Abstract
Background
Many women with early-stage breast cancer are working at the time of diagnosis and survive without recurrence. The short-term impact of chemotherapy receipt on employment has been demonstrated, but the long-term impact merits further research.
Methods
We conducted a longitudinal multicenter cohort study of women diagnosed with non-metastatic breast cancer in 2005–2007, as reported to the population-based Los Angeles and Detroit SEER registries. Of 3133 individuals sent surveys, 2290 (73%) completed a baseline survey soon after diagnosis and 1536 (68%) completed a four-year follow-up questionnaire.
Results
Of the 1026 patients aged <65 at diagnosis whose breast cancer did not recur and who responded to both surveys, 746 (76%) worked for pay before diagnosis. Of these, 236 (30%) were no longer working at follow-up. Women who received chemotherapy as part of initial treatment were less likely to work at follow-up (38% vs. 27%, p=0.003). Chemotherapy receipt at the time of diagnosis (OR 1.4, p=0.04) was independently associated with unemployment during survivorship in a multivariable model. Many women who were not employed in the survivorship period wanted to work: 50% reported that it was important for them to work and 31% were actively seeking work.
Conclusions
Unemployment among breast cancer survivors four years after diagnosis is often undesired and appears related to the receipt of chemotherapy during initial treatment. These findings should be considered when patients decide whether to receive adjuvant chemotherapy, particularly when expected benefit is low.
Keywords: employment, breast cancer, chemotherapy, survivorship, work, survey, SEER
Introduction
Over 225,000 women are diagnosed with invasive breast cancer in the US each year,(1) most of whom are of working age and survive through the typical age for retirement. Some work loss during the treatment period is common as patients balance an arduous treatment schedule and acute side effects with work and family life. However, less is known about long-term impact of cancer treatments on paid employment. Because work may be intrinsically rewarding and is also an important source of income, insurance, and social interactions, loss of work may profoundly affect quality of life in addition to causing economic losses for society, particularly when it extends beyond the treatment period. Therefore, understanding the long-term effects of treatment on employment status is a critical focus of survivorship research (2).
Previous studies have primarily evaluated the employment trajectory of breast cancer patients during treatment and soon thereafter. In a population-based study of U.S. patients 9 months after breast cancer diagnosis, we previously reported that 24% had missed over a month of work and 32% had stopped working altogether due to breast cancer or its treatment (3). Similarly, a Dutch study found that only 70% of workers with breast cancer had even partially returned to work one year after breast cancer diagnosis (4). Other studies have suggested that women do eventually return to work. In a longitudinal U.S. study in 2001–02, only 17% of previously employed breast cancer survivors were not working at 18 months (5,6). In a population-based study of Swedish breast cancer patients, only 11% of those who worked prior to diagnosis were not working 16 months later (7). Thus, existing data suggests substantial effects of cancer diagnosis and treatment on employment during the first year after diagnosis but a possible waning of impact by the second year.
Less is known about the long-term employment outcomes of breast cancer survivors, and specifically whether certain subgroups of cancer patients are particularly vulnerable to loss of desired employment during the long-term survivorship period (8). Previous research has suggested that long-term breast cancer survivors are, in general, less likely to be employed than their non-breast cancer counterparts (9,10). Cancer survivors may experience a change in taste for work, prioritizing volunteerism, family, or leisure more after facing a life-threatening illness (11). Survivors might also face discrimination from employers (12–14). Long-term morbidity related to either treatment or disease recurrence may reduce survivors’ ability to work (15–19). Moreover, treatments may have led to periods of missed work that may have lasting consequences on survivors’ subsequent ability to maintain long-term employment.
The potential impact of chemotherapy on long-term employment outcomes, in particular, requires further investigation. We previously found that patients who received chemotherapy were more likely to stop working in the short-term (3), and in a sample of low-income breast cancer survivors, others have found that very poor women who stop working during chemotherapy are at risk of not returning to work in the longer term.(20) Yet others have found no effect of chemotherapy on return to work (6, 21). Moreover, little is known about whether those who fail to return to work are actively seeking work.
Experts in the field have identified desirable methodologic criteria for studies of work after cancer (22), including population-based sampling, longitudinal design, detailed measures, and adequate sample size. We developed a study that fulfilled these criteria and conducted a longitudinal study inquiring about work outcomes in the population-based sample of breast cancer patients we had previously surveyed near the time of diagnosis (3), seeking specifically to investigate whether chemotherapy receipt as part of initial treatment was associated with the employment outcomes of long-term breast cancer survivors.
