Abstract
Objectives
People with bipolar disorder or schizophrenia are at greater risk for obesity and other cardio-metabolic risks, and several prior studies have linked these risks to poorer cognitive ability. In a large ethnically homogenous outpatient sample, we examined associations among variables related to obesity, treated hypertension and/or diabetes, and cognitive abilities in these two patient populations.
Methods
In a study cohort of outpatients with either bipolar disorder (n = 341) or schizophrenia (n = 417), we investigated the association of self-reported body mass index and current use of medications for hypertension or diabetes with performance on a comprehensive neurocognitive battery. We examined sociodemographic and clinical factors as potential covariates.
Results
Patients with bipolar disorder were less likely to be overweight or obese than patients with schizophrenia, and also less likely to be prescribed medication for hypertension or diabetes. However, obesity and treated hypertension were associated with worse global cognitive ability in bipolar disorder (as well as with poorer performance on individual tests of processing speed, reasoning/problem-solving, and sustained attention), with no such relationships observed in schizophrenia. Obesity was not associated with symptom severity in either group.
Conclusions
Although less prevalent in bipolar disorder compared to schizophrenia, obesity was associated with substantially worse cognitive performance in bipolar disorder. This association was independent of symptom severity and not present in schizophrenia. Better understanding of the mechanisms and management of obesity may aid in efforts to preserve cognitive health in bipolar disorder.
Keywords: bipolar disorder, diabetes, health risk factors, hypertension, neuropsychology, obesity, psychosis
People with schizophrenia or bipolar disorder experience substantially higher rates of medical problems such as obesity, hypertension, and diabetes than the population as a whole. Population-based studies indicate that the presence of diagnoses of schizophrenia or bipolar disorder is associated with roughly twice the risk of obesity compared to non-clinical controls, and that the prevalence of obesity is as much as 60% among people with these disorders (1–6). Obesity and other cardiovascular risk factors negatively impact illness course in both bipolar disorder and schizophrenia, but several recent reports have provided inconsistent evidence regarding the presence of obesity and other components of the cardio-metabolic syndrome and their association with cognitive impairments in both disorders (7–9). Bipolar disorder and schizophrenia share a number of risk factors for obesity and other cardio-metabolic risk factors, including unhealthy lifestyles and diet along with the iatrogenic effects of medications. However, what remains unclear is the extent to which the association of obesity and other cardio-metabolic risk factors and cognitive abilities differ across these diagnoses and which specific domains of cognitive function, if any, are differentially affected.
The connection between cardiovascular risk factors and worse cognitive functioning has been established in non-psychiatric samples (10–12). A handful of studies have examined obesity and cognition in serious mental illnesses. Friedman et al. (9) found, in a sample of 100 patients with schizophrenia, that presence of body mass index (BMI) > 25 was associated with worse performance on delayed recall memory on a word list learning test, but not with a number of other cognitive domains. Similarly, in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, the effect of BMI over and above the presence of diabetes was negligible across the cognitive domains measured (7). In another study in schizophrenia, higher BMI was associated with higher prevalence of the metabolic syndrome, which was associated with lower attention/vigilance, processing speed, and reasoning/problem solving (8). To our knowledge, in bipolar disorder there has been only one published study that has reported on the relationship between obesity and cognitive ability. Yim et al. (13) examined performance on a neuropsychological test battery in 67 euthymic patients with bipolar disorder. Compared to patients with normal weight, overweight/obese (BMI ≥ 25) patients had lower performance on tests of verbal fluency and symbol-digit coding. However, limitations to generalization of this study include that subjects were participants in a study that excluded patients with a BMI > 40, untreated medical illnesses, Axis I comorbidity, or substance abuse.
The exact mechanisms underlying the relationships among cardiovascular risk factors and cognitive functioning are unknown. In non-clinical populations, obesity is associated with increased risk of cardiovascular disease (CVD) that adversely impacts cognitive function (14). Moreover, secretions from adipose tissue, especially from intra-abdominal adiposity, including hormones and cytokines, create a chronic pro-inflammatory response that may lead to alterations to the hypothalamic-pituitary-adrenal (HPA) axis, the serotonin system, and/or neurogenesis (14). In bipolar disorder and schizophrenia, the relationship between cognition and obesity is complicated by symptoms such as disorganization and depression, which are themselves associated with overeating and subsequent weight gain (15, 16). Moreover, greater cognitive impairment may result in placement in treatment facilities that might alter, positively or negatively, access to health-promoting diets and lifestyle activities (17). A more severe course of illness may lead to greater exposure to psychotropic medications at higher doses, which can be associated with weight gain and metabolic complications (18). As such, establishing a link between obesity and cognition in bipolar disorder or schizophrenia requires consideration of socio-demographic, treatment, and illness contributions. Moreover, as recent work has indicated that the potential dose effect of obesity may not be linear, gradations within BMI must be investigated (19).
