Abstract
Background
Desktop dust has been studied as a source of food allergen, but not as a source of potential aeroallergen exposure.
Methods
36 wiped samples from desktop surfaces were collected from preschools and schools. Samples were analyzed for detectable levels of common aeroallergens including Alternaria, cockroach, dog, dust mite, cat, mouse, and rat allergens by immunoassay.
Results
Mouse allergen was the most prevalent, detectable in 97.2% of samples. Cat allergen was detectable in 80.6% of samples and dog allergen was detectable in 77.8% of samples. Other allergens were not as prevalent. Mouse was the only allergen that was highly correlated with settled floor dust collected from the same rooms (r=0.721, p<0.001).
Conclusion
This is the first study to detect aeroallergens on desktop surfaces by using moist wipes. Allergens for mouse, cat, and dog were highly detectable in wipes with mouse desktop surface levels correlating with levels in vacuumed floor dust.
Keywords: Allergen, asthma, inner-city children, school, desktop
Many studies have demonstrated that indoor allergen exposure is a risk factor for asthma development and asthma morbidity (1-5). Environmental assessments typically measure allergens by vacuuming floor dust or sampling air. We present a method of using moist table wipes to detect the presence of aeroallergens on surfaces. This method has previously been used to evaluate the presence of food allergens on desktop surfaces (6), and in one study nearly two decades ago was evaluated in wall wipes (7). Desktop allergen contact may represent a unique and important clinical exposure via the cutaneous route. In this school environment study, we evaluate the usefulness of table and surface wipes in determining levels of indoor aeroallergens as compared to levels measured in simultaneously collected vacuum dust samples from the same rooms.
Methods
This is a sub-study of the School Inner-City Asthma Study (SICAS) which is an observational, prospective study evaluating school-specific environmental risk factors for asthma morbidity while controlling for the home environment (8). A subset of 17 schools and 4 preschools in inner-city Northeastern U.S. area were included in this sub-study. In total, 36 distinct rooms were analyzed by collecting surface wipe samples. This included table wipes from 21 different elementary school cafeterias and table/desk wipes from 15 different preschool classrooms. As a control, 3 blank unused wipes were tested. Briefly, one square foot area was wiped by fiber filter (Kimwipes4x8, cat no.S47299) moistened with endotoxin-free extract solution (PBS and 1%Tween-20, Sigma-Aldrich, St Louis, MO). Samples were immediately stored at −20°C until extraction. All samples were sent to INDOOR Biotechnologies (Charlottesville, VA) for analysis. Table wipes were extracted in extract solution and rocked for 2 hours at room temperature. After that, 2ml aliquot of each extract was collected and analyzed for detectable levels of common indoor allergens by immunoassay. The lower limits of detection for wipe extracts were 0.02 ng/ml for Alt a 1, Rat n 1, and Fel d 1; 0.06 ng/ml for Der p 1, Der f 1, and Can f 1; 0.01 ng/ml for Mus m 1; and 0.98 ng/ml for Bla g 2. Further details of methods, number of rooms per school, and analysis are described in this article’s online supplements.
Results
Table I shows the prevalence, medians, and ranges of allergens detected by desktop/table wipes. Mouse allergen was the most prevalent and was detectable in 100% of preschool samples (median 0.54 ng/ml) and 95.2% of school samples (median 0.29 ng/ml). Dog and cat allergen were also found to be highly detectable by wipes. For cat allergen, 93.3% of preschool samples and 71.4% of school samples were positive. Dog allergen was found to be positive in 53.3% and 95.2% of preschool and school samples, respectively. Allergens for dust mites, cockroach, rat, and Alternaria mold were not commonly detected, if detected at all. For comparison, vacuumed floor dust samples collected from the same rooms demonstrated similar detectable rates for mouse allergen (100% preschools, 88.9% schools), cat allergen (53.3% preschools, 77.8% schools), and dog allergen (53.3% preschools, 33.3% schools). Similar to surface wipes, Alternaria mold, dust mite, cockroach, and rat allergen were not commonly detected in vacuumed floor dust samples as seen in Table E1.
Table I.