Methods
Study sample
We conducted a longitudinal, multicenter cohort study of women diagnosed with breast cancer in metropolitan Los Angeles and Detroit. A major prespecified objective of this study was to examine racial/ethnic differences in disruption of paid work for patients with breast cancer into the survivorship period. Patients aged 20–79 years and diagnosed with stage 0–III breast cancer between June 2005 and February 2007, as reported to the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) population-based program registries in those regions, were eligible for sample selection. Using a population-based registry allows for a study sample that is generally representative of the population of incident cancer cases in the respective geographic area in terms of sex, race or ethnicity, age, and other demographic characteristics. We used the rapid case ascertainment method, which allows the SEER registries to identify patients within 1 month of their diagnosis.(23)
Patients were excluded if they had stage IV breast cancer or could not complete a questionnaire in English or Spanish. Asian women in Los Angeles were excluded because of enrollment in other studies (Los Angeles SEER protocol limits patient enrollment into multiple concurrent studies). Latina (in Los Angeles) and black (in both Los Angeles and Detroit) patients were oversampled to ensure sufficient minority representation.
Questionnaire Design and Content
We developed original questionnaires after considering existing literature, measures previously developed to assess relevant constructs (3,24), and theoretical models. Measures in the survey were pretested to maximize reliability and validity and were based on a priori hypotheses generated from preliminary studies which suggested gaps in return to paid work after treatment of breast cancer. Survey content included extensive batteries of questions addressing paid work, financial issues, and other quality of life factors. Additional content of the 38-page initial survey questionnaire and 42-page follow-up survey questionnaire addressed other treatment and care issues relevant during the survivorship period. To avoid response bias, survey recipients received survey questionnaires simply entitled “A Study of Women’s Experiences with Treatment for Breast Cancer.”
Data Collection
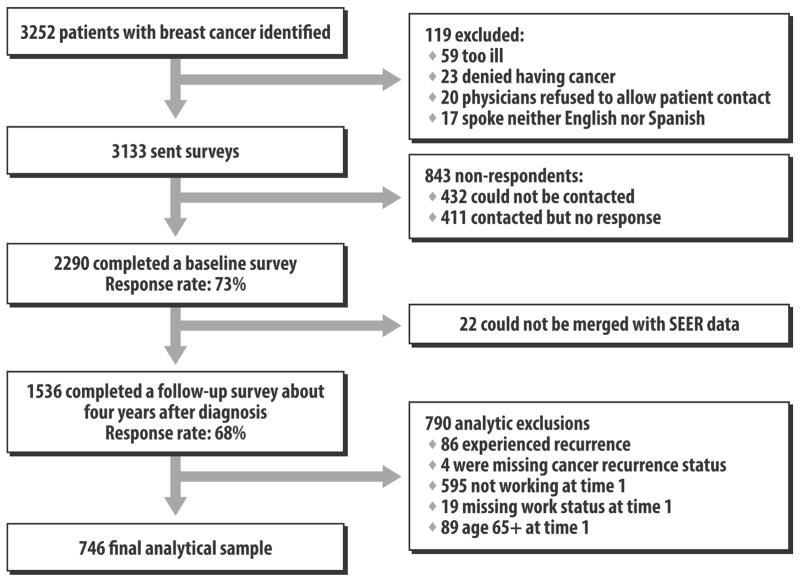
After Institutional Review Board (IRB) approval, eligible patients were identified and informed of all aspects and intent of the study in the survey materials. The IRB approved a waiver of a written signature of informed consent, with the return of a completed survey taken to indicate informed consent. After notifying physicians, we first recruited and surveyed patients a mean of nine months after diagnosis (mean time from diagnosis to survey response 284 days, SD 96). We then contacted all respondents approximately four years later (mean time from diagnosis to survey response 1524 days, SD 143). To encourage response, we provided a $10 cash incentive along with the paper survey mailing and used a modified Dillman method (25), including reminders to non-respondents. All materials were sent in English and Spanish to those with Spanish surnames (26). Responses to the baseline and follow-up surveys were combined into a single dataset, into which clinical data from SEER was merged. The evolution of the sample is detailed in Figure 1.
Figure 1. Flow of Patients into the Study.
This figure depicts the flow of patients into the study from those initially identified to the final analytic sample.
Measures
Our primary dependent variable for analysis was defined by selecting those women who reported working (regardless of whether full- or part-time) prior to diagnosis (as reported in the baseline questionnaire) and then determining which of these reported in the follow-up survey that they were not working at that time.
We considered a number of independent variables. Clinical factors included SEER-reported clinical stage (AJCC Stage 0, I, II, or III) and patient-reported comorbidity and treatment (chemotherapy, radiotherapy, and surgery) measured in the baseline survey. Sociodemographic factors were determined in the baseline questionnaire, including age, race/ethnicity, educational status, family income, marital status, work hours at diagnosis (full time versus less than full-time), and employment support (having a job with sick leave and/or flexible schedule). Geographic site (Los Angeles vs. Detroit) was also included in the analyses.
We measured in the follow-up survey patients’ perceptions of whether, since the time of diagnosis, they were worse off regarding health insurance, employment status, and financial status. We also evaluated, among those not working at the time of the follow-up survey, how important it was for them to work and whether they were actively seeking employment.
