Abstract
Background
Chemotherapy-induced peripheral neuropathy (CIPN) is a significant side effect of taxane and platinum based chemotherapy. Several studies have supported the potential benefit of glutathione for the prevention of platinum-induced CIPN. The current trial was designed to determine whether glutathione would prevent CIPN as a result of carboplatin/paclitaxel therapy.
Methods
185 patients undergoing treatment with paclitaxel and carboplatin were accrued between 12/04/2009 and 12/19/2011. Patients were randomized to receive either placebo (n=91) or 1.5 g/m2 glutathione (n=94) over 15 minutes immediately prior to chemotherapy. CIPN was assessed using the EORTC-CIPN20 sensory subscale and the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.
Results
There were no statistically significant differences between the two study arms with regard to 1) peripheral neurotoxicity, assessed utilizing both EORTC-QLQ-CIPN20 (p=0.21) and CTCAE scales (p=0.449 for grade 2+ neurotoxicity; p=0.039 for time to development of grade 2+ neuropathy, in favor of the placebo); 2) the degree of the paclitaxel acute pain syndrome (p=0.30 for patients who received every 3–4 week paclitaxel vs. p=0.002, in favor of the placebo, for patients who received weekly paclitaxel); 3) the time to disease progression (p=0.63); or 4) apparent toxicities. Subgroup analysis did not reveal any evidence of benefit in any particular subgroup.
Conclusion
This study does not support the use of glutathione for the prevention of paclitaxel/carboplatin-induced CIPN.
Keywords: glutathione, chemotherapy induced peripheral neuropathy, prevention, paclitaxel, carboplatin
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a significant chemotherapy side effect, which manifests as numbness, tingling and/or pain, generally beginning in the hands and/or feet with proximal progression in a “stocking and glove” type manner1,2. It is common with platinum-based agents (e.g., cisplatin, oxaliplatin, and carboplatin), vinca alkaloids, taxanes and other agents3, with an estimated incidence of 30–40%2–6. CIPN can significantly impact patient quality of life; it may lead to dose reductions, dose delays and/or early termination of chemotherapy. Given that there are limited options available to effectively treat CIPN once it is established, efforts have been made to study means to prevent CIPN from developing.
Glutathione is a naturally occurring compound consisting of three amino acids: glutamic acid, cysteine and glycine. Glutathione is an important scavenger molecule, which participates in many detoxification reactions to protect the body from intracellular oxidants such as free radicals and reactive oxygen species. Platinum-induced neurotoxicity is thought to be secondary to the accumulation of platinum within the dorsal root ganglion7,8. Glutathione is a non-toxic agent that has been shown to reduce the accumulation of platinum within the dorsal root ganglion9, supporting that this may provide an underlying mechanism to prevent neurotoxicity.
Multiple previous studies have been performed to investigate the efficacy of glutathione for the prevention of CIPN. One small placebo-controlled randomized trial, involving 33 patients, reported that this regimen was safe; however, there were minimal changes in sensory neuropathy10. Another placebo-controlled randomized trial11, involving 33 patients with relapsed ovarian cancer, reported that higher cisplatin doses could be administered with glutathione. Cascinu et al.12 performed a double-blind, placebo-controlled, randomized trial evaluating the ability of glutathione to prevent CIPN among a cohort of 50 patients with gastric cancer receiving a cisplatin-based regimen, reporting that there was a decreased incidence of neuropathy in the glutathione arm. Cascinu et al.13 performed a second small (n=52) randomized, double-blind, placebo-controlled trial to evaluate the efficacy of glutathione for the prevention of oxaliplatin-induced peripheral neuropathy among a cohort of patients with colorectal cancer, which also showed promising- appearing results. Similar findings were observed by Milla et al.14 (n=27) in patients receiving FOLFOX4. Smyth et al.15 conducted a randomized, double-blind, placebo-controlled study in 151 patients receiving cisplatin, and reported numerically reduced neurotoxicity among those that received glutathione (p=0.22). Lin et al.16 reported a small trial consisting of 14 patients receiving adjuvant FOLFOX who were randomized to receive either 1200 mg of oral N-acetylcysteine, a glutathione precursor, or placebo and found a reduced incidence of oxaliplatin-induced neuropathy among those that received N-acetylcysteine. Further evidence to support the role of glutathione in the reduction of neurotoxicity was reported by Periera et al.,17 who found that neuronal glutamate toxicity was secondary to the inhibition of cysteine uptake and thus depletion of glutathione stores and resulting oxidative stress/damage.
Pursuant to this extensive body of preliminary data, the current study was developed to evaluate the efficacy of glutathione for preventing CIPN among a cohort of patients receiving paclitaxel/carboplatin chemotherapy.
Patients and Methods
Patients considered for this trial were adults scheduled to undergo treatment with paclitaxel at 150–200 mg/m2 and carboplatin (CBDCA) at AUC=5–7 every 21 or 28 days for at least 12 weeks. Alternatively, paclitaxel could be prescribed at 80 mg/m2 weekly for at least 12 weeks, with the same CBDCA dose and schedule. Patients needed an ECOG performance status (PS) of 0–2 and had a life expectancy of at least 6 months. Baseline laboratory values (including white blood cell count [WBC] ≥3.4 ×109/L, absolute neutrophil count [ANC] ≥1500/µL, platelets ≥100 ×109/L, hemoglobin ≥10.0 g/dL and creatinine ≤1.5 times the upper normal limit) were required. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with United States federal and institutional guidelines.
Patients were excluded from study participation for1) a pre-existing history of peripheral neuropathy greater than grade 1 (National Cancer Institute Common Terminology Criteria for Adverse Events [NCI CTCAE] version 4.0) due to any cause (chemotherapy, diabetes, alcohol, toxin, hereditary, etc.); 2) other medical conditions which would make study participation unreasonably hazardous; 3) prior receipt of paclitaxel and/or carboplatin chemotherapy treatment; or 4) concurrent use of any agents to try to prevent or treat neuropathy including, gabapentin, glutamine, vitamin B6 and vitamin E.
