Abstract
Resistance to aromatase inhibitors is a major concern in the treatment of breast cancer. Long-term letrozole cultured (LTLC) cells represent a model of resistance to aromatase inhibitors. The LTLC cells were earlier generated by culturing MCF-7Ca, the MCF-7 human breast cancer cell line stably transfected with human placental aromatase gene for a prolonged period in the presence letrozole. In the present study was investigated the effect of RAMBA, VN/14-1 on the sensitivity of LTLC cells upon multiple passaging and the mechanisms of action of VN/14-1 in such high passage LTLC (HP-LTLC) cells. We report that multiple passaging of LTLC cells (HP-LTLC cell clones) led to profound decrease in their sensitivity to VN/14-1. Additionally, microarray studies and protein analysis revealed that VN/14-1 induced marked endoplasmic reticulum (ER) stress and autophagy in HP-LTLC cells. We further report that VN/14-1 in combination with thapsigargin exhibited synergistic anti-cancer effect in HP-LTLC cells. Preliminary pharmacokinetics in rats revealed that VN/14-1 reached a peak plasma concentration (Cmax) within 0.17 h after oral dosing. Its absolute oral bioavailability was >100%. Overall these results indicate potential of VN/14-1 for further clinical development as a potential oral agent for the treatment of breast cancer.
Keywords: RAMBA-VN/14-1, LTLC, pharmacokinetics (PK), breast cancer, ER stress, autophagy
1. Introduction
The role of estrogens in the progression of breast cancer in both pre-menopausal and post-menopausal women is well established (Jensen and Jordan, 2003). The effects of estrogens on tumor growth are mediated by the estrogen receptor mainly estrogen receptor α (ERα). Synthesis of estrogens from androgens is catalyzed by the enzyme aromatase. Inhibition of this conversion by selective aromatase inhibitors (AIs) is a well-known approach for reducing pro-survival effects of estrogens in breast cancer (Barnadas et al., 2011). We previously reported that MCF-7Ca cells (MCF-7 cells stably transfected with the human aromatase gene) when grown chronically in presence of 1μm letrozole (Femara®, an AI in clinical use) lose their ability to respond to letrozole. This long-term letrozole cultures cells are designated LTLC cells (Belosay et al., 2006). By virtue of their acquired resistance to letrozole, these cells represent a model of endocrine resistant breast cancer (Belosay et al., 2006).
The structural modification of ATRA to develop novel retinoic acid metabolism blocking agents (RAMBAs) with improved potency and metabolic stability has been the focus of our group (Belosay et al., 2006; Godbole et al., 2012a, 2012b; Njar, 2002; Njar et al., 2006; Patel et al., 2007a, 2007b, 2004). Our RAMBAs are considered to be atypical, because in addition to being potent inhibitors of ATRA metabolism, they also possess potent intrinsic multiple anticancer activities. In our previous studies we reported that one of our lead RAMBAs, VN/14-1 (Fig. 1A) potently inhibited MCF7Ca cells (IC50 = 10.5 nM), but unexpectedly the letrozole resistant MCF7Ca cells (LTLC cells) were exquisitely sensitive to VN/14-1 with an IC50 of 0.83 nM (Belosay et al., 2006). We observed that the anticancer effects of VN/14-1 appear to be due to its multiple biological activities, including significant down-regulation of proteins in the mitogen-activated protein kinase (MAPK) pathway in the LTLC cells as well as marked ERα and co-activator amplified in breast cancer 1 (AIB1) down-regulation. In addition, the compound caused induction of apoptosis, differentiation, and cell cycle arrest in vitro and in vivo (Belosay et al., 2006).
Fig.1.
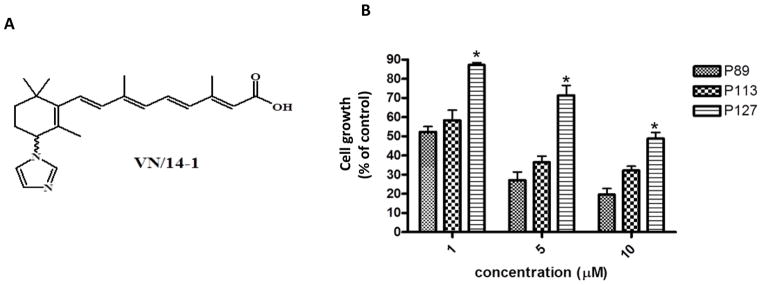
(A) Chemical structure of VN/14-1
(B) Multiple passaging leads to reduced sensitivity of LTLC cells to growth inhibitory effects of VN/14-1: LTLC cells of indicated passages were exposed to the indicated concentrations of VN/14-1 for 96 h. Cell viability was measured with an MTT assay.* P < 0.05
Typically, serial passaging of cancer cells leads to up-regulation of survival signals and development of insensitivity or acquired resistance in cells which are otherwise sensitive to anti-cancer agents (Bose et al., 2011). In the current study, we investigated the effect of VN/14-1 on the sensitivity of LTLC cells upon multiple passaging and the mechanisms of action of VN/14-1 in such high passage LTLC (HP-LTLC) cells. Based on the microarray studies and validation by western blotting analysis, we show that VN/14-1 robustly induced endoplasmic reticulum (ER) stress and autophagy in the HP-LTLC cells. Similar to our previous studies with VN/12-1 versus SKBR-3 breast cancer cells, we identify ERS and autophagy as important pathways up-regulated following treatment of HP-LTLC cells with VN/14-1. We also show that VN/14-1 synergizes with a well-established ERSR inducer, thapsigargin to profoundly inhibit the proliferation of HP-LTLC cells. Furthermore, because of our interest in the further development of VN/14-1 as a novel breast cancer therapeutic, we also determined its oral bioavailability and pharmacokinetics in rats.
