Abstract
Background
Baboons have natural antibodies against pig antigens. We have investigated whether there are differences in anti-nonGal pig antibody levels between baboons maintained under specific pathogen-free (SPF) conditions and those housed under conventional conditions (NonSPF) that might be associated with improved outcome after pig-to-baboon organ transplantation.
Methods
Baboons (n=40) were housed indoors (SPF n=8) or in indoor/outdoor pens (NonSPF n=32) in colonies of similar size and structure. NonSPF colonies harbor a number of pathogens common to nonhuman primate species, whereas many of these pathogens have been eliminated from the SPF colony. Complete blood cell counts (CBC), blood chemistry, and anti-nonGal IgM and IgG levels were monitored.
Results
There were no significant differences in CBC or blood chemistry between SPF and NonSPF baboons. Anti-nonGal IgM levels were significantly lower in the SPF baboons than in the NonSPF baboons (MFI 7.1 vs 8.8, p<0.05). One SPF and two NonSPF baboons had an MFI >20; if these 3 baboons are omitted, the mean MFIs were 4.8 (SPF) vs 7.5 (NonSPF) (p<0.05). Anti-nonGal IgG was minimal in both groups (MFI 1.0 vs 1.0).
Conclusions
As their levels of anti-nonGal IgM are lower, baboons maintained under SPF conditions may be beneficial for xenotransplantation studies as the initial binding of anti-pig IgM to an α1,3-galactosyltransferase gene-knockout pig organ may be less, thus resulting in less complement and/or endothelial cell activation. However, even under identical SPF conditions, an occasional baboon will express a high level of anti-nonGal IgM, the reason for which remains uncertain.
Keywords: Antibody, anti-pig; Baboon; Pig, α1,3-galactosyltransferase gene-knockout; Specific pathogen-free; Xenotransplantation
Introduction
Xenotransplantation could provide an unlimited and elective supply of organs and cells for clinical transplantation. Although advances have been made with the introduction of α1,3-galactosyltransferase gene-knockout (GTKO) pigs (1), even after the removal of this dominant xenoantigen, the anti-nonGal antibody barrier still presents a challenge.
Specific pathogen-free (SPF) baboons were established to reduce colonization with bacteria, viruses, fungi and parasites that might be pathogenic. As it is believed that natural antibodies, including some anti-pig antibodies, develop as a result of microbial colonization of the intestinal tract during infancy (2–10), a reduced level of colonization might be associated with lower levels of anti-pig antibody. We have investigated this hypothesis in baboons.
Methods
Baboons
Baboons (Papio species; SPF n=8; NonSPF n=32), were obtained from the Oklahoma University Health Sciences Center (Oklahoma City, OK). Their age was 3–4 years and weight was 6–9 kg. Baboons came from both conventional (NonSPF) and SPF colonies. The NonSPF colony is housed in indoor/outdoor pens, and the SPF colony is housed in facilities that are all indoors. Breeding groups in both colonies are similar in size and structure (multi-male and multi-female) with 40–80 individuals in each group. The NonSPF colony is known to harbor the normal endogenous viral pathogens present in baboon colonies (Table 1), including HVP1, HVP2, SVV, BaCMV, HHV6, BaRV, SFV, SRV, SIV, STLV, SV40, measles, and monkeypox. However, these have been eliminated from the SPF colony. Furthermore, the two internal parasites, Strongyloides sp. and Trichuris trichiura, endemic in the NonSPF colony, have been eliminated from the SPF colony.
Table 1.
Viruses and parasites present in the NonSPF baboon colony but eliminated from the SPF baboons
| Herpesvirus |
| Herpesvirus papio 1 (HVP1) |
| Herpesvirus papio 2 (HVP2) |
| Simian varicella zoster virus (SVV) |
| Baboon cytomegalovirus (BaCMV) |
| Human herpes virus 6 (HVP1) |
| Baboon rhadinovirus (BaRV) |
| Retrovirus |
| Simian foamy virus (SFV) |
| Simian retrovirus/D (SRV) |
| Simian immunodeficiency virus (SIV) |
| Simian T lymphotropic virus (STLV) |
| Papovavirus |
| Simian virus 40 (SV40) |
| Paramyxovirus |
| Morbillivirus (measles) |
| Orthopoxvirus |
| Monkeypox virus |
| Internal parasites |
| Trichuris trichuria (whipworms) |
| Stongyloides sp. (threadworms) |
Monitoring
Before the baboons had undergone any surgical or immunomodulatory intervention, blood was collected by venepuncture for measurement of hematologic, biochemical, and coagulation parameters using standard methods (Central Laboratory of Presbyterian Hospital of the University of Pittsburgh Medical Center, Pittsburgh, PA) (11).
