Abstract
Background
One hypothesis suggests that the differential response to ondansetron and serotonin specific re-uptake inhibitors (SSRIs) may be due to a functional polymorphism of the 5′-HTTLPR promoter region in SLC6A4, the gene that codes for the serotonin transporter (5-HTT). The LL 5′-HTTLPR genotype is postulated to be specifically sensitive to the effects of ondansetron with SS/SL 5′-HTTLPR genotypes sensitive to SSRIs. This study tests this hypothesis by matching non-treatment seeking alcohol dependent (AD) individuals with LL genotype to ondansetron and SS/SL genotypes to the SSRI sertraline, and mis-matching them assessing naturalistic and bar-laboratory alcohol drinking.
Methods
Seventy-seven AD individuals were randomized to one of two counterbalanced arms to receive sertraline 200mg/day or ondansetron 0.5 mg/day for three weeks followed by an alcohol self-administration experiment (ASAE), then received placebo for three weeks followed by a second ASAE. Individuals then received the alternate drug for three weeks followed by a third ASAE. Drinks per drinking day (DDD with drinks in SDUs) for 7 days prior to each ASAE and milliliters consumed during each ASAE were the primary outcomes.
Results
Fifty-five participants completed the study. The genotype x order interaction was significant [F(1,47) = 8.42, p = .006] for DDD. Three ANCOVAs were conducted for DDD during the week before each ASAE. Ondansetron compared to sertraline resulted in a significant reduction in DDD during the week before the first [F(1,47) = 7.64, p = .008] but not the third ASAE. There was no difference in milliliters consumed during each ASAE.
Conclusion
This study modestly supports the hypothesis that ondansetron may reduce DDD in AD individuals with the LL genotype as measured naturalistically. By contrast there was no support that ondansetron reduces drinking during the ASAEs or that sertraline reduces alcohol use in individuals who have SS/SL genotypes. We provide limited support that ondansetron may reduce drinking in non-treatment seeking individuals with the LL genotype.
Keywords: alcoholism, ondansetron, sertraline, genotype, 5′-HTTLPR
Introduction
Pre-clinical and clinical research suggests that serotonin (5-HT) is affected by and associated with the development and maintenance of alcohol dependence (AD), (Kenna, 2010). The role of 5-HT in neural mechanisms underlying mood and stress responses was clearly demonstrated (e.g. Ressler and Nemeroff, 2000). Furthermore, increasing synaptic 5-HT by blocking re-uptake is shown to reduce alcohol consumption (Daoust et al., 1985; McBride et al., 1989). Therefore, pharmacotherapies targeting the serotonergic system have been studied as potential treatments for alcoholism (Schuckit, 1996). Unfortunately, results of clinical trials using serotonin specific re-uptake inhibitors (SSRIs) and the 5-HT3 antagonist ondansetron, have been inconsistent particularly for heterogeneous groups of alcoholics (Johnson et al., 2000; Johnson et al., 2011; Kranzler et al., 1995; Kranzler et al., 2011; Pettinati et al., 2000).
Given that AD has approximately 50 to 60% heritability (Enoch and Goldman, 1999; Prescott and Kendler, 1999) the prospect that the positive outcomes to drug therapy are at least partly dependent on genetic predisposition for alcoholism is strong (Naranjo et al., 2002). Additionally, there is substantial evidence that alcohol use differs among various subtypes of individuals based on heredity (Cloninger et al., 1981). To explain the inconsistent results of serotonergic drugs as treatments for alcoholism, a “subtype hypothesis” suggests that alcoholics, generally who begin drinking heavily by the age of 25 years old, have a biological predisposition for alcoholism from a dysregulation of 5-HT primarily associated with 5-HT transporter (5-HTT) function. The 5-HTT is responsible for 5-HT reuptake into presynaptic neurons and regulates the concentration of 5-HT in the synaptic cleft. Control of the 5-HT system may be regulated by genetic differences in the SLC6A4 gene (Johnson, 2000).
Two 5′-HTTLPR polymorphic variations are designated as long (L) and short (S) (Heils et al, 1996), resulting in three biallelic genotypes: LL, SS and SL. The LL genotype has a higher transcriptional activity in vitro compared to SS and SL (Heils et al., 1996; Lesch et al., 1996). A difference in the distribution of these genotypes is proposed to result in the variation of the rate of removal of 5-HT from the synapse (Heils et al., 1996), and the variation is putatively associated with the psychopathology of alcoholism (Johnson, 2000). Johnson et al. (2008) demonstrated that 5-HTT expression varies with current and lifetime alcohol consumption in people with the LL but not SS/SL genotypes. The LL genotype is hypothesized to moderate the effectiveness of ondansetron, a 5-HT3 antagonist, in contrast with, the SS/SL genotypes that are hypothesized to moderate the effect of the SSRI class drugs (Johnson, 2000).