Analytic Approach
To allow statistical inferences to be more representative of the original targeted population, we applied survey weights and implemented a multiple imputation method to the calculation of percentages and regression analyses. (27) All percentages reported in the text below are so weighted and reported alongside unweighted Ns. Design weights compensated for the disproportionate selection across race and SEER sites; survey unit non-response weights compensated for the fact that women with certain characteristics were not as likely to respond to the surveys (patients who did not respond to both surveys were more likely to be African American—35.2% v. 26.7%, P<0.001; to be Latina—17.2% vs. 13.3%, p=0.002; to have stage II–III disease—54.9% v. 37.8%, P<0.001; and to have had mastectomy—37.5% vs. 30.8%, P<0.001). Among patients who responded to both surveys, missing data due to survey item non-response constituted 10% of the analytic sample when all covariates in the final model were considered simultaneously. To address missing data from item non-response, we first multiply imputed the data five times followed by combining the results from statistical analyses on these five imputed data sets using Rubin’s formula (28,29). We limited our analytic sample to patients aged <65 at diagnosis, whose breast cancer did not recur before the follow-up survey, who responded to both surveys and reported working for pay before diagnosis in the baseline survey. We examined patterns and correlates of paid work at the time of the follow-up survey using chi-squared tests for univariate analyses and logistic regression for multivariable analyses which included the following theoretically selected independent variables: age, comorbidity, race, education, family income, work hours at diagnosis, employment support, marital status, stage, chemotherapy receipt, surgery type, radiation receipt, and geographic site. In the logistic regression, we tested for interactions between chemotherapy use and other covariates in the model as well as between family income and geographic site. These interactions were not significantly associated with work loss and we subsequently eliminated them from the final model. Collinearity of the covariates was assessed using variance inflation factors (30). All analyses were conducted using SAS 9.2 software (Cary, NC).
Results
Of the 1026 patients aged <65 at diagnosis whose breast cancer did not recur and who responded to both surveys, 746 (76%) reported working for pay before diagnosis in the baseline survey. Of these, 236 (30%) were no longer working at the time of the follow-up survey.
Table 1 describes the clinical and sociodemographic characteristics of the sample, and Table 2 presents the bivariate correlates of employment at the time of the follow-up survey. As shown in the tables, 61% of respondents had received chemotherapy. Women who received chemotherapy as part of their initial cancer treatment were more likely to report that they were not working at the time of the follow-up survey (38% vs. 27%, p=0.003). There was no difference by chemotherapy receipt in the proportion of respondents who considered themselves to be retired at the time of the follow-up survey (13% of patients receiving chemotherapy and 14% of those not receiving chemotherapy, p=0.48).
Table 1.
Characteristics of Patient Sample (n=746)
| Characteristic | N | % of sample* |
|---|---|---|
| Age at Diagnosis | ||
| <46 | 169 | 25.5 |
| 46–55 | 328 | 41.7 |
| 56+ | 248 | 32.7 |
| Comorbidity | ||
| 0 | 186 | 26.5 |
| 1 | 205 | 26.5 |
| 2 or more | 355 | 47.0 |
| Race | ||
| White | 353 | 42.4 |
| Black | 191 | 17.7 |
| Latina | 185 | 38.0 |
| Other | 17 | 1.9 |
| Education | ||
| High school or less | 185 | 29.5 |
| Some college | 282 | 37.0 |
| College graduate or greater | 273 | 33.5 |
| Family Income at Baseline Survey | ||
| <$20,000 | 82 | 21.0 |
| $20,000–$69,999 | 292 | 39.1 |
| $70,000+ | 301 | 39.9 |
| Work Status at Diagnosis | ||
| Employed Full Time | 606 | 80.5 |
| Employed Part Time or Occasional | 140 | 19.5 |
| Employment Support | ||
| Sick leave and/or flexible schedule | 478 | 59.2 |
| None | 268 | 40.8 |
| Marital Status | ||
| Not married or partnered | 293 | 39.7 |
| Married or partnered | 453 | 60.3 |
| Stage | ||
| 0 | 184 | 18.8 |
| I | 255 | 32.5 |
| II | 237 | 37.1 |
| III | 67 | 11.7 |
| Chemotherapy Receipt | ||
| No | 338 | 39.4 |
| Yes | 389 | 60.6 |
| Surgery Type | ||
| Lumpectomy | 478 | 60.5 |
| Mastectomy | 268 | 39.5 |
| Radiation Receipt | ||
| No | 208 | 30.6 |
| Yes | 522 | 69.4 |
| Geographic Site | ||
| Los Angeles | 417 | 79.5 |
| Detroit | 329 | 20.5 |
percentages are weighted and missing values have been imputed.
Table 2.