Measurement of anti-nonGal IgM and IgG by flow cytometry
Baboon serum samples were incubated for 30min at 56°C to inactivate complement. GTKO pig aortic endothelial cells were used as target cells. IgM and IgG antibodies directed to antigen targets other than galactose-α1,3-galactose (anti-nonGal antibodies) were measured by immunofluorescence intensity. Measurement of mean fluorescence intensity (MFI) was accomplished by CellQuest software (BD Biosciences, San Jose, CA) using LSR flow cytometry (San Jose, CA) and relative MFI was calculated by Flowjo software (Ashland, OR).
Statistical analyses
The results were analyzed by Student t-test or analysis of variance (ANOVA) where appropriate. The t-test was used to assess whether the mean values of the SPF and NonSPF groups were statistically different. A p value of <0.05 was considered to be statistically significant. Correlation of MFI was calculated by linear regression analysis. Significance at the 95% or the 99% level was calculated using prism-4 software (Graphpad Software, San Diego, CA).
Results
There were no significant differences in complete blood count or blood chemistry between SPF and NonSPF baboons (Table 2). Anti-nonGal IgM antibody levels were significantly lower in the SPF baboons than in the NonSPF baboons (MFI 7.1 vs 8.8, p<0.05) (Figure 1). There was one SPF baboon with a particularly high level of anti-nonGal IgM (MFI 23.2) and two NonSPF baboons with a MFI >20; if these 3 baboons are omitted from the calculations, the mean MFIs were 4.8 (SPF) vs 7.5 (NonSPF) (p<0.05). Anti-nonGal IgG was minimal in both groups (MFI 1.0 vs 1.0, NS) (Figure 1).
Table 2.
Hematologic and blood chemistry parameters in SPF (n=8) and NonSPF (n=32) baboons
| Hematologic parameter (units) | SPF | NonSPF |
|---|---|---|
| WBC (103/μl) | 6.32 | 6.58 |
| RBC(106/μl) | 5.16 | 4.97 |
| Hct(%) | 39.5 | 37.7 |
| Hb(g/dL) | 12.8 | 12.3 |
| Plt(103/μl) | 304 | 298 |
| Blood chemistry parameters (units) | SPF | NonSPF |
|---|---|---|
| Alanine transaminase (IU/L) | 35 | 34 |
| Aspartate transaminase (IU/L) | 30 | 28 |
| Gamma glutamyl transferase (IU/L) | 51 | 51 |
| Blood urea nitrogen (mg/dl) | 12 | 14 |
| Creatinine (mg/dL) | 0.6 | 0.7 |
| Total protein (g/dL) | 6.9 | 6.8 |
| Albumin (g/dL) | 4.1 | 3.9 |
Figure 1.
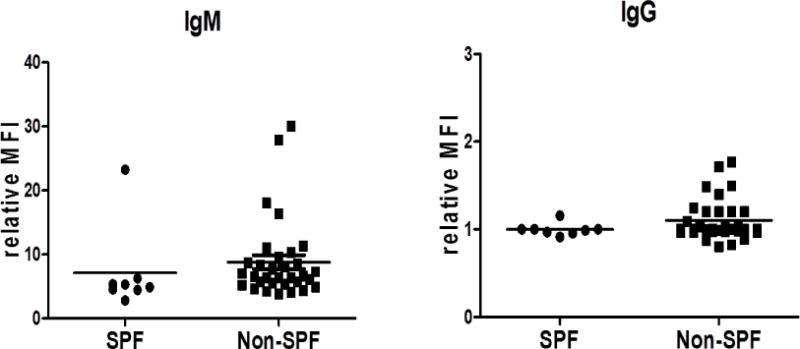
Anti-nonGal IgM and IgG levels in SPF (n=8) and NonSPF (n=32) baboons.