2. Materials and methods
2.1. Chemicals and reagents
VN/14-1 (Fig. 1A) and internal standard (VN/4-1, structure not shown) were synthesized in our laboratory as previously (Patel et al., 2004). The purities of VN/14-1 and VN/4-1 were determined to be >98% pure by a combination of HPLC, NMR and HPLC as previously described. Heparin sodium injection was purchased from Abraxis Pharmaceutical Products (Schaumburg, IL). Isofluorane was generously provided from the animal care facility of University of Maryland School of Medicine, Baltimore. Vacutainer coated with sodium heparin were purchased from Becton Dickinson (Franklin lakes, NJ). Saline (0.9 % sodium chloride) was obtained from Baxter (Deerfield, IL). HPLC grade methanol and acetonitrile were purchased from American Bioanalytical (Natick, MA). HPLC grade water, methanol, acetonitrile and β-cyclodextrin (β-CD) were purchased from Sigma (St. Louis, MO).
2.2. Cell lines
The long-term letrozole cultured (LTLC) was a generous gift from Dr. Angela Brodie, University of Maryland School of Medicine, Baltimore, USA.. LTLC cells were cultured in steroid-depleted medium (phenol red–free improved MEM) supplemented with 5% dextran-coated charcoal-treated serum, 1% penicillin/streptomycin, 700 μg G418, 25 nM of aromatase substrate androstenedione, and 1 μm of aromatase inhibitor letrozole (Belosay et al., 2006).
2.3. MTT cell viability assay
The cells were seeded in 96-well plates (Corning Costar) at a density of 5 × 103 cells per well. Cells were allowed to adhere to the plate for 24 h and then treated with various concentrations of VN/14-1 or thapsigargin dissolved in 95% EtOH. Cells were treated for 96 h with renewal of test compound and media on day 2. On the fourth day, medium was renewed and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) (Sigma, St Louis, MO, USA) solution (0.5 mg MTT per ml of media) was added to the medium such that the ratio of MTT: medium was 1 : 10. The cells were incubated with MTT for 2 h. The medium was then aspirated and DMSO was added to solubilize the violet MTT-formazan product. The absorbance at 560 nm was measured by spectrophotometry.
2.4. Microarray experiment
We performed a comparative microarray experiment to compare the effects of VN/14-1 in the endocrine resistant cell line LTLC. The cell line was treated with 20 μm of VN/14-1 or equal volume of vehicle for 24 h. Experiments were carried out in duplicate, with triplicate samples from each experiment pooled for analysis on a single chip. Samples were hybridized to a GeneChip Human Genome Focus Array (Affymetrix, Inc.) and analyzed using an Affymetrix Genechip Scanner 3000 according to manufacturer’s protocol.The online database for annotation, visualization, and integrated discovery (DAVID) was utilized to identify clusters of related genes affected by VN/14-1. Only genes that were up-regulated or down-regulated by an average of ≥2-fold and had a minimum 1.5-fold change (up or down) per array were used for this analysis. Significant changes in gene expression were evaluated using the program Significance Analysis of Microarrays (SAM 3.0; Stanford University Laboratories).
2.5. Western blot analysis
For immunoblot detection of various proteins, LTLC cells were cultured as described above in T75 flasks. Cells were treated with VN/14-1 and whole cell lysates were prepared using RIPA lysis buffer (Sigma Aldrich) and protease and phosphatase inhibitors (Sigma Aldrich). Protein content was determined using the Bradford Assay (Bio-Rad, Hercules, CA, USA). Samples were run on SDS–PAGE and transferred onto nitrocellulose membrane. The membrane was then blocked with 4% bovine serum albumin for 2 h and was incubated with 1:1000 dilution of primary antibody (Cell Signaling Technology) overnight at 4 °C. After washing, the membranes were then incubated with secondary antibody (Santa Cruz Biotechnology, USA) at room temperature for 1 h. Bands were visualized by chemiluminescence (Millipore). Protein expression was normalized to β-actin and densitometry was carried out using ImageQuant 5.0 (Molecular Dynamics, Sunnyvale, CA, USA).
2.6. Pharmacokinetic Study
2.6.1. Animals
All studies were performed according to the guidelines and approval of the Animal Care Committee of the University of Maryland School of Medicine, Baltimore, USA and also followed NIH guidelines. Female Sprague Dawley Rats (200–224 g) with carotid cannulas were obtained from Harlan (Indianapolis, IN). Rats were maintained in a controlled environment of light, humidity and temperature and were given food and water ad libitum. Cannula patency was confirmed every day and 100 μl saline and 150 μl heparinized (100 IU/mL) saline was flushed into the cannula.
2.6.2. Dosing and Sampling
Female SD rats were used for the PK studies (n = 6 per time point). For oral studies, rats were fasted overnight and were given a single 20 mg/kg dose of VN/14-1 formulated in 30% β-CD in saline. For IV studies, rats were subjected to light anesthesia using isofluorane and were given a single 20 mg/kg bolus of VN/14-1 in the same formulation. Whole blood was collected via the carotid cannula at various time points in heparinized Eppendorf tubes. After sampling 100 μl saline and 150 μl heparinized (100 IU/ml) saline was flushed into the cannula. Blood was centrifuged at 15,339 g for 5 minutes and plasma was transferred to a clean Eppendorf tube for storage at −20°C.