At study baseline and prior to each cycle of chemotherapy, patients were required to undergo a history and physical exam and laboratory evaluation (including complete blood count [CBC], creatinine, aspartate aminotransferase [AST], and bilirubin).
Procedures for measuring CIPN were performed at baseline and one week after each dose of chemotherapy. The EORTC-QLQ-CIPN20, used to measure the primary endpoint in this clinical trial, is a 20-item, CIPN-specific, patient- reported outcome questionnaire that includes three subscales to assess sensory, motor and autonomic symptoms and functioning; each item measured on a 1–4 scale (1 – not at all; 4 – very much). This questionnaire can be completed in five minutes or less and has been well received in previous clinical trials. Additionally, the NCI CTCAE; version 4.0 was utilized to quantify the chronic neurotoxicity associated with chemotherapy, with standardized questions regarding neurotoxic symptoms and examples of answers (Appendix 1), to allow a more accurate classification of patient symptoms as grade 1, 2, 3, or 4.
Paclitaxel-associated acute pain syndrome (P-APS) symptoms were measured by asking patients to keep a daily symptom log on days 2 through 7 following each paclitaxel dose, with a tool used to define this syndrome18,19.
Functional Assessment of Cancer Therapy for Patients with Ovarian Cancer (FACT-O) assessments were obtained at baseline and one week after each dose of chemotherapy. The FACT-O is a questionnaire utilized to assess quality of life in patients, with particular emphasis on patients with ovarian cancer20.
Protocol Treatment
Patients received glutathione 1.5g/m2 or placebo (100 mL of 0.9% NaCl) IV over 15 minutes immediately before chemotherapy. Patients, ideally, were to begin glutathione prior to their first dose of this chemotherapy, but were required to begin prior to their second dose of chemotherapy. Glutathione was obtained from Biomedica Foscarna, a company that makes the product that was used in the positive studies conducted by Cascinu et al.12,13. It was reconstituted from glutathione sodium salt (equivalent to 600 mg glutathione) with sterile water.
In the event that a patient developed a CTCAE grade ≥ 3 neurotoxicity, paclitaxel was held until the patient recovered to CTCAE grade ≤2 toxicity, then treatment was resumed at a 10% dose reduction. Modification or discontinuation of CBDCA due to neurotoxicity was at the discretion of the clinician. If CBDCA was discontinued but paclitaxel was continued, the patient continued glutathione/placebo therapy. If a patient developed any clinically significant adverse event (AE) attributed to glutathione/placebo, the glutathione/placebo was stopped. In the event that glutathione/placebo was stopped for an AE, the patient continued to be followed according to protocol criteria. If the patient required additional chemotherapeutic agents due to chemotherapy toxicity and/or disease progression, the patient was taken off study treatment.
Statistical methodology
This clinical trial employed a single-stage parallel group design. The dynamic allocation procedure21 to balance the marginal distributions of baseline neuropathy, debulked status and cancer type was adopted for randomization.
The primary endpoint was sensory chemotherapy-induced peripheral neuropathy as repeatedly measured by the sensory subscale of the EORTC QLQ-CIPN20 during the first six cycles of chemotherapy. The sensory subscale of EORTC QLQ-CIPN20 was computed by standard scoring algorithm and then converted to 0–100 scale, where higher scores means less symptoms and better quality of life. A repeated measures model was used to compare the primary endpoint between glutathione and placebo arms for the primary statistical analysis. Descriptive statistics such as mean (SD), median (range) and frequency (percentage) were used to summarize all clinical data including the adverse event profile. Two-sample t-tests and Wilcoxon rank-sum tests22 were used for comparing continuous secondary endpoints, while Kaplan-Meier23,24 methodology and the log-rank test were adopted for time-to-event secondary endpoints.
Based on a two-sided test of the time-averaged QLQ-CIPN20 sensory subscale scores with an assumption of moderate correlation (rho=0.5), it was calculated that a sample size of 154 patients (77 patients per arm) was required to provide 90% power to detect a difference of 6 points in QLQ-CIPN20 sensory subscale score (SD = 15 points) between the glutathione and placebo arms25. This sample size was further inflated by 20% to account for patient ineligibility, cancellation, or major violations.
This trial was monitored at least twice annually by a Data and Safety Monitoring Board, composed of individuals from within and outside the Alliance. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson, following Alliance policies. All analyses were based on the study database frozen on January 22, 2013.
Results
Baseline characteristics
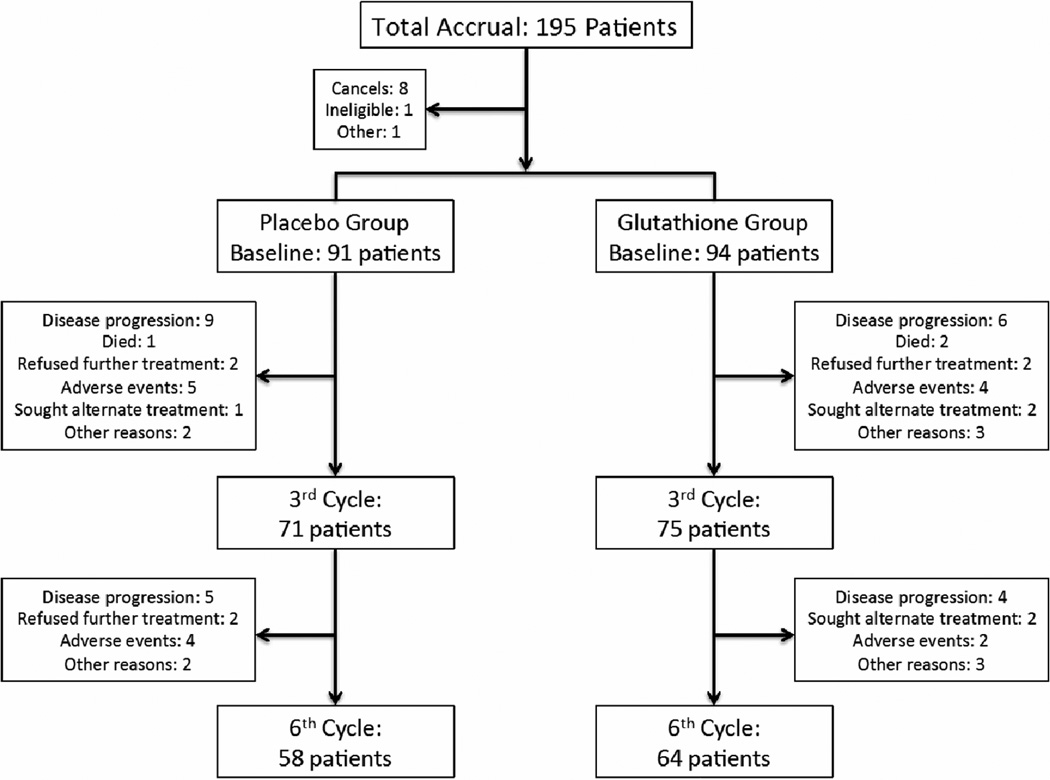
This study accrued 195 patients between 12/04/2009 and 12/19/2011 from over 50 individual sites. Baseline patient characteristics, detailed in Table 1, were similar in the two treatment groups. Patient study flow is illustrated in Figure 1.