One way to test such a hypothesis is to assess alcohol consumption when the same 5′-HTTLPR genotyped individuals with AD are matched and mismatched to both ondansetron and to an SSRI class drug, in this case sertraline. Along these lines, the primary objective of a pilot study we performed was to assess alcohol consumption by matching individuals with the LL genotype to ondansetron and individuals with SS/SL genotypes to sertraline, and then mismatching them (LL to sertraline and SS/SL to ondansetron) using a within and between group design (Kenna et al., 2009). Fifteen non-treatment seeking alcohol dependent individuals were randomized to one of two counterbalanced arms to receive either sertraline 200 mg daily or ondansetron 0.25 mg twice daily for three weeks followed by an alcohol self-administration experiment (ASAE), then received placebo for three weeks followed by a second ASAE. Participants then received the alternate drug for three weeks followed by a third ASAE (see Figure 1.). At the first ASAE compared to sertraline, ondansetron improved drinking outcomes for the LL genotype and volume (mls) of alcohol consumed during the ASAE [t(5) = 2.35, p = 0.07], and significantly for DDD during the seven days prior to the ASAE [t(5) = 4.34, p = 0.007]. Logically we considered given the short three-week period of drug exposure, that the seven-day period prior to the first and third ASAEs maximized the pharmacodynamic effects of each drug and subsequent effect on drinking. Our original design called for threesix-week periods (19 weeks overall) however in our experience we were concerned with drop-outs, given that that these were non-treatment seeking individuals who may not have the strongest motivation to complete the study.
Figure 1.
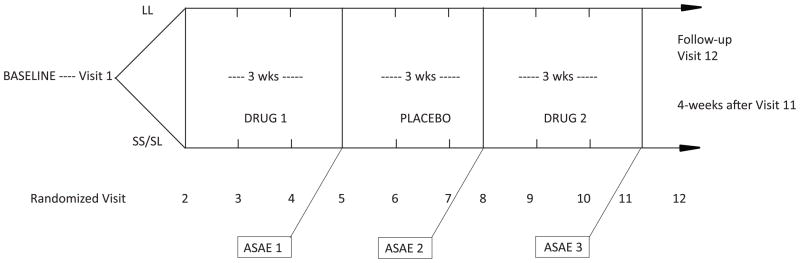
Study design. Randomization was at Visit 2 and a follow-up occurred at Visit 12, 4 weeks after Visit 11.
Compared with ondansetron, outcomes at ASAE 1 for sertraline and SS or SL genotypes were opposite what was hypothesized. Overall, as there was an order effect, as individuals reduced both naturalistic and ASAE drinking across their participation in the trial. Nevertheless, this study suggests that ondansetron may reduce alcohol consumption in alcohol-dependent individuals who have the LL genotype. By contrast there was no support that sertraline reduces alcohol use in individuals who have SS or SL genotypes. However, a major limitation of this pilot study was the small sample (Kenna et al., 2009).
In the current study, we present data from a larger trial using the exact same paradigm. Utilizing genotypes to match (LL to ondansetron, SS/SL to sertraline) and mismatch (LL to sertraline, SS/SL to ondansetron) individuals to examine two important hypotheses: (1) the efficacy of ondansetron for reducing drinking in non-treatment seeking participants who carry the LL genotype compared to sertraline, would result in a significant reduction in alcohol consumption as measured by naturalistic alcohol consumption in the seven-day (one week) period leading up to the first and third ASAEs and alcohol consumed during these two ASAEs; and (2) the efficacy of sertraline compared to ondansetron for reducing drinking in participants who carry either the SL/SS genotypes in the seven-day period leading up to the first and third ASAEs and alcohol consumed during these ASAEs. Additionally we looked at these results by gender and also sought to determine if urge, compulsions or obsessions might be potential mechanisms for how these drugs work.
Materials and Methods
Participants
The present sample was recruited with local advertisements in the Providence, RI area. The study was conducted at the Brown University Center for Alcohol and Addiction Studies and approved by the Brown University Institutional Review Board and listed on clinicaltrial.gov (NCT01113164).
Inclusion criteria
Participants were men or women between 21 and 65 years old (inclusive); in good health as confirmed by medical history, physical examination, electrocardiogram, laboratory tests, urinalysis and vital signs; female participants were: postmenopausal for at least one year, surgically sterile, or practicing an effective method of birth control before entry and throughout the study; had a negative urine pregnancy test at baseline screening and prior to the three alcohol challenge sessions; participants understood that this was not a treatment study; were diagnosed with AD as assessed by the Structured Clinical Instrument for the Diagnostic and Statistical Manual of Mental Disorders -IV- (SCID-I/P; First et al., 2002), were drinking at least ≥ 35 standard drinking units (SDUs)/week for men or ≥ 28 SDUs/week for women on average during the 90-day period before screening, and were not seeking treatment for AD; an Alcohol Use Disorders Identification Test (AUDIT; Bohn et al., 1995) score ≥ 12; willing to take oral medication, adhere to the medication regimen and to return for weekly visits and the alcohol challenge sessions; be able to read and comprehend written instructions at an 8th grade level and comprehend and complete all scales and inventories required by the protocol; must have signed an informed consent and were instructed not to consume alcohol in the 24 hour period prior to the alcohol challenge sessions.