2.6.3. Sample preparation
Aliquots of thawed plasma (200 μl) were transferred to glass test tubes. Plasma samples were spiked with 0.25 μg/ml of internal standard (VN/4-1). Plasma samples were briefly vortexed after which 3 ml of ethyl acetate was added and samples were further vortexed for 3 min at full speed. Tubes were then centrifuged for 15 min at 704 g at 4°C. 2.7 ml of the supernatant was transferred to a clean glass test tube and samples were air dried while submerged in a warm water bath. Samples were then reconstituted in 100 μl of methanol and vortexed for 20 sec. The solution was then transferred to a clean Eppendorf tube and samples were centrifuged for 3 min at 15,339 g at 4°C. Supernatant was transferred to a HPLC vial for analysis. Sample handling and preparation was performed in a room with dim lighting.
2.6.4. Equipment and chromatographic conditions
The chromatographic system consisted of a 1535 pump, 717 autosampler, and a 996 detectors (Waters, Miliford, MA). Chromatographic separation was achieved on a Nova-Pak C18 column (3.9 mm × 150 mm × 4 μm) and a C18 guard column (3.9 mm × 20 mm × 4 μm) (Waters). The isocratic mobile phase consisted of water, methanol, and acetonitrile (50:25:25 v/v) with 20 mM ammonium acetate buffer and 0.01% triethylamine and was run at a flow rate of 0.8 ml/min. The eluate was monitored with UV detection from 200 to 400 nm and chromatograms were extracted at 330 nm.
2.7. Statistical analysis
All data are presented as the mean ± S.E.M. The statistical significance of differences between two groups was assessed with Student’s t-test. A probability value of less than 0.05 was considered significant.
3. Results
3.1. High passage LTLC cells show reduced response to cytotoxic effects of VN/14-1
In order to assess the effects of multiple passaging on VN/14-1 induced cell growth inhibition, we analyzed the anti-proliferative activity (AP) using MTT assay. Our group had previously reported an IC50 of 0.83 nM for early passage (< 60) LTLC cells (Belosay et al., 2006). As shown in Fig. 1B, the AP activity of passage 89 (P89) was 50% and 80 % for concentrations of 1 μm and 10 μm respectively. However, higher passage cells were less sensitive to the growth inhibitory effects of VN/14-1. For passage 127 (P127), the AP activity was only ~13% at 1 μm and only 53% at 10 μm (Fig. 1B). This clearly indicates that the HP- LTLC cells became less responsive to anti-proliferative effects of VN/14-1 compared to the early passage LTLC cells.
3.2. Microarray Analysis Reveals VN/14-1 Induces Up-regulation of Genes Involved in Cellular Response to Stress and intracellular organelle formation and the Down-regulation of Cell Cycle Related Genes in LTLC Cells
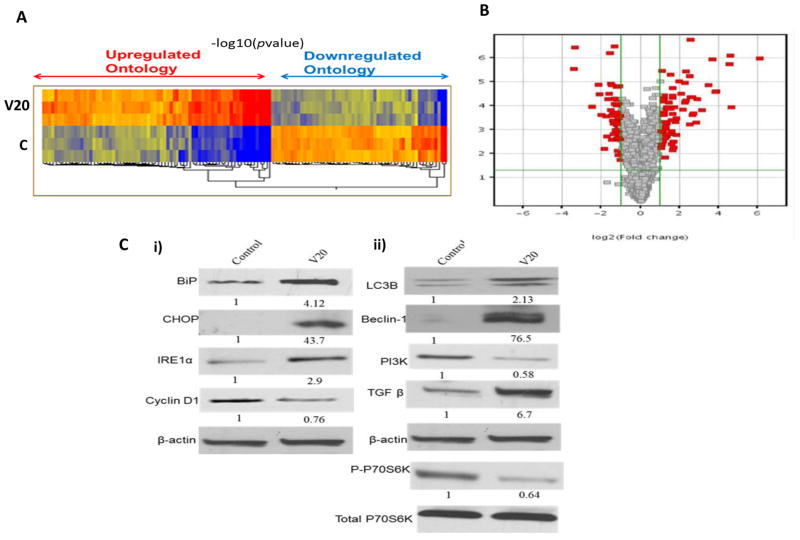
To shed light on the loss of sensitivity and to investigate the mechanism of action of VN/14-1, LTLC cells (P127) were treated with 20 μm (GI80) of VN/14-1 for 24 h and global gene expression changes were measured using a GeneChip Human Genome Focus Array. We used the online Database for Annotation, Visualization, and Integrated Discovery (DAVID) to identify clusters of related genes affected by VN/14-1. Our data analysis focused on comparing and contrasting the set of significantly deregulated genes for each treatment group Fig. 2A shows heat diagram and Fig. 2B shows volcano plot of deregulated genes. In Fig. 2A, red or blue rectangles indicate genes that were at least 2 fold up or down-regulated respectively. In Fig. 2B, grey squares indicate genes that either did not change in expression compared to control or were less than 2 fold up or down regulated. 127 Genes were differentially expressed (> 2 fold) in VN/14-1 treated group compared to control. 72 genes are more than 2 fold up regulated and 55 genes are more than 2 fold down-regulated. As shown in (Table 1), the most enriched up-regulated ontologies are those relating to stress and metabolism, particularly amino acid metabolism and intracellular organelle formation such as autophagy. The down-regulated ontologies included starch, carbohydrate metabolism, signaling cascade and DNA replication (Table 2). Interestingly, induction of genes involved in amino acid metabolism has been shown to occur after induction of ERS (Bruno et al., 2008; Lecca et al., 2005). These preliminary findings led us to hypothesize that VN/14-1 at concentrations ≤ 20 μm may induce adaptive mechanisms that support survival and resistance of HP-LTLC cells via induction of ERS and autophagy.