Bivariate Analyses of Four-Year Unemployment
| Characteristic | % With Four-Year Unemployment * | p |
|---|---|---|
| Age at Diagnosis | .005 | |
| <46 | 30.2 | |
| 46–55 | 28.5 | |
| 56+ | 43.2 | |
| Comorbidity | <.001 | |
| 0 | 17.4 | |
| 1 | 24.7 | |
| 2 or more | 48.1 | |
| Race | <.001 | |
| White | 26.4 | |
| Black | 31.0 | |
| Latina | 43.0 | |
| Other | 39.7 | |
| Education | <.001 | |
| High school or less | 48.6 | |
| Some college | 29.8 | |
| College graduate or greater | 25.0 | |
| Family Income at Baseline Survey | <.001 | |
| <$20,000 | 52.1 | |
| $20,000–$69,999 | 34.7 | |
| $70,000+ | 23.3 | |
| Work Status at Diagnosis | .48 | |
| Employed Full Time | 33.0 | |
| Employed Part Time or Occasional | 36.7 | |
| Employment Support | <.001 | |
| Sick leave and/or flexible schedule | 24.7 | |
| None | 46.8 | |
| Marital Status | .44 | |
| Not married or partnered | 35.7 | |
| Married or partnered | 32.5 | |
| Stage | .020 | |
| 0 | 29.4 | |
| I | 26.8 | |
| II | 39.6 | |
| III | 41.9 | |
| Chemotherapy Receipt | .003 | |
| No | 26.7 | |
| Yes | 38.3 | |
| Surgery Type | .039 | |
| Lumpectomy | 30.4 | |
| Mastectomy | 38.8 | |
| Radiation Receipt | .75 | |
| No | 34.6 | |
| Yes | 33.4 | |
| Geographic Site | .59 | |
| Los Angeles | 34.2 | |
| Detroit | 32.0 |
percentages are weighted and missing values have been imputed.
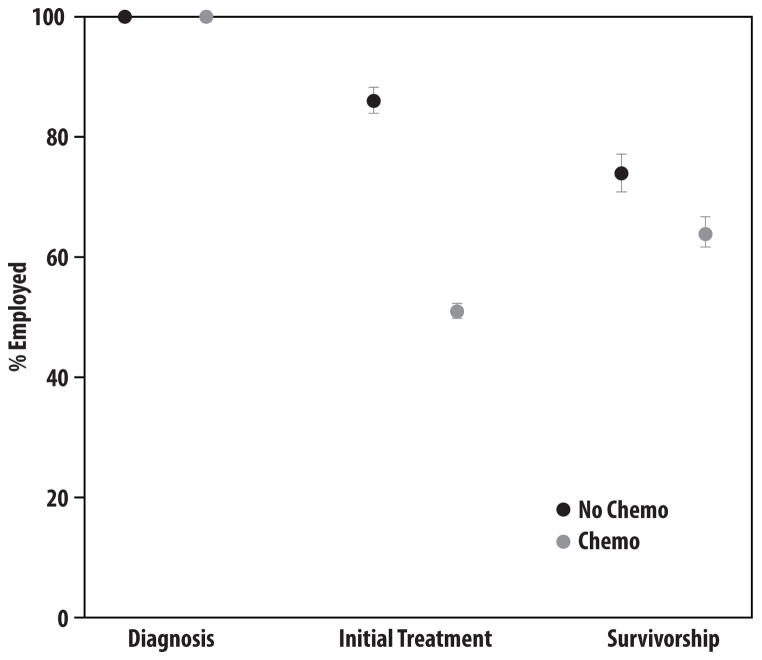
Figure 2 depicts the pattern of employment among women who were employed at the time of breast cancer diagnosis. Women who were employed at diagnosis were substantially less likely to be employed after initial treatment if they had received chemotherapy. Long-term survivors were also less likely to be employed four years after diagnosis if they had received chemotherapy as part of initial treatment.
Figure 2. Employment Outcomes.
This figure depicts the proportion of women working prior to diagnosis, at the time of the baseline survey (approximately 9 months after diagnosis, “the initial treatment period”), and at the time of the follow-up survey (approximately four years later, “the survivorship period”), by chemotherapy receipt.
The excess unemployment observed for women who received chemotherapy began soon after diagnosis. Compared to women who did not get chemotherapy, women who did were more likely to report stopping work 2 or more years prior to the follow-up survey (30% vs. 14%, p<0.001) and more likely to have stopped work during the initial course of therapy (56% vs. 13%, p<0.001). Overall, 26% of chemotherapy patients and 9% of others were not working both after initial treatment and in the long-term; 22% of chemotherapy patients and 7% of others were not working after initial treatment but were working again in the long-term; 11% of chemotherapy patients and 17% of others had not stopped work after initial treatment but were not working in the long-term; and 41% of chemotherapy patients and 67% of others continued working both after initial treatment and in the long-term.
Table 3 presents a multivariable model for four-year unemployment. Chemotherapy recipients at the time of diagnosis were significantly more likely to report unemployment at four years (OR: 1.42, 95% CI: 1.03–1.98). Other significant correlates of four-year unemployment were older age (OR 1.42 for age 56+ compared with <46, 95% CI 1.03–1.95), greater comorbidity (OR 2.16 for 2 or more versus none, 95% CI 1.59–2.94), and lack of employment support (OR 1.33, 95% CI 1.08–1.67).
Table 3.
