. doi: 10.1016/s0165-5728(96)00786-2. [DOI] [PubMed] [Google Scholar]
- 16.Haider S, Nafziger D, Gutierrez JA, Brar I, Mateo N, Fogle J. Progressive multifocal leukoencephalopathy and idiopathic CD4+lymphocytopenia: a case report and review of reported cases. Clin Infect Dis. 2000;31(4):E20–2. doi: 10.1086/318120. [DOI] [PubMed] [Google Scholar]
- 17.Parsons LM, Webb HE. Identification of immunoglobulin-containing cells in the central nervous system of the mouse following infection with the demyelinating strain of Semliki Forest virus. Br J Exp Pathol. 1989;70(3):247–55. [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson S, Shida H, McFarlin DE, Fauci AS, Koenig S. Circulating CD8+ cytotoxic T lymphocytes specific for HTLV-I pX in patients with HTLV-I associated neurological disease. Nature. 1990;348(6298):245–8. doi: 10.1038/348245a0. [DOI] [PubMed] [Google Scholar]
- 19.Haring J, Perlman S. Mouse hepatitis virus. Curr Opin Microbiol. 2001;4(4):462–6. doi: 10.1016/S1369-5274(00)00236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller SD, Olson JK, Croxford JL. Multiple pathways to induction of virus-induced autoimmune demyelination: lessons from Theiler’s virus infection. J Autoimmun. 2001;16(3):219–27. doi: 10.1006/jaut.2000.0489. [DOI] [PubMed] [Google Scholar]
- 21.Osorio Y, La Point SF, Nusinowitz S, Hofman FM, Ghiasi H. CD8+-dependent CNS demyelination following ocular infection of mice with a recombinant HSV-1 expressing murine IL-2. Exp Neurol. 2005;193(1):1–18. doi: 10.1016/j.expneurol.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Mott KR, Gate D, Zandian M, Allen SJ, Rajasagi NK, Van Rooijen N, et al. Macrophage IL-12p70 signaling prevents HSV-1-induced CNS autoimmunity triggered by autoaggressive CD4+ Tregs. Invest Ophthalmol Vis Sci. 2011;52:2321–2333. doi: 10.1167/iovs.10-6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zandian M, Belisle R, Mott KR, Nusinowitz S, Hofman FM, Ghiasi H. Optic neuritis in different strains of mice by a recombinant HSV-1 expressing murine interleukin-2. Invest Ophthalmol Vis Sci. 2009;50(7):3275–82. doi: 10.1167/iovs.08-3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zandian M, Mott KR, Allen SJ, Chen S, Arditi M, Ghiasi H. IL-2 suppression of IL-12p70 by a a recombinant HSV-1 expressing IL-2 induces T cells auto-reactivity and CNS demyelination. PLoS ONE. 2011;6:e16820. doi: 10.1371/journal.pone.0016820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zandian M, Mott KR, Allen SJ, Dumitrascu O, Kuo JZ, Ghiasi H. Use of cytokine immunotherapy to block CNS demyelination induced by a recombinant HSV-1 expressing murine interleukin-2. Gene Ther. 2011;18:734–742. doi: 10.1038/gt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mott KR, Zandian M, Allen SJ, Ghiasi H. Role of IL-2 and HSV-1 in CNS demyelination in mice. J Virol. 2013;87:12102–12109. doi: 10.1128/JVI.02241-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fletcher JM, Lalor SJ, Sweeney CM, Tubridy N, Mills KH. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin Exp Immunol. 2010;162(1):1–11. doi: 10.1111/j.1365-2249.2010.04143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hafler DA. Multiple sclerosis. J Clin Invest. 2004;113(6):788–94. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of monocyte chemoattractant protein-1 and other beta- chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol. 1998;84(2):238–49. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- 30.Li H, Cuzner ML, Newcombe J. Microglia-derived macrophages in early multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1996;22(3):207–15. [PubMed] [Google Scholar]
- 31.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, et al. Expression of costimulatory molecules B7–1 (CD80), B7–2 (CD86), and interleukin 12 cytokine in multiple sclerosis lesions. J Exp Med. 1995;182(6):1985–96. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trotter JL, Clifford DB, McInnis JE, Griffeth RC, Bruns KA, Perlmutter MS, et al. Correlation of immunological studies and disease progression in chronic progressive multiple sclerosis. Ann Neurol. 1989;25(2):172–8. doi: 10.1002/ana.410250211. [DOI] [PubMed] [Google Scholar]
- 33.Wu GF, Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune- mediated demyelination following murine infection with a neurotropic coronavirus. J Virol. 1999;73(10):8771–80. doi: 10.1128/jvi.73.10.8771-8780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori Y, Murakami M, Arima Y, Zhu D, Terayama Y, Komai Y, et al. Early pathological alterations of lower lumbar cords detected by ultrahigh-field MRI in a mouse multiple sclerosis model. Int Immunol. 2013 doi: 10.1093/intimm/dxt044. [DOI] [PubMed] [Google Scholar]