Allergen levels in table wipe samples from schools and preschools
| Elementary School Samples |
Preschool Samples |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allergen | Total Wipes |
Detectable Samples %(N) |
Median | Range | Total Wipes |
Detectable Samples %(N) |
Median | Range | ||
| Mus ml | 21 | 95.20% | (n=20) | 0.29 ng/ml | 0.06 -77.13 ng/ml | 15 | 100.00% | (n=15) | 0.54 ng/ml | 0.04 - 10.34 ng/ml |
| Fel d1 | 21 | 71.40% | (n=15) | 0.14 ng/ml | 0.03 - 2.02 ng/ml | 15 | 93.30% | (n=14) | 0.20 ng/ml | 0.03 - 1.89 ng/ml |
| Can f1 | 21 | 95.20% | (n=20) | 1.61 ng/ml | 0.14 - 4.80 ng/ml | 15 | 53.30% | (n=8) | 0.22 ng/ml | 0.09 - 1.76 ng/ml |
| Altai | 21 | 9.50% | (n=2) | 0.28 ng/ml | 0.15-0.41 ng/ml | 15 | 13.30% | (n=2) | 0.10 ng/ml | 0.06 - 0.14 ng/ml |
| Der f1 | 21 | 0.00% | - | - | 15 | 6.70% | (n=1) | 0.15 ng/ml | 0.15 ng/ml | |
| Der p1 | 21 | 4.80% | (n=1) | 0.07 ng/ml | 0.07 ng/ml | 15 | 6.70% | (n=1) | 0.09 ng/ml | 0.09 ng/ml |
| Bla g2 | 21 | 4.80% | (n=1) | 1.50 ng/ml | 1.50 ng/ml | 15 | 0.00% | - | - | |
| Rat n1 | 21 | 0.00% | - | - | 15 | 0.00% | - | - | ||
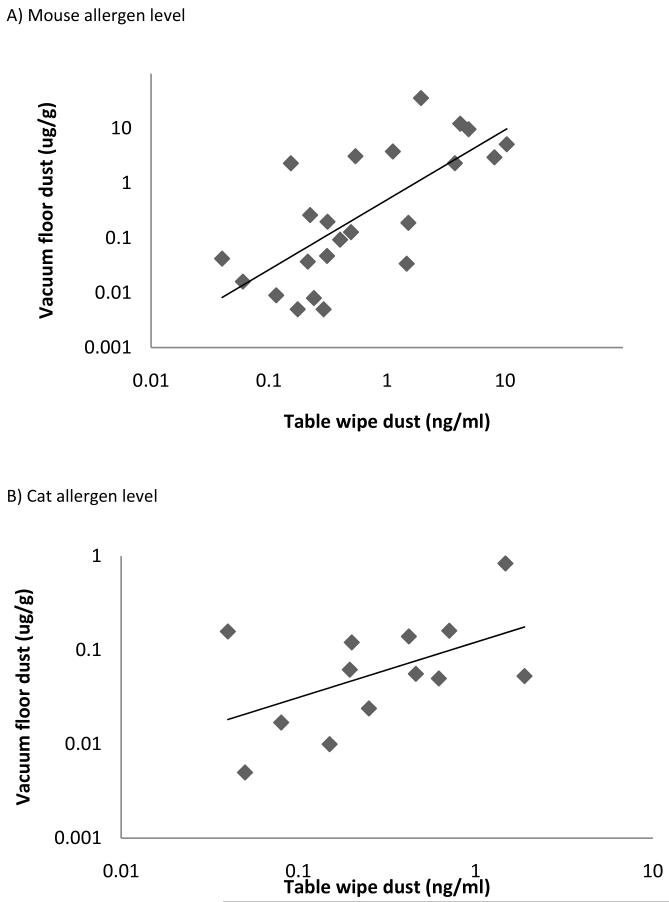
A correlation analysis was used to test the relationship between allergen levels measured in table/desk wipe samples to those levels measured in vacuumed floor dust within the same rooms. This analysis demonstrated that mouse allergen levels from surface wipes were highly correlated with mouse allergen levels collected from settled floor dust (r=0.721, p<0.001) as seen in Figure 1. Correlation of cat allergen levels between the two procedures was found to be moderately correlated (r=0.407, p=0.168). No significant correlation was detected for dog allergen (r= −0.180, p=0.670). After adjusted analysis for within school correlation using a generalized estimating equation, the mouse correlation remained significant (p<0.001).