Table 1.
Patient Demographics and Clinical Characteristics
| Glutathione (N=94) |
Placebo (N=91) |
Total (N=185) |
p-value | |
|---|---|---|---|---|
| Age | 0.411 | |||
| Median | 63.0 | 63.0 | 63.0 | |
| Age >50 | 81 (86%) | 79 (87%) | 160 (87%) | 0.902 |
| Race | 0.792 | |||
| White | 88 (94%) | 84 (92%) | 172 (93%) | |
| Black or African-American | 5 (5%) | 5 (6%) | 10 (5%) | |
| Native Hawaiian or Other Pacific Islander | 0 (0%) | 1 (1%) | 1 (1%) | |
| Asian | 1 (1%) | 1 (1%) | 2 (1%) | |
| Gender | 0.412 | |||
| Female | 74 (79%) | 76 (84%) | 150 (81%) | |
| Baseline Neuropathy | 0.942 | |||
| None | 83 (88%) | 80 (88%) | 163 (88%) | |
| Grade 1 | 11 (12%) | 11 (12%) | 22 (12%) | |
| De-bulked Status3 | 0.692 | |||
| No gross residual disease | 22 (49%) | 20 (49%) | 42 (49%) | |
| Optimal4 | 15 (33%) | 11 (27%) | 26 (30%) | |
| Sub-optimally de-bulked | 8 (18%) | 10 (24%) | 18 (21%) | |
| Cancer Type | 0.892 | |||
| Ovarian/fallopian tube/primary peritoneal | 45 (48%) | 41 (45%) | 86 (47%) | |
| Lung | 27 (29%) | 26 (29%) | 53 (29%) | |
| Other | 22 (23%) | 24 (26%) | 46 (25%) | |
| Group | 0.302 | |||
| Weekly | 12 (13%) | 9 (10%) | 21 (11%) | |
| Every 3 weeks | 82 (87%) | 80 (88%) | 162 (88%) | |
| Every 4 weeks | 0 (0%) | 2 (2%) | 2 (1%) | |
| ECOG Performance Score | 0.802 | |||
| 0 | 42 (45%) | 39 (43%) | 81 (44%) | |
| 1 | 47 (50%) | 45 (50%) | 92 (50%) | |
| 2 | 5 (5%) | 7 (8%) | 12 (7%) | |
| Diabetes | 0.142 | |||
| Yes | 8 (9%) | 14 (16%) | 22 (12.0%) | |
| No | 86 (92%) | 76 (84%) | 162 (88%) |
Kruskal Wallis test,
Chi-Square test,
Applicable only to ovarian/fallopian tube/primary peritoneal,
No residual tumor mass greater than 1 cm.
Figure 1.
CONSORT diagram
Neuropathy Data
Paclitaxel acute pain syndrome data
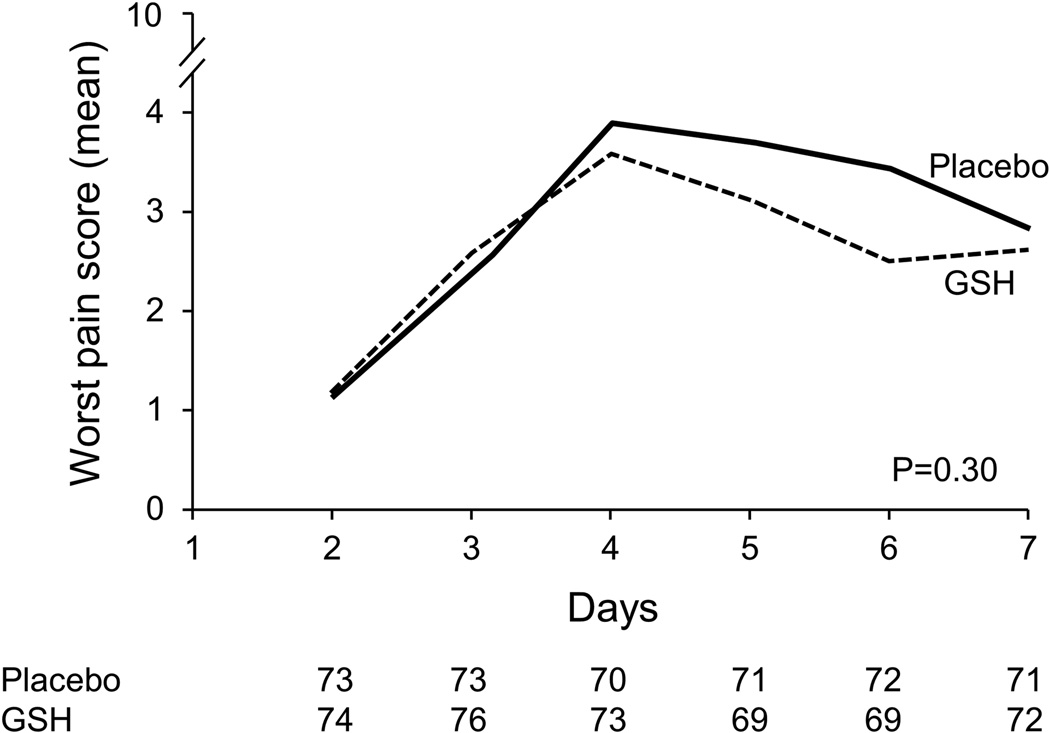
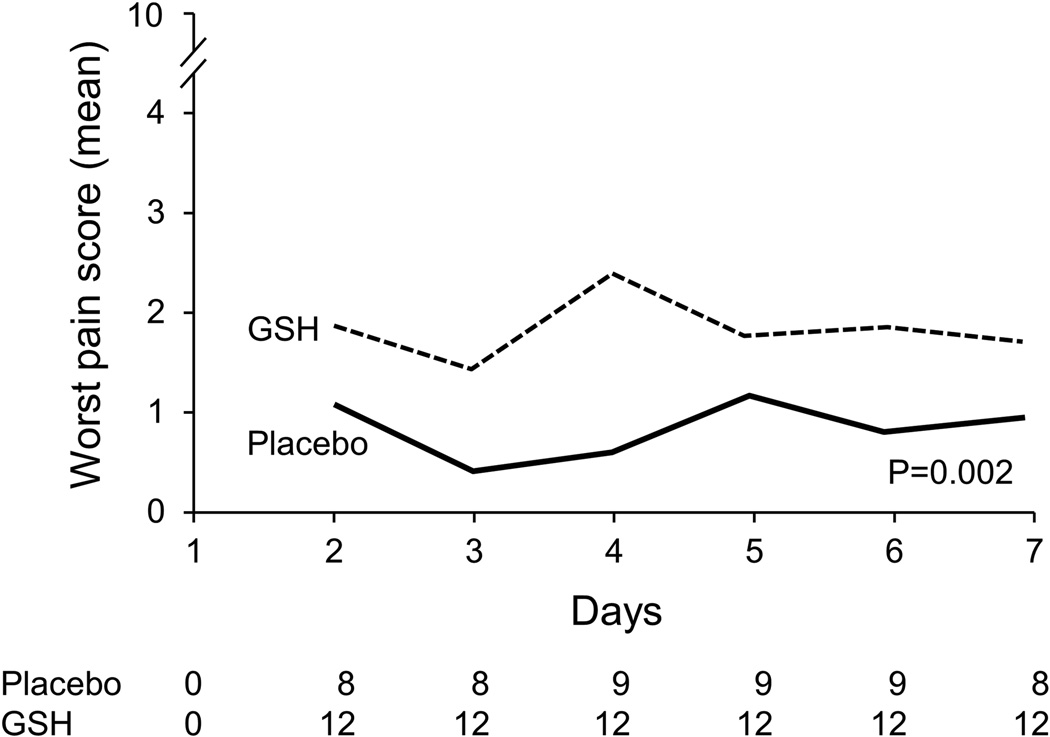