In this study, we examined the relationship of overweight and obesity as well as pharmacologically treated diabetes and hypertension with performance-based assessments of cognitive abilities in bipolar disorder and schizophrenia. In a large ethnically homogenous sample (persons of Ashkenazi descent) of 804 adults with schizophrenia or bipolar I disorder, we assessed the relationship between commonly defined categories of BMI (defined as normal: 18.5–25.0 kg/m2, overweight: 25–30 kg/m2, and obese: > 30 kg/m2) (20) and global cognitive ability and individual cognitive domains measured by a comprehensive and well-normed neuropsychological test battery, adjusting for demographic/socioeconomic, clinical, and medication exposure covariates. We hypothesized that there would be significant relationships between BMI, treated hypertension, and diabetes and global cognitive functioning that would persist after adjustment for relevant covariates in both bipolar disorder and schizophrenia. We explored whether this relationship differed across diagnoses, and whether BMI was differentially associated with individual cognitive domains that were examined as part of the overall cognitive assessment.
Methods
Sample
All participants were originally enrolled in a parent study focusing on the genetics of schizophrenia and bipolar disorder. Participants were of full or mixed Ashkenazi Jewish descent, determined on the basis of ancestry of four grandparents. The purpose of restriction to this population subgroup was to take advantage of potential founder effects in this population for genetic studies (21). Participants were recruited via advertisements, websites, and publications marketed toward Jewish people. Enrollment in the parent study, which took place between 1996 and 2006, included the completion of an in-person clinical interview [the Diagnostic Interview for Genetics Studies (DIGS) (22)], blood draws, and a family history interview. Most of the participants in the parent study were evaluated in their homes, with only a small subset being evaluated in an institutional setting. The in-person assessment was completed by Ph.D.-level clinical psychologists. Previous reports have described the purpose and methodology of the parent study in detail (23, 24).
Between 2007 and 2012, subjects diagnosed with bipolar I or schizophrenia in the parent study were re-contacted to participate in a follow-up study that involved administration of a battery of neurocognitive and functional capacity measures. All participants signed written informed consent to participate in this follow-up study, which was approved by the Johns Hopkins Medicine Institutional Review Board. Participants were once again seen in their place of residence for administration of the follow-up study measures.
In the present analysis, data were available for a total of 368 participants with bipolar disorder and 436 participants with schizophrenia. For the present study, we only included participants who completed the neurocognitive battery and had available BMI data, which excluded 27 patients with bipolar disorder and 19 participants with schizophrenia. There were no differences in demographic or clinical variables between patients included and excluded in this study. The final sample for analysis contained 341 patients with bipolar disorder and 417 patients with schizophrenia (22.5% had diagnoses of schizoaffective disorder).
Diagnoses
Participants were diagnosed at the time of their enrollment in the parent study. Diagnoses were based on an independent review of all available information (i.e., the in-person clinical interview, information reported by informants, treatment records) by two members of a Diagnostic Committee (psychiatrists, Ph.D.-level clinical psychologists). Diagnoses were made according to DSM-IV criteria, and required consensus between the two independent reviewers. The remaining variables relevant to the present study were ascertained during the follow-up study.
BMI
BMI was calculated based on self-reported height and weight, and subsequently categorized according to the Centers for Disease Control as normal weight (BMI: 18.5–24.9), overweight (BMI: 25.0–29.9), and obese (BMI: > 30). Three participants with bipolar disorder had a BMI < 18.5 (underweight) and were not included in this analysis. For descriptive purposes, we also reported the proportion of the sample meeting criteria for levels of obesity (Grade 1: BMI 30.0–34.9, Grade 2: BMI 35.0–35.9, Grade 3: BMI ≥ 40).
Diabetes, hypertension, and psychotropic medications
A concurrent record of physician or self-reported diagnoses of diabetes or hypertension was not available, but we did have available medications at the time of the follow-up study. Subjects were asked to provide information about all of their current medications, and since subjects were seen in their homes, they were asked to show the interviewer bottles of their medications so that accurate information regarding type of medication and dosage could be recorded. From each subject’s list of medications, we recorded the presence of at least one medication for hypertension including all diuretics, beta blockers, calcium channel blockers, ACE inhibitors, or combination medications. Similarly, for diabetic medications, we recorded presence of any of insulin, sulfonylurea, meglitidine, byguanide, thiazolidine, alpha-glucosidase inhibitors, or combination drugs. We also recorded the presence of antipsychotic medication (atypical, typical), mood stabilizers, antidepressants, benzodiazepines, and anticholinergics.
Neurocognitive ability
We developed a Neurocognitive Composite Score to assess global cognitive ability, derived from a set of commonly used neuropsychological tests addressing verbal memory [Rey Auditory Verbal Learning Test (RAVLT)], processing speed [Trail Making Test–Part A, Wechsler Adult Intelligence Scale–Third edition (WAIS-III) Digit Symbol], switching (Trail Making Test–Part B), working memory (WAIS-III Letter Number Sequencing), verbal fluency (Animal Fluency), problem-solving (Wisconsin Card Sorting Test Perseverative Errors), and sustained attention [Continuous Performance Test (CPT) Identical Pairs version, d′]. Normative scores on these eight measures were derived from raw scores on the basis of published normative data. To obtain the Composite Neurocognitive Score we transformed variables to z-scores and then obtained an average z-score across all tests, as in multiple previously published studies. The internal consistency of the neuropsychological variables used to create the composite score was Cronbach’s alpha = 0.81 in bipolar disorder and 0.83 in schizophrenia. [See our previous publications on this sample for the validity of this composite measure, e.g., Bowie et al. (25)].