Discussion
Natural antibody levels, e.g., anti-Gal antibody, are believed to be associated with microbial colonization of the gastrointestinal tract of the animal during the first few months of life (2–10). The development of antibody is generally considered to be T cell-independent (12), although there is some evidence that it may be T cell-dependent (13, 14). Although several factors, such as age, gender, ABO blood type, and vaccination history, may affect the levels of these antibodies (15), the level may be influenced by the extent of colonization of the animal. Baboons housed under SPF conditions, in which many of the usual microorganisms have been eliminated, might therefore be anticipated to express lower levels of natural antibody. With regard to anti-nonGal IgM, this proved to be the case in the SPF baboons in the present study.
As anti-Gal antibody is no longer of significance in xenotransplantation studies because of the availability of GTKO pigs, we did not measure anti-Gal IgM or IgG levels. However, the mean level of anti-nonGal IgM (though not of IgG) was significantly lower in the SPF baboons than in the NonSPF baboons. Baboons maintained under SPF conditions may therefore be beneficial for xenotransplantation studies as the initial binding of anti-pig IgM to a GTKO pig organ may be less, thus resulting in less complement deposition and/or endothelial cell activation. However, even under identical SPF conditions, an occasional baboon will express a high level of anti-nonGal IgM, the reason for which remains uncertain.
To our knowledge, there has been only one previous study comparing antibody levels in baboons housed under SPF or NonSPF conditions (16). Although there were differences in the assays (resulting in higher MFIs in the previous study), the results were similar to those reported here, with anti-nonGal IgM being lower in SPF baboons, with little difference in IgG. It may be significant that the longest survivals to date of GTKO pig heart grafts have been reported in baboons that had been housed under SPF conditions (16–17).
It is of interest to compare the levels of anti-nonGal IgM and IgG in baboons with those in humans (15, 16). The lower levels in SPF baboons are closer to those in humans and, although there are some variations in humans related to geographic environment (15), this may reflect the reduced colonization of the gastro-intestinal tract in SPF baboons and humans.
In the USA, the current cost of purchasing SPF baboons is significantly higher than purchasing NonSPF baboons (>$8,500 vs <$7,000), but this discrepancy is likely to be reduced in the future. The cost of establishing an SPF colony is high (18), but, once established, maintaining a baboon in such a facility is minimally more than under conventional conditions, the only extra costs being related to twice yearly testing for pathogens on the exclusion list. The purchase price should fall as the colony reaches full maturity.
Acknowledgments
Burcin Ekser, MD, is a recipient of NIH NIAID T32 AI 074490 training grant. Mohamed Ezzelarab, MD, is supported in part by the Joseph A. Patrick Fellowship at the Thomas E. Starzl Transplantation Institute. Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is, or has been, supported in part by NIH grants #U19 AI090959, #U01 AI068642, and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Inc., Blacksburg, VA. The baboons used in the study were from the Oklahoma University Health Sciences Center, Baboon Research Resources, which is supported by the Office Of The Director, NIH under Award Number P40OD010431 and P40OD010988.
Abbreviations
- Gal
galactose-α1,3-galactose
- GTKO
α1,3-galactosyltransferase gene-knockout
- SPF
specific pathogen-free
Footnotes
Disclosure of conflict of interest:
No author has a conflict of interest.