Figure 1.
Scatter plot of detectable table wipe and vacuumed floor dust samples from both preschools and elementary schools for A) mouse allergen level, r=0.721, p=0.0001, B) cat allergen level, r=0.407, p=0.168.
Discussion
To our knowledge, this study is the first to evaluate the utility of moist surface wipes in measuring aeroallergens on school desktop surface. We found that aeroallergens were detectable on table and desk wipes of schools and preschools. Additionally, we found that the percentages of detectable allergens in surface wipes were similar to the findings of vacuumed floor dust samples simultaneously collected from the same rooms. More specifically, a strong correlation between the levels of mouse allergen was discovered between the surface wipe samples and the vacuumed floor samples. Therefore, surface wipes may provide a simple and efficient method of analyzing environments for the presence of aeroallergens.
Surface wipes may be useful in environmental allergen studies by either enhancing or replacing current standard evaluation practices that include vacuum settled dust and air sampling. Wood et al demonstrated that cat allergen on wall surfaces was detected by wipe sampling and mentioned that this method would provide a significant advance due to its simplicity (7). The collection of these table wipes is simple and efficient. The process does not require a lot of training or heavy equipment. In addition, surface aeroallergens are important to evaluate because these exposures may affect humans via the cutaneous route. This cutaneous exposure may be particularly important for individuals with a disrupted skin barrier, such as those with eczema (9).
Our findings of mouse allergen in the schools agree with previous studies (1, 10). Furthermore, visible mouse infestation in schools has been reported with signs of mouse infestation being associated with higher levels of detectable mouse allergen (11). In contrast, cat and dog allergens were likely brought into schools on clothing and shoes of students and staffs with pets at their homes (12, 13). Interestingly, we found significant correlation for mouse allergen between floors and surface tops. This may be due to the more easily aerosolized mouse allergen with direct sources within the schools (1, 10, 11). Regarding Alt a 1, a recent study using air samplers for mold collection found that Alt a1 was commonly found in schools (14). However, the immunoassay in this study required high spore concentrations in order to detect Alt a 1. These high spore concentrations are probably greater than that likely to be found in indoor environment (15). This might be the reason for low detectable Alt a 1 in our study samples. In addition, allergen exposure may vary by season for a variety of factors including cleaning frequency. For example, this study was primarily done during the winter, flu season, months. While speculative, possible frequent cleaning during the flu season did not appear to eliminate exposure levels to certain allergens, such as mouse. It is possible in other (non-flu) seasons that exposure levels may even be higher if cleaning frequency is decreased.
Our study is limited in that this was a pilot study with a small number of samples collected. Larger studies would be needed to appropriately determine if there is a correlation between surface measurements and floor measurements for other allergens. However, this study provides important insight to an objective way to measure allergen exposure, especially in a school environment where infestation may not be well known. We expect that schools with the presence of higher allergen levels or any specific allergen infestation would have findings that are generalizable from this study. Another limitation to consider is the possible drawback of using a moist sample wipe. For example, the collected wet sample likely needs to be processed immediately to prevent any possible degradation of the allergen sample by bacterial or fungal proteases. There is a chance for contamination, either from an investigator’s hands and clothing or from another site. This may be a disadvantage as compared to dry dust sampling that would not be expected to be susceptible to such immediate degradation.
In summary, we present that surface wipe sampling is an efficient method of measuring aeroallergens in the environment and demonstrated that mouse, cat, and dog allergens were highly prevalent in school desktop dust. Further studies are required to validate this method and assess the effect of this surface allergen exposure on morbidity in atopic diseases.
Supplementary Material
Acknowledgments
This work was conducted with the support from Harvard Catalyst/The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Center for Research Resources, or the National Institutes of Health
Funding: This study was supported by grants R01 AI 073964, R01 AI 073964-02S1, K24 AI 106822, U10HL098102 from the National Institutes of Health (PI Phipatanakul) and the Division of Immunology Clinical Research Advisory Group Pilot Research Grant (PI Sheehan).