Affective and psychotic symptoms
Depressive symptoms were assessed with the self-report Beck Depression Inventory–II (BDI) (26) and psychotic symptoms with the clinician-rated Positive and Negative Syndrome Scale (PANSS) (27). The BDI is a widely used 21-item self-report measure of depressive symptoms, with good internal consistency (schizophrenia: Cronbach’s alpha = 0.892; bipolar disorder: Cronbach’s alpha = 0.932). The PANSS was rated by doctoral-level clinicians and also evidenced acceptable internal consistency for both the positive (schizophrenia: Cronbach alpha = 0.710; bipolar disorder: Cronbach alpha = 0.718) and negative scores (schizophrenia: Cronbach alpha = 0.851; bipolar disorder: Cronbach alpha = 0.654). To assess the severity of manic symptoms, we also calculated the PANSS Excitement Subscale score, which is the sum of four items (impulse control, uncooperativeness, hostility, and excitement) that is highly correlated with standardized rating scales for mania (28).
Statistical analysis
All continuous variables were transformed when not normally distributed (i.e., skewness or kurtosis ± 3). We first compared the bipolar and schizophrenia samples on demographic and clinical characteristics by way of chi-square or t-tests. For our independent variables, we assessed BMI as both a categorical (normal, overweight, and obese) and continuous variable in these analyses, and we also reported the proportion of participants who met Grade 1, 2, and 3 obesity for descriptive purposes. We computed the proportion of subjects prescribed at least one hypertensive or diabetes medication. We then assessed the effects of potential covariates by assessing for significant bivariate associations between sociodemographic, clinical, and medication variables with global cognitive ability (our dependent variable) within each of the diagnostic groups. We then assessed the association between BMI and global cognitive ability in the bipolar disorder and schizophrenia samples with unadjusted and adjusted models using the general linear model procedure in SPSS Version 21. In categorical analyses, Tukey post-hoc analyses were used to assess pairwise differences. With BMI treated as a continuous variable, we compared the difference in strength of correlation between diagnostic groups by way of Fischer r-to-z transformation and subsequent z-tests. Finally, we conducted a stepwise multivariate hierarchical regression to examine the relative association between BMI and treated hypertension/diabetes and cognition after entering covariates in the first block. The alpha level for the study was set to 0.05.
Results
Sample characteristics (Table 1)
Table 1.
Sample characteristics
| Variable | Bipolar disorder (n = 341) | Schizophrenia (n = 417) | Test statistic (t or χ2) | p-value |
|---|---|---|---|---|
| Age, years, mean (SD) | 48.3 (12.9) | 50.1 (9.8) | 5.4 | 0.020 |
| Sex, female, % | 51.9 | 34.1 | 24.5 | < 0.001 |
| Education, years, mean (SD) | 15.0 (2.5) | 14.4 (2.5) | 14.4 | < 0.001 |
| In residential treatment facility, % | 1.8 | 10.1 | 99.8 | < 0.001 |
| Age of onset, mean (SD) | 19.0 (7.4) | 20.1 (5.6) | 2.1 | 0.029 |
| BDI score, mean (SD) | 10.7 (9.9) | 10.1 (9.0) | 0.8 | 0.352 |
| PANSS Positive score, mean (SD) | 13.5 (6.2) | 12.9 (5.5) | 2.1 | 0.145 |
| PANSS Negative score, mean (SD) | 12.8 (5.5) | 12.9 (6.8) | 2.2 | 0.136 |
| PANSS Excitement score, mean (SD) | 6.4 (3.1) | 6.1 (3.0) | 2.1 | 0.142 |
| Psychotropic medications, %a | ||||
| Atypical antipsychotic | 41.3 | 81.1 | ||
| Typical antipsychotic | 3.2 | 18.1 | ||
| Antidepressant | 37.5 | 42.9 | ||
| Mood stabilizer | 66.9 | 41.2 | ||
| Anticholinergic | 2.4 | 13.0 | ||
| Benzodiazepine | 32.7 | 32.7 | ||
| Hypertension medications, %a | 17.9 | 25.7 | 6.6 | 0.011 |
| Diabetes medications, %a | 7.3 | 12.2 | 4.9 | 0.029 |
| Cognitive Ability Composite (z-score) | −0.37 (0.84) | −1.11 (0.97) | 122.0 | < 0.001 |
| Body mass index, mean (SD) | 28.2 (6.1) | 29.9 (6.5) | 13.1 | < 0.001 |
| Normal, %b | 34.0 | 23.7 | 13.2 | 0.001 |
| Overweight, %c | 34.0 | 33.1 | ||
| Obese, %d | 32.0 | 43.2 |
SD = standard deviation; BDI = Beck Depression Inventory; PANSS = Positive and Negative Syndrome Scale; BMI = body mass index.
At least one.
