Abstract
For decades, southern China has been considered to be an important source for emerging influenza viruses since key hosts live together in high densities in areas with intensive agriculture. However, the underlying conditions of emergence and spread of avian influenza viruses (AIV) have not been studied in detail, particularly the complex spatiotemporal interplay of viral transmission between wild and domestic ducks, two major actors of AIV epidemiology. In this synthesis, we examine the risks of avian influenza spread in Poyang Lake, an area of intensive free-ranging duck production and large numbers of wild waterfowl. Our synthesis shows that farming of free-grazing domestic ducks is intensive in this area and synchronized with wild duck migration. The presence of juvenile domestic ducks in harvested paddy fields prior to the arrival and departure of migrant ducks in the same fields may amplify the risk of AIV circulation and facilitate the transmission between wild and domestic populations. We provide evidence associating wild ducks migration with the spread of H5N1 in the spring of 2008 from southern China to South Korea, Russia, and Japan, supported by documented wild duck movements and phylogenetic analyses of highly pathogenic avian influenza H5N1 sequences. We suggest that prevention measures based on a modification of agricultural practices may be implemented in these areas to reduce the intensity of AIV transmission between wild and domestic ducks. This would require involving all local stakeholders to discuss feasible and acceptable solutions.
Keywords: avian influenza virus, wild birds, migration, interface, contact, ecology, epidemiology, China, Poyang, telemetry, remote sensing, GPS
Introduction
South China has been considered the epicenter for the emergence of new avian influenza strains and an area of high risk for emergence of human pandemic strains (Shortridge 1982; Webster et al. 1992; Webster et al. 2006; Jones et al. 2008). Persistence of highly pathogenic H5N1 (hereafter H5N1) in southern China since its emergence in 1997(Guan et al. 2002) has been associated with risk factors including high human population densities, high densities of domestic ducks raised on water ponds and intensively irrigated paddy fields that are also areas that attract wild ducks and waterfowl (Martin et al. 2011). Interestingly, the role of domestic ducks in the epidemiology of avian influenza viruses (AIV) was already pointed out in South China in the 1980s (Shortridge 1982).
Wild and domestic ducks are thought to play a major role in the epidemiology of low pathogenic avian influenza (LPAI) as well as H5N1. Wild ducks, especially dabbling ducks from the Anas genera, are considered reservoirs of LPAI viruses (Webster et al. 1992; Olsen et al. 2006; Munster et al. 2007). Studies show that LPAI viruses are the precursors of highly pathogenic avian influenza (HPAI) viruses of gallinaceous poultry including H5N1 (Webster et al. 1992; Munster et al. 2005; Chen et al. 2006; Alexander 2007). Furthermore, some wild duck species that were experimentally infected with H5N1 of wildfowl origin remained asymptomatic, indicating that wild ducks may have the potential to spread the virus (Brown et al. 2008; Keawcharoen et al. 2008; Gaidet et al. 2010; Newman et al. 2012; Nemeth et al. 2013). Domestic ducks may also shed H5N1 asymptomatically and are considered to play a key role in the persistence of H5N1 in Asia (Chen et al. 2004; Hulse-Post et al. 2005; Sturm-Ramirez et al. 2005; Kim et al. 2008; Martin et al. 2011), especially free-grazing ducks that have been associated with H5N1 outbreaks (Gilbert et al. 2006; Songserm et al. 2006; Gilbert et al. 2008).
The recent emergence of a zoonotic H7N9 LPAI in China highlighted how domestic ducks act as a key intermediate host by serving as a mixing vessel for a variety of AIV from migratory birds and transmitting the different combinations to chickens, with potential emergence into domestic mammals and humans (Lam et al. 2013). The domestic – wild duck interface is thus key to the emergence and spread of potentially zoonotic AIV. A better understanding of its spatial and temporal characteristics could identify hot spots and periods for targeted surveillance. Furthermore, areas with high emergence, spread, or pandemic potential may allow deployment of targeted preventative measures that reduce potential transmission of AIV between wild and domestic ducks.
In southern China, Poyang Lake in Jiangxi Province and Dongting Lake in Hunan Province are of great concern for risk of H5N1 persistence (Martin et al. 2011; Prosser et al. 2013) (Supporting Fig. 1). Both sites are characterized by high densities of domestic ducks raised in paddy fields that were historically converted from natural wetlands, and they support large numbers of resident and wild migratory ducks in the nearby wetlands. We hypothesize that Poyang Lake and Dongting Lake exemplify agro-ecological systems where the extensive interface between natural wetlands and paddy rice fields act as key sites to facilitate AIV transmission between wild and domestic ducks. We further hypothesize that certain HPAI strains may then spread intermittently across the continent through wild duck migration.