- 35.Karp CL, van Boxel-Dezaire AH, Byrnes AA, Nagelkerken L. Interferon-beta in multiple sclerosis: altering the balance of interleukin-12 and interleukin-10? Curr Opin Neurol. 2001;14(3):361–8. doi: 10.1097/00019052-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Gran B, Zhang GX, Yu S, Li J, Chen XH, Ventura ES, et al. IL-12p35-Deficient Mice Are Susceptible to Experimental Autoimmune Encephalomyelitis: Evidence for Redundancy in the IL-12 System in the Induction of Central Nervous System Autoimmune Demyelination. J Immunol. 2002;169(12):7104–10. doi: 10.4049/jimmunol.169.12.7104. [DOI] [PubMed] [Google Scholar]
- 37.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–8. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 38.Vollmer TL, Wynn DR, Alam MS, Valdes J. A phase 2, 24-week, randomized, placebo-controlled, double-blind study examining the efficacy and safety of an anti-interleukin-12 and -23 monoclonal antibody in patients with relapsing-remitting or secondary progressive multiple sclerosis. Mult Scler. 2011;17(2):181–91. doi: 10.1177/1352458510384496. [DOI] [PubMed] [Google Scholar]
- 39.Poliani PL, Brok H, Furlan R, Ruffini F, Bergami A, Desina G, et al. Delivery to the central nervous system of a nonreplicative herpes simplex type 1 vector engineered with the interleukin 4 gene protects rhesus monkeys from hyperacute autoimmune encephalomyelitis. Hum Gene Ther. 2001;12(8):905–20. doi: 10.1089/104303401750195872. [DOI] [PubMed] [Google Scholar]
- 40.Furlan R, Poliani PL, Marconi PC, Bergami A, Ruffini F, Adorini L, et al. Central nervous system gene therapy with interleukin-4 inhibits progression of ongoing relapsing-remitting autoimmune encephalomyelitis in Biozzi AB/H mice. Gene Ther. 2001;8(1):13–9. doi: 10.1038/sj.gt.3301357. [DOI] [PubMed] [Google Scholar]
- 41.Broberg E, Setala N, Roytta M, Salmi A, Eralinna JP, He B, et al. Expression of interleukin-4 but not of interleukin-10 from a replicative herpes simplex virus type 1 viral vector precludes experimental allergic encephalomyelitis. Gene Ther. 2001;8(10):769–77. doi: 10.1038/sj.gt.3301465. [DOI] [PubMed] [Google Scholar]
- 42.Kuerten S, Angelov DN. Comparing the CNS morphology and immunobiology of different EAE models in C57BL/6 mice - a step towards understanding the complexity of multiple sclerosis. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 2008;190(1):1–15. doi: 10.1016/j.aanat.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Soderstrom M, Ya-Ping J, Hillert J, Link H. Optic neuritis: prognosis for multiple sclerosis from MRI, CSF, and HLA findings. Neurology. 1998;50(3):708–14. doi: 10.1212/wnl.50.3.708. [DOI] [PubMed] [Google Scholar]
- 44.Sandberg-Wollheim M, Bynke H, Cronqvist S, Holtas S, Platz P, Ryder LP. A long-term prospective study of optic neuritis: evaluation of risk factors. Ann Neurol. 1990;27(4):386–93. doi: 10.1002/ana.410270406. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez M, Siva A, Cross SA, O’Brien PC, Kurland LT. Optic neuritis: a population-based study in Olmsted County, Minnesota. Neurology. 1995;45(2):244–50. doi: 10.1212/wnl.45.2.244. [DOI] [PubMed] [Google Scholar]
- 46.O’Riordan JI, Losseff NA, Phatouros C, Thompson AJ, Moseley IF, MacManus DG, et al. Asymptomatic spinal cord lesions in clinically isolated optic nerve, brain stem, and spinal cord syndromes suggestive of demyelination. J Neurol Neurosurg Psychiatry. 1998;64(3):353–7. doi: 10.1136/jnnp.64.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghezzi A, Martinelli V, Torri V, Zaffaroni M, Rodegher M, Comi G, et al. Long-term follow-up of isolated optic neuritis: the risk of developing multiple sclerosis, its outcome, and the prognostic role of paraclinical tests. J Neurol. 1999;246(9):770–5. doi: 10.1007/s004150050453. [DOI] [PubMed] [Google Scholar]
- 48.Optic Neuritis Study G. Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol. 2008;65(6):727–32. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hardmeier M, Hatz F, Naegelin Y, Hight D, Schindler C, Kappos L, et al. Improved Characterization of Visual Evoked Potentials in Multiple Sclerosis by Topographic Analysis. Brain topography. 2013 doi: 10.1007/s10548-013-0318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steinman L. Myelin-specific CD8 T cells in the pathogenesis of experimental allergic encephalitis and multiple sclerosis. J Exp Med. 2001;194(5):F27–30. doi: 10.1084/jem.194.5.f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Traugott U, Reinherz EL, Raine CS. Multiple sclerosis. Distribution of T cells, T cell subsets and Ia- positive macrophages in lesions of different ages. J Neuroimmunol. 1983;4(3):201–21. doi: 10.1016/0165-5728(83)90036-x. [DOI] [PubMed] [Google Scholar]