BMI 18.5–25.0.
BMI 25–30.
BMI > 30.
As seen in Table 1, patients with schizophrenia were slightly older, less likely to be female, and had lower mean educational attainment than patients with bipolar disorder. Patients with schizophrenia were also more likely to live in residential care settings. There were no significant differences between diagnostic groups on severity of depressive or psychotic symptoms as indicated by the BDI, PANSS Positive, or PANSS Negative scores. The mean level of symptom severity revealed on each of these instruments was in the minimal to mild severity range. As would be expected, patients with schizophrenia were more likely to be prescribed antipsychotic medication, whereas, a greater proportion of the group with bipolar disorder was receiving mood stabilizers. Patients with schizophrenia had substantially worse performance on the Global Cognitive Composite.
Prevalence of obesity and use of hypertension/diabetes medications
Patients in the schizophrenia group had higher proportions of overweight and obesity, higher BMI, and were more likely to be prescribed medications for hypertension or diabetes than patients with bipolar disorder. The distribution of gradations of obesity in the samples was as follows: 23.8% of the schizophrenia sample had Grade 1 Obesity (BMI: 30.0–34.9), 12.7% had Grade 2 Obesity (BMI: 35.0–39.9), and 6.7% Grade 3 Obesity (BMI: ≥ 40). For subjects with bipolar disorder, Grade 1 Obesity was evident in 18.6% of subjects, Grade 2 in 9.2% of subjects, and Grade 3 in 4.4% of subjects.
In terms of inter-relationships among metabolic risk factors, the presence of overweight or obesity was associated with greater rates of treatment for hypertension in schizophrenia (normal: 14.1%, overweight: 23.2%, and obese: 33.9%; χ2(2): 13.7, p < 0.001), but not in bipolar disorder (normal: 14.1%, overweight: 18.1%, obese: 21.1%; χ2(2): 1.5, p = 0.450). Overweight and obesity were associated with greater rates of treatment for diabetes in both bipolar disorder (normal: 5.2%, overweight: 2.6%, and obese: 14.7%; χ2(2): 13.1, p = 0.001) and schizophrenia (normal: 7.1%, overweight: 8.3%, and obese: 18.3%; χ2(2): 11.0, p = 0.003).
Non-cognitive associations with BMI and hypertension/diabetes medication
Few of the sociodemographic or clinical variables were associated with the BMI or hypertension/diabetes medication use. Female patients with schizophrenia had a significantly higher BMI than male patients with schizophrenia [females: mean = 31.0, standard deviation (SD) = 6.5; males: mean = 29.2, SD = 5.7] [F(1,415) = 7.4, p = 0.007]. In contrast, female patients with bipolar disorder had a slightly lower BMI than their male counterparts (females: mean = 27.5, SD = 6.5; males: mean = 28.9, SD = 5.5) [F(1,339) = 4.7, p = 0.030]. Despite the large sample size, BMI was not significantly correlated with age, education, BDI, or PANSS Positive, Negative, or Excitement Subscale scores in bipolar disorder or schizophrenia. Use of atypical antipsychotics was associated with a higher BMI in bipolar disorder [not taking atypical = 27.5, SD = 6.0 versus taking atypical = 29.1, SD = 6.1; F(1,339) = 5.5, p = 0.019], but not in schizophrenia. The use of mood stabilizers was associated with a higher BMI in schizophrenia [not taking mood stabilizers: mean BMI = 29.2, SD = 6.8 versus taking mood stabilizers: mean BMI = 30.7, SD = 5.8; F(1,415) = 5.0, p = 0.026] but not in bipolar disorder. Use of typical antipsychotics, antidepressants, benzodiazepines, or anticholinergics was not associated with BMI in either diagnostic group.
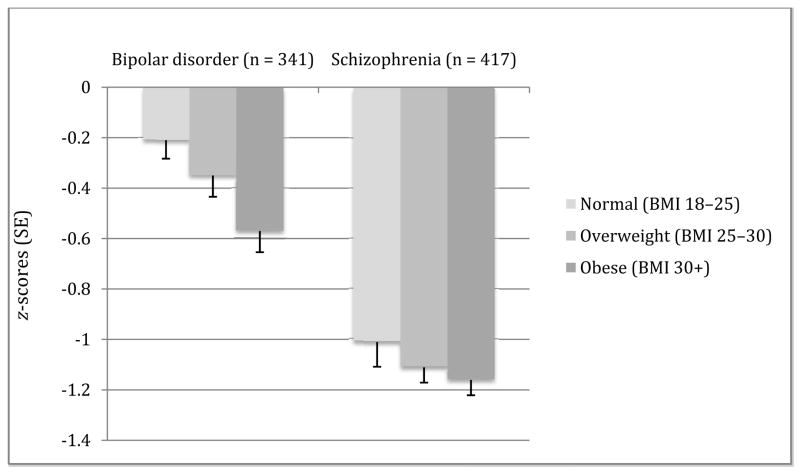
Neurocognitive ability and BMI (Fig. 1, Table 2)
Fig. 1.