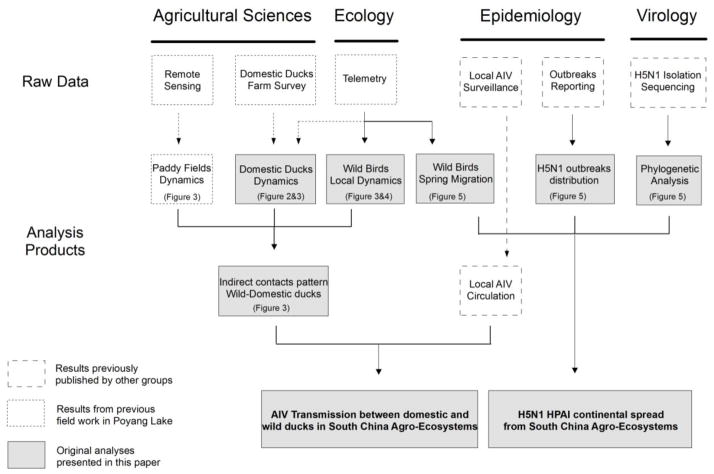
The main objective of this synthesis was to characterize the spatial and temporal relationships between wild and domestic ducks in these high-interface areas by integrating previously published and original interdisciplinary data (Fig. 1). We combined field-collected data on free-grazing duck farms, remotely-sensed environmental indicators on rice cropping systems, satellite-tracking data of resident and migratory wild ducks and epidemiological and phylogenetic data of H5N1 isolates in East Asia. More precisely, we compared the temporal pattern of free-grazing domestic duck farming in the Poyang Lake area with the seasonal presence of wild ducks in paddy fields to identify a period with potential increased risk of AIV transmission. We implemented farm surveys and used satellite tracking data to investigate the potential for harvested paddy fields to act as a favorable environment for AIV transmission between wild and domestic ducks. Finally, we combined satellite tracking data and phylogenetic analyses of H5N1 sequences to suggest the role played by wild migratory ducks in the long-distance spread of HPAI strains. We showed that spatial and temporal relationships between wild ducks and free-ranging farmed ducks increased the potential for contact and subsequent risk of AIV transmission as well as the potential long-distance spread of certain AIV strains through wild duck migration.
Fig. 1. Conceptual model of integrated study components.
The original analyses integrated here rely on original and historic agricultural and ecological field studies conducted in Poyang Lake and on original and historic epidemiological and virological analyses.
Materials and Methods
Spatiotemporal characterization of the domestic and wild duck interface
Free-grazing duck farming in paddy fields
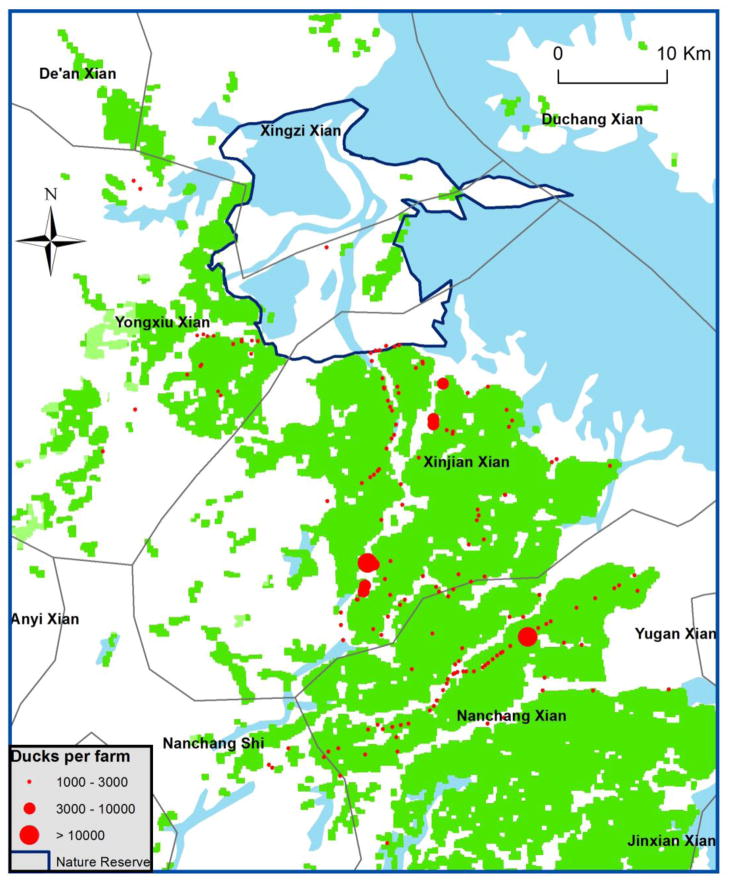
Field investigations were undertaken in wetlands and paddy fields surrounding Poyang Lake (see Supporting information for study site details) to document characteristics and spatiotemporal patterns of free-grazing domestic duck farming. To estimate population dynamics and population size of free-grazing ducks, we combined different sources of information including data from the provincial agricultural yearbooks and the results of two field studies of local duck farming. During October of 2007, a total of 42 duck farms participated in interviews and provided data on the number of free-grazing ducks raised and the timing of production cycles. A “road survey” was also implemented, including GPS coordinates and number of duck shelters observed per farm along the roads within our study area. During March of 2011, a total of 216 farms were investigated in the Yongxiu (n=50), Nanchang (n =112) and De’an (n = 54) counties (Fig. 2) as part of a larger study on the risk of AIV transmission at the domestic poultry – human interface implemented by Chinese Center for Disease Control and Prevention. We extracted data related to the number of adult and juvenile ducks, chickens, and pigs raised on the farms. We classified farms with > 100 ducks as duck farms (n=150) and farms with < 40 ducks as Other (n=66).