Fig. 2. Gene expression profiles in VN/14-1 treated LTLC cells.
(A) LTLC (P127) cells were treated with vehicle or 20 μm VN/14-1 and microarray was performed as described in materials section. Representative heat map showing differential expression of genes in VN/14-1 treated LTLC cells vs. vehicle treated LTLC cells. Red color indicates up-regμation and blue indicates down-regulation. Stronger intensity of the color indicates the higher fold change in gene expression. The experiment was done in triplicate. Each column indicates one sample. First 3 columns on the left represent vehicle treatment groups and last 3 columns represent VN/14-1 treatment groups. V20 stands for 20μm concentration of VN/14-1. (B) Volcano plot of microarray of LTLC cells treated with VN/14-1 showing differential gene expression. Red squares indicate the genes that are at least 2 fold up / down-regulated after the treatment of 10 μM VN/14-1 in LTLC cells while grey squares represent the unchanged genes. (C) LTLC (P127) cells were treated with 20 μm of VN/14-1 for 24 h, whole cell lysates were tested for markers for (i) ER stress such as BiP, CHOP, IRE1α and (ii) markers for autophagy LC3B, Beclin-1, p-p70S6K and PI3K, TGFβ. β-actin and total p70S6K were used as control. V20 stands for 20μm concentration of VN/14-1.
Table 1.
Up-regulated ontology in VN/14-1 treated LTLC cells
| Up-regulated Ontology | Count | % | P-Value |
|---|---|---|---|
| Cellular metabolic process | 46 | 63 | 1.20E-02 |
| Intracellular membrane-bound organelle | 40 | 54.8 | 1.70E-02 |
| Response to stimulus | 19 | 26 | 9.40E-02 |
| Transcription regulator activity | 18 | 24.7 | 8.50E-05 |
| Cell differentiation | 17 | 23.3 | 4.00E-03 |
| Negative regulation of cellular process | 12 | 16.4 | 8.40E-03 |
| Negative regulation of biological process | 12 | 16.4 | 1.10E-02 |
| Homeobox | 8 | 11 | 1.50E-05 |
| Cellular lipid metabolic process | 7 | 9.6 | 5.10E-02 |
| Generation of precursor metabolites and energy | 7 | 9.6 | 6.00E-02 |
| Regulation of protein metabolic process | 5 | 6.8 | 4.30E-02 |
| Proteasome | 3 | 4.1 | 2.00E-02 |
| Endonuclease activity | 3 | 4.1 | 6.90E-02 |
| Retinoic acid metabolic process | 2 | 2.7 | 2.60E-02 |
Up-regulated ontologies were identified using Database for Annotation, Visualization, and Integrated Discovery, as described in Materials and Methods. P values are based on an enrichment (EASE) score.
Table 2.
Down-regulated ontology in VN/14-1 treated LTLC cells
| Down-regulated ontology | Count | % | P-value |
|---|---|---|---|
| Intracellular signaling cascade | 11 | 16.2 | 6.30E-02 |
| UDP-glycosyltransferase activity | 10 | 14.7 | 6.40E-10 |
| Starch and sucrose metabolism | 9 | 13.2 | 1.60E-08 |
| calcium ion binding | 9 | 13.2 | 3.70E-02 |
| Androgen and estrogen metabolism | 9 | 13.2 | 6.50E-10 |
| Response to chemical stimulus | 7 | 10.3 | 3.00E-02 |
| Nucleobase, nucleoside and nucleotide metabolic process | 4 | 5.9 | 7.50E-02 |
| DNA replication | 4 | 5.9 | 7.40E-02 |
| Metalloprotein | 3 | 4.4 | 8.10E-02 |
| Positive regulation of phosphoinositide 3-kinase activity | 2 | 2.9 | 1.60E-02 |
Down-regulated ontologies were identified using Database for Annotation, Visualization, and Integrated Discovery, as described in Materials and Methods. P values are based on an enrichment (EASE) score.
3.3. VN/14-1 up-regulates protein markers of ER stress and autophagy
We next sought to validate the gene expression changes of specific genes found to be altered by VN/14-1 in the microarray experiment by using western blot analysis (Fig. 2C). We treated LTLC (P127) with VN/14-1 (20 μm) for 24 h and analyzed the changes in protein expression of ER stress and autophagy markers. There was an up-regulation of BiP (~ 4 fold), CHOP (~ 43-fold), IRE1α (~ 3-fold) compared to vehicle control confirming that VN/14-1 induces ER stress in LTLC cells. There was also an up-regulation of LC3B (~ 2-fold), Beclin-1 (~ 76-fold), TGFβ (~ 6-fold) and down-regulation of PI3K (~0.58-fold) and phosphorylation of p70S6K (~ 0.64-fold) compared to vehicle control. This clearly shows that VN/14-1 also induces autophagy in these cells and that these two mechanisms may contribute to the reduced sensitivity of the HP-LTLC cells to VN/14-1. VN/14-1 treatment also led to a down-regulation of cyclin D1.
