Abstract
Recently we have shown that the highly conserved herpes simplex virus glycoprotein K (gK) binds to signal peptide peptidase (SPP), also known as minor histocompatibility antigen H13. In this study we have demonstrated for the first time that inhibitors of SPP, such as L685,458, (Z-LL)2 ketone, aspirin, ibuprofen and DAPT, significantly reduced HSV-1 replication in tissue culture. Inhibition of SPP activity via (Z-LL)2 ketone significantly reduced viral transcripts in the nucleus of infected cells. Finally, when administered during primary infection, (Z-LL)2 ketone inhibitor reduced HSV-1 replication in the eyes of ocularly infected mice. Thus, blocking SPP activity may represent a clinically effective and expedient approach to the reduction of viral replication and the resulting pathology.
Keywords: virus replication, glycoprotein K (gK), (Z-LL)2 Ketone, nucleus
INTRODUCTION
HSV-1 encodes at least 85 genes and 12 of these genes code for glycoproteins (Barnett et al., 1992; Ghiasi et al., 1998; McGeoch et al., 1988; Spear, 2004; Spear et al., 2000), one of which is glycoprotein K (gK) (Ghiasi et al., 1994b; Hutchinson et al., 1992; McGeoch et al., 1988). gK is encoded by the UL53 open reading frame and is a highly hydrophobic 338-amino-acid protein with a predicted molecular mass of 37-kDa (McGeoch et al., 1988). gK from HSV-1 and HSV-2 are both 338 amino acids long with approximately 84% amino acid homology (Dolan et al., 1998; McGeoch et al., 1991; McGeoch et al., 1988). gK has a cleavable 30-amino-acid amino-terminal signal sequence and is N-glycosylated on amino acids 48 and 58 (Debroy et al., 1985; McGeoch et al., 1988; Ramaswamy and Holland, 1992). In HSV-1-infected cells gK is expressed as a 39 kDa high-mannose precursor polypeptide, designated precursor gK, which is further glycosylated to produce a 41 kDa mature glycoprotein (Hutchinson et al., 1992).
When we expressed gK using a recombinant baculovirus system, four gK-related baculovirus-expressed polypeptides of 29-, 35-, 38- and 40-kDa were detected (Ghiasi et al., 1994b). The 35-, 38-, and 40-kDa species were susceptible to tunicamycin treatment revealing that they were N-linked glycosylated, the 35-kDa protein represented the cleaved and partially glycosylated peptide, while the 29-kDa protein represented the cleaved unglycosylated protein. gK translated in vitro has a molecular mass of 36-kDa with three (Mo and Holland, 1997; Ramaswamy and Holland, 1992) to four (Foster et al., 2003) predicted membrane-spanning regions.
Studies using insertion/deletion mutants have shown the importance of gK in virion morphogenesis and egress (Foster and Kousoulas, 1999; Hutchinson and Johnson, 1995; Hutchinson et al., 1995). gK is also required for virus replication (Foster and Kousoulas, 1999; Hutchinson and Johnson, 1995), a concept that is supported by the observation that gK-deficient virus can only be propagated on complementing cells which express gK (Foster and Kousoulas, 1999; Hutchinson and Johnson, 1995). Although gK is not involved in virus attachment or penetration, it is involved in virus entry as entry substantially slower in the absence of gK (Foster and Kousoulas, 1999; Hutchinson and Johnson, 1995; Jambunathan et al., 2011). Recently we have shown that the virus replication function of gK is dependent on signal peptide peptidase (SPP) (Allen et al., 2014). SPP, also known as minor histocompatibility antigen H13, is a member of the intramembrane cleaving proteases family. SPP cleaves peptide bonds within the plane of the lipid bilayer (Lemberg and Martoglio, 2002; Weihofen et al., 2002) and is highly conserved between human and mouse (Golde et al., 2009). SPP localizes predominantly to the endoplasmic reticulum and exists in different forms depending on its glycosylation status (Grigorenko et al., 2002). Unlike other family members, SPP appears to achieve enzyme activity in the absence of protein cofactors (Sato et al., 2006; Weihofen et al., 2002). SPP has been linked to pathogenic conditions such as Alzheimer’s disease (Esler et al., 2002), certain cancers (Taniguchi et al., 2003), and HCV infection (McLauchlan et al., 2002; Okamoto et al., 2004).
Recently we have shown that SPP dominant negative mutants and shRNA against SPP significantly reduced HSV-1 replication in vitro (Allen et al., 2014). In addition to the use of dominant negative mutants and shRNA (Okamoto et al., 2004), blocking the interaction of viral protein with SPP using SPP inhibitors has been suggested as an alternative anti-viral treatment (Dovey et al., 2001; Lanz et al., 2003; Li et al., 2000; Seiffert et al., 2000; Targett-Adams et al., 2006). Thus, in this study we used a panel of different SPP inhibitors to evaluate their potential to block or reduce HSV-1 infectivity in vitro and in vivo and we have shown for the first time that: 1) inhibitors of SPP enzyme catalysis significantly reduced HSV-1 replication in vitro by blocking the transcription of viral DNA in the nucleus of infected cells; and 2) SPP is required for virus infectivity in vivo. These results highlight the importance of SPP in HSV-1 infectivity in vitro and in vivo. Thus, blocking SPP activity may represent an alternative approach to the reduction of viral replication and the resulting pathology.
Materials and Methods
Virus, cells, and mice
Triple plaque-purified HSV-1 strain McKrae was grown in RS (rabbit skin) cell monolayers as described previously (Ghiasi et al., 1994b). RS cells were grown in minimal essential medium (MEM) plus 5% fetal calf serum (FCS). Female BALB/c and C57BL/6 mice (6-weeks of age) were purchased from The Jackson Laboratories. All animal procedures adhered to the Association for Research in Vision and Ophthalmology (ARVO) statement for the Use of Animals in Ophthalmic and Vision Research and according to institutional animal care and use guidelines.