Fig. 2. Free-grazing-duck farms investigated within the study area.
This map shows results from the survey of duck shelters conducted in October 2007. We estimated number of ducks per farm by counting the number of duck shelters per farm and estimated 1500 ducks per shelter. The grey lines delineate county boundaries.
Ecology of resident and migratory wild ducks and potential contact with domestic ducks
We used telemetry data from 14 resident and 19 migratory wild ducks that were captured and equipped with satellite transmitters in Poyang Lake in March and November 2007 (Supporting Table 1). From the Poyang Lake area, we obtained 4266 GPS locations (±18.5 m accuracy) and 1905 Argos Doppler locations (~1–10 km accuracy) from resident ducks and 357 Argos locations from migrating ducks (for more details, see (Takekawa et al. 2010).
We estimated the intensity of indirect contacts between wild and domestic ducks by calculating the percentage of GPS locations (available for resident ducks only) transmitted within areas identified as rice paddy using remotely sensed data (Torbick et al. 2011). We also implemented a generalized linear mixed model to test the influence of daily (night vs day) and seasonal (wintering period vs other) temporal variables on the distribution of wild resident birds in paddy fields (Supporting Information).
Spread of H5N1 through wild duck migration
To assess the spatiotemporal relationship between wild duck migration and H5N1 outbreaks during the spring migration, we overlaid satellite tracking data along the East Asian Flyway from South China and the location and date of H5N1 outbreaks along this flyway. We used the satellite tracking dataset described above and a dataset from wild ducks captured in Hong Kong. This additional dataset contained 3,275 locations from 23 wild ducks captured in December 2009 and 2010 (Takekawa et al. 2010). We included in the study all H5N1 outbreaks officially reported to the World Organization for Animal Health (OIE) in East Asia during the satellite tracking programs in Poyang Lake and HongKong (2007 to 2010). We considered only the first reported outbreak (primary outbreak) for a given geographical area, since subsequent local outbreaks may have been spread through local poultry movements or trade.
To assess the role played by wild ducks in the spread of H5N1, we reviewed the official epidemiological reports provided for each outbreak to OIE. Outbreak reports were collected using the OIE World Animal Health Information Database (WAHID) and the United Nations Food and Agriculture Organization Emergency Prevention System (FAO EMPRES-i) interfaces. We also compared the duration of the long-distance movements of satellite-marked wild ducks with the duration of the asymptomatic excretion period of the virus by experimentally infected wild ducks as detailed by Gaidet et al. (2010).
Phylogenetic analysis
We searched in GenBank for the full-length (1704 nucleotide) HA sequences of the viruses isolated for each H5N1 outbreak. We inferred the phylogenetic relationships using maximum likelihood methods available via PAUP* (Swofford 2003), from the viruses in the outbreaks, viruses isolated from Poyang and Dongting Lakes, related influenza viruses from clade 2.3.2, and representative viruses from other H5N1 clades and subclades (Chen et al. 2006; Smith et al. 2009; Kang et al. 2011). A best-fit model was inferred directly from this data set (n = 73 sequences) using MODELTEST: GTR + gamma (Posada and Crandall 1998). Support for individual nodes was estimated by bootstrap resampling (1,000 replicates) using the neighbor-joining method with incorporation of the ML substitution model. The analysis was repeated for NA gene sequences of the viruses.