Patient-reported acute pain syndrome data, which have been described as being primarily a manifestation of acute paclitaxel neuropathy18,19 but have commonly been labeled as paclitaxel-induced arthralgias/myalgias, are illustrated in Figure 2. This figure illustrates that, for 7 days after each chemotherapy dose, there was no significant advantage for glutathione between the two study arms (p=0.30 for the every 3 week subset; p= 0.002 for the weekly subset, in favor of the placebo arm).
Figure 2.
Worst daily paclitaxel-induced acute pain scores by treatment arm for patients who received every 3–4 week paclitaxel (cycle 1) (A) and weekly paclitaxel (B). Lower scores are better.
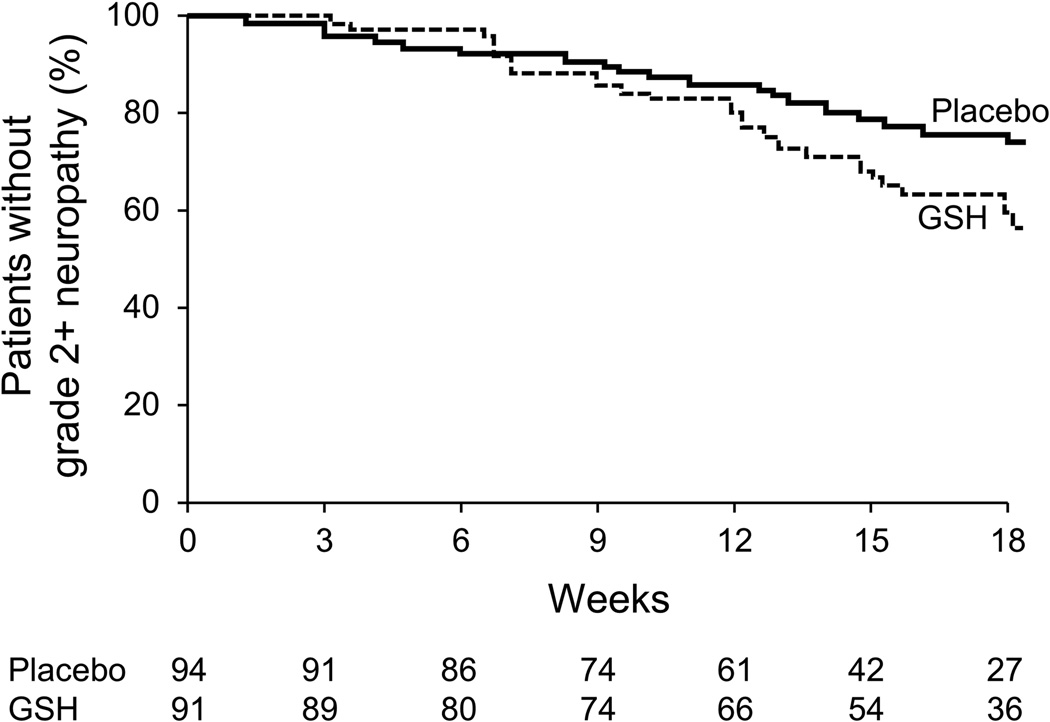
Cumulative peripheral neurotoxicity
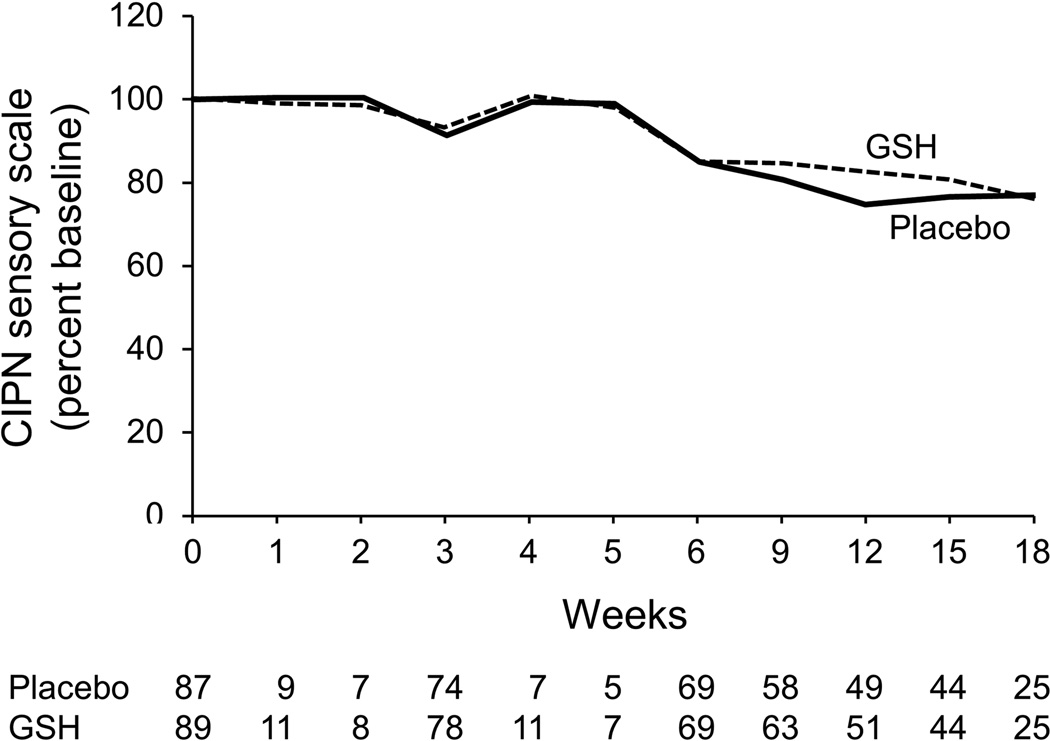
The presented data regarding peripheral neuropathy include patients receiving weekly and every three week paclitaxel, since the data were quite similar in these two subsets. Peripheral neuropathy data for the two study arms, using the QLQ-CIPN20 sensory neuropathy scale (primary endpoint, Figure 3), illustrate that there were no statistically significant differences in the AUC between the two study arms (p= 0.21). The median follow-up time for these patients was 326 days.
Figure 3.
Percent of baseline neuropathy as measured by the EORTC QLQ-CIPN20 sensory scale. Higher scores are better.
Additionally, no significant benefits for glutathione are illustrated when neurotoxicity was assessed by physicians using the CTCAE scale for determining grade 2+ neurotoxicity (p=0.449) or the time to the development of grade 2+ neurotoxicity (p= 0.039, in favor of placebo arm) (Table 2 and Figure 4).
Table 2.
Percentage of patients with grade 2+ and grade 3+ paclitaxel/carboplatin-induced CIPN by CTCAE
| Glutathione (N=94) |
Placebo (N=91) |
Total (N=185) |
p-value | |
|---|---|---|---|---|
| Indicator: Grade 2+ CIPN | 0.451 | |||
| No | 58 (62%) | 61 (67%) | 119 (64%) | |
| Yes | 36 (38%) | 30 (33%) | 66 (36%) | |
| Indicator: Grade 3+ CIPN | 0.771 | |||