3.4. VN/14-1 Synergizes with Known ERSR Inducer Thapsigargin
Increase in levels of ER stress beyond the tolerance of the cells leads to an apoptotic response (Apel et al., 2009; Schoenlein et al., 2009; White and DiPaola, 2009). To investigate whether VN/14-1 would synergize with a known ER stress inducer, thapsigargin to result in an enhancement in its anti-cancer effects, we combined these two agents and assessed their effects using MTT assays. We found that VN/14-1, when combined with thapsigargin resulted in profound synergistic AP effects, as assessed by isobologram analysis using the median-effect principle of Chou and Talalay (Fig. 3A) (Chou, 2010). These data suggest that VN/14-1 and thapsigargin elaborate unique effects that are complementary when these compounds are used together. In order to quantitatively evaluate the effect of the combination of VN/14-1 with thapsigargin, the dose reduction index (DRI) and combination index (CI) were calculated from the data in Table 3 using a Calcusyn software program at 50%, 75%, and 95% inhibition levels of cell viability. DRI values indicate that the synergic combination of VN/14-1 and thapsigargin can result in 1.7- to 11-fold reduction of the VN/14-1 dose and 11- to 172-fold reduction of thapsigargin dose in order to achieve 50–95% cell growth inhibition compared to the dose if they were used as single agents (Table 3). The values of CI suggest that the combination of VN/14-1 and thapsigargin was synergistic at 50%, 75% and 95% of cell growth inhibition. It also shows that the degree of synergism increases as the percentage inhibition of cell viability increases from 50% to 95% inhibition.
Fig. 3. Isobologram analysis and plasma concentration time profiles of VN/14-1.

(A) Normalized isobolograms are derived from MTT assay data involving VN/14-1 and thapsigargin in a fixed-ratio combination (1:1000) analyzed using the median-effect principle of Chou and Talalay (Calcusyn). The diagonal lines represent lines of additivity. Circles with dots indicate paired values of drug concentrations assessed for synergism at ED50 (Red), ED75 (Green), ED95 (Blue). (B) Female Sprague Dawley rats (n=6) were given of 20 mg/kg VN/14-1 (oral or intravenous) and their plasma was collected at various time points as indicated. The plasma was analyzed using reverse phase HPLC as mentioned in the methods section. Plasma concentration time profiles of VN/14-1 after a 20 mg/kg oral dose (red line) or a 20 mg/kg IV bolus (green line) are as shown in the figure.
Table 3.
Computer simulated CI and DRI values for VN/14-1 and thapsigargin
| Inhibition % | CI | DRI VN/14-1 | DRI Thapsigargin |
|---|---|---|---|
| 50 | 0.658 | 1.751 | 11.498 |
| 75 | 0.317 | 3.503 | 31.584 |
| 95 | 0.095 | 11.235 | 172.494 |
The synergism analysis of VN/14-1 with thapsigargin was performed with Chou–Talalay’s combination index and multiple drug-dose effect analysis method as described in Methods section. The values were determined using computer program from the data from the MTT assays. Combination index (CI): CI < 1, =1, and >1 indicates synergism, additive effect, and antagonism, respectively. Dose reduction index (DRI): fold of dose reduction for each drug in combination, for a given degree of inhibition, when compared with the dose of each drug alone for the same degree of inhibition.
3.5. Pharmacokinetics and oral bioavailability of VN/14-1
Oral pharmacokinetic properties of compounds play a vital role in their development for clinical use. We performed a preliminary pharmacokinetic analysis of VN/14-1 in female Sprague Dawley rats. Fig. 3B displays the VN/14-1 plasma concentration versus time profile in female Sprague Dawley rats upon oral administration of 20 mg/kg VN/14-1. The pharmacokinetic parameters are listed in Table 4. In rats, VN/14-1 exhibited rapid absorption upon oral administration, reaching highest its plasma concentration within 0.5 h post dose [mean time to achieve maximum plasma concentration, tmax 0.5 h]. The highest plasma concentration achieved upon oral administration of a 20 mg/kg of VN/14-1 was 38.2 mg/mL [mean highest plasma concentration achieved, Cmax (range 21.5 mg/mL to 44.8 mg/mL; n = 6). This concentration is higher than concentration required to cause anti-cancer effects in vitro. These pharmacokinetic results indicate that therapeutically relevant plasma exposures of VN/14-1 can be rapidly achieved upon oral administration of VN/14-1.
Table 4.
Pharmacokinetic properties of VN/14-1 (oral vs intravenous)
| Route Dose | Cmax (mg/ml) | tmax (h) | t1/2 (h) | AUC (0-∞) (h*mg/ml) | Bioavailability |
|---|---|---|---|---|---|
| Oral (20 mg/kg) | 38.2 | 0.50 | 1.41 | 93.9 | 1.34 |
| Intravenous (20 mg/kg) | 107 | - | 0.98 | 70.3 |
Pharmacokinetic parameters of VN/14-1 in female rats after oral or i.v. dose of 20 mg/kg. Cmax maximum plasma concentration, tmax time taken to achieve Cmax, t1/2 elimination half-life, AUC area under the curve.
