Abstract
Background
Evidence indicating an increase in dopamine D2 receptor (D2R) density and occupancy in patients with schizophrenia comes from positron emission tomography studies using ligands that bind both D2Rs and dopamine D3 receptors (D3Rs), questioning the role of D3Rs in the pathophysiology of the disease. Dopamine D3 receptor positron emission tomography ligands have recently been developed and antagonists with preferential affinity for D3R versus D2R are undergoing clinical evaluation. To determine if an increase in D3Rs in the striatum could produce phenotypes relevant to schizophrenia, we generated a transgenic model of striatal D3R overexpression.
Methods
A bi-transgenic system was used to generate mice with increased D3Rs selectively in the striatum. Mice with overexpression of D3R were subjected to an extensive battery of behavioral tests, including several relevant to schizophrenia. Ligand binding and quantitative reverse transcription polymerase chain reaction methods were used to quantify the effect of D3R overexpression on dopamine D1 receptors (D1Rs) in the striatum.
Results
Mice with overexpression of D3R show no abnormalities in basic behavioral functions or cognitive tests but do display a deficit in incentive motivation. This was associated with a reduction in striatal D1R ligand binding, driven by a downregulation at the level of transcription. Both motivation and D1R expression were rescued by switching off the transgene in adulthood.
Conclusions
Overexpression of D3Rs in the striatum of mice does not elicit cognitive deficits but disrupts motivation, suggesting that changes in D3Rs may be involved in the negative symptoms of schizophrenia. These data imply that it will be important to evaluate the effects of D3R antagonists on motivational symptoms, which are not improved by currently available antipsychotic medications.
Keywords: Cognition, dopamine D3 receptor, motivation, schizophrenia, striatum, transgenic model
Several lines of evidence support the idea that the symptoms of schizophrenia result from dysfunction of the dopamine system [for review see (1)], including disruption of both presynaptic and postsynaptic components (2). While it is generally assumed that the dopamine D2 receptor (D2R) is the critical dopamine receptor subtype for the postsynaptic component of the disease, it is also possible that dopamine D3 receptors (D3Rs) play a role. For example, early studies found that the clinical efficacy of antipsychotic medications correlates with their occupancy of D2 receptors (3,4). However, because antipsychotics show limited preference for D2Rs over D3Rs, a similar relationship between D3R receptor occupancy and clinical efficacy is also likely to exist (5). While the clinical efficacy of any drug does not prove that its target is involved in the etiology of the disease, other lines of evidence suggest that dopamine D2 and D3 receptors could play a role.
A meta-analysis of 17 positron emission tomography (PET) imaging studies revealed a small (12%) but significant elevation of striatal D2-type receptor level in patients with schizophrenia (6). A more recent meta-analysis confirmed this finding and also showed it to be influenced by exposure to antipsychotic medication and the imaging methods used (2). However, the radio-labeled ligands used in these studies do not distinguish between the structurally similar D2R and D3R (7) and therefore do not provide the necessary molecular specificity. Developing PET ligands with high selectivity for the D3R over D2R has been notoriously difficult (8). Recently, one PET ligand, [11C]-(+)-PHNO, which has higher affinity for D3Rs than D2Rs, was developed for in vivo use; however the selectivity is not very high (9–11). One study did not find an increase in D3R binding in schizophrenia (12); however, this may not be conclusive, because [11C]-(+)-PHNO cannot measure D3Rs independently of D2Rs in some regions of the brain, including the striatum. Ligands with much higher selectively for D3R/D2R have been developed for in vitro use, including [125I]trans-7-OH-PIPAT (13), and postmortem studies with this ligand identified an increase in the number of D3Rs in the basal ganglia of patients with schizophrenia who were drug free for more than 1 month before death compared with medicated patients or control subjects (14).
To determine the consequences of D3R upregulation in the striatum, we developed a new transgenic model of regionally restricted and temporally regulated overexpression of wild-type D3R messenger RNA (mRNA) using the tetracycline expression system (D3R-OE mice). These mice show a deficit in incentive motivation, without any deficit in general behavioral functions. Mice with overexpression of D3R also perform normally in several cognitive assays, including tests of spatial memory, associative learning, and working memory. We also found that D1R ligand binding was downregulated in the striatum of D3R-OE mice and propose that this molecular alteration may contribute to the deficit in motivation.
Methods and Materials
Generation of D3R-OE Mice
Mice with overexpression of D3R were generated using the same strategy described in Kellendonk et al. (15). Briefly, mice expressing the human D3 receptor under control of the tet-operator (tetO) (tetO-D3R mice) were crossed to mice expressing the tetracycline transactivator (tTA) transgene under the calcium/calmodulin-dependent kinase IIα (CaMKIIα) promoter (CaMKIIα-tTA mice) (16). Further details are provided in Supplement 1. Mice were housed under standard conditions, consistent with National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee.
Molecular and Biochemical Methods
Molecular and biochemical methods are briefly described below. Further details for each method can be found in Supplement 1.
In situ hybridization was performed using a 40-base antisense oligonucleotide complementary to the transgenic mRNA as described in Kellendonk et al. (15). We also used a digoxigenin-labeled complementary RNA probe against the transgene using the method described in Schaeren-Wiemers and Gerfin-Moser (17).
Ligand Binding for striatal dopamine D1 receptor (D1R) was performed as described in Kellendonk et al. (15), using six different concentrations of [3H]SCH23390 (75.5 Ci/1 mmol; GE Healthcare, Piscataway, New Jersey), 10 μmol/L Ketanserin (Sigma, St. Louis, Missouri) to block serotonin receptors. Unspecific binding was measured with 1 μmol/L (±) butaclamol (Sigma).
Immunoblotting for the D1R was performed as previously described in Biezonski and Meyer (18) using a rat-generated anti-D1R antibody (Sigma, Catalog # D2944).