| No | 89 (95%) | 87 (96%) | 176 (95%) | |
| Yes | 5 (5%) | 4 (4%) | 9 (5%) |
Chi-Square test
Figure 4.
Time to grade 2+ peripheral neuropathy, as measured by the NCI CTCAE scale
FACT-O data, evaluating changes from baseline values, did not reveal any substantial difference between the two study arms.
Effect of glutathione on cancer outcome
There were no significant differences between the two study arms with regards to the time to disease progression in the gynecologic patients per CA-125-determined disease progression, defined as an elevation of greater than two times the upper limit of normal on two occasions, separated by at least one week, when the CA-125 level had normalized during, or upon completion of therapy.
Evaluation of glutathione toxicity
There were no statistically significant or clinically apparent toxicity differences between the two study arms with regard to multiple evaluated toxicities (including fatigue, nausea, vomiting, diarrhea, rash, anaphylaxis, anemia and leukopenia).
Sub-group analyses
Sub-group analyses by age, gender, tumor type, and specific paclitaxel regimens, revealed no compelling evidence of benefit in any subgroup.
Discussion
The negative findings from this current trial contrast with the positive pilot findings10–17 that led to its development. Of the data available to investigate the efficacy of glutathione as a CIPN preventative agent, most of the studies have been conducted in patients receiving either oxaliplatin- or cisplatin-based therapy. In comparing the neurotoxicity of the agents involved in the current trial, carboplatin is the least neurotoxic of the platinum agents and is less neurotoxic than paclitaxel. While the results of this current study support that glutathione is not an effective agent in the prevention of taxane-induced CIPN when given in combination with carboplatin, the current results may not be applicable for cisplatin- or oxaliplatin-induced neurotoxicity.