Global cognitive ability by body mass index (BMI) level clustered by diagnosis. Error bars are standard errors (SE). Group comparisons: bipolar disorder: F(2,338) = 5.2, p = 0.006 [adjusted for education, Positive and Negative Syndrome Scale negative score, atypical antipsychotic use, and residential status: F(2,320) = 18.2, p = 0.035] Tukey post-hoc normal > obese; schizophrenia: F(2,413) = 0.70, p = 0.482. Effect size from lowest to highest BMI in bipolar disorder is Cohen’s d = 0.43 compared to d = 0.16 for schizophrenia.
Table 2.
Neurocognitive measures and body mass index in bipolar disorder and schizophrenia
| Bipolar disorder (n = 341) | Schizophrenia (n = 417) | |||
|---|---|---|---|---|
| Pearson’s r | p-value | Pearson’s r | p-value | |
| Verbal fluency | −0.075 | 0.164 | −0.054 | 0.269 |
| RAVLT Learning | −0.143 | 0.008 | 0.001 | 0.979 |
| CPT d′ | −0.178 | 0.001 | −0.045 | 0.362 |
| Trail Making Test–Part A | −0.140 | 0.010 | 0.017 | 0.736 |
| Trail Making Test–Part B | −0.104 | 0.058 | −0.078 | 0.118 |
| WCST Perseverative Errors | −0.114 | 0.038 | −0.064 | 0.064 |
| WAIS Digit Symbol | −0.184 | 0.001 | −0.026 | 0.593 |
| WAIS Letter Number Sequencing | −0.082 | 0.130 | −0.006 | 0.895 |
RAVLT = Rey Auditory Verbal Learning Test; CPT = Continuous Performance Test; WCST = Wisconsin Card Sorting Test; WAIS = Wechsler Adult Intelligence Scale.
As depicted in Figure 1, in an unadjusted model, cognitive ability differed significantly by normal, overweight, and obese groups in bipolar disorder (with a significant Tukey post-hoc pairwise difference between normal and obese subjects). This effect remained significant after adjusting for the following covariates, based on their bivariate association with global cognitive ability: educational attainment, residential status, PANSS Negative Syndrome Scale score, and use of atypical antipsychotics. There were no significant interaction effects (e.g., atypical antipsychotics × BMI in predicting cognitive impairment). In bipolar disorder, the correlation between BMI and global neurocognitive ability was r = −0.185, p = 0.001 (partial correlation with covariates r = −0.144, p = 0.009). In contrast, in schizophrenia there were no significant associations either with BMI as a categorical variable in unadjusted model or adjusted for education, PANSS Negative Syndrome Scale, and residential status. There was also no significant association with BMI as a continuous variable and cognitive ability (r = −0.052, p = 0.294), partial correlation r = −0.022, p = 0.716). Using r-to-z transformation and comparison by z-test, the difference in magnitude of correlations between diagnoses was not significant (z = 1.84, p = 0.065).
Examination of individual subtests revealed that in the bipolar disorder group, three of the eight subtests were significantly more impaired at different levels of BMI (normal, overweight, obese): Digit Symbol [F(1,339) = 6.3, p = 0.001], RAVLT Learning [F(1,339) = 3.3, p = 0.036], and CPT d′ [F(1,339) = 4.6, p = 0.011] and five of the subtests showed greater impairments at higher weights when BMI was treated as a continuous variable (see Table 2). After inclusion of covariates, Digit Symbol and RAVLT Learning remained significant when BMI was treated as a categorical variable, and Digit Symbol, RAVLT Learning, and CPT d′ when treated as a continuous variable. None of the subtests showed significant associations with BMI (either categorical or continuous) in schizophrenia in unadjusted or adjusted models.
Neurocognitive ability and presence of hypertension or diabetes medication
Among patients with bipolar disorder, the use of hypertension medication was associated with worse performance on the Global Cognitive Composite, which remained after adjusting for covariates as above [no antihypertensive medication mean z-score = −0.32, SD = 0.83 versus anti-hypertension medication = −0.60, SD = 0.83; F(1,339) = 7.5, p = 0.006]. Among individual subtests, worse performance on Digit symbol [F(1,339) = 4.7, p = 0.031] and Trail Making Test–Part A (F = 19.5, p = 0.001) was seen among patients using anti-hypertensive medications. There was no significant effect of anti-hypertensives in schizophrenia on the Global Cognitive Composite [F(1,415) = 0.001, p = 0.891], nor any of the subtests. The use of diabetes medication was not associated with the Cognitive Composite score in either bipolar disorder [F(1,339) = 0.28, p = 0.596] or schizophrenia [F(1,415) = 0.78, p = 0.378].
Multivariate regression models with BMI and treated hypertension/diabetes
Finally, to assess the relative impact of BMI and treated hypertension and diabetes on the Global Cognitive Composite, we first conducted a stepwise regression with covariates entered in the first block and BMI, treated hypertension, and treated diabetes in the second block. In bipolar disorder, BMI was the first variable selected [R2 change =0.023, F(1,323) = 8.9, p = 0.003], followed by hypertension treatment [R2 change = 0.020, F(1,323) = 8.1, p = 0.005]. To ascertain the impact of BMI in bipolar disorder after adjusting for hypertension and diabetes treatment in the second block, we entered BMI in the third block. We found that BMI remained a significant predictor of cognitive ability [R2 change = 0.020, F(1,322) = 8.0, p = 0.005]. In schizophrenia, none of the cardio-metabolic variables emerged as significant in the second block.