In order to determine the extent of oral absorption, another group of female Sprague Dawley rats was administered 20 mg/kg VN/14-1 directly into systemic circulation (intravenously, IV) thereby eliminating any absorption steps. The plasma concentration versus time profile of VN/14-1 in female Sprague Dawley rats upon IV administration of 20 mg/kg VN/14-1 is shown in Fig. 3B and the pharmacokinetic parameters are listed in Table 4. As shown in Table 4, the absolute bioavailability of VN/14-1 is greater than 100%, indicating complete absorption of VN/14-1 upon oral administration. These data are in accordance with a number of literature reports of drugs that have absolute oral bioavailability of more than 100% (Gardelli et al., 2007; Hwang et al., 2007; Strandgarden et al., 1999; Ward et al., 2004a; Ward et al., 2004b).
VN/14-1 exhibited short plasma elimination half-lives (t1/2) in rats. The mean t1/2 upon oral administration was 1.41 h and upon IV administration was 0.98 h. Since, it takes approximately 5 elimination half-lives for almost complete (> 99%) elimination of an agent, VN/14-1 will be almost completely eliminated 7 h post oral administration. These results indicate that VN/14-1 will not linger in the body for long term and thus will not cause long term toxicological effects from prolonged presence of VN/14-1 in biological tissues. Also, the short t1/2 will prevent any meaningful accumulation of VN/14-1 upon repeated multiple administrations, thus making it suitable for repeated administration without any concerns of toxicity due to systemic accumulation of VN/14-1.
4. Discussion
Although ATRA has made some progress in the treatment of cancer, development of resistance and the toxicity of ATRA in patients represent the main problems of the therapy (Altucci et al., 2007; Njar et al., 2006). The development of RAMBAs represents a promising way to counter this problem (Njar, 2002; Njar et al., 2006). Aromatase inhibitors are an important class of clinically used drugs that are used to treat ERα positive breast cancers. However, development of resistance to aromatase inhibitors is also a major problem that leads to failure of treatment (Wong and Chen, 2012). In our previous studies, we reported LTLC cells as a model for resistance to aromatase inhibitors. We also reported that our RAMBA VN/14-1 exquisitely inhibited LTLC cells with an IC50 of 0.83 nM (Belosay et al., 2006). However, we observed that serial passaging lead to reduced sensitivity of LTLC cells to AP effects of VN/14- 1. The purpose of this study was to investigate the loss of sensitivity of the HP-LTLC to the anti-proliferative primary mechanism of action of VN/14-1 and to investigate its oral pharmacokinetic properties.
The microarray studies performed on LTLC cells revealed that VN/14-1 up-regulated several genes that are linked with cellular metabolism and intracellular membrane-bound organelle formation. Literature survey of these ontologies showed that ER stress and autophagy could be pathways involved. As the center of protein-folding, the ER is extremely sensitive to disruptions in homeostasis, including disruptions in calcium concentrations. The ER stress response (ERSR) pathway is an evolutionarily conserved pathway that seeks to relieve the buildup of unfolded proteins in the ER. To achieve this, the cell first up-regulates ER resident molecular chaperones, such as glucose-regulated protein 78 (gp78/BiP) (Malhotra and Kaufman, 2011), and reduces ER load through phosphorylation of the α subunit of the eukaryotic translation initiation factor 2α (eIF2α) (Harding et al., 1999). Phosphorylation of eIF2α results in attenuation of translation of nonessential proteins, including growth related proteins, such as cyclin D1 (Harding et al., 1999). Although the ER stress (ERS) is a survival pathway, prolonged stimulation of the ERS results in growth arrest and apoptosis via the up-regulation of apoptotic-related proteins, including the CCAAT/enhancer-binding protein homologous transcription factor (CHOP) (Zinszner et al., 1998). Autophagy is an intracellular process in which proteins and cytoplasmic organelles are degraded (Liu et al., 2011). It has been implicated in various physiological processes such as survival in stress, response to starvation and pathogenesis (Tschan and Simon, 2010; Xi et al., 2011). It is also reported to be a mechanism of cell protection against drug-induced apoptosis (Fan et al., 2010; Maycotte and Thorburn, 2011; Vazquez-Martin et al., 2009). Western blot analysis of the various proteins involved in ER stress and autophagy confirmed the up-regulation of these pathways in VN/14-1 treated LTLC cells. Furthermore, normalized isobologeam analysis showed that VN/14-1 acts synergistically with a known ER stress inducer, thapsigargin to inhibit proliferation of the resistant HP-LTLC breast cancer cells.
The findings from pharmacokinetic studies of VN/14-1 indicate rapid and complete absorption for VN/14-1 upon oral administration and a short half-life. The closely related ATRA has limited oral bioavailability (F ~40%) (Saadeddin et al., 2004) and thus VN/14-1 with an apparent absolute oral complete bioavailability in excess of 100% in rat indicates significant improvement in the biopharmaceutics properties of this class. These results favor development of VN/14-1 as an oral agent. The oral route is by far the most convenient route for administration of therapeutic agents. The oral route has the highest patient compliance and it is also the most cost effective route for drug administration, since it eliminates the need for subject admittance to the clinic for drug administration as required with parenteral agents. The short elimination half-life of VN/14-1 as seen in these pharmacokinetic studies provides further assurance towards a better safety profile of VN/14-1 as an anti-cancer agent. No meaningful systemic accumulation of VN/14-1 is expected upon repeated multiple dosing of VN/14-1 due to its short elimination half-life. Furthermore, VN/14-1 should also not stay for prolonged periods in the systemic circulation and thus should not have long term toxicological implications due to prolonged systemic exposure of VN/14-1.