A recently published study by Smith et al.26 supports that therapies for chemotherapy-induced neuropathy may be different for different chemotherapy agents. Their manuscript reported data from a randomized, double-blind, placebo-controlled, crossover trial to investigate the efficacy of duloxetine for the treatment of established CIPN among a cohort of patients with either taxane- or oxaliplatin-induced CIPN. These authors found a significant decrease in patient-reported average pain among those that received duloxetine, compared to placebo. However, in a subgroup analysis, it appeared that duloxetine was efficacious in patients with oxaliplatin-induced CIPN but not efficacious in those with taxane-induced CIPN. This may explain the differences between the findings from the present study and what has been previously suggested in other pilot trials looking at oxaliplatin- or cisplatin-based therapies.
Despite substantial efforts, there are no recommended agents for preventing chemotherapy-induced neuropathy at this time. A recent large trial illustrated that intravenous calcium/magnesium was not helpful for oxaliplatin induced neuropathy27 despite substantial previous enthusiasm for this approach. Similarly, despite pilot reports suggesting the utility of vitamin E, a larger, placebo-controlled, double-blinded randomized trial was not able to substantiate benefit. Acetyl-L-carnitine, despite preliminary reports and supporting animal tumor data, actually appeared to worsen chemotherapy-induced peripheral neuropathy in patients receiving paclitaxel-based therapy.28 Two reasonably sized, placebo-controlled, double-blinded clinical trials showed no benefit for an ACTH derivative29,30 despite four smaller pilot trials suggesting benefit.31–34
While this series of negative trials is disappointing, so is the substantial neuropathy caused by commonly-used neurotoxic chemotherapy agents. This calls for ongoing scientific methods to identify ways of utilizing these agents for their anti-tumor activity while preventing unwanted neuropathy.
Along this line, we are excited about the potential utility of minocycline35–52 and selective serotonin norepinephrine reuptake inhibitors such as venlafaxine53 and duloxetine.26 Efforts are ongoing to address the potential utility of these agents. Additionally, work is being done to address the role of genetic factors as a means of identifying which patients are at increased risk for developing chemotherapy-induced peripheral neuropathy.
In conclusion, this study does not support the use of glutathione for the prevention of taxane-induced CIPN. There was no suggestion of glutathione-associated toxicity or interference with antitumor activity. Further inquiries into the efficacy of this drug in patients receiving oxaliplatin- or cisplatin-based therapy would be of interest.
Acknowledgments
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group/Alliance and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-63848, CA-35090, CA-35431, CA-63849, CA-35272, CA-35195, CA-35103, CA-35267, CA-35269, CA-63844, CA-52352, and CA-35448. The study was also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Additional participating institutions include: Siouxland Hematology-Oncology Associates, Sioux City, IA 51105 (Donald Wender, M.D.); Medical College of Georgia, Augusta, GA 30912 (Anand P. Jillella M.D.); Colorado Cancer Research Program, Denver, CO 80224 (Eduardo R. Pajon, Jr., M.D.); Mayo Clinic Arizona, Scottsdale, AZ 85259-5404 (Michele Y. Halyard, M.D.); Medcenter One Health Systems, Bismarck, ND 58506 (Keren Sturtz, M.D.) Carle Cancer Center CCOP, Urbana, IL 61801 (Kendrith M. Rowland, Jr, M.D.); Essentia Duluth CCOP, Duluth, MN 55805 (Daniel A. Nikcevich, M.D.); Metro-Minnesota Community Clinical Oncology Program, St. Louis Park, MN 55416 (Daniel M. Anderson, M.D.); Missouri Valley Cancer Consortium, Omaha, NE 68106 (Gamini S. Soori, M.D.); St. Vincent Regional Cancer Center CCOP, Green Bay, WI 54303 (Anthony J. Jaslowski, M.D.); Hematology & Oncology of Dayton, Inc, Dayton, OH 45415 (Howard M. Gross, M.D.); Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882 (Shaker R. Dakhil, M.D.); Edward Comprehensive Cancer Center, Huntington, WV 25701 (Maria Rosalia B. Tri Tirona, M.D.); Ochsner CCOP, New Orleans, LA 70121 (Jyotsna Fuloria, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Miroslaw Mazurczak, M.D.); Virginia Mason CCOP, Seattle, WA 98101 (Craig R. Nichols, M.D.); Hawaii Minority-Based CCOP, Honolulu, HI 96813 (Jeffrey L. Berenberg, M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Deborah Weil Wilbur, M.D.); Geisinger Clinic & Medical Center CCOP, Danville, PA 17822 (Maged Khalil, M.D.)