Discussion
In this sample of relatively well-educated and minimally symptomatic patients, 66% of patients with bipolar I disorder and 76% of patients with schizophrenia or schizoaffective disorder were overweight or obese. In bipolar disorder, a BMI in the obese range was associated with worse cognitive functioning than a normal BMI, with the magnitude of the difference approaching a moderate effect size (Cohen’s d = 0.43), even after adjusting for age, sex, and antipsychotic medication use. Individual cognitive test scores that were negatively correlated with BMI in bipolar disorder included those assessing sustained attention, processing speed, memory, and reasoning/problem solving. Additionally, bipolar participants who were concurrently medicated for hypertension had worse cognitive function than participants not treated for hypertension. In contrast, there were no significant associations between global or individual measures of cognitive ability and BMI, nor concurrent use of hypertension or diabetes medications in schizophrenia. Therefore, our study indicates that while obesity, hypertension, and diabetes are more prevalent in schizophrenia, the relative importance of these risk factors with respect to cognitive ability seems greater in bipolar disorder than in schizophrenia.
There are several strengths of this study. We drew from a large sample of patients with different serious mental illnesses to examine obesity and related cardio-metabolic risk factors in relation to cognitive ability as assessed by a comprehensive neuropsychological test battery. The sample was generally well educated, minimally symptomatic, and largely independent in residence, thus mitigating the influence of potential confounders such as poverty and institutional care. However, there were several limitations that must be noted. The sample was ethnically homogenous (all of Ashkenazi descent) and thus the results may not generalize to ethnically diverse samples. Furthermore, because almost all of the participants were outpatients and, on average, minimally symptomatic, the results may not generalize to inpatients or to patients with more refractory illness or more severe affective symptoms. This observational study lacked several other measures of metabolic syndrome such as blood pressure, lipid profiles, or anthropomorphic assessments, as well as the duration and severity of cardiovascular illnesses, which may have been more sensitive to variations in cognitive ability. We also lacked a standardized measure of manic symptoms and so we cannot rule out manic symptoms as a confounding variable in the observed findings, although the Excitement subscale of the PANSS was not associated with BMI or hypertension/diabetes treatment in either diagnosis. BMI was collected by self-report, which in prior reports has been found to be valid in non-clinical samples with a small bias toward underestimation (29). Finally, we used medication usage as a proxy measure to indicate the presence of treated illnesses. It is acknowledged that a substantial proportion of patients with hypertension or diabetes are not receiving treatment (30, 31), and therefore interpretation of the findings related to hypertension or diabetes medication should be made with caution.
Nonetheless, the rate of overweight observed in our sample in bipolar disorder roughly approximates that of the U.S. population (68.5%), and was still higher in schizophrenia by approximately 10% (compared to 2009–2010 data on US Population from the Centers for Disease Control; http://www.CDC.gov/nchs/fastats/overwt.htm). Fewer patients were overweight or obese in our sample than observed in other clinical samples (15). In addition to being at higher risk for obesity, patients with schizophrenia were also more likely to be prescribed medications for hypertension or diabetes than patients with bipolar disorder. These differences were present in the absence of marked sample differences in symptom severity.
To our knowledge, this is the second study to find a significant association between greater BMI and worse cognitive functioning in bipolar disorder. To place the magnitude of the effect identified in our study in context, the difference in cognitive ability between normal weight and obesity in bipolar disorder is approximately the same as the difference observed in cognitive ability between healthy comparisons and euthymic patients with bipolar disorder [see meta analysis (32)]. This effect persisted after adjusting for the identified covariates age, gender, and atypical antipsychotic usage. Similar effects were seen in the relationship between treatment for hypertension and cognitive ability variables in bipolar disorder. In contrast, cognitive ability, while substantially worse in schizophrenia than in bipolar disorder, was not associated with any BMI, hypertension, or diabetes in schizophrenia.
We do not know why cognitive ability was associated with BMI in bipolar disorder but not in schizophrenia. One speculation is that metabolic dysfunction and cognitive impairment may be best conceptualized as systemic aspects of schizophrenia because most patients experience both metabolic dysfunction and cognitive impairment, and many experience them prior to illness onset (33). Further, obesity may contribute to disability in schizophrenia in a manner independent of the effects of cognitive impairment (16), much like the contributions of depression to disability in schizophrenia. Also, the greater cognitive impairments seen in schizophrenia may have affected the potential for correlations with other risk factors; the cognitive impairment signal in schizophrenia approaches that seen in Alzheimer’s disease or other dementias and is considered a core feature of the illness. In contrast, metabolic dysfunction and cognitive impairment may be better characterized as comorbidities in bipolar disorder that emerge post onset, and add considerable excess disability beyond mood symptoms. There is little evidence of premorbid cognitive impairment in bipolar disorder (34, 35) (to our knowledge, no study has described the extent to which cardio-metabolic problems precede the onset of bipolar disorder).