Quantitative reverse transcription polymerase chain reaction (RT-PCR) was performed as described in Simpson et al. (19) using D1R specific primers and glyceraldehyde-3-phosphate dehydrogenase primers for reference. Mean normalized expression (MNE) levels were calculated using the equation MNE = Cycle Threshold(reference)Efficiency(reference)/Cycle Threshold(target)Efficiency(target) (20).
Behavioral Methods and Data Analysis
The open field, elevated plus, prepulse inhibition, water maze, and delayed-nonmatch to sample T-maze tasks were all conducted as described in Kellendonk et al. (15). The inhibitory avoidance paradigm was conducted as described in Pittenger et al. (21). The conditional associative learning task was performed as described in Bach et al. (22). The progressive-ratio (PR) task was performed as described in Drew et al. (23). Details of all these tests, as well as the test for SKF81297-induced hyper-locomotion, are provided in Supplement 1. Mean ± SEM are presented. Statistical analyses included unpaired t tests, one-way analysis of variance (ANOVA), two-way ANOVA, or log ranks tests, as indicated.
Results
Selective Overexpression of Dopamine D3 Receptor mRNA in the Striatum
To achieve striatal-specific D3R upregulation with temporal regulation (D3R-OE mice), we used the same strategy that we previously employed to generate mice with selective upregulation of D2 receptors in the striatum, D2R-OE mice (15). We generated transgenic mice with the human D3 receptor open reading frame under control of the tetracycline response element tetO. We then crossed these animals to mice expressing the tetracycline transactivator, tTA, under the CaMKIIα promoter (16). Although CaMKIIα is endogenously expressed in the entire fore-brain, CaMKIIα-tTA mice have been found, fortuitously, to sometimes target subregions of the forebrain, including the striatum, because site of genomic integration of the tetO response element can further restrict transgene expression (15,16,21).
We generated several tetO-D3R lines and screened for and found two lines, which when crossed to CaMKIIα-tTA mice, resulted in striatum-specific expression. One of these lines was used in the current study. Figure 1A shows expression of the D3R-OE transgene in a sagittal section of the entire mouse brain (gene on) and a section from a control littermate. Higher resolution images of coronal sections labeled with a digoxigenin labeled riboprobe, which is complementary to a portion of the D3R transgene, revealed that transgenic mRNA is strongly expressed in the striatum (Figure 1B), including the dorsal striatum (caudate putamen) and nucleus accumbens (Figure 1C), as well as the olfactory tubercle (Figure 1B). By contrast, there is very sparse expression in hippocampus and neocortex (Figure 1D). The percentage of D3R-OE transgene-positive cells in the caudate putamen appears to be approximately 30%. Within the striatum, the CaMKII-tTA mouse drives tetO-transgene expression in striatonigral and striatopallidal mediums spiny neurons (24), both of which express D3Rs (25,26). Using the CaMKII-tTA mouse, we previously did not find any tetO driven response in cholinergic interneurons (23). Thus, transgene expression is limited to the main output neurons of the striatum.
Figure 1.
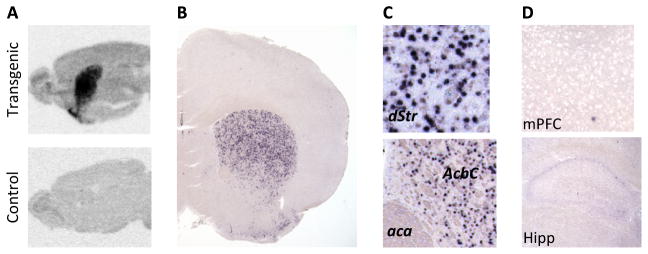
(A) Transgenic expression was limited to the striatum, shown by oligonucleotide in situ hybridization with a transgene specific probe. (B) Within the striatum, transgene expression included both dorsal and ventral striatum, shown by in situ hybridization with a digoxigenin labeled RNA riboprobe. Section shown (20×) is approximately 1.0 mm anterior to bregma (B +1.0 mm). (C) Higher magnification images from the same section reveal the density of transgene expression in the dorsal striatum (dStr) (B +1.0 mm, 200×) and nucleus accumbens core (AcbC) (B +1.0 mm, 150×). (D) Expressions in the medial prefrontal cortex (mPFC) (B +1.6 mm, 100×) and hippocampus (Hipp) (B −1.7 mm, 100×) are very sparse. aca, anterior commissure, anterior part.
Striatal D3R-Overexpression Does Not Affect Locomotor Activity, Anxiety-Related Behavior, or Sensorimotor Gating
Because striatal dopamine signaling can affect locomotion, we measured locomotor activity and stereotypic behavior in control and D3R-OE mice in an open field. We found no significant differences between the genotypes for total path length. Repeated measures (RM) ANOVA revealed no significant effect of genotype (F1,25 = 1.58, p = .22), a significant effect of time (F11,275 = 50.74, p < .0001), and no genotype by time interaction (F11,275 = .99, p = .45) n = 15 control mice, n = 12 D3R-OE mice (Figure 2A). We also measured anxiety-related behaviors in the mice using an elevated plus maze and found no effect of genotype on the ratio of entries into the closed arms (CA) and open arms (OA), control CA/OA ratio = 1.53 ± .12 n = 13, D3R-OE CA/OA ratio = 1.64 ± .15 n = 12; t23 = .59, p = .56 (Figure 2B). There was also no effect of genotype on the ratio of time spent in the closed arms and open arms, control CA/OA ratio = 2.74 ± .57, D3R-OE CA/OA ratio = 2.74 ± .43; t23 = .0004, p = .99. We measured sensorimotor gating by testing prepulse inhibition and found no effect of genotype on the attenuation of the response to an acoustic stimulus of 120 dB after any of the prepulse stimuli tested (3, 6, or 12 dB). A two-way ANOVA revealed no overall effect of genotype (F1,69 = 1.35, p = .25), a significant effect of prepulse magnitude (F2,69 = 68.8, p < .0001), and no genotype by stimulus interaction (F2,69 = .14, p = .87), Figure 2C. There was also no difference in the magnitude of startle response to the 120 dB acoustic stimulus (control mice = 896.4 ± 61.64 lb/m2/sec, n = 13, D3R-OE mice = 873.9 ± 103.6 lb/m2/sec, n = 12; t23 = .19, p = .851). Habituation to the acoustic stimulus on repeated exposure was also normal. Repeated measures ANOVA revealed no effect of genotype, (F1,23 = .04, p = .85), a significant effect of stimulus order (F2,46 = 15.43, p < .0001), and no interaction (F2,46 = .30, p = .74). Mice with overexpression of D3R, therefore, showed normal locomotion, anxiety-related behaviors, and sensorimotor gating, allowing us to use this model to study cognitive and motivational functions without concern for the confound of a more general behavioral deficit.