Exclusion criteria
Excluded were individuals with Axis I DSM-IV diagnoses other than alcohol and nicotine dependence; pregnancy or breast feeding women; positive urine drug screen at baseline for any illegal substance other than marijuana; participants were excluded if they had: (a) clinically significant medical abnormalities as determined by the study physician; medical contraindications or allergy to sertraline or ondansetron; drugs that interfered with the metabolism of either; might be institutionalized during the course of the trial or pending legal charges; participants who had significant alcohol withdrawal symptoms, Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) revised score >10 (Sullivan et al., 1989); lifetime depression or history of suicide, seizures (e.g. epilepsy) or migraine headaches; current use of psychotropic medications that could not be discontinued. Persons with medical conditions that were adequately controlled by their primary care physician were not excluded.
Study design
The experimental design was a placebo-controlled mixed two-factor design in which the 5′-HTTLPR genotypes (LL vs. SS/SL) were crossed with the medication conditions (within-subjects factor). Baseline drinking and gender were statistically controlled for. The order of the active medications was controlled for by randomly assigning half the subjects to receive 200 mg/day of sertraline for the first active medication phase, and the other half to receive 0.25 mg twice/day of ondansetron for the first medication phase. The researchers were blinded to the two medication conditions, and individuals were blinded to all conditions. All received three weeks of either ondansetron or sertraline, followed by three weeks of placebo, followed by three weeks of the other active medication (see Figure 1). There was an up-titration of sertraline over 12-days and a taper over seven-days when giving sertraline. The placebo period was essentially used as a washout in order to taper sertraline down when administered first. When sertraline was administered last, the down-taper occurred during the week following the third ASAE. Individuals taking ondansetron during the last period, received placebo capsules during the week after the third ASAE. The proportion of genotypes is about 35% (LL), 49% (SL) and 16% (SS) in the alcoholic population (Parsian and Cloninger 2001). When compared to the general alcoholic population there were slightly more LL participants (44%) than SS/SL participants (56%) anticipated in this study. There were nine participants with the LL genotype that received ondansetron first and 15 with the LL genotype that received sertraline first. There were 18 participants with the SS or SL genotypes that received ondansetron first and 13 with the SS or SL genotypes that received sertraline first. Therefore, 40% of the sample received the hypothetical matching medication first (ondansetron + LL genotype and sertraline + SS/SL genotypes) and 60% of the sample received a hypothetical mismatched (sertraline + LL genotype and ondansetron + SS/SL genotypes) medication first.
Statistical Analysis
Analyses consisted of repeated measures ANCOVAs on the two dependent measures (DDD the week prior and square root of mls consumed during the ASAEs) for the 3 ASAE sessions, and then the two active medication phases (the first and third ASAE) of the study. Terms for genotype and order of medication, a 2-way interaction, and 3-way interaction (genotype X order X time), along with the covariates of baseline DDD and gender were in the model. ANCOVAs on each of the time points examined separately were conducted, subsequently. Further, medication, genotype, and genotype X medication interaction were examined during the first ASAE on the Alcohol Urge Questionnaire (AUQ; Bohn et al., 1995) assessed throughout the ASAE. A similar analysis was conducted using the Obsessive Compulsive Drinking Scale (OCDS; Anton et al., 1995), and these two analyses were conducted to test for a possible mechanism of action of the medications. Finally, we examined hypothesized medication matches and mismatches separately for each gender during the first ASAE through t-tests. Consistent with our aims, matches were individuals with the LL-genotype who received ondansetron and SS/SL-genotypes who received sertraline. Matches were predicted to drink less than mismatches. Mismatches were individuals with the LL-genotype who received sertraline or SS/SL-genotypes and received ondansetron. In addition, given the considerable pharmacodynamic carry-over for sertraline and short medication intervals, we did not perform outcomes involving comparisons to placebo.
Procedure
Complete descriptions of the Study Design and Procedure were previously published and are identical to that study (Kenna et al., 2009). In brief, an ASAE was conducted at the end of each of the three medication phases. The procedure involved administration of a priming drink that had to be consumed. The volume of alcohol for all drinks was adjusted for gender, body mass and age. The AUQ and OCDS were administered at the start and every 30 minutes during each ASAE. During each ASAE, individuals were offered two trays of four drinks each, each tray followed by a 45 minute drinking period. As an alternative reinforcement, participants received $3.00 for each drink they chose not to consume.