Multivariable Model of Long-Term Work Loss
| Characteristic | Odds Ratio | P |
|---|---|---|
| Age at Diagnosis | 0.031 | |
| <46 | 1 (ref) | |
| 46–55 | 0.76 (0.57–1.01) | |
| 56+ | 1.42 (1.03–1.95) | |
| Comorbidity | <.001 | |
| 0 | 1 (ref) | |
| 1 | 0.84 (0.61–1.17) | |
| 2 or more | 2.16 (1.59–2.94) | |
| Race | 0.161 | |
| White | 1 (ref) | |
| Black | 0.86 (0.53–1.38) | |
| Latina | 1.42 (0.87–2.33) | |
| Other | 0.94 (0.34–2.56) | |
| Education | 0.31 | |
| High school or less | 1 (ref) | |
| Some college | 0.86 (0.65–1.15) | |
| College graduate or greater | 0.98 (0.7–1.37) | |
| Family Income at Baseline Survey | 0.081 | |
| <$20,000 | 1 (ref) | |
| $20,000–$69,999 | 1.00 (0.74–1.34) | |
| $70,000+ | 0.73 (0.51–1.04) | |
| Work Status at Diagnosis | 0.89 | |
| Employed Full Time | 1 (ref) | |
| Employed Part Time or Occasional | 0.98 (0.75–1.28) | |
| Employment Support | 0.011 | |
| Sick leave and/or flexible schedule | 1 (ref) | |
| None | 1.33 (1.08–1.67) | |
| Marital Status | 0.95 | |
| Not married or partnered | 1 (ref) | |
| Married or partnered | 1.01 (0.63–1.61) | |
| Stage | 0.183 | |
| 0 | 1 (ref) | |
| I | 0.81 (0.55–1.2) | |
| II | 1.04 (0.69–1.55) | |
| III | 1.01 (0.48–2.12) | |
| Chemotherapy Receipt | 0.038 | |
| No | 1 (ref) | |
| Yes | 1.42 (1.03–1.98) | |
| Surgery Type | 0.28 | |
| Lumpectomy | 1 (ref) | |
| Mastectomy | 0.82 (0.57–1.18) | |
| Radiation Receipt | 0.56 | |
| No | 1 (ref) | |
| Yes | 1.12 (0.75–1.68) | |
| Geographic Site | 0.27 | |
| Los Angeles | 1 (ref) | |
| Detroit | 1.14 (0.9–1.43) |
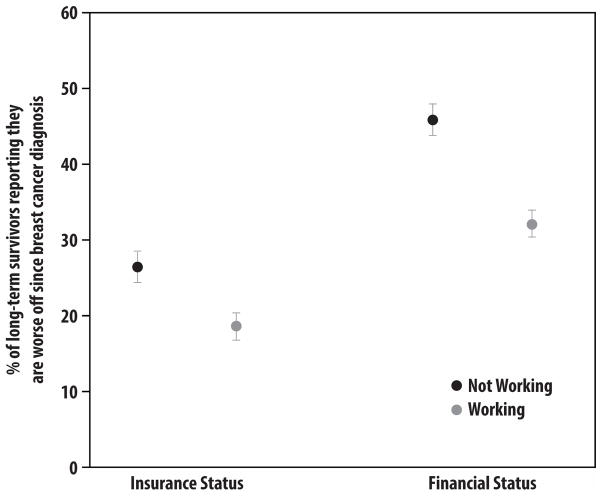
Many women who were not employed in the survivorship period wanted to work. Of the 127 who had not worked since diagnosis, 63 (55%) reported that it was important for them to work and 39 (39%) were actively looking for work. These figures were similar for patients who did and did not receive chemotherapy in the initial treatment period: 31% vs 32% were actively looking for work (p=0.96); and 50% vs 49% reported that work remained important to them (p=0.76). Moreover, those who were no longer working were significantly more likely to report that they were worse off regarding their insurance status and financial status, as depicted in Figure 3 (each p<0.001).
Figure 3. Impact of Employment Status.
This figure demonstrates, by current employment status, the perceptions of long-term breast cancer survivors regarding their insurance status and financial status. Bars represent the proportion of survivors who report being worse off at the time of the follow-up survey (approximately four years after diagnosis).
Discussion
In this longitudinal survey in two diverse U.S. metropolitan areas, about half of the women diagnosed with early stage breast cancer were of working age and had paid employment at time of diagnosis. We found that nearly a third of those employed before diagnosis were no longer working four years later, and many of these women continued to desire employment. Patients who had received chemotherapy as part of their initial course of therapy were less likely to be working four years after diagnosis than patients who did not receive chemotherapy, after controlling for other factors.
Published studies of cancer and employment outcomes have provided limited information about the long-term impact of diagnosis and treatment on breast cancer survivors. In analyses of the Health and Retirement Study (10, 11) and the National Health Interview Study (31), cancer survivors were less likely to work than non-cancer controls. However, absent information on key clinical characteristics such as cancer stage and treatment, the mechanisms by which cancer diagnosis affects long-term employment have remained uncertain.