It is also unclear why hypertension medication use was associated with cognitive ability (negatively) in bipolar disorder but not in schizophrenia. There are several possibilities as to why different effects were seen across diagnoses. For one, the portion of the sample not taking anti-hypertensive or diabetes medications includes patients without these illnesses and untreated patients who had these illnesses yet who were not receiving treatment. It is possible that in schizophrenia a larger proportion of untreated patients with these risk factors that may balance out the use of hypertensive medications as a marker for the presence of illness. As above, the larger magnitude of cognitive impairment in schizophrenia compared to bipolar disorder may diminish a subtler signal produced by factors such as hypertension medication. Another possibility is that poorer performance on cognitive tests among patients with bipolar disorder taking anti-hypertensives may be a result of overtreatment. We believe this possibility is remote as there is little evidence from non-psychiatric samples of negative cognitive effects of anti-hypertensive medications. Moreover, it was notable that while rates of hypertension treatment were higher among patients with schizophrenia who were overweight or obese, no such relationship was seen in bipolar disorder. High rates of under-treatment for cardiovascular risk factors have been described in schizophrenia; the results of our study suggest that a similar if not greater problem may exist in bipolar disorder (31). Prospective longitudinal studies including patients in premorbid states would be needed to understand the temporal course of cognitive impairment and obesity and concomitant medication for cardio-metabolic illnesses, in order to understand causal influences in bipolar disorder and schizophrenia.
The clinical implications of this study are that obesity, and perhaps hypertension, may contribute to cognitive impairment in bipolar disorder. Moreover, only one in four patients with schizophrenia and one in three in bipolar disorder in our study had a BMI in the normal range. The addition of cognitive impairment to the other negative consequences of obesity makes it imperative for clinicians to incorporate weight loss strategies into routine clinical care for these patients. (36, 37). Selection of weight neutral medications may be particularly important in bipolar disorder, as we found that atypical antipsychotic medication use was associated with significantly higher BMI in this sample. Emerging evidence suggests that behavioral weight management strategies are effective in both bipolar disorder and schizophrenia, especially when tailored to cognitive impairments that are evident in this population (38, 39). Our study suggests that successful weight loss may be a way to mitigate a potentially substantial risk factor for cognitive impairment in bipolar disorder.
Acknowledgments
Supported by NIMH grant R34MH091260, R01MH100417 (CAD), R01MH079784 (AEP), grant RO1MH78775 (PDH), and grant R01MH078737 (TLP).
Footnotes
Disclosures
CRB has served on a scientific advisory board and as a consultant for Abbott Labs; and as a speaker for Janssen Cilag. In the past year, TLP has served as a consultant for Abbott Labs and Amgen. During the past year, PDH has received consulting fees from Abbott Labs, Amgen, Boehringer Ingelheim, Genentech, Otsuka America Pharma, Pharma Neuroboost, Roche Pharma, Sunovion Pharma, Takeda Pharma, and Teva Pharma. CAD, MS, BTM, PW, MHT, JRL, JAM, and AEP do not have any commercial associations that might pose a conflict of interest in connection with this manuscript.
References
- 1.McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63:207–213. doi: 10.4088/jcp.v63n0306. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Portilla MP, Saiz PA, Benabarre A, et al. The prevalence of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106:197–201. doi: 10.1016/j.jad.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Fagiolini A, Frank E, Scott JA, Turkin S, Kupfer DJ. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7:424–430. doi: 10.1111/j.1399-5618.2005.00234.x. [DOI] [PubMed] [Google Scholar]
- 4.McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80:19–32. doi: 10.1016/j.schres.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Allison DB, Newcomer JW, Dunn AL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med. 2009;36:341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein BI, Fagiolini A, Houck P, Kupfer DJ. Cardiovascular disease and hypertension among adults with bipolar I disorder in the United States. Bipolar Disord. 2009;11:657–662. doi: 10.1111/j.1399-5618.2009.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takayanagi Y, Cascella NG, Sawa A, Eaton WW. Diabetes is associated with lower global cognitive function in schizophrenia. Schizophr Res. 2012;142:183–187. doi: 10.1016/j.schres.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindenmayer JP, Khan A, Kaushik S, et al. Relationship between metabolic syndrome and cognition in patients with schizophrenia. Schizophr Res. 2012;142:171–176. doi: 10.1016/j.schres.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Friedman JI, Wallenstein S, Moshier E, et al. The effects of hypertension and body mass index on cognition in schizophrenia. Am J Psychiatry. 2010;167:1232–1239. doi: 10.1176/appi.ajp.2010.09091328. [DOI] [PubMed] [Google Scholar]