Genotyping
The cheeks and gums were rubbed for 20 seconds with three sterile, cotton-tipped wooden swabs. The analysis was performed at the Veterans Memorial Hospital in Providence, RI. A complete description of the genotyping procedure was previously described (Kenna et al., 2009).
RESULTS
Seventy-seven participants were urn-randomized to receive ondansetron first or sertraline first, based on gender and baseline DDD. The sample was 35% female, and consisted of 5.2% Hispanic, 1.3% American Indian or Alaskan Indian, 20.8% African-American, 64.9% Caucasian and 7.8% were individuals of more than one ethnicity. Fourteen percent of the sample was employed full-time, 31% part-time, 5% were students, 5% were retired or disabled, 5% were homemakers, and 40% were unemployed. Twenty-nine percent were married or cohabiting, 3% were widowed, 2% were separated, 16% were divorced, and 48% were single and never married. The age ranged from 21 through 65 years old (M = 43.4, SD = 10.4). The average AUDIT score at baseline was 16.2 (SD = 7.3). The mean DDD during a 28-day baseline period was 12.87(SD = 7.16) SDUs. Forty six percent of the sample had the LL genotype. Genotype frequencies were in Hardy–Weinberg equilibrium.
Data were available for 55 participants who completed the entire study including the two active medication phases. The majority of dropouts (28.5%) were lost to follow-up, or did not complete the study because they started a new job or for family or other personal reasons. The attriters and completers were compared on baseline drinks per drinking day, age, gender, and ethnicity/race. There was no statistical difference between the completers and attriters on any of these comparisons. Those receiving ondansetron first and those receiving sertraline first, were equivalent on gender [44% women and 32% women, respectively; χ2(1, N = 55) = 0.88, p = .35]. In regard to genotype and randomization, the 4 cells were: n of 9 = o1g1; 18=o1g2; 15=o2g1; and 13=o2g2 (see Table 1). Women were evenly distributed across the four cells. There were four to six women in each of the four cells [χ2 (1, N = 21) = .06, p = .80]. However, the men were not as balanced in the four cells, with three in the o1g1 cell and nine to 12 in the remaining cells [χ2 (1, N = 34) = 3.78, p = .052]. Baseline drinking was equivalent across gender [t(53) = 0.55, p = .58] and genotype [t(53) = 1.36, p = 0.18]. Likewise, baseline DDD was equivalent across order for the two groups [ondansetron first: 11.35 DDD (SD = 7.03), sertraline first: 13.52 DDD (SD = 6.52), t(53) = 1.19, p = .24].
Table 1.
Order of drug administration by genotype
| N=55 | O (order) x g (gene) | Order of drug administration x genotype |
|---|---|---|
| 9 | o1g1 | ondansetron, placebo, sertraline for those with the LL genotype |
| 18 | o1g2 | ondansetron, placebo, sertraline for those with the SS/SL genotype |
| 15 | o2g1 | sertraline, placebo, ondansetron for those with the LL genotype |
| 13 | o2g2 | sertraline, placebo, ondansetron for those with the SS/SL genotype |
Drinks per Drinking Day
Naturalistic drinking was assessed as DDD throughout the study by the Time-line Follow Back (TLFB; Sobell and Sobell,1992) and approximated normal distributions (kurtosis and skew <±2>). A repeated-measures ANCOVA was conducted using the TLFB data for the DDD data. Subjects were included if they had TLFB data for the seven-day periods leading up to the two active (first and third ASAEs) medication periods. In this cross-over design a genotype x order x time interaction and/or a genotype x order interaction were hypothesized. Figure 2 shows there were four potential treatment conditions. Analyses examined the two outcomes, DDD in the seven days prior to the two active ASAEs and milliliters (mls) of alcohol consumed during the two active ASAEs by medication condition (ondansetron x sertraline) and 5′-HTTLPR genotypes (LL vs. SS/SL) across the ASAEs. With all three experimental periods included (the first active medication, the placebo, and the second active medication), and gender and baseline DDD entered as covariates the three-way interaction (genotype x order x time) was not significant [F(1,47) = 0.92, p = .34]. The two-way interaction however (genotype X order) was significant [F(1,47) = 8.42, p = .006]. There were four main statistical tests conducted: genotype x order x time interaction, and the genotype x order interaction, crossed by the two primary dependent variables. To test for Type I error we conducted a Bonferroni correction that remained significant [i.e, p = .006 < p = .0125]. When the first and third ASAEs were assessed, a two-way interaction was still significant [F(1,47) = 7.40, p = .009].
Figure 2.