Results
Spatiotemporal characterization of the domestic and wild duck interface
Free-grazing duck farming and rice paddy cropping
We estimated that ca. 14,000,000 ducks (both free-grazing and non-free-grazing) were raised yearly in the Poyang Lake area on the basis of the 2004 to 2006 Jiangxi Province agricultural yearbooks (Supporting Table 2). In Xinjina and Yongxiu counties that comprise most of the nature reserve (Fig. 2), approximately 2,000,000 domestic ducks were raised, documenting high densities of domestic ducks in this area.
Farm interviews and investigations confirmed the high densities of free-grazing ducks reported in the yearbooks. The average number of ducks per farm was ca 2,000 (Table 1, Fig. 2). In October 2007, all 42 farmers had juvenile domestic ducks and all indicated the start of the free-grazing period after the harvest of the second rice crop in October (Fig. 3). In March 2011, 24% of the 150 duck farms had juvenile ducks, which reflected the start of a new production cycle. The period when ducks were sold varied depending on market prices which in turn induced variability in the length of production cycles. Of the 150 duck farms surveyed 8% of the farms also housed swine and 45% housed other types of poultry (Table 1).
Table 1.
Summary of duck farmer surveys in the Poyang Lake area.
| Study type | Date | Number of farms | Ducks per farm*
|
Number per farm
|
||||
|---|---|---|---|---|---|---|---|---|
| Mean | sd | Range | Juvenile ducks | swine | Other poultry | |||
| Duck farms | 10/2007 | 42 | 2374 | 1519 | 300–8000 | 42 (100%) | - | - |
| Duck shelters | 10/2007 | 166 | 2115* | 2764* | 1500–30000* | - | - | - |
| Duck farms | 03/2011 | 150 | 1893 | 2632 | 120–23000 | 36 (24%) | 12 (8%) | 67 (44.67%) |
|
| ||||||||
| Other farms | 03/2011 | 66 | 7.24 | 6.95 | 0–40 | 2 (3%) | 22 (33.34%) | 60 (90.9%) |
For the survey of duck shelters conducted in October 2007, we estimated the number of ducks per farm by multiplying the number of duck shelters per farm by an estimated 1500 ducks per shelter.
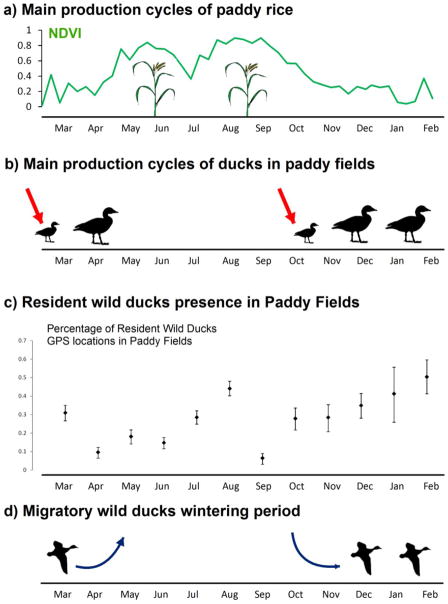
Fig. 3. Seasonal fluctuations of the potential indirect contacts between wild and domestic ducks in rice paddy fields surrounding Poyang Lake, China.
Rice production systems influence periods when domestic ducks are free-grazing and in indirect contact with wild ducks sharing the same paddy fields. Illustrations include a) the double crop rice production calendar. A single crop system also occurs in the area, although the double crop system is predominant in the vicinity of Poyang Lake (Li et al. 2012); b) free-grazing periods for domestic ducks in two cycles from March to April before planting of early rice and from October to February after harvesting of late rice(red arrows show the two yearly pulses of juvenile ducks in the paddy fields); c) wild duck presence in rice paddy fields estimated for resident and migratory ducks by the percentage of monthly satellite locations (95% binomial confidence interval was calculated with the binom.test function in R.); d) timing of wild duck departure and arrival for the wintering period.
We identified two annual production cycles for free-grazing ducks both associated with the double-cropping system of rice production (Fig. 3). The first cycle started in February–March before planting of early rice, and the second cycle started in October–November after the harvest of late rice (Fig. 3a,b). Thus, large numbers of juvenile ducks are released twice yearly into the paddy fields: a month before the arrival of migrating wild ducks and a month prior to their spring departure (Fig. 3b,d).