SPP chemical inhibitors
There are several chemicals that have been shown to inhibit the proteolytic activity of SPP. We selected the following SPP inhibitors for analysis of their effects on HSV-1 replication: A) L685,458 (1S-Benzyl-4R-[1-(1S-carbamoyl-2-phenethylcarbamoyl)-1S-3-methylbutylcarbamoyl]-2R-hydroxy-5-phenylpentyl) carbamic Acid tert-butyl Ester) (Tocris Bioscience, Ellisville, MO); B) (Z-LL)2 ketone (Calbiochem, San Diego, CA); C) DAPT (N-[(3,5-Difluorophenyl)acetyl]-L-alanyl-2-phenyl]glycine-1,1-dimethylethyl ester) (Tocris Bioscience, Ellisville, MO); D) Aspirin (Sigma Aldrich, Saint Louis, MO); and E) Ibuprofen (Sigma Aldrich). We tested different concentrations of each inhibitor and chose concentrations specific for SPP inhibition which caused no toxicity in Vero, HeLa or RS cell lines as determined by tryptan blue staining and direct observation of cytotoxicity from 0 to 48 hours (hr) post-treatment. Concentrations of inhibitors used were as follows: 1μm L685,458; 20.0 μm (Z-LL)2 ketone; 150 μm DAPT; 250 μm aspirin; and 100 μm ibuprofen. Ibuprofen was diluted in ethanol, while the rest of the inhibitors were diluted in DMSO. No toxicity was reported with the use of these compounds in mice, rats, or tissue cultures (Dev et al., 2006; Dovey et al., 2001; Jeong et al., 2011; Li et al., 2009; Shearman et al., 2000; Weihofen et al., 2000; Zara et al., 2013). In addition, all of the compounds that we have used in this study have been shown to have high membrane permeability (Gutknecht, 1992; Kang et al., 2004; Marks and Berg, 2008; Weihofen et al., 2000).
RS cells were pre-treated with each inhibitor 2 hr prior to HSV-1 infection with 0.1 or 1.0 PFU/cell of HSV-1 strain McKrae for 1 hr at 37°C. C ells were then washed 3 times with 1X PBS and medium plus inhibitor was added back to the infected cells. Infected cells were harvested at 12, 24 and 36 hr post-infection (PI) and virus titers determined by standard plaque assay on RS cells. In addition, as a negative control for the above experiments the effect of each inhibitor on HSV-1 replication was measured by direct incubation with different concentrations of each inhibitor with HSV-1. Briefly, HSV-1 was pre-treated with each inhibitor for 1 hr at 37°C. After incubation, virus was added to RS cells without any inhibitor for 48 hr and titers were determined by standard plaque assay.
In vivo administration of inhibitors
Mice received 100 μg of (Z-LL)2 ketone or DAPT as an eye drop in 5 μl of DMSO 1 hr before ocular infection and at 2, 4, 6 and 8 hr PI. (Z-LL)2 ketone administration was repeated 5 times daily for 4 consecutive days. Sham control mice were treated similarly using 5 μl of DMSO alone. For ocular infection, mice were infected in both eyes without scarification or anesthesia by placing eye drops containing 2 × 104 PFU of HSV-1 strain McKrae in 2 μl of tissue culture medium. Eyes were swabbed once daily with a Dacron swab (Spectrum type 1) prior to administering the (Z-LL)2 ketone. The swab was transferred to a culture tube containing 1 ml of medium, frozen, thawed, and virus titers determined by standard plaque assay on RS cells as above.
Cell fractionation
RS cells were cultured in MEM containing 5% FCS. The day before the experiment, approximately 8 × 108 cells were plated on 100-mm tissue culture dishes and cultured overnight in regular culture medium or medium containing 20 μm (Z-LL)2 ketone. The following day the medium was replaced with fresh medium with or without (Z-LL)2 ketone and the cells were infected with 0.1 PFU/cell of HSV-1 strain McKrae. At one hr PI, cells were washed to remove free virus and fresh medium was added with or without (Z-LL)2 ketone. At 2, 4, and 12 hr PI, cells were harvested and partitioned into nuclear and cytoplasmic fractions with subsequent isolation of total RNA using the Protein and RNA Isolation System (PARIS Kit AM1921, Life Technologies, Grand Island, NY) as per manufacturer protocol.
Gene expression analyses
Quantitative real-time PCR (qRT-PCR) was performed as we have described previously (Allen et al., 2011). The differences in the expression levels of mRNAs were evaluated using custom-made TaqMan gene expression primers against ICP0, gB and gK with optimized primer and probe concentrations (Life Technologies, Grand Island, NY). Primer probe sets consisted of two unlabeled PCR primers and the FAM™ dye-labeled TaqMan MGB probe formulated into a single mixture. Additionally, all cellular amplicons included an intron-exon junction to eliminate signal from genomic DNA contamination. The assays used in this study were as follows: 1) gB specific primers (forward, 5′-AACGCGACGCACATCAAG-3′; Reverse - 5′-CTGGTACGCGATCAGAAAGC-3′; and Probe - 5′-FAM-CAGCCGCAGTACTACC-3′) with amplicon length of 72 bp; 2) ICP0 specific primers (forward, 5′- CGGACACGGAACTGTTCGA-3′; reverse, 5′-CGCCCCCGCAACTG-3′; and probe, 5′-FAM-CCCCATCCACGCCCTG-3′) with amplicon length of 111 bp; and 3) gK specific primers (forward, 5′-GGCCACCTACCTCTTGAACTAC-3′; reverse primer, 5′-CAGGCGGGTAATTTTCGTGTAG-3′; and probe, 5′-FAM-CAGGCCGCATCGTATC-3′) with amplicon length of 82 bp. As an internal control, a set of GAPDH primers from Applied Biosystems (ASSAY I.D. m999999.15_G1 - Amplicon Length = 107 bp) was used.
The relative copy numbers for ICP0, gB, and gK mRNAs were calculated using standard curves generated from the plasmids pGem-ICP0, pAc-gB1, and pAC-gK1. In all experiments, GAPDH was used for normalization of transcripts. The inhibitors had no effect on GAPDH mRNA expression. qRT-PCR was performed using an ABI ViiA7 sequence detection system (Applied Biosystems). The threshold cycle values, which represent the PCR cycles at which there is a noticeable increase in the reporter fluorescence above baseline, were determined using ViiA7 RUO software.
Statistical analysis
Student’s t-tests were performed using the computer program Instat (GraphPad, San Diego). Results were considered statistically significant when the P value was <0.05.