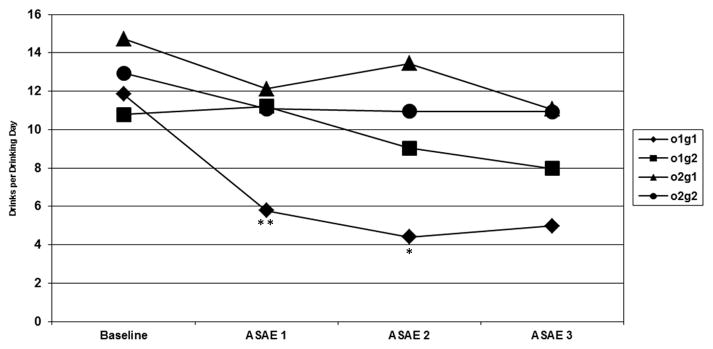
*p < .05; **p <.01; Drinks per drinking day by order and genotype in the seven days prior to the Alcohol Self Administration Experiments (ASAEs). o1g1: ondansetron-long; o1g2: ondansetron-short; o2g1: sertraline- long; o2g2: sertraline-short. The two-way interaction (genotype X order) was significant [F(1,47) = 8.42, p = .006]. Further analysis of this interaction was significant for the DDD in the week before the first ASAE [F(1,47) = 7.64, p = .008] and second ASAE [F(1,47) = 5.46, p = .02], but not the third ASAE [F(1,47) = 1.45, p = .24].
Note: *p < .05; **p <.01; o1g1: ondansetron-long; o1g2: ondansetron-short; o2g1: sertraline- long; o2g2: sertraline-short
Three separate dependent measure ANCOVAs were conducted for DDD during the week before the first, second (though not important for these analyses), and third ASAEs respectively (with gender and baseline DDD as covariates), and the interaction terms examined. This interaction was significant for the first ASAE [F(1,47) = 7.64, p = .008] and the second ASAE [F(1,47) = 5.46, p = .02], but not the third ASAE [F(1,47) = 1.45, p = .24] (see Table 2 and Figure 2).
Table 2.
Drinks per Drinking Day (DDD) during the 7-day period before each ASAE
| ASAE1 | ASAE2 | ASAE3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| F | p | F | p | F | p | |
| Gene | 3.41 | .07 | 0.47 | .50 | 1.18 | .28 |
| Order | 6.72 | .01 | 12.36 | .001 | 11.46 | .001 |
| Gene X Order | 7.64 | .008 | 5.46 | .02 | 1.45 | .24 |
| Baseline DDD | 41.87 | <.001 | 14.57 | <.001 | 4.81 | .03 |
| Gender | 1.84 | .18 | 0.05 | .83 | 0.07 | .79 |
| Estimated Marginal | Estimated Marginal | Estimated Marginal | |
|---|---|---|---|
| M (SE) | M (SE) | M (SE) | |
| LL O first | 5.79 (1.50) | 4.40 (1.91) | 4.98 (1.63) |
| LL S first | 12.13 (1.07) | 13.46 (1.39) | 11.07 (1.19) |
| d for LL | 1.59 | 1.76 | 1.32 |
| SSSL O first | 11.21 (1.00) | 9.03 (1.30) | 7.98 (1.11) |
| SSSL S first | 11.08 (1.13) | 10.95 (1.47) | 10.93 (1.26) |
| d for SS/SL | 0.03 | 0.37 | 0.67 |
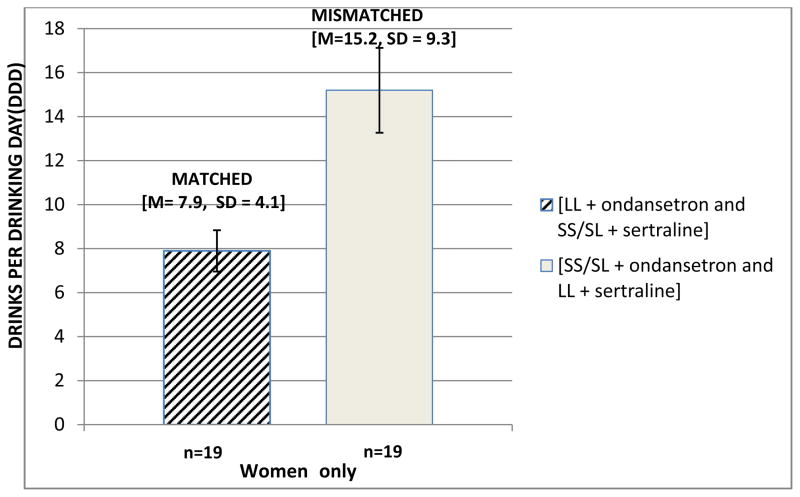
We also examined participants drinking outcomes by gender when they were matched (LL + ondansetron and SS/SL + sertraline) compared to mismatched (LL + sertraline and SS/SL + ondansetron). Women with the LL 5′-HTTLPR genotype receiving ondansetron and women with SS/SL 5′-HTTLPR genotypes receiving sertraline (matched) [M= 7.9, SD = 4.1] drank significantly fewer DDD during the seven-days prior to the first ASAE than women receiving the mismatched medications (i.e. sertraline to LL and ondansetron to SS/SL) [M= 15.2, SD = 9.3; t(14.3)=2.35, p=.03] (see Figure 3). Alcohol consumed by the matched vs. mismatched groups during each ASAE for women and both drinking outcomes for men were not significant.