Because of the heterogeneous nature of breast cancer (Thacher et al., 1999), combination therapies or multi-target agents are required for effective therapy. We have previously reported that VN/12-1, a methyl ester derivative of VN/14-1, induced autophagy and ER stress and that the combination of a VN/12-1 with chloroquine, an autophagy inhibitor, resulted in potent anti-cancer activity against SKBR-3 breast cancer cells (Godbole et al., 2012a). Recently, we also demonstrated that VN/12-1 has favorable pharmacokinetic properties (Godbole et al., 2012b). Based on our current studies, we certainly envision further advanced pre-clinical and clinical development of our RAMBAs particularly, VN/14-1 to treat breast cancers that have developed the resistance to aromatase inhibitors.
Acknowledgments
Funding
This research study was supported by grants from the US Department of Defense under the Peer Reviewed Medical Research Program (PRMRP, W81XW-04-1-0101, Njar, VCO), the US National Institutes of Health and National Cancer Institute (NIH/NCI, 1R01 CA12379-01A2, Njar, VCO). We thank all these agencies for their generous support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Van der Geer J, Hanraads JAJ, Lupton RA. The art of writing a scientific article. J Sci Commun. 2010;163:51–59. [Google Scholar]
- Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- Apel A, Zentgraf H, Buchler MW, Herr I. Autophagy-A double-edged sword in oncology. International journal of cancer. Int J Cancer. 2009;125:991–995. doi: 10.1002/ijc.24500. [DOI] [PubMed] [Google Scholar]
- Barnadas A, Estevez LG, Lluch-Hernandez A, Rodriguez-Lescure A, Rodriguez-Sanchez C, Sanchez-Rovira P. An overview of letrozole in postmenopausal women with hormone-responsive breast cancer. Adv Ther. 2011;28:1045–1058. doi: 10.1007/s12325-011-0075-4. [DOI] [PubMed] [Google Scholar]
- Belosay A, Brodie AM, Njar VC. Effects of novel retinoic acid metabolism blocking agent (VN/14-1) on letrozole-insensitive breast cancer cells. Cancer Res. 2006;66:11485–11493. doi: 10.1158/0008-5472.CAN-06-2168. [DOI] [PubMed] [Google Scholar]
- Bose D, Zimmerman LJ, Pierobon M, Petricoin E, Tozzi F, Parikh A, Fan F, Dallas N, Xia L, Gaur P, Samuel S, Liebler DC, Ellis LM. Chemoresistant colorectal cancer cells and cancer stem cells mediate growth and survival of bystander cells. Br J Cancer. 2011;105:1759–1767. doi: 10.1038/bjc.2011.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RD, Gover TD, Burger AM, Brodie AM, Njar VC. 17alpha-Hydroxylase/17,20 lyase inhibitor VN/124-1 inhibits growth of androgen-independent prostate cancer cells via induction of the endoplasmic reticulum stress response. Mol Cancer Ther. 2008;7:2828–2836. doi: 10.1158/1535-7163.MCT-08-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T, Haas-Kogan D, James CD, Oakes SA, Debnath J, Shokat KM, Weiss WA. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal. 2010;3:ra81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardelli C, Nizi E, Muraglia E, Crescenzi B, Ferrara M, Orvieto F, Pace P, Pescatore G, Poma M, del Ferreira MR, Scarpelli R, Homnick CF, Ikemoto N, Alfieri A, Verdirame M, Bonelli F, Paz OG, Taliani M, Monteagudo E, Pesci S, Laufer R, Felock P, Stillmock KA, Hazuda D, Rowley M, Summa V. Discovery and synthesis of HIV integrase inhibitors: development of potent and orally bioavailable N-methyl pyrimidones. J Med Chem. 2007;50:4953–4975. doi: 10.1021/jm0704705. [DOI] [PubMed] [Google Scholar]
- Godbole AM, Purushottamachar P, Martin MS, Daskalakis C, Njar VC. Autophagy inhibition synergistically enhances anticancer efficacy of RAMBA, VN/12-1 in SKBR-3 cells, and tumor xenografts. Mol Cancer Ther. 2012a;11:898–908. doi: 10.1158/1535-7163.MCT-11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbole AM, Purushottamachar P, Martin MS, Njar VC. Murine toxicology and pharmacokinetics evaluation of retinoic acid metabolism blocking agent (RAMBA), VN/12-1. Cancer Chemother Pharmacol. 2012b;70:339–344. doi: 10.1007/s00280-012-1877-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Hwang SH, Tsai HJ, Liu JY, Morisseau C, Hammock BD. Orally bioavailable potent soluble epoxide hydrolase inhibitors. J Med Chem. 2007;50:3825–3840. doi: 10.1021/jm070270t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. Clin Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- Lecca MR, Wagner U, Patrignani A, Berger EG, Hennet T. Genome-wide analysis of the unfolded protein response in fibroblasts from congenital disorders of glycosylation type-I patients. Faseb J. 2005;19:240–242. doi: 10.1096/fj.04-2397fje. [DOI] [PubMed] [Google Scholar]