Figure 2.
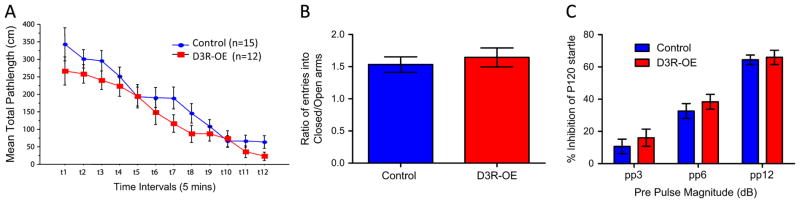
(A) Mice with an overexpression of dopamine D3 receptors (D3R-OE mice) traveled the same mean path length in an open field arena over a 60-minute period. (B) D3R-OE mice expressed the same ratio of entries into closed arms/open arms in the elevated plus maze test of anxiety. (C) D3R-OE mice showed normal sensorimotor gating in a test of prepulse inhibition of startle response to an auditory stimulus. P120, pulse stimulus of 120dB; pp, prepulse.
D3R Striatal Overexpression Does Not Affect Cognitive Performance on Tests of Reference Memory, Working Memory, Conditional Associative Learning, or Inhibitory Avoidance
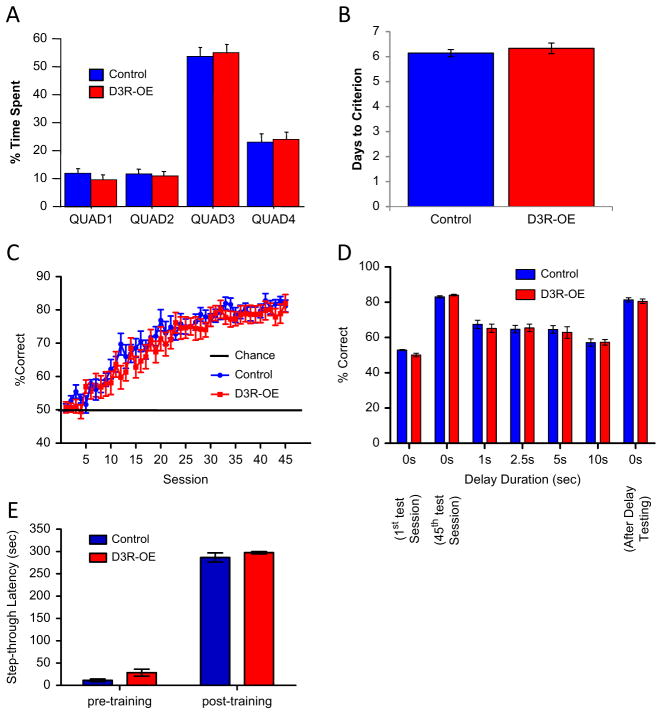
Patients with schizophrenia exhibit deficits in explicit memory (27–29), working memory (30–32), and conditional associative learning (33–35), all of which can be assayed in mice. In the Morris water maze test of hippocampal-dependent spatial reference memory, D3R-OE mice performed normally on all variables measured during both the learning of the first location of an invisible platform and then a second location of the invisible platform during the transfer phase of the task. For latency to find the invisible platform, swim speed, time spent floating, and time spent in the correct target quadrant during probe trials, all RM ANOVA ps > .05, n = 15 control mice, n = 12 D3R-OE mice (Figure 3A).
Figure 3.
(A) Mice with an overexpression of dopamine D3 receptors (D3R-OE mice) spent the same percentage of time in the target quadrant during a probe trial on the hidden version of the spatial water maze task, demonstrating normal spatial memory. (B) D3R-OE mice took the same number of days to reach criterion level performance in a delayed-nonmatch to sample T-maze task. (C) D3R-OE mice learned the conditional associative learning task at the same rate as control mice and reached the same level of performance. (D) D3R-OE mice performed equally well as control mice when delays of different lengths were imposed in the conditional associative learning task. (E) D3R-OE mice displayed normal learning in a passive avoidance paradigm. QUAD, quadrant.
We then tested D3R-OE mice on a spatial working memory task using a delayed-nonmatch to sample T-maze protocol and found no effect of genotype on the number of training sessions required to reach criteria, control mice = 6.143 ± .143, n = 7, D3R-OE mice = 6.333 ± .2108, n = 6; p = .46 (Figure 3B). We also tested D3R-OE and control mice on a two-choice conditional associative learning paradigm that required the mice to press a lever in response to one specific auditory cue and to withhold from lever pressing in response to a different auditory cue. Figure 3C shows that D3R-OE mice acquired this task normally (RM ANOVA for effect of genotype p = .451, n = 10 control mice, n = 11 D3R-OE mice). After all mice had acquired this task, we tested each animal’s ability to hold information during short intervals by inserting short delays between the offset of the auditory cue and the presentation of the response levers. Performance on this task was affected by the insertion of a delay period (RM ANOVA for effect of delay p < .0001), but there was no significant effect of genotype (RM ANOVA p = .855, n = 8 control mice, n = 9 D3R-OE mice) and no interaction (RM ANOVA p = .77) (Figure 3D). Therefore, D3R-OE mice displayed no deficits in either spatial or nonspatial working memory tasks.