References
- 1.Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, et al. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science. 2003;299(5605):411–414. doi: 10.1126/science.1078942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida IC, Krautz GM, Krettli AU, Travassos LR. Glycoconjugates of Trypanosoma cruzi: a 74 kD antigen of trypomastigotes specifically reacts with lytic anti-alpha-galactosyl antibodies from patients with chronic Chagas disease. J Clin Lab Anal. 1993;7(6):307–316. doi: 10.1002/jcla.1860070603. [DOI] [PubMed] [Google Scholar]
- 3.Avila JL, Rojas M, Garcia L. Persistence of elevated levels of galactosyl-alpha(1-3)galactose antibodies in sera from patients cured of visceral leishmaniasis. J Clin Microbiol. 1988;26(9):1842–1847. doi: 10.1128/jcm.26.9.1842-1847.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avila JL, Rojas M, Galili U. Immunogenic Gal alpha 1—3Gal carbohydrate epitopes are present on pathogenic American Trypanosoma and Leishmania. J Immunol. 1989;142(8):2828–2834. [PubMed] [Google Scholar]
- 5.Avila JL. alpha-Galactosyl-bearing epitopes as potent immunogens in Chagas’ disease and leishmaniasis. Subcell Biochem. 1999;32:173–213. doi: 10.1007/978-1-4615-4771-6_8. [DOI] [PubMed] [Google Scholar]
- 6.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26(4):347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 7.Galili U, Mandrell RE, Hamadeh RM, Shohet SB, Griffiss JM. Interaction between human natural anti-alpha-galactosyl immunoglobulin G and bacteria of the human flora. Infect Immun. 1988;56(7):1730–1737. doi: 10.1128/iai.56.7.1730-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisowska E, Duk M. Diversity of natural anti-alpha-galactosyl antibodies in human serum. Adv Exp Med Biol. 2011;705:571–583. doi: 10.1007/978-1-4419-7877-6_30. [DOI] [PubMed] [Google Scholar]
- 9.Mangold A, Lebherz D, Papay P, Liepert J, Hlavin G, Lichtenberger C, et al. Anti-Gal titers in healthy adults and inflammatory bowel disease patients. Transplant Proc. 2011;43(10):3964–3968. doi: 10.1016/j.transproceed.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 10.Rood PP, Tai HC, Hara H, Long C, Ezzelarab M, Lin YJ, et al. Late onset of development of natural anti-nonGal antibodies in infant humans and baboons: implications for xenotransplantation in infants. Transpl Int. 2007;20(12):1050–1058. doi: 10.1111/j.1432-2277.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 11.Ekser B, Bianchi J, Ball S, et al. Comparison of hematologic, biochemical, and coagulation parameters in 1,3-galactosyltransferase gene-knockout pigs, wild-type pigs, and four primate species. Xenotransplantation. 2012;19:342–354. doi: 10.1111/xen.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohdan H, Yang YG, Swenson KG, Thall AD, Sykes M. In vivo T-cell depletion enhances production of anti-GALalpha1,3GAL natural antibodies in alpha1,3-galactosyltransferase-deficient mice. Transplantation. 2000;69(5):910–913. doi: 10.1097/00007890-200003150-00041. [DOI] [PubMed] [Google Scholar]
- 13.Cretin N, Bracy J, Hanson K, Iacomini J. The role of T cell help in the production of antibodies specific for Gal alpha 1-3Gal. J Immunol. 2002;168(3):1479–1483. doi: 10.4049/jimmunol.168.3.1479. [DOI] [PubMed] [Google Scholar]
- 14.Dons EM, Montoya C, Long CE, Hara H, Echeverri GJ, Ekser B, et al. T-cell-based immunosuppressive therapy inhibits the development of natural antibodies in infant baboons. Transplantation. 2012;93(8):769–776. doi: 10.1097/TP.0b013e3182481168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar G, Satyananda V, Fang J, Zhou H, Fujita M, Ekser B, et al. Is there a correlation between anti-pig antibody levels in humans and geographic location during childhood? Transplantation. 2013;96(4):387–393. doi: 10.1097/TP.0b013e3182992a84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohiuddin MM, Corcoran PC, Singh AK, Azimzadeh A, Hoyt RJ, Thomas ML, et al. B-cell depletion extends the survival of GTKO.hCD46Tg pig heart xenografts in baboons for up to 8 months. Am J Transplant. 2012;12(3):763–771. 307–316. doi: 10.1111/j.1600-6143.2011.03846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper DKC. A milestone in xenotransplantation research. Xenotransplantation. 2014 doi: 10.1111/xen.12079. (Commentary) in press. [DOI] [PubMed] [Google Scholar]
- 18.Wolf RF, Eberle R, White GL. Generation of a specific-pathogen free baboon colony. JAALAS. 2010;49:814–820. [PMC free article] [PubMed] [Google Scholar]