RESULTS
SPP inhibitors reduce HSV-1 replication in vitro
Recently we have shown that both SPP shRNA and SPP dominant negative mutants reduced virus replication in vitro (Allen et al., 2014). Many forms of γ-secretase inhibitors interfere with SPP activity and are of potential interest as therapeutics for Alzheimer’s disease (Wolfe, 2009). The majority of commercially available SPP inhibitors, including the ones that we have chosen, have been studied extensively in cell lines (Bihel et al., 2004; Das et al., 2003; Esler et al., 2002; Weggen et al., 2001). Based on the reported safety and efficacy studies (Okamoto et al., 2008; Weihofen et al., 2003), we have selected aspirin, ibuprofen, (Z-LL)2 ketone, L685,458, and DAPT to test our hypothesis that SPP inhibitors would reduce HSV-1 replication similar to the SPP shRNA and SPP dominant negatives that we reported recently (Allen et al., 2014).
We tested different concentrations of each inhibitor and chose concentrations which caused no toxicity in HeLa, Vero or RS cell lines as determined by trypan blue staining and direct observation of cytotoxicity from 0 to 48 hr post-treatment. To determine the effect of SPP inhibitors on virus replication in vitro, RS cells were incubated with inhibitor before and after infection with 0.1 PFU/cell of HSV-1 strain McKrae and titer was determined by plaque assay at various times PI. Virus yield in the presence of aspirin (Fig. 1A), ibuprofen (Fig. 1B), (Z-LL)2 ketone (Fig. 1C), L685,458 (Fig. 1D), and DAPT (Fig. 1E) were reduced as compared to mock-treated control cells. Our results also suggest that ibuprofen had the greatest effect on reducing virus replication (Fig. 1B). Similar results were also obtained using 1 PFU/cell of HSV-1 (data not shown). In addition, HSV-1 was incubated alone with each inhibitor to verify that the observed effects were not due to inactivation of the virus by the inhibitor. As expected direct incubation of HSV-1 with each inhibitor showed no side effect on virus titer (not shown). Thus, these results demonstrate that HSV-1 replication requires functional SPP in vitro and that chemical inhibitors are able to reduce HSV-1 replication in vitro. Similar to our finding, previously it was shown that both (Z-LL)2 ketone and L-685,458 effectively inhibited malaria parasite invasion as well as growth in human erythrocytes (Li et al., 2009).
Fig. 1. SPP Inhibitors reduce HSV-1 replication in infected cells.
Confluent RS cells were incubated with SPP inhibitors 2 hr prior to infection with 0.1 PFU/cell of HSV-1 strain McKrae. Infected cells were harvested 12, 24 and 36 hr PI and titered on fresh RS cells. Virus yield in the presence of each inhibitor is shown for A) Aspirin; B) Ibuprofen; C) (Z-LL)2; D) L685,458, and E) DAPT. Each point represents the mean titer ± SEM from three independent experiments. “*”; Indicates significantly different from no inhibitor control group (p<0.01, Student’s t-test).
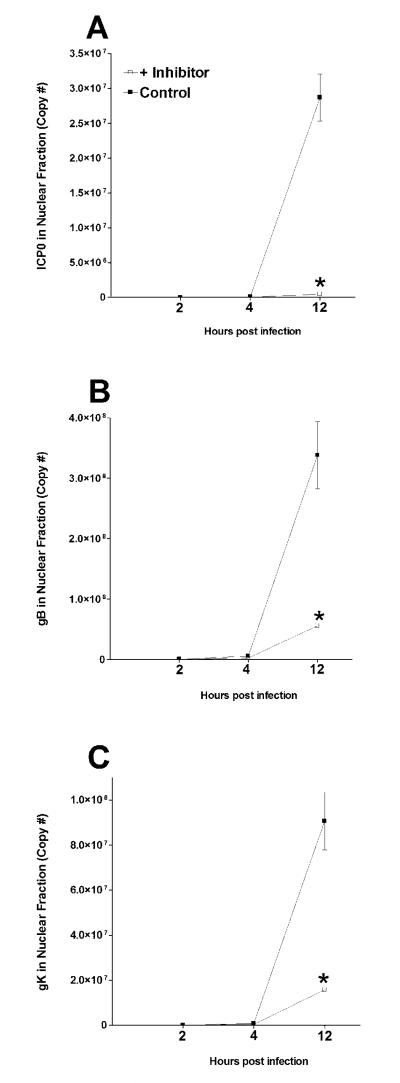
Viral gene expression is reduced in the nucleus of infected cells in the presence of SPP inhibitor
The transcription of viral DNA takes place in the nucleus of infected cells and our in vitro results suggest that SPP inhibitors reduced virus replication in infected RS cells (Fig. 1). To determine if this significant reduction in virus replication specifically involved viral gene expression, we sought to determine if SPP inhibition altered transcription of viral genes in the nucleus of infected cells. As (Z-LL)2 ketone was the most specific SPP inhibitor in our panel (Nyborg et al., 2006; Okamoto et al., 2008), we infected RS cells in the presence and absence of (Z-LL)2 ketone. At various times PI, infected cells were fractionated into nuclear and cytoplasmic fractions. qRT-PCR was performed on total RNA isolated from each fraction as described in Materials and Methods. We detected significant reductions in ICP0 (Fig. 2A), gB (Fig. 2B), and gK (Fig. 2C) expressions in the presence of (Z-LL)2 ketone compared with mock-treated control cells. Since ICP0 is a transcriptional regulator of gene expression its reduced expression may also reduce gB and gK expressions. However, this reduction in gB and gK expressions is probably independent of ICP0, as our published results suggest that inhibition of SPP directly suppresses HSV-1 replication by blocking the binding of gK to SPP (Allen et al., 2014). In contrast to the differences that we observed in expression of viral transcripts in the nuclear fraction of infected cells in the presence of (Z-LL)2 ketone, expression of ICP0 (Fig. 3A), gB (Fig. 3B), and gK (Fig. 3C) mRNAs in the cytoplasmic fraction of infected cells were not reduced in the presence of (Z-LL)2 ketone compared with mock-treated control cells. Interestingly, the levels of ICP0 (Fig. 3A) and gK (Fig. 3C) but not gB (Fig. 3B) increased by 12 hr PI in the presence of inhibitor compared with control group. The results indicate that selective cytoplasmic accumulation of some of the viral transcripts correlates with blocking SPP synthesis. Thus, our results with regards to the cytoplasmic fraction suggest that the net mRNA transport to the cytoplasm was not adversely affected at the time points tested in our study. Taken together, our results show that HSV-1 gene expression is impaired in the nucleus but not cytoplasm of infected cells when SPP activity is inhibited.