Abbreviations
- SICAS
School Inner-City Asthma Study
References
- 1.Permaul P, Hoffman E, Fu C, Sheehan W, Baxi S, Gaffin J, et al. Allergens in urban schools and homes of children with asthma. Pediatr Allergy Immunol. 2012;23(6):543–549. doi: 10.1111/j.1399-3038.2012.01327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torjusen EN, Diette GB, Breysse PN, Curtin-Brosnan J, Aloe C, Matsui EC. Dose-response relationships between mouse allergen exposure and asthma morbidity among urban children and adolescents. Indoor Air. 2012 doi: 10.1111/ina.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsui EC, Eggleston PA, Buckley TJ, Krishnan JA, Breysse PN, Rand CS, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97(4):514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- 4.Almqvist C, Wickman M, Perfetti L, Berglind N, Renstrom A, Hedren M, et al. Worsening of asthma in children allergic to cats, after indirect exposure to cat at school. Am J Respir Crit Care Med. 2001;163(3 Pt 1):694–698. doi: 10.1164/ajrccm.163.3.2006114. [DOI] [PubMed] [Google Scholar]
- 5.Perry T, Matsui E, Merriman B, Duong T, Eggleston P. The prevalence of rat allergen in inner-city homes and its relationship to sensitization and asthma morbidity. J Allergy Clin Immunol. 2003;112(2):346–352. doi: 10.1067/mai.2003.1640. [DOI] [PubMed] [Google Scholar]
- 6.Perry TT, Conover-Walker MK, Pomes A, Chapman MD, Wood RA. Distribution of peanut allergen in the environment. J Allergy Clin Immunol. 2004;113(5):973–976. doi: 10.1016/j.jaci.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 7.Wood RA, Mudd KE, Eggleston PA. The distribution of cat and dust mite allergens on wall surfaces. J Allergy Clin Immunol. 1992;89(1 Pt 1):126–130. doi: 10.1016/s0091-6749(05)80049-1. [DOI] [PubMed] [Google Scholar]
- 8.Phipatanakul W, Bailey A, Hoffman EB, Sheehan WJ, Lane JP, Baxi S, et al. The school inner-city asthma study: design, methods, and lessons learned. J Asthma. 2011;48(10):1007–1014. doi: 10.3109/02770903.2011.624235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck LA, Leung DY. Allergen sensitization through the skin induces systemic allergic responses. J Allergy Clin Immunol. 2000;106(5 Suppl):S258–263. doi: 10.1067/mai.2000.110159. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan WJ, Rangsithienchai PA, Muilenberg ML, Rogers CA, Lane JP, Ghaemghami J, et al. Mouse allergens in urban elementary schools and homes of children with asthma. Ann Allergy Asthma Immunol. 2009;102(2):125–130. doi: 10.1016/S1081-1206(10)60242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Permaul P, Sheehan WJ, Baxi SN, Gaffin JM, Fu C, Petty CR, et al. Predictors of indoor exposure to mouse allergen in inner-city elementary schools. Ann Allergy Asthma Immunol. 2013;111(4):299–301. doi: 10.1016/j.anai.2013.07.028. e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berge M, Munir AK, Dreborg S. Concentrations of cat (Fel d1), dog (Can f1) and mite (Der f1 and Der p1) allergens in the clothing and school environment of Swedish schoolchildren with and without pets at home. Pediatr Allergy Immunol. 1998;9(1):25–30. doi: 10.1111/j.1399-3038.1998.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 13.Almqvist C, Larsson PH, Egmar AC, Hedren M, Malmberg P, Wickman M. School as a risk environment for children allergic to cats and a site for transfer of cat allergen to homes. J Allergy Clin Immunol. 1999;103(6):1012–1017. doi: 10.1016/s0091-6749(99)70172-7. [DOI] [PubMed] [Google Scholar]
- 14.Baxi SN, Muilenberg ML, Rogers CA, Sheehan WJ, Gaffin J, Permaul P, et al. Exposures to molds in school classrooms of children with asthma. Pediatr Allergy Immunol. 2013;24(7):697–703. doi: 10.1111/pai.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vailes L, Sridhara S, Cromwell O, Weber B, Breitenbach M, Chapman M. Quantitation of the major fungal allergens, Alt a 1 and Asp f 1, in commercial allergenic products. J Allergy Clin Immunol. 2001;107(4):641–646. doi: 10.1067/mai.2001.114118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.