Figure 3.
Drinks per Drinking Day by women matched by genotype to drug [LL + ondansetron and SS/SL + sertraline] vs mismatched [SS/SL + ondansetron and LL + sertraline] during the 7-days before the first ASAE [t(14.3)=2.35, p=.03].
Alcohol consumed during the ASAEs
Milliliters of alcohol consumed after the priming drink during the ASAEs had a kurtosis > +2 so these outcome variables were transformed using a square root transformation. An ANCOVA with gender and baseline DDD as covariates, revealed a non-significant three-way interaction (genotype x order x time) and two-way interaction (genotype x order) when all three ASAEs were considered. When only the first and third ASAEs were used as outcome measures the three-way and two-way interactions were also not significant.
To further evaluate the data, three separate ANCOVAs were conducted for each of the ASAEs: none of the 2-way interactions were significant. Gamma glutamyl-transferase (GGT) was assessed at four time points and GGT at baseline was found to be correlated with baseline DDD [r(45) = .43, p = .003] lending some support to the validity of the TLFB data.
Assessing mechanisms of action
Alcohol Urge Questionnaire (AUQ)
We focused on the first ASAE since this is where the ondansetron effect was most robust. A repeated-measures ANCOVA for AUQ assessed throughout the ASAE session was conducted and medication did not predict AUQ score (p = .20). In another analyses, the AUQ score at Week 0 was dropped, and the first AUQ score (T0) at Week 4 was entered as a covariate instead of an outcome variable, with similar results (p = .20).
Obsessive Compulsive Drinking Scale (OCDS)
A parallel set of analyses were conducted for the OCDS scale. Medication did not predict OCDS scores when the seven time points for the first ASAE were used (p = .23). When the OCDS score from Week 0 was dropped as a covariate, the medication effect was also not-significant (p = .12).
DISCUSSION
The primary aim of this study was to test the “subtype hypothesis” (Johnson, 2000) and evaluate the potential for ondansetron to reduce drinking in non-treatment seeking participants with AD who carry the LL genotype and sertraline to reduce drinking in participants who carry the SS/SL genotypes. Our results suggest that there is modest support for ondansetron improving drinking outcomes in participants with the LL genotype. There was however, no support that individuals with SS/SL genotypes receiving sertraline reduced naturalistic drinking. Further, neither drug reduced alcohol consumed during any of the ASAEs. The mechanism of action for ondansetron to reduce drinking in this study was not mediated by urge, compulsions or obsessions to consume alcohol.
The positive effect of ondansetron reducing drinking in our individuals leading up to the first ASAE with the LL genotype is consistent with our previous results demonstrating the efficacy of ondansetron in non-treatment seeking subjects (Kenna et al., 2009). Outcomes for alcohol consumption by individuals with SS/SL genotypes receiving sertraline were not significant thus providing little support for the use of sertraline by individuals who carry the SS and SL genotypes. One should however, not draw conclusions regarding sertraline’s efficacy from this study given our short administration periods and population. We were also cognizant of the importance of the 5′-HTTLPR tri-allele LALA (also called L′L′ see Kranzler et al., 2011) however we had only 18 LALA participants with 0 participants in a cell and were unable to complete any planned analyses.
While our results provide some support for our aims, it is notable that there has been an evolution in recognizing the importance of genetics involving serotonergic treatments for alcoholism. Johnson and colleagues (2011) for example, demonstrated how a single-nucleotide polymorphism (SNP) could influence treatment in a trial with AD patients with the LL genotype. Patients with the LL genotype who received ondansetron 4 mcg/kg twice a day reported significantly fewer DDD and greater percent days abstinent compared to placebo. Further, patients with the LL genotype and SNP (T/G), rs1042173 variants interacted significantly. Individuals with the combined LL/TT genotypes in the ondansetron group had a significantly lower number of DDD and significantly greater percent days abstinent than all other genotype and treatment groups combined.
By contrast, while scientific evidence continues to grow that attributes the effectiveness of ondansetron to the LL genotype and potentially amplified by other polymorphisms, the influence of age of onset of alcoholism and gender has waned despite treatment studies that potentially demonstrate some importance with specific subgroups (Johnson et al., 2000; Pettinati et al., 2004; Kranzler et al., 2011).
In our study, we also demonstrated a significant reduction in alcohol consumption in women but not men, whose genotype was matched to the hypothesized serotonergic drug compared to mismatched (see Figure 3). Taken together, one possible explanation why men and women as well as adolescents and adults may differ on genetic associations could be related to changes in gene expression as a consequence of neuroendocrine influences that change developmentally over time (Munafò et al., 2005; Edelman et al., 2012). Additionally, there are strong and consistent differences in sex and stress hormones between men and women that affect or are affected by alcohol consumption. Therefore there is enough extant evidence to suggest that temporal and gender differences in response to treatment for AD with sertraline and ondansetron could occur and that these differences could at least be partially moderated by genetic polymorphisms and polymorphic combinations that affect treatment efficacy at the time of study (Kenna et al., 2012; Kranzler et al., 2012; Mendelson and Mello, 1988; Pettinati et al., 2004; Roache et al., 2008).