To probe an alternative form of learning that is mediated by the striatum, we used an inhibitory (passive) avoidance paradigm in which mice learned to avoid entering a chamber in which they had previously experienced a mild foot shock. Two-way ANOVA identified a significant effect of training F1,46 = 1029.8, p < .0001 but no effect of genotype F1,46 = 2.73, p = .11 and no genotype by training interaction F1,46 = .16, p = .69, control mice n = 17, D3R-OE mice n = 8 (Figure 3E).
Striatal D3R-Overexpression Impairs Incentive Motivation
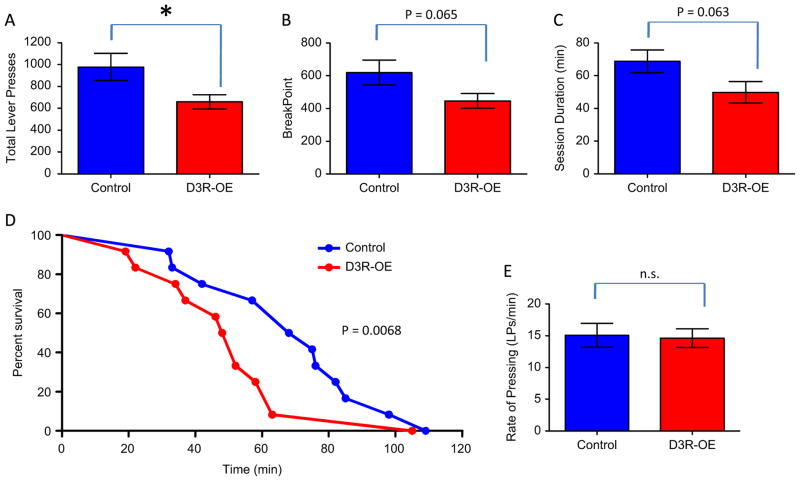
The negative symptoms of schizophrenia include disruption in incentive motivation. We therefore tested the D3R-OE mice on an operant PR schedule, in which the number of lever presses required to earn food rewards doubles following each reward earned. Therefore, this assay provides a measure of how much effort the subject is willing to expend to gain the food reinforcer. Each session ended after 2 hours or after 3 minutes had elapsed without a lever press. Figure 4A shows that the D3R-OE mice made, on average, significantly fewer lever presses in this task (control mice = 976.8 ± 123.7 n = 12, D3R-OE mice = 659.4 ± 64.60 n = 12, p = .0371). Figure 4B shows that D3R-OE mice showed a trend for a lower break point, defined as the first lever press criterion they were unable to successfully complete (control mice = 620.1 ± 75.56, D3R-OE mice = 446.4 ± 45.06, p = .0649). There was also a trend for the D3R-OE mice to quit the task sooner than control mice based on session duration in minutes (control mice = 68.75 ± 7.012, D3R-OE mice = 49.92 ± 6.528, p = .0627; Figure 4C). This reduction in working for rewards is also reflected in a lower survival rate for D3R-OE mice; the median survival rate for control mice was 71.5 minutes and for D3R-OE mice only 50 minutes (Figure 4D). These survival curves were compared using a log-rank (Mantel-Cox) test, which again revealed a trend effect, p = .0684. The observed modest effect on incentive motivation in D3R-OE mice was not likely due to effects on motor performance, as there was no effect of genotype on rate of lever pressing (control mice = 15.08 ± 1.877 lever presses/minute, D3R-OE mice = 14.64 ± 1.461 lever presses/minute, p = .857; Figure 4E).
Figure 4.
Mice with an overexpression of dopamine D3 receptors (D3R-OE mice) are impaired in a progressive ratio (PR × 2) measure of incentive motivation. (A) D3R-OE mice made significantly fewer total lever presses (LP) compared with control mice (*p = .0371). (B) D3R-OE mice also exhibited a trend for a lower break point and (C) shorter session duration. (D) A plot of the percentage of mice still working on the task as a function of time reveals a trend for the D3R-OE group to have a lower survival curve. (E) D3R-OE mice lever pressed at the same rate as control mice in the progressive ratio task. n.s., nonsignificant.