- 10.Farr SA, Yamada KA, Butterfield DA, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinol. 2008;149:2628–2636. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 12.Sabia Sv, Kivimaki M, Shipley MJ, Marmot MG, Singh-Manoux A. Body mass index over the adult life course and cognition in late midlife: the Whitehall II Cohort Study. Am J Clin Nutri. 2009;89:601–607. doi: 10.3945/ajcn.2008.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yim C, Soczynska J, Kennedy S, Woldeyohannes H, Brietzke E, McIntyre R. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder. Europ Psychiatry. 2012;27:223–228. doi: 10.1016/j.eurpsy.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Naderali EK, Ratcliffe SH, Dale MC. Review: Obesity and Alzheimer’s disease: a link between body weight and cognitive function in old age. Am J Alzheimer’s Dis Other Demen. 2009;24:445–449. doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElroy SL, Keck PE. Obesity in bipolar disorder: an overview. Curr Psychiatry Rep. 2012;14:650–658. doi: 10.1007/s11920-012-0313-8. [DOI] [PubMed] [Google Scholar]
- 16.Strassnig MT, Caceda R, Newcomer JW, Harvey PD. Cognitive deficits, obesity and disability in schizophrenia. Trans Neurosci. 2012;3:345–354. [Google Scholar]
- 17.Weiser P, Becker T, Losert C, et al. European network for promoting the physical health of residents in psychiatric and social care facilities (HELPS): background, aims and methods. BMC Public Health. 2009;9:315. doi: 10.1186/1471-2458-9-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Nikolics L, Abbasi F, et al. Relationship between body mass index and insulin resistance in patients treated with second generation antipsychotic agents. J Psychiatric Res. 2010;44:493–498. doi: 10.1016/j.jpsychires.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flegal K, Kit B, Orphana H, Graubard B. Association of all-cause mortality with overweight and obesity using standard body mass index categories: A systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quickstats: from the National Center for Health Statistics: Prevalence of obesity* among adults aged ≥ 20 years, by race/ethnicity and sex – National Health and Nutrition Examination Survey, United States, 2009–2010. JAMA. 2012;308:1084. [Google Scholar]
- 21.Bray SM, Mulle JG, Dodd AF, Pulver AE, Wooding S, Warren ST. Signatures of founder effects, admixture, and selection in the Ashkenazi Jewish population. Proceed Natl Acad Sci U S A. 2010;107:16222–16227. doi: 10.1073/pnas.1004381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nurnberger JI, Jr, Blehar MC, Kaufmann CA, et al. Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 23.Fallin MD, Lasseter VK, Wolyniec PS, et al. Genomewide linkage scan for bipolar-disorder susceptibility loci among Ashkenazi Jewish families. Am J Hum Genet. 2004;75:204–219. doi: 10.1086/422474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fallin MD, Lasseter VK, Avramopoulos D, et al. Bipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish case-parent trios. Am J Hum Genet. 2005;77:918–936. doi: 10.1086/497703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowie CR, Depp C, McGrath JA, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatry. 2010;167:1116–1124. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dozois DJA, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory. Psychological Assess. 1998;10:83–89. [Google Scholar]
- 27.Kay S, Opler L, Lindenmayer J. Reliability and validity of the Positive and Negative Syndrome Scale for Schizophrenia. Schizophr Bull. 1988;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 28.Lindenmayer J, Brown E, Baker R, et al. An excitement subscale of the Positive and Negative Syndrome Scale. Schizophr Res. 2004;68:331–337. doi: 10.1016/S0920-9964(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 29.Roberts R. Can self-reported data accurately describe the prevalence of overweight? Pub Health. 1995;109:275–284. doi: 10.1016/s0033-3506(95)80205-3. [DOI] [PubMed] [Google Scholar]
- 30.De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10:52. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res. 2006;86:15–22. doi: 10.1016/j.schres.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 32.Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl. 2007;(434):17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 33.Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull. 2008;34:1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zammit S, Allebeck P, David AS, et al. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Archiv Gen Psychiatry. 2004;61:354–360. doi: 10.1001/archpsyc.61.4.354. [DOI] [PubMed] [Google Scholar]
- 35.Reichenberg A, Weiser M, Rabinowitz J, et al. A population-based cohort study of premorbid intellectual, language, and behavioral functioning in patients with schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. Am J Psychiatry. 2002;159:2027–2035. doi: 10.1176/appi.ajp.159.12.2027. [DOI] [PubMed] [Google Scholar]
- 36.McIntyre R. Managing weight gain in patients with severe mental illness. J Clin Psychiatry. 2009;70:e23. doi: 10.4088/jcp.7075cc4c. [DOI] [PubMed] [Google Scholar]
- 37.Ganguli R, Strassnig M. Prevention of metabolic syndrome in serious mental illness. Psychiatr Clin N Am. 2011;34:109–125. doi: 10.1016/j.psc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Daumit G, Dalcin A, Jerome G, et al. A behavioral weight-loss intervention for persons with serious mental illness in psychiatric rehabilitation centers. Int J Obesity. 2010;35:1114–1123. doi: 10.1038/ijo.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown C, Goetz J, Hamera E. Weight loss intervention for people with serious mental illness: a randomized controlled trial of the RENEW program. Psychiatr Ser. 2011;62:800–802. doi: 10.1176/appi.ps.62.7.800. [DOI] [PMC free article] [PubMed] [Google Scholar]