Consistent with our pilot study, non-treatment seeking alcoholic individuals reduced drinking across their participation in the trial as there was a significant order effect. Though these individuals were not seeking treatment for their AD, perhaps given the opportunity to receive a pharmacological treatment they were more motivated to reduce their drinking as the trial progressed. Alternately, the order effect may have been a function of repeated administrations of the TLFB assessments and ASAEs. Initially there may have been a novelty effect for the first ASAE as participants may not be used to an “open bar” experiment in a research setting. Moreover, after experiencing the first ASAE, participants may have thought that not drinking during the ASAEs would allow them to leave earlier. However, we tried to minimize this effect as everyone was required to stay a minimum of two hours after each ASAE. We also considered performing a baseline ASAE but felt that not only was the study design valid as performed, by using each person as their own control, an additional ASAE would have imposed additional study burden on the individuals. Ultimately however, using this design in a non-treatment seeking population presents significant challenges in terms of keeping participants fully engaged over an extended period of time and to repeatedly drink in an artificial bar-lab. Perhaps over a short period of time leading up to one ASAE remains a valid and reliable way to assess a drinking response to pharmacotherapy, however we would not recommend this particular within-subjects design be used in the future with non-treatment seeking alcoholics.
One of the strengths of our design was the careful measurement of the amount of alcohol consumed during controlled environmental conditions (the ASAEs). Further, the use of the same individual as their own control taking both drugs was a unique contribution to alcohol pharmacotherapy research and also increased our statistical power. Significant limitations to this research though should also be noted. First, while the design itself was a strength the 3-week segments resulted in short treatment periods, as well as a short placebo period that we were concerned may provide inaccurate data given the sertraline taper and could therefore not use it for any meaningful analysis. Second, while larger than our pilot trial, the small sizes of the cells could have contributed to instability of the results. A subsequent lack of statistical power may still have limited our ability to consider the full importance of this research within the context of endophenotype response to medications. Third, since this study was conceived, several other important polymorphisms were identified with less emphasis on age of onset as a treatment moderator(Johnson et al., 2011; Johnson et al., 2013), but were not possible to test in our study. Moreover, we attempted to examine our results in light of the importance of the LALA genotype however our cell sizes were incomplete. Therefore there is much more that could be done to improve this research and study design.
In conclusion, the present findings modestly support the hypothesis of ondansetron as a medication with efficacy in adult alcoholic individuals with the LL 5′-HTTLPR genotype. While these results must be considered within the framework of our limitations, we suggest that future research in larger samples proceed by assessing both genetic and alcoholic subtypes. Several studies with serotonergic medications have highlighted the importance of moderation of alcohol use by gender and age as well as the 5′-HTTLPR and other polymorphisms. Resultant clinical matching of AD patients both genetically and by subtype to the proper therapy may still be critical to successful treatment outcomes for a broader group of alcoholic individuals.
Acknowledgments
This work was supported by the National Institute on Alcohol Abuse and Alcoholism grant R01-AA016079 (PI: Kenna), Shared Equipment grants (ShEEP) from the Medical Research Service Department of Veteran Affairs, and 1S10RR023457-01A1 (PI: McGeary). Dr. Swift has received travel and honorarium from D&A Pharma and Drs. Kenna and Swift have received consultant fees from CT Laboratories.
Footnotes
The other authors report no financial interests or potential conflicts of interest to declare.