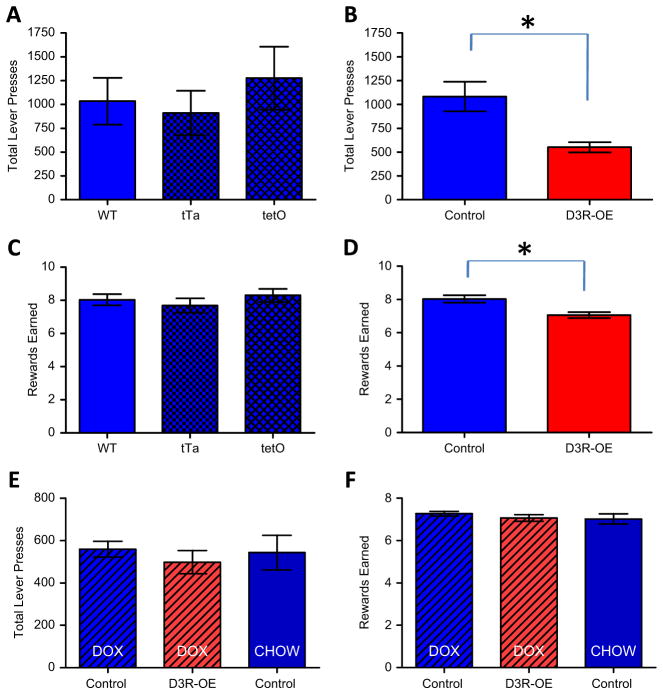
Because our transgenic model involves two independent transgenic lines of mice bred together (CaMKIIα-tTA and tetO-D3R), it is important to check that any phenotype observed is not due to the insertion of either of the single transgenes, rather than the transgenic expression driven by the combination of both transgenes. We therefore ran a second PR experiment including sufficient numbers of mice with each single genotype to make a statistical comparison. Because we used female mice in the first PR experiment, in this second experiment we used male mice so that we could also test for potential sexual dimorphism of the phenotype. A one-way ANOVA found no differences in the number of lever presses made per session by mice carrying either single transgene or no transgene (F2,14 = .44, p = .65, wild-type (no transgene) n = 6, tTA n = 5, tetO n = 6; Figure 5A). Because these three groups did not differ, we combined them for comparison with double transgenic (D3R-OE) mice and found a significant decrease in D3R-OE mice (t22 = 2.15, p = .042, combined control mice n = 17, D3R-OE mice n = 7; Figure 5B). We also found no significant effect of either single transgene on the average number of rewards earned (F2,14 = .62, p = .55; Figure 5C) and a significant difference between all control groups combined and D3R-OE mice (t22 = 2.67, p = .014; Figure 5D). These results confirm that striatal overexpression of D3R reduces motivated behavior in both male and female mice.
Figure 5.
(A) There were no significant differences between mice carrying either single transgene, tetracy-cline transactivator (tTA) (calcium/calmodulin-dependent kinase IIα-tTA), tet-operator (tetO) (tetO-dopamine D3 receptor), or no transgene (wild-type [WT]) in lever pressing in the progressive ratio (PR × 2) task. (B) Male mice with an overexpression of dopamine D3 receptors (D3R-OE mice) made significantly fewer lever presses in the task compared with the control groups combined (*p < .042). (C) Single transgenes also had no effect on the number of rewards earned. (D) Male D3R-OE mice earned significantly fewer rewards compared with the control groups combined (*p < .014). (E) Switching off the D3R-OE transgene in adult mice was sufficient to rescue the deficit in lever pressing in the PR × 2 test for motivation. (F) Doxycycline (DOX) also rescued the number of rewards earned in the task. CHOW, regular homecage chow diet (Non-DOX).
The deficit in motivation observed in D3R-OE mice could be due to concurrent expression of the D3R transgene or it could be due to persistent changes that result from increased striatal D3R expression during development. To test this, we treated a new cohort of adult D3R-OE and control littermate mice with doxycycline (dox) for 2 weeks to switch off the transgene before they ran on the same PR test described above. We also included a group of control mice that ate regular (non-dox) chow, to control for the effect of dox on behavior. A one-way ANOVA revealed no significant differences in the average number of total lever presses made in the PR × 2 task between these three groups, F2,20 = .25, p = .77, control dox mice n = 7, D3R-OE dox mice n = 8, control chow mice n = 8; Figure 5E). We also found no differences in the number of rewards earned (F2,20 = .51, p = .61; Figure 5F). Therefore, switching off the transgene rescued the deficit in PR performance.
Overexpression of D3 Receptors in the Striatum Results in a Transient Downregulation of Striatal D1Rs
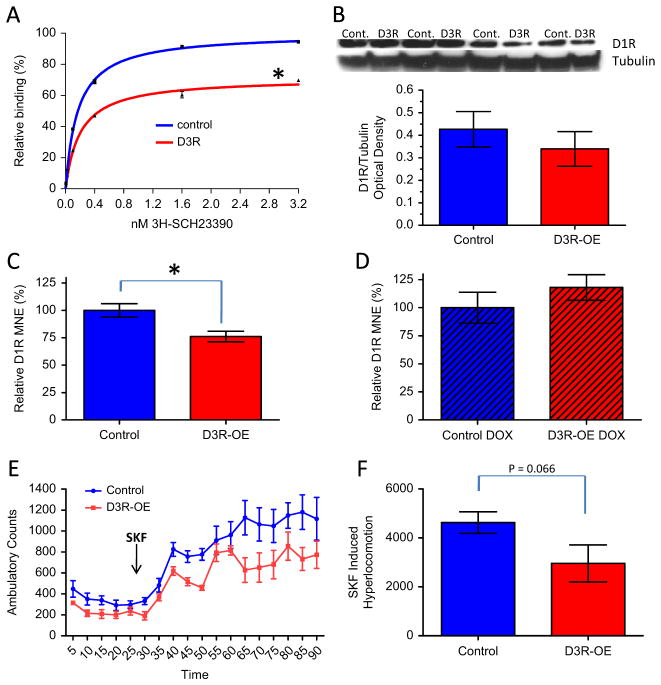
Our PR task provides a measure of willingness to work or the perception of effort-related cost (36), which has been found to be reduced by striatal dopamine depletion as well as striatal D1R antagonist infusions (37–39). We therefore measured D1R level in the striatum of D3R-OE mice to determine if D3R overexpression in the striatum had any impact on the level of D1 receptors. We performed a saturation ligand binding assay with membrane preparations from striatum of D3R-OE and control mice using the D1 ligand SCH23390. The striata from five animals of the same genotype were pooled per sample and we ran two independent binding experiments, each comparing one control and one D3R-OE sample. A total of 20 mice were used, 10 mice of each genotype. We fit the data from both experiments to a nonlinear hyperbola (single binding site) model to determine the best-fit values for Bmax (fmol/mg) and Kd (nmol/L) for each genotype: Bmax control mice = 1301 ± 11.25, Bmax D3R-OE mice = 927.4 ± 20.47, Kd control mice = .171 ± .006, Kd D3R-OE mice = .199 ± .019. An unpaired t test of the relative Bmax values obtained in each experiment revealed that D3R-OE mice have approximately 30% lower D1 receptor binding capacity than their control littermates (relative Bmax [%]: control mice 99.99 ± .01, D3R-OE mice = 71.24 ± 1.88; t2 = 15.25, p = .0043, n = 2 experiments per genotype; Figure 6A).