Fig. 2. (Z-LL)2 ketone reduces viral gene expression in the nucleus of infected cells.
RS cells were incubated with (Z-LL)2 ketone as in Figure 1 above. Infected cells were harvested 2, 4 and 12 hr PI and separated into nuclear and cytoplasmic fractions followed by RNA extraction and cDNA synthesis. Expression of ICP0, gB and gK in the nuclear fraction were measured using qRT-PCR and presented as copy number per time point. Each point represents the mean ± SEM from 3 experiments. Panels: A) ICP0 in nuclear fraction; B) gB in nuclear fraction; and C) gK in nuclear fraction. “*”; Indicates significantly different from no inhibitor control group (p<0.01, Student’s t-test).
Fig. 3. (Z-LL)2 ketone does not reduce viral gene expression in the cytoplasm of infected cells.
RS cells were incubated with (Z-LL)2 ketone as in Figure 2 above. Infected cells were harvested 2, 4 and 12 hr PI and separated into nuclear and cytoplasmic fractions followed by RNA extraction and cDNA synthesis. Expression of ICP0, gB and gK in the cytoplasmic fraction were measured as in Figure 2 above. Each point represents the mean ± SEM from 3 experiments. Panels: A) ICP0 in cytoplasmic fraction; B) gB in cytoplasmic fraction; and C) gK in cytoplasmic fraction.
SPP inhibitor reduces virus replication in vivo
Collectively, our in vitro results suggest that SPP inhibitors reduced virus replication in infected RS cells (Fig. 1). We next tested whether the most specific SPP inhibitor, (Z-LL)2 ketone would also reduce HSV-1 replication in vivo. (Z-LL)2 ketone was given to C57BL/6 mice (100 μg/eye as an eye drop, 5X/day for 4 consecutive days), starting 1 hr before ocular infection with HSV-1 strain McKrae. Control mice received DMSO as an eye drop. (Z-LL)2 ketone significantly decreased virus replication in the eyes of infected mice on days 1-5 PI (Fig. 3A) (P <0.001, Student’s t-test compared to the sham control). Similar results were obtained with BALB/c mice treated with (Z-LL)2 ketone compared with sham control mice on both days 1 and 3 PI (Fig. 3B). Thus, consistent with our in vitro results (Fig. 1), SPP inhibition by (Z-LL)2 ketone treatment decreased viral replication in tears of ocularly infected mice.
DISCUSSION
HSV-1-induced corneal scarring (CS), also broadly referred to as herpes stromal keratitis, can lead to blindness and HSV-1 is the leading cause of corneal blindness due to an infectious agent in developed countries (Barron et al., 1994; Dawson, 1984; Hill, 1987; Liesegang, 1999, 2001; Wilhelmus et al., 1996). In the U.S., approximately 30,000 people suffer recurrent ocular HSV episodes annually, requiring doctor visits, medication, and in severe cases, corneal transplants. It is estimated that 70-90% of American adults have antibodies to HSV-1 and/or HSV-2 and about 25% of these individuals have clinical symptoms upon routine clinical inquiry (Barron et al., 1994; Dawson, 1984; Hill, 1987; Liesegang, 1999, 2001; Wilhelmus et al., 1996) with HSV-1 being responsible for >90% of ocular HSV infections. A significant proportion (15-50%) of primary genital herpes is caused by HSV-1 and recent studies indicate that the proportion of clinical first episode genital herpes due to HSV-1 is increasing (Auslander et al., 2005; Roberts et al., 2003; Singh et al., 2005). It was recently reported that the global incidence of HSV keratitis is roughly 1.5 million, including 40,000 new cases of severe monocular visual impairment or blindness each year (Farooq and Shukla, 2012).
It is well established that HSV-1-induced CS, and thus HSV-1-induced corneal blindness, are the result of immune responses triggered by the virus (Brandt, 2005; Dix, 2002; Hendricks and Tumpey, 1990; Ksander and Hendricks, 1987; Metcalf and Kaufman, 1976; Thomas and Rouse, 1997). However, the exact identity of the immune responses, including the fine specificity of the potentially harmful T cell effectors expressing classic TCRαβ antigen receptors that lead to CS, remains an area of intense controversy (Banerjee et al., 2002; Huster et al., 2002; Zhao et al., 1998).
At present time there is no vaccine available to control HSV-1 infection and spread. In the past two decades several large clinical HSV vaccine trials were performed but both vaccine studies failed to reach their goals (Awasthi and Friedman, 2014; Farooq and Shukla, 2012). Despite the seriousness of recurrent ocular herpes, no drug has been FDA approved for prevention of ocular recurrences. In addition, the wide use of acyclovir and its homolog in the treatment of both HSV-1 and HSV-2 has raised concern over the development of resistant subtypes of HSV (Antoine et al., 2013; Farooq and Shukla, 2012). Previously it was shown that there is a high prevalence of acyclovir-resistant HSV-1 isolates in patients with HSK (Duan et al., 2008). Thus, the absence of a viable vaccine against the virus and the increase in incidence of resistance to acyclovir and its homolog adds to the critical need for the development of alternative approaches for the prevention and control of serious HSV-1-induced ocular diseases.