Understanding which subgroups of cancer patients are most vulnerable to long-term work loss is critical for clinicians and policy-makers seeking to develop appropriate interventions (32). In particular, the impact of treatments and social supports are important considerations, as these are potentially modifiable. Previous studies have suggested an important influence of employment support (3, 6, 7, 33) or chemotherapy receipt (21,34–35) on short-term employment outcomes of breast cancer survivors, including missed work, work hours, and short-term job loss. Our results suggest that both of these factors may also have a long-lasting negative impact on paid employment.
We were particularly interested in chemotherapy as a risk factor for long-term unemployment because of the potential for impact of long-term toxicity such as neuropathy or neurocognitive effects, as well as potential downstream effects of missed work during treatment due to acute toxicity. Few other studies have examined the long-term impact of chemotherapy on employment outcomes. In a study of patients diagnosed with lymphoma, endometrial, or prostate cancer from 1989–1998 in the Netherlands, chemotherapy receipt appeared to increase the risk of work loss (36). In contrast, researchers who interviewed breast cancer patients diagnosed in Quebec in 1996 and 1997 three years later found no association between receipt of chemotherapy and employment status (21). The contrast with our findings may result from changes in chemotherapy regimens and dose intensity by the time of the current study or differences in social policies and employer accommodations in the two countries. The timing of our study, which spanned a period of national economic recession, may also have accentuated the adverse impact of chemotherapy upon employment outcomes. In a recent study of a low-income sample of patients in the U.S., chemotherapy receipt was an independent predictor of long-term failure to return to work, consistent with the current study.(20)
Our study has a number of strengths, including its large, diverse sample, longitudinal design, and access to both clinical data and patient reports of treatment, socioeconomic characteristics, and policy-relevant outcomes. Several limitations also merit comment. First, the study was located in two large metropolitan areas, which may limit the generalizability of the findings, particularly to more rural areas. Second, many of our measures were drawn from patient self-report, which may have introduced bias. However, evidence supports the validity of self-report in this context (37). Third, although we had access to some clinical information, we did lack information on the specific chemotherapy regimens utilized, precluding our ability to differentiate whether certain approaches have greater impact upon employment outcomes. We also lacked sufficient detail on the nature of women’s jobs to include this in the analysis, nor did we have information on spousal employment. Fourth, although the response rate to our surveys was high, it is possible that response bias may also have influenced our results. However, we believe it is very unlikely that correcting non-response bias would attenuate the association of chemotherapy with long-term employment status observed in our study. Although we did not have valid information about chemotherapy receipt at time of sampling, patients who received chemotherapy may have been less likely to complete our baseline survey because it was administered during the treatment period. However, we do not believe that chemotherapy patients who did not respond were less vulnerable to work loss than those who responded. In fact, the opposite may be more plausible, to the extent that those experiencing the greatest acute toxicity from chemotherapy might have been less likely to complete a survey at 9 months post-diagnosis and might in fact have been those most vulnerable to employment loss related to treatment. Thus, we may actually have under-estimated the negative impact of chemotherapy on paid work outcomes. Finally, we also lacked information on the employment outcomes of women without cancer during the time of our study, which spanned a major recession. Although this information was not necessary to address our primary research question regarding the association between adjuvant chemotherapy and long-term employment outcomes, it might have provided potentially interesting context if available. Moreover, as noted above, because the recession may have accentuated any relationship between chemotherapy and subsequent unemployment, the findings of this study should not be generalized to settings in which the economic environment differs substantially from that experienced by the survivors we studied.
As in any observational study, challenges exist in interpreting causation. However, it seems unlikely that women with higher risk of job loss for other reasons would have been more likely to receive chemotherapy. Indeed, we explored other potential explanatory or confounding factors for differential job loss by chemotherapy groups and did not observe an association with chemotherapy receipt, including insurance status, reasons for stopping work (e.g. retirement) or less motivation to continue work (e.g. less importance of work or job-seeking) into the survivorship period. It is, of course, possible that an unmeasured factor might play a confounding role. However, the most plausible candidates for unmeasured factors associated with both chemotherapy receipt and with work loss act in a direction to strengthen rather than weaken the association observed. For example, one unmeasured factor might be the geographic microenvironment. Individuals who live in less populated areas would be expected to have less access to chemotherapy and also less access to jobs.
In sum, this study suggests that loss of paid employment after breast cancer diagnosis may be common, often undesired, not restricted to the treatment period, and potentially related to treatment administered. Many clinicians believe that although patients may miss work during treatment, they will “bounce back” in the longer term. Our study suggests otherwise and highlights a possible adverse consequence of adjuvant chemotherapy. Our findings support current efforts to reduce the morbidity and burden of treatments for breast cancer (38). Indeed, initiatives to reduce the morbidity and burden of treatments for breast cancer are actively being evaluated, including better strategies to identify patients who might omit adjuvant chemotherapy because the marginal benefit is small (39–41). Our study reinforces the need to advance these evaluative strategies to help physicians “first, do no harm.”