Figure 6.
(A) Dopamine D1 receptor (D1R) ligand binding capacity is significantly lower in the striatum of mice with an overexpression of dopamine D3 receptors (D3R) (D3R-OE mice) compared with control (Cont.) mice (*p = .0043). (B) D1R protein level in the cortex was not significantly different in the D3R-OE mice compared with control littermates. (C) The mean normalized expression (MNE) value for the D1R transcript in the striatum of D3R-OE mice is significantly lower compared with control mice (*p = .016). (D) This downregulation in D1R expression level is rescued when the transgene is switched off by doxycycline (DOX) treatment. (E) D3R-OE mice displayed less of an increase in locomotor activity in response to 5 mg/kg of the D1R agonist SKF 81297 (SKF) (time of injection is indicated by the black arrow). (F) SKF-induced hyperlocomotion was calculated as total ambulation counts in the last 30 minutes of the assay − total ambulation counts in the first 30 minutes of the assay (before intraperitoneal drug injection).
Positron emission tomography imaging studies with D1R ligands have suggested that there are abnormalities in the availability of D1 receptors in the cortex of patients with schizophrenia, with both increases and decreases being reported in different patient populations (40,41). To determine if D1R binding is altered in the cortex of D3R-OE mice, we attempted saturation ligand binding experiments using cortical membrane preparations. However, in the cortex, D1R levels are relatively very low compared with serotonin receptor levels, and these receptors share affinity with SCH23390. Therefore, we could not achieve reliable saturation curves from cortical samples. As an alternative approach, we performed immunoblots on cortical homogenates using a D1R specific antibody. Figure 6B shows that we did not identify a significant difference in D1R protein levels in the cortex of D3R-OE mice (t6 = .79, p = .46), n = 4 mice per genotype. This result should be perhaps interpreted with caution, because we observed a high level of across-subject variability and were limited to n = 4 per genotype in an 8-well western blot assay. Therefore, it is possible that this assay is not sensitive enough to detect small (yet physiologically important) changes in the level of D1Rs in cortical tissue.
The reduction in D1R ligand binding that we observed in striatal membranes may be due to a decrease in the transcription, translation, or membrane localization of the receptors. To examine the first possibility, we performed a quantitative RT-PCR assay with striatal tissue to measure the level of D1R mRNA transcript. Figure 6C shows that in D3R-OE mice, the MNE level of D1R mRNA is approximately 25% lower than it is in control mice (relative D1R MNE [% ± SEM], control mice: 100.0 ± 6.052, n = 5, D3R-OE mice: 76.21 ± 4.924, n = 5, t8 = 3.054, p = .0157). If this downregulation of D1R expression is related to the motivation phenotype, then it would similarly be rescued by switching off the transgene with dox. Indeed, when we repeated the quantitative RT-PCR assay on a separate cohort of mice that had been treated with dox for 2 weeks, we now found no significant effect of genotype (relative D1R MNE [% ± SEM], control mice: 100.0 ± 13.81, n = 6, D3R-OE mice: 118.0 ± 11.43, n = 5, t9 = .976, p = .355; Figure 6D).
To determine if the observed transient decrease in D1 receptor expression is of functional consequence, we subjected D3R-OE and control mice to an acute challenge with a D1R agonist (SKF 81297, 5.0 mg/kg) and monitored their locomotor activity. As previously described (42), we found that this drug treatment results in a robust increase in locomotor activity in mice (Figure 6E). Mice with overexpression of D3R displayed a trend for a reduction in SKF 81297-induced hyperlocomotion (total ambulatory counts in the last 30 minutes of the assay − total ambulatory counts in the first [predrug] 30 minutes of the assay), control mice: 4627 ± 441.8, n = 8, D3R-OE mice: 2958 ± 755.6, n = 6, t12 = 2.02, p = .066 (Figure 6F). This result suggests that D3R-OE mice may be less sensitive to the hyperlocomotor effects of D1 receptor stimulation.
Discussion
The dysregulation of dopamine function in schizophrenia is now an indisputable fact, yet the detailed nature of the dysregulation in the etiology of the disease remains elusive. This is, in part, due to a lack of research tools for use in vivo, which are sufficiently selective on a molecular level. In vivo PET imaging has revealed an increase in the occupancy of D2 or D3 receptors in patients with schizophrenia, though the ligands used are not selective enough to provide signal from either receptor subtype independently.
To date, there have been no reports of altered D3R mRNA levels in the striatum of patients with schizophrenia, which may reflect the poor sensitivity of postmortem assays or it may reflect a true lack of change at the level of transcription. Receptors may instead be affected at the level of translation, membrane localization, or functional state. Indeed, dissociation between mRNA level and receptor binding has previously been found. For example, chronic treatment with antipsychotic medication increases striatal D2R levels in rats without affecting the level of D2R mRNA (43). Genetic studies have the potential to implicate changes in specific dopamine receptors, but so far, the genetic evidence supports both D2 and D3 receptors playing minor roles in disease risk (for a listing of D2R and D3R genetic studies, see http://www.szgene.org/).
One reason that much more attention has been paid to the D2 rather than D3 receptors is because D2Rs are more broadly and abundantly expressed in the striatum compared with D3Rs (44). This disparity in expression levels results in the dominance of D2R signal in PET studies with D2/D3 ligands, resulting in the dominance of the idea that D2R function is critically disturbed. However, despite being expressed in a smaller neuronal population, it is possible that an upregulation of D3Rs could contribute to symptoms. It is also possible that the population of striatal neurons expressing D3Rs may be increased in patients, because D3R expression has previously been found to expand under certain conditions, specifically in an animal model of levodopa-induced behavioral sensitization (45). Though it is important to note that there are differences in the endogenous expression patterns of D3R between primates and rodents (including in the dorsal striatum), the strongest expression in both orders is in the Islands of Calleja followed by the ventral striatum (46).