Recently we have shown that HSV-1 gK binds to SPP and that blocking of SPP through dominant negative mutants or SPP shRNA reduced viral replication in vitro (Allen et al., 2014). Our published study also demonstrated that gK is the only HSV-1 gene that binds to SPP in vitro. There are several chemicals that have been shown to inhibit the function of SPP. Many forms of γ-secretase inhibitors interfere with SPP activity and are of potential interest as therapeutics in Alzheimer’s disease, HCV, pestivirus and malarial infection (Dovey et al., 2001; Heimann et al., 2006; Lanz et al., 2003; Li et al., 2009; Okamoto et al., 2008; Seiffert et al., 2000; Weihofen et al., 2003; Wolfe, 2009). The γ-secretase inhibitor LY-450139 is one of the most potent inhibitors and is currently in phase II clinical trials and it appears to be well tolerated in human Alzheimer’s patients (Siemers et al., 2006). While this inhibitor is the only one currently being tested in humans, other SPP inhibitors, such as LY-411,575 (Eli Lilly), BMS-299,897 (Bristol-Meyers Squibb) and DAPT (Eli Lilly) have been studied for their effect in vivo (Best et al., 2005; Dovey et al., 2001; Siemers et al., 2005; Siemers et al., 2006). In this study we have shown for the first time that SPP chemical inhibitors are able to block HSV-1 infectivity in vitro. Similarly our published results clearly demonstrated that SPP and gK can colocalize and bind with one another and blocking this interaction with shRNA reduced virus replication in vitro (Allen et al., 2014). Thus, our results suggest that inhibition of SPP activity reduced virus infectivity. Since gK is involved in exacerbation of CS and eye disease in mice, rabbits and humans (Ghiasi et al., 1997; Ghiasi et al., 1994a; Ghiasi et al., 1994b; Mott et al., 2009; Mott et al., 2007a; Mott et al., 2007b; Osorio et al., 2007), this HSV-1 dependence on SPP may be considered as a specific therapeutic target for the prevention of corneal infection in patients at risk. Overall, the global result of these studies suggests that these inhibitors could be an alternative and expedient anti-viral treatment for control of ocular HSV-1 infection.
Fig. 4. SPP inhibitor (Z-LL)2 ketone reduces virus replication in vivo.
One hour before ocular infection with 2 × 104 PFU per eye of HSV-1 strain McKrae, C57BL/6 or BALB/c mice received 100 ug/eye of (Z-LL)2 ketone in DMSO as eye drops. Sham control mice received DMSO alone. (Z-LL)2 ketone treatments were repeated as described in Materials and Methods. In C57BL/6 mice, tear swabs were collected from infected eyes on days 1-5 PI, while in BALB/c infected mice swabs were collected on day 1 and day 3 PI. Virus titers were determined by standard plaque assay. Each point represents the mean titer from 20 eyes from two independent experiments. Panels: A) C57BL/6 mice; and B) BALB/c mice. “*”; Indicates significantly different from sham control group (p<0.001, Student’s t-test).
ACKNOWLEDGEMENTS
This work was supported by Public Health Service grant 1 RO1 EY13615.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen SJ, Hamrah P, Gate DM, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, Benmohamed L, Ahmed R, Wechsler SL, Ghiasi H. The role of LAT in increased CD8+ T cell exhaustion in trigeminal ganglia of mice latently infected with herpes simplex virus type 1. J Virol. 2011;85:4184–4197. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SJ, Mott KR, Matsuura Y, Moriishi K, Kousoulas KG, Ghiasi H. Binding of HSV-1 Glycoprotein K (gK) to Signal Peptide Peptidase (SPP) Is Required for Virus Infectivity. PLoS One. 2014;9:e85360. doi: 10.1371/journal.pone.0085360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoine TE, Park PJ, Shukla D. Glycoprotein targeted therapeutics: a new era of anti-herpes simplex virus-1 therapeutics. Reviews in medical virology. 2013;23:194–208. doi: 10.1002/rmv.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auslander BA, Biro FM, Rosenthal SL. Genital herpes in adolescents. Semin Pediatr Infect Dis. 2005;16:24–30. doi: 10.1053/j.spid.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Friedman HM. A paradigm shift: vaccine-induced antibodies as an immune correlate of protection against herpes simplex virus type 1 genital herpes. J Infect Dis. 2014;209:813–815. doi: 10.1093/infdis/jit658. [DOI] [PubMed] [Google Scholar]
- Banerjee K, Deshpande S, Zheng M, Kumaraguru U, Schoenberger SP, Rouse BT. Herpetic stromal keratitis in the absence of viral antigen recognition. Cell Immunol. 2002;219:108–118. doi: 10.1016/s0008-8749(02)00601-9. [DOI] [PubMed] [Google Scholar]
- Barnett BC, Dolan A, Telford EA, Davison AJ, McGeoch DJ. A novel herpes simplex virus gene (UL49A) encodes a putative membrane protein with counterparts in other herpesviruses. J Gen Virol. 1992;73:2167–2171. doi: 10.1099/0022-1317-73-8-2167. [DOI] [PubMed] [Google Scholar]
- Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, Jones DB, Wilhelmus KR, Kaufman HE, Sugar J, Hyndiuk RA, et al. Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1871–1882. doi: 10.1016/s0161-6420(13)31155-5. [DOI] [PubMed] [Google Scholar]
- Best JD, Jay MT, Otu F, Ma J, Nadin A, Ellis S, Lewis HD, Pattison C, Reilly M, Harrison T, Shearman MS, Williamson TL, Atack JR. Quantitative measurement of changes in amyloid-beta(40) in the rat brain and cerebrospinal fluid following treatment with the gamma-secretase inhibitor LY-411575 [N2-[(2S)-2-(3,5-difluorophenyl)-2-hydroxyethanoyl]-N1-[(7S)-5-methyl-6-ox o-6,7-dihydro-5H-dibenzo[b,d]azepin-7-yl]-L-alaninamide] J Pharmacol Exp Ther. 2005;313:902–908. doi: 10.1124/jpet.104.081174. [DOI] [PubMed] [Google Scholar]