- Liu JJ, Lin M, Yu JY, Liu B, Bao JK. Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett. 2011;300:105–114. doi: 10.1016/j.canlet.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. ER stress and its functional link to mitochondria: role in cell survival and death. Cold Spring Harb Perspect Biol. 2011;3:a004424. doi: 10.1101/cshperspect.a004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maycotte P, Thorburn A. Autophagy and cancer therapy. Cancer Biol Ther. 2011;11:127–137. doi: 10.4161/cbt.11.2.14627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njar VC. Cytochrome p450 retinoic acid 4-hydroxylase inhibitors: potential agents for cancer therapy. Mini Rev Med Chem. 2002;2:261–269. doi: 10.2174/1389557023406223. [DOI] [PubMed] [Google Scholar]
- Njar VC, Gediya L, Purushottamachar P, Chopra P, Vasaitis TS, Khandelwal A, Mehta J, Huynh C, Belosay A, Patel J. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg Med Chem. 2006;14:4323–4340. doi: 10.1016/j.bmc.2006.02.041. [DOI] [PubMed] [Google Scholar]
- Patel JB, Huynh CK, Handratta VD, Gediya LK, Brodie AM, Goloubeva OG, Clement OO, Nanne IP, Soprano DR, Njar VC. Novel retinoic acid metabolism blocking agents endowed with multiple biological activities are efficient growth inhibitors of human breast and prostate cancer cells in vitro and a human breast tumor xenograft in nude mice. J Med Chem. 2004;47:6716–6729. doi: 10.1021/jm0401457. [DOI] [PubMed] [Google Scholar]
- Patel JB, Khandelwal A, Chopra P, Handratta VD, Njar VC. Murine toxicology and pharmacokinetics of novel retinoic acid metabolism blocking agents. Cancer Chemother Pharmacol. 2007a;60:899–905. doi: 10.1007/s00280-007-0438-3. [DOI] [PubMed] [Google Scholar]
- Patel JB, Mehta J, Belosay A, Sabnis G, Khandelwal A, Brodie AM, Soprano DR, Njar VC. Novel retinoic acid metabolism blocking agents have potent inhibitory activities on human breast cancer cells and tumour growth. Br J Cancer. 2007b;96:1204–1215. doi: 10.1038/sj.bjc.6603705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadeddin A, Torres-Molina F, Carcel-Trullols J, Araico A, Peris JE. Pharmacokinetics of the time-dependent elimination of all-trans-retinoic acid in rats. AAPS PharmSci. 2004;6:E1. doi: 10.1208/ps060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenlein PV, Periyasamy-Thandavan S, Samaddar JS, Jackson WH, Barrett JT. Autophagy facilitates the progression of ERalpha-positive breast cancer cells to antiestrogen resistance. Autophagy. 2009;5:400–403. doi: 10.4161/auto.5.3.7784. [DOI] [PubMed] [Google Scholar]
- Strandgarden K, Hoglund P, Nordle O, Polacek J, Wannman H, Gunnarsson PO. Dissolution rate-limited absorption and complete bioavailability of roquinimex in man. Biopharm Drug Dispos. 1999;20:347–354. doi: 10.1002/(sici)1099-081x(199910)20:7<347::aid-bdd194>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Thacher SM, Nagpal S, Klein ES, Arefieg T, Krasinski G, DiSepio D, Agarwal C, Johnson A, Eckert RL, Chandraratna RA. Cell type and gene-specific activity of the retinoid inverse agonist AGN 193109: divergent effects from agonist at retinoic acid receptor gamma in human keratinocytes. Cell Growth Differ. 1999;10:255–262. [PubMed] [Google Scholar]
- Tschan MP, Simon HU. The role of autophagy in anticancer therapy: promises and uncertainties. J Intern Med. 2010;268:410–418. doi: 10.1111/j.1365-2796.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. Autophagy facilitates the development of breast cancer resistance to the anti-HER2 monoclonal antibody trastuzumab. PLoS One. 2009;4:e6251. doi: 10.1371/journal.pone.0006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward KW, Azzarano LM, Evans CA, Smith BR. Apparent absolute oral bioavailability in excess of 100% for a vitronectin receptor antagonist (SB-265123) in rat. I Investigation of potential experimental and mechanistic explanations. Xenobiotica. 2004a;34:353–366. doi: 10.1080/0049825042000205540. [DOI] [PubMed] [Google Scholar]
- Ward KW, Hardy LB, Kehler JR, Azzarano LM, Smith BR. Apparent absolute oral bioavailability in excess of 100% for a vitronectin receptor antagonist (SB-265123) in rat. II Studies implicating transporter-mediated intestinal secretion. Xenobiotica. 2004b;34:367–377. doi: 10.1080/0049825042000205540a. [DOI] [PubMed] [Google Scholar]
- White E, DiPaola RS. The double-edged sword of autophagy modulation in cancer. Clin Cancer Res. 2009;15:5308–5316. doi: 10.1158/1078-0432.CCR-07-5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Chen S. The development, application and limitations of breast cancer cell lines to study tamoxifen and aromatase inhibitor resistance. J Steroid Biochem Mol Biol. 2012;131:83–92. doi: 10.1016/j.jsbmb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi H, Kurtoglu M, Liu H, Wangpaichitr M, You M, Liu X, Savaraj N, Lampidis TJ. 2-Deoxy-D: -glucose activates autophagy via endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother Pharmacol. 2011;67:899–910. doi: 10.1007/s00280-010-1391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D. CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 1998;12:982–995. doi: 10.1101/gad.12.7.982. [DOI] [PMC free article] [PubMed] [Google Scholar]