Appendix I
Neurotoxicity Evaluation
| Grade | I | II | III |
|---|---|---|---|
| NCI-CTC AE v4.0 | Mild symptoms | Moderate symptoms; limiting instrumental activities of daily living | Severe symptoms; limiting self-care activities of daily living |
| Questions | Sample answers for each toxicity grade | ||
| Do you have problems tying your shoelaces, buttoning your shirts, fastening buckles or pulling up zippers? | “No, I might feel some tingling in my hands, but I have no problems tying laces, buttoning shirts, fastening buckles or pulling up zippers” | “It is a bit harder than before, but I can still tie laces, button shirts, fasten buckles or pull up zippers” | “I have severe difficulties tying shoe laces, buttoning shirts, fastening buckles or pulling up zippers” or “I cannot tie laces, button shirts, fasten buckles or pull up zippers anymore” |
| Do you have problems writing? | “No, I might feel some tingling in my hands, but I have no problems writing” | “It is a bit harder than before, but I can still write” | “I have severe difficulties writing” or “I cannot write anymore” |
| Do you have problems putting on your jewelry or your watch? | “No, I might feel some tingling in my hands, but I have no problems putting on my jewelry or my watch” | “It is a bit harder than before, but I can still put on my jewelry or my watch” | “I have severe difficulties putting on my jewelry or my watch” or “I cannot put on my jewelry or my watch anymore” |
| Do you have problems walking? | “No, I might feel some tingling in my feet, but I have no problems walking” | “It is a bit harder than before, but I can still walk” | “I have severe difficulties walking” or “I cannot walk anymore” |
References
- 1.Hammack JE, Michalak JC, Loprinzi CL, et al. Phase III evaluation of nortriptyline for alleviation of symptoms of cis-platinum-induced peripheral neuropathy. Pain. 2002;98(1–2):195–203. doi: 10.1016/s0304-3959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 2.Polomano RC, Bennett GJ. Chemotherapy-evoked painful peripheral neuropathy. Pain Med. 2001;2(1):8–14. doi: 10.1046/j.1526-4637.2001.002001008.x. [DOI] [PubMed] [Google Scholar]
- 3.Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63(15):1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhary UB, Haldas JR. Long-term complications of chemotherapy for germ cell tumours. Drugs. 2003;63(15):1565–1577. doi: 10.2165/00003495-200363150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109(1–2):150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 6.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12(9):619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 7.Gregg RW, Molepo JM, Monpetit VJ, et al. Cisplatin neurotoxicity: the relationship between dosage, time, and platinum concentration in neurologic tissues, and morphologic evidence of toxicity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1992;10(5):795–803. doi: 10.1200/JCO.1992.10.5.795. [DOI] [PubMed] [Google Scholar]
- 8.Cavaletti G, Tredici G, Marmiroli P, Petruccioli MG, Barajon I, Fabbrica D. Morphometric study of the sensory neuron and peripheral nerve changes induced by chronic cisplatin (DDP) administration in rats. Acta Neuropathol. 1992;84(4):364–371. doi: 10.1007/BF00227662. [DOI] [PubMed] [Google Scholar]
- 9.Cavaletti G, Minoia C, Schieppati M, Tredici G. Protective effects of glutathione on cisplatin neurotoxicity in rats. Int J Radiat Oncol Biol Phys. 1994;29(4):771–776. doi: 10.1016/0360-3016(94)90565-7. [DOI] [PubMed] [Google Scholar]
- 10.Bogliun G, Marzorati L, Cavaletti G, Frattola L. Evaluation by somatosensory evoked potentials of the neurotoxicity of cisplatin alone or in combination with glutathione. Ital J Neurol Sci. 1992;13(8):643–647. doi: 10.1007/BF02334967. [DOI] [PubMed] [Google Scholar]
- 11.Colombo N, Bini S, Miceli D, et al. Weekly cisplatin +/− glutathione in relapsed ovarian carcinoma. Int J Gynecol Cancer. 1995;5(2):81–86. doi: 10.1046/j.1525-1438.1995.05020081.x. [DOI] [PubMed] [Google Scholar]
- 12.Cascinu S, Cordella L, Del Ferro E, Fronzoni M, Catalano G. Neuroprotective effect of reduced glutathione on cisplatin-based chemotherapy in advanced gastric cancer: a randomized double-blind placebo-controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1995;13(1):26–32. doi: 10.1200/JCO.1995.13.1.26. [DOI] [PubMed] [Google Scholar]
- 13.Cascinu S, Catalano V, Cordella L, et al. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(16):3478–3483. doi: 10.1200/JCO.2002.07.061. [DOI] [PubMed] [Google Scholar]
- 14.Milla P, Airoldi M, Weber G, Drescher A, Jaehde U, Cattel L. Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs. 2009;20(5):396–402. doi: 10.1097/CAD.0b013e32832a2dc1. [DOI] [PubMed] [Google Scholar]
- 15.Smyth JF, Bowman A, Perren T, et al. Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: results of a double-blind, randomised trial. Ann Oncol. 1997;8(6):569–573. doi: 10.1023/a:1008211226339. [DOI] [PubMed] [Google Scholar]
- 16.Lin PC, Lee MY, Wang WS, et al. N-acetylcysteine has neuroprotective effects against oxaliplatin-based adjuvant chemotherapy in colon cancer patients: preliminary data. Support Care Cancer. 2006;14(5):484–487. doi: 10.1007/s00520-006-0018-9. [DOI] [PubMed] [Google Scholar]
- 17.Pereira CF, Oliveira CR. Oxidative glutamate toxicity involves mitochondrial dysfunction and perturbation of intracellular Ca2+ homeostasis. Neurosci Res. 2000;37(3):227–236. doi: 10.1016/s0168-0102(00)00124-3. [DOI] [PubMed] [Google Scholar]
- 18.Loprinzi CL, Reeves BN, Dakhil SR, et al. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(11):1472–1478. doi: 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reeves BN, Dakhil SR, Sloan JA, et al. Further data supporting that paclitaxel-associated acute pain syndrome is associated with development of peripheral neuropathy: North Central Cancer Treatment Group trial N08C1. Cancer. 2012;118(20):5171–5178. doi: 10.1002/cncr.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basen-Engquist K, Bodurka-Bevers D, Fitzgerald MA, et al. Reliability and validity of the functional assessment of cancer therapy-ovarian. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19(6):1809–1817. doi: 10.1200/JCO.2001.19.6.1809. [DOI] [PubMed] [Google Scholar]
- 21.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 22.Altman D. Practical Statistics for Medical Research. London: Chapman & Hall; 1991. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 24.Cox D. Regression Models and Life-Tables. Journal of the Royal Statistical Society. Series B (Methodological) 1972;34(2):187–220. [Google Scholar]
- 25.Diggle P, Heagerty P, Liang K-Y, et al. Analysis of Longitudinal Data. ed 2nd. Oxford, England: Oxford University Press; 2002. [Google Scholar]