- Bihel F, Das C, Bowman MJ, Wolfe MS. Discovery of a Subnanomolar helical D-tridecapeptide inhibitor of gamma-secretase. J Med Chem. 2004;47:3931–3933. doi: 10.1021/jm049788c. [DOI] [PubMed] [Google Scholar]
- Brandt CR. The role of viral and host genes in corneal infection with herpes simplex virus type 1. Exp Eye Res. 2005;80:607–621. doi: 10.1016/j.exer.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Das C, Berezovska O, Diehl TS, Genet C, Buldyrev I, Tsai JY, Hyman BT, Wolfe MS. Designed helical peptides inhibit an intramembrane protease. J Am Chem Soc. 2003;125:11794–11795. doi: 10.1021/ja037131v. [DOI] [PubMed] [Google Scholar]
- Dawson CR. Ocular herpes simplex virus infections. Clin Dermatol. 1984;2:56–66. doi: 10.1016/0738-081x(84)90066-x. [DOI] [PubMed] [Google Scholar]
- Debroy C, Pederson N, Person S. Nucleotide sequence of a herpes simplex virus type 1 gene that causes cell fusion. Virology. 1985;145:36–48. doi: 10.1016/0042-6822(85)90199-0. [DOI] [PubMed] [Google Scholar]
- Dev KK, Chatterjee S, Osinde M, Stauffer D, Morgan H, Kobialko M, Dengler U, Rueeger H, Martoglio B, Rovelli G. Signal peptide peptidase dependent cleavage of type II transmembrane substrates releases intracellular and extracellular signals. European journal of pharmacology. 2006;540:10–17. doi: 10.1016/j.ejphar.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Dix RD. Pathogenesis of herpes simplex ocular disease. Lippincott, Williams and Wilkins; Philadelphia: 2002. [Google Scholar]
- Dolan A, Jamieson FE, Cunningham C, Barnett BC, McGeoch DJ. The genome sequence of herpes simplex virus type 2. J Virol. 1998;72:2010–2021. doi: 10.1128/jvi.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovey HF, John V, Anderson JP, Chen LZ, de Saint Andrieu P, Fang LY, Freedman SB, Folmer B, Goldbach E, Holsztynska EJ, Hu KL, Johnson-Wood KL, Kennedy SL, Kholodenko D, Knops JE, Latimer LH, Lee M, Liao Z, Lieberburg IM, Motter RN, Mutter LC, Nietz J, Quinn KP, Sacchi KL, Seubert PA, Shopp GM, Thorsett ED, Tung JS, Wu J, Yang S, Yin CT, Schenk DB, May PC, Altstiel LD, Bender MH, Boggs LN, Britton TC, Clemens JC, Czilli DL, Dieckman-McGinty DK, Droste JJ, Fuson KS, Gitter BD, Hyslop PA, Johnstone EM, Li WY, Little SP, Mabry TE, Miller FD, Audia JE. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001;76:173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- Duan R, de Vries RD, Osterhaus AD, Remeijer L, Verjans GM. Acyclovir-resistant corneal HSV-1 isolates from patients with herpetic keratitis. J Infect Dis. 2008;198:659–663. doi: 10.1086/590668. [DOI] [PubMed] [Google Scholar]
- Esler WP, Kimberly WT, Ostaszewski BL, Ye W, Diehl TS, Selkoe DJ, Wolfe MS. Activity-dependent isolation of the presenilin- gamma -secretase complex reveals nicastrin and a gamma substrate. Proc Natl Acad Sci U S A. 2002;99:2720–2725. doi: 10.1073/pnas.052436599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Survey of ophthalmology. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TP, Alvarez X, Kousoulas KG. Plasma membrane topology of syncytial domains of herpes simplex virus type 1 glycoprotein K (gK): the UL20 protein enables cell surface localization of gK but not gK-mediated cell-to-cell fusion. J Virol. 2003;77:499–510. doi: 10.1128/JVI.77.1.499-510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TP, Kousoulas KG. Genetic analysis of the role of herpes simplex virus type 1 glycoprotein K in infectious virus production and egress. J Virol. 1999;73:8457–8468. doi: 10.1128/jvi.73.10.8457-8468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi H, Cai S, Slanina S, Nesburn AB, Wechsler SL. Nonneutralizing antibody against the glycoprotein K of herpes simplex virus type-1 exacerbates herpes simplex virus type-1-induced corneal scarring in various virus-mouse strain combinations. Invest Ophthalmol Vis Sci. 1997;38:1213–1221. [PubMed] [Google Scholar]
- Ghiasi H, Kaiwar R, Nesburn AB, Slanina S, Wechsler SL. Expression of seven herpes simplex virus type 1 glycoproteins (gB, gC, gD, gE, gG, gH, and gI): comparative protection against lethal challenge in mice. J Virol. 1994a;68:2118–2126. doi: 10.1128/jvi.68.4.2118-2126.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiasi H, Nesburn AB, Cai S, Wechsler SL. The US5 open reading frame of herpes simplex virus type 1 does encode a glycoprotein (gJ) Intervirology. 1998;41:91–97. doi: 10.1159/000024919. [DOI] [PubMed] [Google Scholar]
- Ghiasi H, Slanina S, Nesburn AB, Wechsler SL. Characterization of baculovirus-expressed herpes simplex virus type 1 glycoprotein K. J Virol. 1994b;68:2347–2354. doi: 10.1128/jvi.68.4.2347-2354.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golde TE, Wolfe MS, Greenbaum DC. Signal peptide peptidases: a family of intramembrane-cleaving proteases that cleave type 2 transmembrane proteins. Semin Cell Dev Biol. 2009;20:225–230. doi: 10.1016/j.semcdb.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko AP, Moliaka YK, Korovaitseva GI, Rogaev EI. Novel class of polytopic proteins with domains associated with putative protease activity. Biochemistry (Mosc) 2002;67:826–835. doi: 10.1023/a:1016365227942. [DOI] [PubMed] [Google Scholar]
- Gutknecht J. Aspirin, acetaminophen and proton transport through phospholipid bilayers and mitochondrial membranes. Molecular and cellular biochemistry. 1992;114:3–8. doi: 10.1007/BF00240290. [DOI] [PubMed] [Google Scholar]
- Heimann M, Roman-Sosa G, Martoglio B, Thiel HJ, Rumenapf T. Core protein of pestiviruses is processed at the C terminus by signal peptide peptidase. J Virol. 2006;80:1915–1921. doi: 10.1128/JVI.80.4.1915-1921.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks RL, Tumpey TM. Contribution of virus and immune factors to herpes simplex virus type I-induced corneal pathology. Invest Ophthalmol Vis Sci. 1990;31:1929–1939. [PubMed] [Google Scholar]