- 52.Pannemans K, Broux B, Goris A, Dubois B, Broekmans T, Van Wijmeersch B, et al. HLA-E restricted CD8+ T cell subsets are phenotypically altered in multiple sclerosis patients. Mult Scler. 2013 doi: 10.1177/1352458513509703. [DOI] [PubMed] [Google Scholar]
- 53.Wu GF, Dandekar AA, Pewe L, Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus- induced demyelination. J Immunol. 2000;165(4):2278–86. doi: 10.4049/jimmunol.165.4.2278. [DOI] [PubMed] [Google Scholar]
- 54.Zamvil S, Nelson P, Trotter J, Mitchell D, Knobler R, Fritz R, et al. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317(6035):355–8. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 55.Prineas JW, Wright RG. Macrophages, lymphocytes, and plasma cells in the perivascular compartment in chronic multiple sclerosis. Lab Invest. 1978;38(4):409–21. [PubMed] [Google Scholar]
- 56.Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J Immunol. 1998;161(7):3767–75. [PubMed] [Google Scholar]
- 57.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176(5):1387–98. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwarz T. Interleukin-12 and its role in cutaneous sensitization. Res Immunol. 1995;146(7–8):494–9. doi: 10.1016/0923-2494(96)83022-7. [DOI] [PubMed] [Google Scholar]
- 59.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76(2):241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 60.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4- producing cells. J Exp Med. 1990;172(3):921–9. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koehler NK, Genain CP, Giesser B, Hauser SL. The human T cell response to myelin oligodendrocyte glycoprotein: a multiple sclerosis family-based study. J Immunol. 2002;168(11):5920–7. doi: 10.4049/jimmunol.168.11.5920. [DOI] [PubMed] [Google Scholar]
- 62.Kahl KG, Kruse N, Toyka KV, Rieckmann P. Serial analysis of cytokine mRNA profiles in whole blood samples from patients with early multiple sclerosis. J Neurol Sci. 2002;200(1–2):53–5. doi: 10.1016/s0022-510x(02)00136-3. [DOI] [PubMed] [Google Scholar]
- 63.Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, et al. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates ith improved disease course. J Immunol. 1995;154(1):450–8. [PubMed] [Google Scholar]
- 64.Plosker GL. Interferon-beta-1b: a review of its use in multiple sclerosis. CNS drugs. 2011;25(1):67–88. doi: 10.2165/11206430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 65.Ghiasi H, Osorio Y, Perng GC, Nesburn AB, Wechsler SL. Overexpression of interleukin-2 by a recombinant herpes simplex virus type 1 attenuates pathogenicity and enhances antiviral immunity. J Virol. 2002;76(18):9069–78. doi: 10.1128/JVI.76.18.9069-9078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghiasi H, Osorio Y, Perng GC, Nesburn AB, Wechsler SL. Recombinant herpes simplex virus type 1 expressing murine interleukin-4 is less virulent than wild-type virus in mice. J Virol. 2001;75(19):9029–36. doi: 10.1128/JVI.75.19.9029-9036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glorioso JC, Bender MA, Goins WF, Fink DJ, DeLuca N. HSV as a gene transfer vector for the nervous system. Mol Biotechnol. 1995;4(1):87–99. doi: 10.1007/BF02907473. [DOI] [PubMed] [Google Scholar]
- 68.Zwaagstra JC, Ghiasi H, Slanina SM, Nesburn AB, Wheatley SC, Lillycrop K, et al. Activity of herpes simplex virus type 1 latency-associated transcript (LAT) promoter in neuron-derived cells: evidence for neuron specificity and for a large LAT transcript. J Virol. 1990;64(10):5019–28. doi: 10.1128/jvi.64.10.5019-5028.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A. 2000;97(5):2208–13. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirata S, Senju S, Matsuyoshi H, Fukuma D, Uemura Y, Nishimura Y. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol. 2005;174(4):1888–97. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 71.Pham H, Doerrbecker J, Ramp AA, D’Souza CS, Gorasia DG, Purcell AW, et al. Experimental autoimmune encephalomyelitis (EAE) IN C57Bl/6 mice is not associated with astrogliosis. J Neuroimmunol. 2011;232(1–2):51–62. doi: 10.1016/j.jneuroim.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Ghiasi H, Wechsler SL, Kaiwar R, Nesburn AB, Hofman FM. Local expression of tumor necrosis factor alpha and interleukin-2 correlates with protection against corneal scarring after ocular challenge of vaccinated mice with herpes simplex virus type 1. J Virol. 1995;69(1):334–340. doi: 10.1128/jvi.69.1.334-340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.