To determine if increased D3R signaling in the striatum affects brain and behavioral functions related to schizophrenia, we generated mice in which normal D3Rs are overexpressed in striatum. Mice with overexpression of D3R display a mild deficit in incentive motivation, with normal behavior in several cognitive tests and assays of basic behavioral functions. In parallel, we observed a decrease in the binding capacity and mRNA level of D1Rs in the striatum, a factor that may contribute to the deficit in motivation in these animals. Indeed, several earlier studies found that D1R antagonists delivered directly to the ventral striatum resulted in a decreased willingness to work in two different behavioral paradigms (37,39). We found that both the decrease in motivation, as well as the downregulation of D1R expression, was normalized when the transgene was switched off in adulthood. This suggests that both phenotypes are transient and caused by concurrent expression of the D3R transgene, rather than a persistent developmental defect. The present study has not identified the mechanism by which striatal D3R upregulation results in the downregulation of striatal D1Rs; this is an important future goal. We speculate that because D1R and D3R are normally co-expressed within neurons, there may be a reciprocal co-regulation of the activity of these receptors, which may result in altered expression. Indeed, there is preliminary evidence to suggest that they form heterodimers (47).
The fact that overexpression of D3Rs in the striatum and subsequent downregulation of striatal D1Rs did not disrupt performance on several tests of cognition is consistent with previously published data showing no effect of D1R antagonist treatment in the striatum on the cognitive processes required to perform the tasks we have examined here. The incentive salience model suggests that dopamine is not required for all forms of learning but rather for attributing incentive salience or wanting of the reinforcement (48,49). Our data therefore suggest that a modest increase in D3R activity in the striatum is sufficient to contribute to one of the negative symptom of schizophrenia, decreased motivation, and that this may be due to a secondary effect on D1R activity. Our data further suggest that targeting the dopamine D3R selectively may provide relief from the negative symptoms of schizophrenia and could perhaps also be useful in other disorders of motivation such as depression. In rodents, D3R antagonism/partial agonism has an antidepressant effect in stressed animals (50), but the effect of targeting D3R specifically on motivation processes has not yet been investigated in either humans or animals. If a drug with preferential binding to D3Rs could improve motivation in patients with schizophrenia, it would represent a significant advance in treatment because currently available medications do not help patients with this debilitating aspect of the disease.
Supplementary Material
Acknowledgments
This work was funded by the Lieber Institute for Brain Development (EHS, ERK) and the Howard Hughes Medical Institute (ERK) and R01MH093672 (CK, DKB).
We are indebted to Gael Malleret, Mary Elizabeth Bach-Burder, and Svetlana Vronskaya for assistance with the behavioral testing. We are also grateful to Heidi Smith, Tessa Hirschfeld-Stoler, and Tamara Azayeva for maintaining the animal colony.
Eric R. Kandel is on the Scientific Advisory Boards of Pharnext Pharmaceuticals, EMS Pharmaceuticals, and Neurofocus, and receives honoraria from the first two of these companies. Christoph Kellendonk has a research contract with Forest Pharmaceuticals.
Footnotes
All other authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2013.11.023.
References
- 1.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. doi: 10.1016/j.neuron.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69:776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seeman P, Lee T. Antipsychotic drugs: Direct correlation between clinical potency and presynaptic action on dopamine neurons. Science. 1975;188:1217–1219. doi: 10.1126/science.1145194. [DOI] [PubMed] [Google Scholar]
- 4.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz JC, Diaz J, Pilon C, Sokoloff P. Possible implications of the dopamine D(3) receptor in schizophrenia and in antipsychotic drug actions. Brain Res Brain Res Rev. 2000;31:277–287. doi: 10.1016/s0165-0173(99)00043-0. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger DR, Laruelle M. Neurochemical and neuropharmacological imaging in schizophrenia. In: Davis KL, Charney D, Coyle JT, Nemeroff CB, editors. Neuropharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott, Williams, and Wilkins; 2001. [Google Scholar]
- 7.Sibley DR. Cloning of a ‘D3’ receptor subtype expands dopamine receptor family. Trends Pharmacol Sci. 1991;12:7–9. doi: 10.1016/0165-6147(91)90480-g. [DOI] [PubMed] [Google Scholar]
- 8.Prante O, Dorfler M, Gmeiner P. Dopamine receptor subtype-selective drugs: D2-like receptors. In: Neve KA, editor. The Dopamine Receptors. 2. New York: Humana Press; 2010. pp. 101–136. [Google Scholar]
- 9.Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, et al. Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66:489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- 10.Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. Neuroimage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–399. doi: 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, et al. The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: A clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology. 2009;34:1078–1086. doi: 10.1038/npp.2008.199. [DOI] [PubMed] [Google Scholar]
- 13.Kung MP, Kung HF, Chumpradit S, Foulon C. In vitro binding of a novel dopamine D3 receptor ligand: [125I]trans-7-OH-PIPAT-A. Eur J Pharmacol. 1993;235:165–166. doi: 10.1016/0014-2999(93)90839-a. [DOI] [PubMed] [Google Scholar]
- 14.Gurevich EV, Bordelon Y, Shapiro RM, Arnold SE, Gur RE, Joyce JN. Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia. A postmortem study. Arch Gen Psychiatry. 1997;54:225–232. doi: 10.1001/archpsyc.1997.01830150047009. [DOI] [PubMed] [Google Scholar]