- Hill TJ. Ocular pathogenicity of herpes simplex virus. Curr Eye Res. 1987;6:1–7. doi: 10.3109/02713688709020060. [DOI] [PubMed] [Google Scholar]
- Huster KM, Panoutsakopoulou V, Prince K, Sanchirico ME, Cantor H. T cell-dependent and -independent pathways to tissue destruction following herpes simplex virus-1 infection. Eur J Immunol. 2002;32:1414–1419. doi: 10.1002/1521-4141(200205)32:5<1414::AID-IMMU1414>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hutchinson L, Goldsmith K, Snoddy D, Ghosh H, Graham FL, Johnson DC. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J Virol. 1992;66:5603–5609. doi: 10.1128/jvi.66.9.5603-5609.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L, Johnson DC. Herpes simplex virus glycoprotein K promotes egress of virus particles. J Virol. 1995;69:5401–5413. doi: 10.1128/jvi.69.9.5401-5413.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson L, Roop-Beauchamp C, Johnson DC. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J Virol. 1995;69:4556–4563. doi: 10.1128/jvi.69.7.4556-4563.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambunathan N, Chowdhury S, Subramanian R, Chouljenko VN, Walker JD, Kousoulas KG. Site-specific proteolytic cleavage of the amino terminus of herpes simplex virus glycoprotein K on virion particles inhibits virus entry. J Virol. 2011;85:12910–12918. doi: 10.1128/JVI.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JK, Moon MH, Seol JW, Seo JS, Lee YJ, Park SY. Prion peptide-mediated cellular prion protein overexpression and neuronal cell death can be blocked by aspirin treatment. Internat J Mol Med. 2011;27:689–693. doi: 10.3892/ijmm.2011.626. [DOI] [PubMed] [Google Scholar]
- Kang L, Park MO, Jun HW. Two-phase melt systems of ibuprofen for enhanced membrane permeation. Pharmaceutical development and technology. 2004;9:349–357. doi: 10.1081/pdt-200032991. [DOI] [PubMed] [Google Scholar]
- Ksander BR, Hendricks RL. Cell-mediated immune tolerance to HSV-1 antigens associated with reduced susceptibility to HSV-1 corneal lesions. Invest Ophthalmol Vis Sci. 1987;28:1986–1993. [PubMed] [Google Scholar]
- Lanz TA, Himes CS, Pallante G, Adams L, Yamazaki S, Amore B, Merchant KM. The gamma-secretase inhibitor N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester reduces A beta levels in vivo in plasma and cerebrospinal fluid in young (plaque-free) and aged (plaque-bearing) Tg2576 mice. J Pharmacol Exp Ther. 2003;305:864–871. doi: 10.1124/jpet.102.048280. [DOI] [PubMed] [Google Scholar]
- Lemberg MK, Martoglio B. Requirements for signal peptide peptidase-catalyzed intramembrane proteolysis. Mol Cell. 2002;10:735–744. doi: 10.1016/s1097-2765(02)00655-x. [DOI] [PubMed] [Google Scholar]
- Li X, Chen H, Bahamontes-Rosa N, Kun JF, Traore B, Crompton PD, Chishti AH. Plasmodium falciparum signal peptide peptidase is a promising drug target against blood stage malaria. Biochem Biophs Res Commun. 2009;380:454–459. doi: 10.1016/j.bbrc.2009.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luo L, Thomas DY, Kang CY. The HIV-1 Env protein signal sequence retards its cleavage and down-regulates the glycoprotein folding. Virology. 2000;272:417–428. doi: 10.1006/viro.2000.0357. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea. 1999;18:127–143. doi: 10.1097/00003226-199903000-00001. [DOI] [PubMed] [Google Scholar]
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- Marks N, Berg MJ. Neurosecretases provide strategies to treat sporadic and familial Alzheimer disorders. Neurochem internat. 2008;52:184–215. doi: 10.1016/j.neuint.2007.06.020. [DOI] [PubMed] [Google Scholar]
- McGeoch DJ, Cunningham C, McIntyre G, Dolan A. Comparative sequence analysis of the long repeat regions and adjoining parts of the long unique regions in the genomes of herpes simplex viruses types 1 and 2. J Gen Virol. 1991;72:3057–3075. doi: 10.1099/0022-1317-72-12-3057. [DOI] [PubMed] [Google Scholar]
- McGeoch DJ, Dalrymple MA, Davison AJ, Dolan A, Frame MC, McNab D, Perry LJ, Scott JE, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- McLauchlan J, Lemberg MK, Hope G, Martoglio B. Intramembrane proteolysis promotes trafficking of hepatitis C virus core protein to lipid droplets. Embo J. 2002;21:3980–3988. doi: 10.1093/emboj/cdf414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf JF, Kaufman HE. Herpetic stromal keratitis-evidence for cell-mediated immunopathogenesis. Am J Ophthalmol. 1976;82:827–834. doi: 10.1016/0002-9394(76)90057-x. [DOI] [PubMed] [Google Scholar]
- Mo C, Holland TC. Determination of the transmembrane topology of herpes simplex virus type 1 glycoprotein K. J Biol Chem. 1997;272:33305–33311. doi: 10.1074/jbc.272.52.33305. [DOI] [PubMed] [Google Scholar]
- Mott KR, Chentoufi AA, Carpenter D, Benmohamed L, Wechsler SL, Ghiasi H. The role of a glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus on virus replication and pathogenicity. Invest Ophthalmol Vis Sci. 2009;50:2903–2912. doi: 10.1167/iovs.08-2957. [DOI] [PubMed] [Google Scholar]
- Mott KR, Osorio Y, Maguen E, Nesburn AB, Wittek AE, Cai S, Chattopadhyay S, Ghiasi H. Role of anti-glycoproteins D (anti-gD) and K (anti-gK) IgGs in pathology of herpes stromal keratitis in humans. Invest Ophthalmol Vis Sci. 2007a;48:2185–2193. doi: 10.1167/iovs.06-1276. [DOI] [PubMed] [Google Scholar]