Supplementary Material
Acknowledgments
Funding: Grants R01 CA109696 and R01 CA088370 from the National Cancer Institute (NCI) to the University of Michigan. Dr. Jagsi was supported by a Mentored Research Scholar Grant from the American Cancer Society (MRSG-09-145-01). Dr. Katz was supported by an Established Investigator Award from the NCI (K05CA111340). The collection of LA County cancer incidence data used was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the NCI’s Surveillance, Epidemiology and End Results (SEER) Program under contract N01-PC-35139 awarded to the University of Southern California, contract N01-PC-54404 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement 1U58DP00807-01 awarded to the Public Health Institute. The collection of metropolitan Detroit cancer incidence data was supported by the NCI SEER Program contract N01-PC-35145.
Footnotes
Presented in preliminary form as an oral presentation at the ASCO Annual Meeting, Chicago, Illinois, June 2011.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Hewitt M, Greenfield S, Stovall E, editors. From Cancer Patient to Cancer Survivor: Lost in Translation. Institute of Medicine; Washington, DC: The National Academies Press; 2005. [Google Scholar]
- 3.Mujahid MS, Janz NK, Hawley ST, Griggs JJ, Hamilton AS, Katz SJ. The impact of sociodemographic, treatment, and work support on missed work after breast cancer diagnosis. Breast Cancer Res Treat. 2010;119:213–220. doi: 10.1007/s10549-009-0389-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roelen CA, Koopmans PC, van Rhenen W, Groothoff JW, van der Klink JJ, Bültmann U. Trends in return to work of breast cancer survivors. Breast Cancer Res Treat. 2011;128:237–242. doi: 10.1007/s10549-010-1330-0. [DOI] [PubMed] [Google Scholar]
- 5.Bradley CJ, Neumark D, Luo Z, Schenk M. Employment and cancer: findings from a longitudinal study of breast and prostate cancer survivors. Cancer Invest. 2007;25:47–54. doi: 10.1080/07357900601130664. [DOI] [PubMed] [Google Scholar]
- 6.Bouknight RR, Bradley CJ, Luo Z. Correlates of return to work for breast cancer survivors. J Clin Oncol. 2006;24:345–353. doi: 10.1200/JCO.2004.00.4929. [DOI] [PubMed] [Google Scholar]
- 7.Hoyer M, Nordin K, Ahlgren J, et al. Change in working time in a population-based cohort of patients with breast cancer. J Clin Oncol. 2012;30(23):2853–2860. doi: 10.1200/JCO.2011.41.4375. [DOI] [PubMed] [Google Scholar]
- 8.Spelten ER, Sprangers MA, Verbeek JH. Factors reported to influence the return to work of cancer survivors: a literature review. Psychooncology. 2002;11:124–131. doi: 10.1002/pon.585. [DOI] [PubMed] [Google Scholar]
- 9.de Boer AG, Taskila T, Ojajarvi A, van Dijk FJ, Verbeek JH. Cancer survivors and unemployment: a meta-analysis and meta-regression. JAMA. 2009;301(7):753–762. doi: 10.1001/jama.2009.187. [DOI] [PubMed] [Google Scholar]
- 10.Bradley CJ, Bednarek HL, Neumark D. Breast cancer and women’s labor supply. Health Serv Res. 2002;37(5):1309–1328. doi: 10.1111/1475-6773.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley CJ, Bednarek HL, Neumark D. Breast cancer survival, work, and earnings. J Health Econ. 2002;21:757–779. doi: 10.1016/s0167-6296(02)00059-0. [DOI] [PubMed] [Google Scholar]
- 12.Carter BJ. Surviving breast cancer: a problematic work re-entry. Cancer Pract. 1994;2(2):135–140. [PubMed] [Google Scholar]
- 13.Berry DL. Return-to-work experiences of people with cancer. Oncol Nurs Forum. 1993;20(6):905–911. [PubMed] [Google Scholar]
- 14.Maunsell E, Drolet M, Brisson J, Brisson C, Mâsse B, Deschênes L. Work situation after breast cancer: results from a population-based study. J Natl Cancer Inst. 2004;96(24):1813–1822. doi: 10.1093/jnci/djh335. [DOI] [PubMed] [Google Scholar]
- 15.Oberst K, Bradley CJ, Gardiner JC, Schenk M, Given CW. Work task disability in employed breast and prostate cancer patients. J Cancer Surviv. 2010;4(4):322–330. doi: 10.1007/s11764-010-0128-8. [DOI] [PubMed] [Google Scholar]
- 16.Steiner JF, Cavender TA, Nowels CT, et al. The impact of physical and psychosocial factors on work characteristics after cancer. Psychooncology. 2008;17(2):138–147. doi: 10.1002/pon.1204. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JA, Feuerstein M, Calvio LC, Olsen CH. Breast cancer survivors at work. J Occup Environ Med. 2008;50(7):777–784. doi: 10.1097/JOM.0b013e318165159e. [DOI] [PubMed] [Google Scholar]
- 18.Quinlan E, Thomas-MacLean R, Hack T, et al. The impact of breast cancer among Canadian women: disability and productivity. Work. 2009;34(3):285–296. doi: 10.3233/WOR-2009-0926. [DOI] [PubMed] [Google Scholar]