- 15.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 17.Schaeren-Wiemers N, Gerfin-Moser A. A single protocol to detect transcripts of various types and expression levels in neural tissue and cultured cells: In situ hybridization using digoxigenin-labelled cRNA probes. Histochemistry. 1993;100:431–440. doi: 10.1007/BF00267823. [DOI] [PubMed] [Google Scholar]
- 18.Biezonski DK, Meyer JS. Effects of 3,4-methylenedioxymetham-phetamine (MDMA) on serotonin transporter and vesicular mono-amine transporter 2 protein and gene expression in rats: Implications for MDMA neurotoxicity. J Neurochem. 2010;112:951–962. doi: 10.1111/j.1471-4159.2009.06515.x. [DOI] [PubMed] [Google Scholar]
- 19.Simpson EH, Kellendonk C, Ward RD, Richards V, Lipatova O, Fairhurst S, et al. Pharmacologic rescue of motivational deficit in an animal model of the negative symptoms of schizophrenia. Biol Psychiatry. 2011;69:928–935. doi: 10.1016/j.biopsych.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372–1374. 1376, 1378–1379. [PubMed] [Google Scholar]
- 21.Pittenger C, Fasano S, Mazzocchi-Jones D, Dunnett SB, Kandel ER, Brambilla R. Impaired bidirectional synaptic plasticity and procedural memory formation in striatum-specific cAMP response element-binding protein-deficient mice. J Neurosci. 2006;26:2808–2813. doi: 10.1523/JNEUROSCI.5406-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bach ME, Simpson EH, Kahn L, Marshall JJ, Kandel ER, Kellendonk C. Transient and selective overexpression of D2 receptors in the striatum causes persistent deficits in conditional associative learning. Proc Natl Acad Sci U S A. 2008;105:16027–16032. doi: 10.1073/pnas.0807746105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cazorla M, Shegda M, Ramesh B, Harrison NL, Kellendonk C. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci. 2012;32:2398–2409. doi: 10.1523/JNEUROSCI.6056-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci U S A. 1992;89:10178–10182. doi: 10.1073/pnas.89.21.10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aleman A, Hijman R, de Haan EH, Kahn RS. Memory impairment in schizophrenia: A meta-analysis. Am J Psychiatry. 1999;156:1358–1366. doi: 10.1176/ajp.156.9.1358. [DOI] [PubMed] [Google Scholar]
- 28.Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev. 2005;15:73–95. doi: 10.1007/s11065-005-6254-9. [DOI] [PubMed] [Google Scholar]
- 29.Spieker EA, Astur RS, West JT, Griego JA, Rowland LM. Spatial memory deficits in a virtual reality eight-arm radial maze in schizophrenia. Schizophr Res. 2012;135:84–89. doi: 10.1016/j.schres.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 33.Gold JM, Bish JA, Iannone VN, Hobart MP, Queern CA, Buchanan RW. Effects of contextual processing on visual conditional associative learning in schizophrenia. Biol Psychiatry. 2000;48:406–414. doi: 10.1016/s0006-3223(00)00930-6. [DOI] [PubMed] [Google Scholar]
- 34.Kemali D, Maj M, Galderisi S, Monteleone P, Mucci A. Conditional associative learning in drug-free schizophrenic patients. Neuropsychobiology. 1987;17:30–34. doi: 10.1159/000118337. [DOI] [PubMed] [Google Scholar]
- 35.Rushe TM, Woodruff PW, Murray RM, Morris RG. Episodic memory and learning in patients with chronic schizophrenia. Schizophr Res. 1999;35:85–96. doi: 10.1016/s0920-9964(98)00117-0. [DOI] [PubMed] [Google Scholar]
- 36.Salamone JD. Will the last person who uses the term ‘reward’ please turn out the lights? Comments on processes related to reinforcement, learning, motivation and effort. Addict Biol. 2006;11:43–44. doi: 10.1111/j.1369-1600.2006.00011.x. [DOI] [PubMed] [Google Scholar]
- 37.Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 38.Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 39.Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: Contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berl) 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- 40.Kosaka J, Takahashi H, Ito H, Takano A, Fujimura Y, Matsumoto R, et al. Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life Sci. 2010;86:814–818. doi: 10.1016/j.lfs.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, et al. Increased prefrontal cortical D(1) receptors in drug naive patients with schizophrenia: A PET study with [(1)(1)C]NNC112. J Psychopharmacol. 2012;26:794–805. doi: 10.1177/0269881111409265. [DOI] [PubMed] [Google Scholar]
- 42.Napolitano F, Bonito-Oliva A, Federici M, Carta M, Errico F, Magara S, et al. Role of aberrant striatal dopamine D1 receptor/cAMP/protein kinase A/DARPP32 signaling in the paradoxical calming effect of amphetamine. J Neurosci. 2010;30:11043–11056. doi: 10.1523/JNEUROSCI.1682-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Tol HH, Riva M, Civelli O, Creese I. Lack of effect of chronic dopamine receptor blockade on D2 dopamine receptor mRNA level. Neurosci Lett. 1990;111:303–308. doi: 10.1016/0304-3940(90)90279-i. [DOI] [PubMed] [Google Scholar]
- 44.Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: Comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- 45.Guillin O, Diaz J, Carroll P, Griffon N, Schwartz JC, Sokoloff P. BDNF controls dopamine D3 receptor expression and triggers behavioural sensitization. Nature. 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- 46.Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: A therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 47.Fiorentini C, Busi C, Gorruso E, Gotti C, Spano P, Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol Pharmacol. 2008;74:59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- 48.Berridge KC. The debate over dopamine’s role in reward: The case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 49.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adham N, Gyertyan I, Kiss B, Pasztor G, Gupta S, Szombathelyi Z, et al. Antidepressant activity of RGH-188, a potential antipsychotic with D3/D2 antagonist/partial agonist properties in a chronic mild stress-induced anhedonia model. Presented at the Society for Neuroscience Annual Meeting; November 12–17; San Diego, California. San Diego, CA: Society for Neuroscience; 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.