References
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler FR. The Alcohol Use Disorders Test (AUDIT): validation of a screening instrument for use in medical settings. J Std Alcohol. 1995;56:423–32. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–6. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman B, Sigvardsson S. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Daoust M, Chretien P, Moore N, Saligut C, Lhuintre JP, Boismare F. Isolation and striatal (3H) serotonin uptake:role in the voluntary intake of ethanol by rats. Pharmacol Biochem Behav. 1985;22:205–8. doi: 10.1016/0091-3057(85)90378-8. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Wash. DC: 2000. (DSM-IV)-TR. [Google Scholar]
- Edelman S, Shalev I, Uzefovsky F, Israel S, Knafo A, Kremer I, Mankuta D, Kaitz M, Ebstein RP. Epigenetic and genetic factors predict women’s salivary cortisol following a threat to the social self. PLoS One. 2012;7:e48597. doi: 10.1371/journal.pone.0048597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. Genetics of alcoholism and substance abuse. Psychiatr Clin North Am. 1999;22:289–99. doi: 10.1016/s0193-953x(05)70077-0. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. Nov, [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Serotonergic agents and alcoholism treatment: rebirth of the subtype concept--an hypothesis. Alcohol Clin Exp Res. 2000;24:1597–601. [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Seneviratne C, Roache JD, Javors MA, Wang XQ, Liu L, Penberthy JK, DiClemente CC, Li MD. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatry. 2011;168:265–75. doi: 10.1176/appi.ajp.2010.10050755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Javors MA, Roache JD, Seneviratne C, Bergeson SE, Ait-Daoud N, Dawes MA, Ma JZ. Can serotonin transporter genotype predict serotonergic function, chronicity, and severity of drinking? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:209–16. doi: 10.1016/j.pnpbp.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA. 2000;284:963–71. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Seneviratne C, Wang XQ, Ait-Daoud N, Li MD. Determination of Genotype Combinations That Can Predict the Outcome of the Treatment of Alcohol Dependence Using the 5-HT3 Antagonist Ondansetron. Am J Psychiatry. 2013 Jul 30; doi: 10.1176/appi.ajp.2013.12091163. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA. Medications acting on the serotonergic system for the treatment of alcohol dependent patients. Curr Pharm Des. 2010;16:2126–35. doi: 10.2174/138161210791516396. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Swift RM, Hillemacher T, Leggio L. The relationship of appetitive, reproductive and posterior pituitary hormones to alcoholism and craving in humans. Neuropsychol Rev. 2012;22:211–28. doi: 10.1007/s11065-012-9209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna GA, Zywiak WH, McGeary JE, Leggio L, McGeary C, Wang S, Grenga A, Swift RM. A within-group design of nontreatment seeking 5-HTTLPR genotyped alcohol-dependent subjects receiving ondansetron and sertraline. Alcohol Clin Exp Res. 2009;33:315–23. doi: 10.1111/j.1530-0277.2008.00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Armeli S, Tennen H, Covault J, Feinn R, Arias AJ, Pettinati H, Oncken C. A double-blind, randomized trial of sertraline for alcohol dependence: moderation by age of onset [corrected] and 5-hydroxytryptamine transporter-linked promoter region genotype. J Clin Psychopharmacol. 2011;31:22–3. doi: 10.1097/JCP.0b013e31820465fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Burleson JA, Korner P, Del Boca FK, Bohn MJ, Brown J, Liebowitz N. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152:391–397. doi: 10.1176/ajp.152.3.391. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Feinn R, Armeli S, Tennen H. Comparison of alcoholism subtypes as moderators of the response to sertraline treatment. Alcohol Clin Exp Res. 2012;36:509–16. doi: 10.1111/j.1530-0277.2011.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Scott D, Tennen H, Feinn R, Williams C, Armeli S, Taylor RE, Briggs-Gowan MJ, Covault J. The 5-HTTLPR polymorphism moderates the effect of stressful life events on drinking behavior in college students of African descent. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:484–90. doi: 10.1002/ajmg.b.32051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. Serotonin and ethanol preference. Recent Dev Alcohol. 1989;7:187–209. doi: 10.1007/978-1-4899-1678-5_10. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Chronic alcohol effects on anterior pituitary and ovarian hormones in healthy women. J Pharmacol Exp Ther. 1988;245:407–412. [PubMed] [Google Scholar]
- Munafò MR, Lingford-Hughes AR, Johnstone EC, Walton RT. Association between the serotonin transporter gene and alcohol consumption in social drinkers. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:10–14. doi: 10.1002/ajmg.b.30162. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Chu AY, Tremblay LK. Neurodevelopmental liabilities in alcohol dependence: central serotonin and dopamine dysfunction. Neurotox Res. 2002;4:343–61. doi: 10.1080/10298420290034231. [DOI] [PubMed] [Google Scholar]
- Parsian A, Cloninger CR. Serotonergic pathway genes and subtypes of alcoholism: association studies. Psychiatric Genet. 2001;11:89–94. doi: 10.1097/00041444-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Dundon W, Lipkin C. Gender differences in response to sertraline pharmacotherapy in Type A alcohol dependence. Am J Addict. 2004;13(3):236–47. doi: 10.1080/10550490490459906. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24:1041–9. [PubMed] [Google Scholar]
- Ressler KJ, Nemeroff CB. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Roache JD, Wang Y, Ait-Daoud N, Johnson BA. Prediction of serotonergic treatment efficacy using age of onset and Type A/B typologies of alcoholism. Alcohol Clin Exp Res. 2008;32:1502–12. doi: 10.1111/j.1530-0277.2008.00717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Recent developments in the pharmacotherapy of alcohol dependence. J Consult Clin Psychol. 1996;64:669–76. doi: 10.1037//0022-006x.64.4.669. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychological and Biological Methods. Humana Press; Towota, NJ: 1992. pp. 41–72. [Google Scholar]
- Sullivan JT, Sykora K, Schneidermann J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]