- 19.Lindbohm ML, Taskila T, Kuosma E, et al. Work ability of survivors of breast, prostate, and testicular cancer in Nordic countries: a NOCWO study. J Cancer Surviv. 2012;6(1):72–81. doi: 10.1007/s11764-011-0200-z. [DOI] [PubMed] [Google Scholar]
- 20.Blinder V, Patil S, Eberle C, Griggs J, Maly RC. Early predictors of not returning to work in low-income breast cancer survivors: a 5-year longitudinal study. Breast Cancer Res Treat. 2013;140(2):407–416. doi: 10.1007/s10549-013-2625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drolet M, Maunsell E, Mondor M, et al. Work absence after breast cancer diagnosis: a population-based study. CMAJ. 2005 Sep 27;173(7):765–771. doi: 10.1503/cmaj.050178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steiner JF, Cavender TA, Main DS, Bradley CJ. Assessing the impact of cancer on work outcomes: what are the research needs? Cancer. 2004 Oct 15;101(8):1703–1711. doi: 10.1002/cncr.20564. [DOI] [PubMed] [Google Scholar]
- 23.Hutchinson CL, Menk HR, Burch M, Gottschalk R, Lowenstein C. Rapid case ascertainment. In: Hutchinson CL, Menk HR, Burch M, Gottschalk R, editors. Cancer Registry Management Principles and Practice. 2. Dubuque, IA: Kendall/Hunt Publishing Co; 1997. [Google Scholar]
- 24.Bradley C, Neumark D, Bednarek HL, Schenk M. Short-term effects of breast cancer on labor market attachment: results from a longitudinal study. J Health Econ. 2005;24(1):137–160. doi: 10.1016/j.jhealeco.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Dillman DA. Mail and Internet Surveys: The Tailored Design Method. 2. New York, NY: John Wiley and Sons; 2007. [Google Scholar]
- 26.Hamilton AS, Hofer TP, Hawley ST, et al. Latinas and breast cancer outcomes: Population-based sampling, ethnic identity, and acculturation assessment. Cancer Epidemiol Biomarkers Prev. 2009;18:2022–2029. doi: 10.1158/1055-9965.EPI-09-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groves RM, Fowler FJ, Couper MP, Lepkoswski JM, Singer E, Tourangeau R. Survey Methodology. 2. Hoboken, NJ: John Wiley & Sons; 2013. [Google Scholar]
- 28.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 29.Berglund PA. SAS Global Forum. 2010. An Introduction to Multiple Imputation of Complex Sample Data using SAS®. Paper 265–2010. [Google Scholar]
- 30.Allison PD. Logistic Regression Using SAS: Theory and Application. 2. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 31.Dowling E, Yabroff KR, Mariotto A, McNeel T, Zeruto C, Buckman D. Burden of illness in adult survivors of childhood cancers: findings from a population-based national sample. Cancer. 2010;116(15):3712–3721. doi: 10.1002/cncr.25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeBoer AG, Taskila T, Tamminga SJ, Frings-Dresen MH, Feuerstein M, Verbeek JH. Interventions to enhance return-to-work for cancer patients. Cochrane Database Syst Rev. 2011 Feb 16;(2):CD007569. doi: 10.1002/14651858.CD007569.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Mujahid MS, Janz NK, Hawley ST, et al. Racial/ethnic differences in job loss for women with breast cancer. J Cancer Surviv. 2011;5(1):102–111. doi: 10.1007/s11764-010-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balak F, Roelen CA, Koopmans PC, Ten Berge EE, Groothoff JW. Return to work after early-stage breast cancer: a cohort study into the effects of treatment and cancer-related symptoms. J Occup Rehabil. 2008;18(3):267–272. doi: 10.1007/s10926-008-9146-z. [DOI] [PubMed] [Google Scholar]
- 35.Hassett MJ, O’Malley AJ, Keating NL. Factors influencing changes in employment among women with newly diagnosed breast cancer. Cancer. 2009;115(12):2775–2782. doi: 10.1002/cncr.24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mols F, Thong MS, Vreugdenhil G, van de Polll-Franse LV. Long-term cancer survivors experience work changes after diagnosis: results of a population-based study. Psychooncology. 2009;18(12):1252–1260. doi: 10.1002/pon.1522. [DOI] [PubMed] [Google Scholar]
- 37.Maunsell E, Drolet M, Ouhoummane N, Robert J. Breast cancer survivors accurately reported key treatment and prognostic characteristics. J Clin Epidemiol. 2005;58(4):364–369. doi: 10.1016/j.jclinepi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Katz SJ, Morrow M. The challenge of individualizing treatments for patients with breast cancer. JAMA. 2012;307(13):1379–1380. doi: 10.1001/jama.2012.409. [DOI] [PubMed] [Google Scholar]
- 39.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 40.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SWOG S1007 RxPONDER trial. 12 Oct 26; Available from URL: http://www.swog.org/Visitors/S1007/patients.asp.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.