- Mott KR, Perng GC, Osorio Y, Kousoulas KG, Ghiasi H. A Recombinant Herpes Simplex Virus Type 1 Expressing Two Additional Copies of gK Is More Pathogenic than Wild-Type Virus in Two Different Strains of Mice. J Virol. 2007b;81:12962–12972. doi: 10.1128/JVI.01442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyborg AC, Ladd TB, Jansen K, Kukar T, Golde TE. Intramembrane proteolytic cleavage by human signal peptide peptidase like 3 and malaria signal peptide peptidase. Faseb J. 2006;20:1671–1679. doi: 10.1096/fj.06-5762com. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Mori Y, Komoda Y, Okamoto T, Okochi M, Takeda M, Suzuki T, Moriishi K, Matsuura Y. Intramembrane processing by signal peptide peptidase regulates the membrane localization of hepatitis C virus core protein and viral propagation. J Virol. 2008;82:8349–8361. doi: 10.1128/JVI.00306-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Moriishi K, Miyamura T, Matsuura Y. Intramembrane proteolysis and endoplasmic reticulum retention of hepatitis C virus core protein. J Virol. 2004;78:6370–6380. doi: 10.1128/JVI.78.12.6370-6380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio Y, Mott KR, Jabbar AM, Moreno A, Foster TP, Kousoulas KG, Ghiasi H. Epitope mapping of HSV-1 glycoprotein K (gK) reveals a T cell epitope located within the signal domain of gK. Virus Res. 2007;128:71–80. doi: 10.1016/j.virusres.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy R, Holland TC. In vitro characterization of the HSV-1 UL53 gene product. Virology. 1992;186:579–587. doi: 10.1016/0042-6822(92)90024-j. [DOI] [PubMed] [Google Scholar]
- Roberts CM, Pfister JR, Spear SJ. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex Transm Dis. 2003;30:797–800. doi: 10.1097/01.OLQ.0000092387.58746.C7. [DOI] [PubMed] [Google Scholar]
- Sato T, Nyborg AC, Iwata N, Diehl TS, Saido TC, Golde TE, Wolfe MS. Signal peptide peptidase: biochemical properties and modulation by nonsteroidal antiinflammatory drugs. Biochemistry. 2006;45:8649–8656. doi: 10.1021/bi060597g. [DOI] [PubMed] [Google Scholar]
- Seiffert D, Bradley JD, Rominger CM, Rominger DH, Yang F, Meredith JE, Jr., Wang Q, Roach AH, Thompson LA, Spitz SM, Higaki JN, Prakash SR, Combs AP, Copeland RA, Arneric SP, Hartig PR, Robertson DW, Cordell B, Stern AM, Olson RE, Zaczek R. Presenilin-1 and -2 are molecular targets for gamma-secretase inhibitors. J Biol Chem. 2000;275:34086–34091. doi: 10.1074/jbc.M005430200. [DOI] [PubMed] [Google Scholar]
- Shearman MS, Beher D, Clarke EE, Lewis HD, Harrison T, Hunt P, Nadin A, Smith AL, Stevenson G, Castro JL. L-685,458, an aspartyl protease transition state mimic, is a potent inhibitor of amyloid beta-protein precursor gamma-secretase activity. Biochemistry. 2000;39:8698–8704. doi: 10.1021/bi0005456. [DOI] [PubMed] [Google Scholar]
- Siemers E, Skinner M, Dean RA, Gonzales C, Satterwhite J, Farlow M, Ness D, May PC. Safety, tolerability, and changes in amyloid beta concentrations after administration of a gamma-secretase inhibitor in volunteers. Clin Neuropharmacol. 2005;28:126–132. doi: 10.1097/01.wnf.0000167360.27670.29. [DOI] [PubMed] [Google Scholar]
- Siemers ER, Quinn JF, Kaye J, Farlow MR, Porsteinsson A, Tariot P, Zoulnouni P, Galvin JE, Holtzman DM, Knopman DS, Satterwhite J, Gonzales C, Dean RA, May PC. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006;66:602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- Singh AE, Romanowski B, Wong T, Gourishankar S, Myziuk L, Fenton J, Preiksaitis JK. Herpes simplex virus seroprevalence and risk factors in 2 Canadian sexually transmitted disease clinics. Sex Transm Dis. 2005;32:95–100. doi: 10.1097/01.olq.0000151415.78210.85. [DOI] [PubMed] [Google Scholar]
- Spear PG. Herpes simplex virus: receptors and ligands for cell entry. Cell Microbiol. 2004;6:401–410. doi: 10.1111/j.1462-5822.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- Spear PG, Eisenberg RJ, Cohen GH. Three classes of cell surface receptors for alphaherpesvirus entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC) J Biol Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- Targett-Adams P, Schaller T, Hope G, Lanford RE, Lemon SM, Martin A, McLauchlan J. Signal peptide peptidase cleavage of GB virus B core protein is required for productive infection in vivo. J Biol Chem. 2006;281:29221–29227. doi: 10.1074/jbc.M605373200. [DOI] [PubMed] [Google Scholar]
- Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science. 2002;296:2215–2218. doi: 10.1126/science.1070925. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Lemberg MK, Friedmann E, Rueeger H, Schmitz A, Paganetti P, Rovelli G, Martoglio B. Targeting presenilin-type aspartic protease signal peptide peptidase with gamma-secretase inhibitors. J Biol Chem. 2003;278:16528–16533. doi: 10.1074/jbc.M301372200. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Lemberg MK, Ploegh HL, Bogyo M, Martoglio B. Release of signal peptide fragments into the cytosol requires cleavage in the transmembrane region by a protease activity that is specifically blocked by a novel cysteine protease inhibitor. J Biol Chem. 2000;275:30951–30956. doi: 10.1074/jbc.M005980200. [DOI] [PubMed] [Google Scholar]
- Wilhelmus KR, Dawson CR, Barron BA, Bacchetti P, Gee L, Jones DB, Kaufman HE, Sugar J, Hyndiuk RA, Laibson PR, Stulting RD, Asbell PA, Herpetic Eye Disease Study Group Risk factors for herpes simplex virus epithelial keratitis recurring during treatment of stromal keratitis or iridocyclitis. Br J Ophthalmol. 1996;80:969–972. doi: 10.1136/bjo.80.11.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS. gamma-Secretase in biology and medicine. Semin Cell Dev Biol. 2009;20:219–224. doi: 10.1016/j.semcdb.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Zara S, De Colli M, Rapino M, Pacella S, Nasuti C, Sozio P, Di Stefano A, Cataldi A. Ibuprofen and lipoic acid conjugate neuroprotective activity is mediated by Ngb/Akt intracellular signaling pathway in Alzheimer’s disease rat model. Gerontology. 2013;59:250–260. doi: 10.1159/000346445. [DOI] [PubMed] [Google Scholar]
- Zhao ZS, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–1347. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]