- 26.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309(13):1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loprinzi CL. Phase III Randomized, Placebo-Controlled, Double-Blind Study of Intravenous Calcium/Magnesium (CaMg) to Prevent Oxaliplatin-Induced Sensory Neurotoxicity, N08CB (Alliance) In press at JCO. 2013 doi: 10.1200/JCO.2013.52.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hershman DL, Unger JM, Crew KD, et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(20):2627–2633. doi: 10.1200/JCO.2012.44.8738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts JA, Jenison EL, Kim K, Clarke-Pearson D, Langleben A. A randomized, multicenter, double-blind, placebo-controlled, dose-finding study of ORG 2766 in the prevention or delay of cisplatin-induced neuropathies in women with ovarian cancer. Gynecol Oncol. 1997;67(2):172–177. doi: 10.1006/gyno.1997.4832. [DOI] [PubMed] [Google Scholar]
- 30.Koeppen S, Verstappen CC, Korte R, et al. Lack of neuroprotection by an ACTH (4–9) analogue. A randomized trial in patients treated with vincristine for Hodgkin's or non-Hodgkin's lymphoma. J Cancer Res Clin Oncol. 2004;130(3):153–160. doi: 10.1007/s00432-003-0524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Hoop RG, Vecht CJ, van der Burg ME, et al. Prevention of cisplatin neurotoxicity with an ACTH(4–9) analogue in patients with ovarian cancer. N Engl J Med. 1990;322(2):89–94. doi: 10.1056/NEJM199001113220204. [DOI] [PubMed] [Google Scholar]
- 32.van Gerven JM, Hovestadt A, Moll JW, et al. The effects of an ACTH (4–9) analogue on development of cisplatin neuropathy in testicular cancer: a randomized trial. J Neurol. 1994;241(7):432–435. doi: 10.1007/BF00900961. [DOI] [PubMed] [Google Scholar]
- 33.Hovestadt A, van der Burg ME, Verbiest HB, van Putten WL, Vecht CJ. The course of neuropathy after cessation of cisplatin treatment, combined with Org 2766 or placebo. J Neurol. 1992;239(3):143–146. doi: 10.1007/BF00833914. [DOI] [PubMed] [Google Scholar]
- 34.van Kooten B, van Diemen HA, Groenhout KM, et al. A pilot study on the influence of a corticotropin (4–9) analogue on Vinca alkaloid-induced neuropathy. Arch Neurol. 1992;49(10):1027–1031. doi: 10.1001/archneur.1992.00530340043016. [DOI] [PubMed] [Google Scholar]
- 35.Boyette-Davis J, Xin W, Zhang H, Dougherty PM. Intraepidermal nerve fiber loss corresponds to the development of taxol-induced hyperalgesia and can be prevented by treatment with minocycline. Pain. 2011;152(2):308–313. doi: 10.1016/j.pain.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu CC, Lu N, Cui Y, et al. Prevention of paclitaxel-induced allodynia by minocycline: Effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol Pain. 2010;6:76. doi: 10.1186/1744-8069-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Yoon SY, Dougherty PM. Evidence that spinal astrocytes but not microglia contribute to the pathogenesis of Paclitaxel-induced painful neuropathy. J Pain. 2012;13(3):293–303. doi: 10.1016/j.jpain.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yong VW, Wells J, Giuliani F, Casha S, Power C, Metz LM. The promise of minocycline in neurology. Lancet Neurol. 2004;3(12):744–751. doi: 10.1016/S1474-4422(04)00937-8. [DOI] [PubMed] [Google Scholar]
- 39.Lampl Y, Boaz M, Gilad R, et al. Minocycline treatment in acute stroke: an open-label, evaluator-blinded study. Neurology. 2007;69(14):1404–1410. doi: 10.1212/01.wnl.0000277487.04281.db. [DOI] [PubMed] [Google Scholar]
- 40.Cho IH, Chung YM, Park CK, et al. Systemic administration of minocycline inhibits formalin-induced inflammatory pain in rat. Brain Res. 2006;1072(1):208–214. doi: 10.1016/j.brainres.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 41.LeBlanc BW, Zerah ML, Kadasi LM, Chai N, Saab CY. Minocycline injection in the ventral posterolateral thalamus reverses microglial reactivity and thermal hyperalgesia secondary to sciatic neuropathy. Neurosci Lett. 2011;498(2):138–142. doi: 10.1016/j.neulet.2011.04.077. [DOI] [PubMed] [Google Scholar]
- 42.Ledeboer A, Sloane EM, Milligan ED, et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain. 2005;115(1–2):71–83. doi: 10.1016/j.pain.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Marchand F, Tsantoulas C, Singh D, et al. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13(7):673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Mika J, Rojewska E, Makuch W, Przewlocka B. Minocycline reduces the injury-induced expression of prodynorphin and pronociceptin in the dorsal root ganglion in a rat model of neuropathic pain. Neuroscience. 2010;165(4):1420–1428. doi: 10.1016/j.neuroscience.2009.11.064. [DOI] [PubMed] [Google Scholar]
- 45.Pabreja K, Dua K, Sharma S, Padi SS, Kulkarni SK. Minocycline attenuates the development of diabetic neuropathic pain: possible anti-inflammatory and anti-oxidant mechanisms. Eur J Pharmacol. 2011;661(1–3):15–21. doi: 10.1016/j.ejphar.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Padi SS, Kulkarni SK. Minocycline prevents the development of neuropathic pain, but not acute pain: possible anti-inflammatory and antioxidant mechanisms. Eur J Pharmacol. 2008;601(1–3):79–87. doi: 10.1016/j.ejphar.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306(2):624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 48.Stirling DP, Khodarahmi K, Liu J, et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24(9):2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mei XP, Xu H, Xie C, et al. Post-injury administration of minocycline: an effective treatment for nerve-injury induced neuropathic pain. Neurosci Res. 2011;70(3):305–312. doi: 10.1016/j.neures.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Kim TH, Kim HI, Kim J, Park M, Song JH. Effects of minocycline on Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2011;1370:34–42. doi: 10.1016/j.brainres.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 51.Cata JP, Weng HR, Dougherty PM. The effects of thalidomide and minocycline on taxol-induced hyperalgesia in rats. Brain Res. 2008;1229:100–110. doi: 10.1016/j.brainres.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Casha SZD, McGowan D, yong VW, JR H. Neuroprotection with minocycline after spinal cord injury: results of a double blind, randomized, controlled pilot study. Neurosurgery. 2009;65:410–411. [Google Scholar]
- 53.Durand JP, Deplanque G, Montheil V, et al. Efficacy of venlafaxine for the prevention and relief of oxaliplatin-induced acute neurotoxicity: results of EFFOX, a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2012;23(1):200–205. doi: 10.1093/annonc/mdr045. [DOI] [PubMed] [Google Scholar]