Ecology of resident and migratory wild ducks and potential contact with domestic ducks
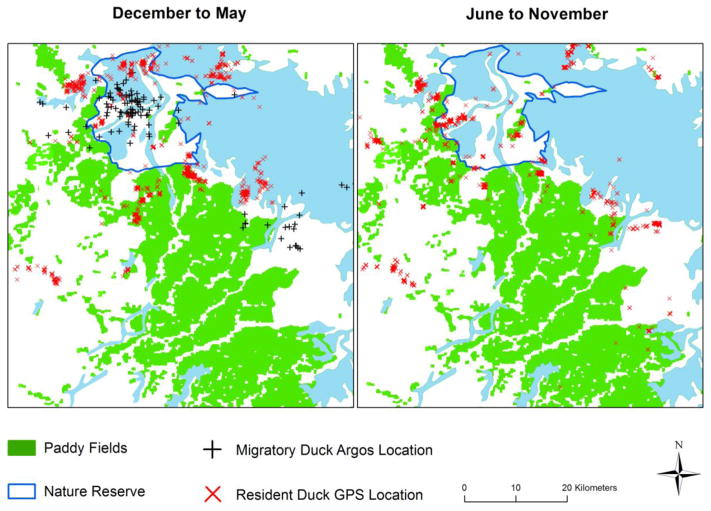
Results from satellite telemetry showed spatial and temporal overlap between free-grazing ducks and resident wild ducks presence in paddy fields, especially in the close vicinity of the nature reserve (Fig. 4). During October to March, more than 30% of the resident duck GPS locations were in paddy fields (Fig. 3c). Furthermore, both resident and migratory wild ducks were present in the area from December to May with several overlapping locations, especially within the nature reserve (Fig. 4).
Fig. 4. Locations of wild ducks at Poyang Lake.
This figure shows the best Argos locations (classes 1 to 3) of migratory birds and GPS locations of resident wild birds in the Poyang Lake area for two periods of the year. Resident species included spot-billed ducks and mallards. Migratory species included Baikal teal, common teal, Eurasian wigeon, falcated teal, garganey and northern pintail (Supporting Table 1).
The results from the generalized linear mixed model discerned a significant impact of night time and wintering period on the probability of presence of resident wild ducks in rice paddy fields (Table 2). Night locations were more often located in paddy fields than those during the day (Supporting Fig. 2).
Table 2.
Results from a generalized linear mixed model indicating a significant difference in presence of resident wild ducks in rice paddy fields during the winter and at night.
(GLMM Model: Presence in Paddy fields ~ Wintering + Night + (1 | bird ID))
| Variables | Coefficient | Sd | p |
|---|---|---|---|
| Intercept | −1.321 | 0.5763 | 0.0219* |
| Wintering | 0.3774 | 0.1004 | 0.00017*** |
| Night | 0.5838 | 0.0819 | 1.02E-12*** |
Spread of H5N1 through wild duck migration
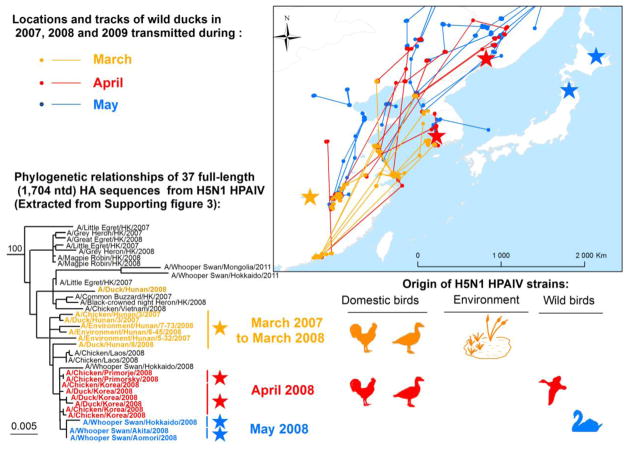
Wild migratory ducks showed spatial and temporal correspondence between stopover sites used and H5N1 outbreaks that occurred in the East Asian Flyway during the spring of 2008 (Fig. 5). One duck flew from Hong Kong to South Korea in less than three days, within the average 4-day asymptomatic excretion period for H5N1 viruses by wild Anatidae (Gaidet et al. 2010), indicating the possibility of migration with the virus.
Fig. 5. Potential spread of highly pathogenic H5N1 strains by wild migratory ducks.
H5N1 strains isolated from outbreaks in South Korea, Russia and Japan from April to May 2008 were closely related to each other and to strains isolated from Dongting Lake in March 2008 from domestic chickens, ducks, and water.
Results of the phylogenetic analysis indicated that the viruses isolated during the outbreaks were all members of the 2.3.2 sub-clade which had previously been isolated from wild ducks in South China (Smith et al. 2009) (Supporting Fig. 3). They were phylogenetically closely related to the viruses isolated in Dongting Lake in both water and poultry during the months preceding the outbreaks (Fig. 5 and Supporting Figs. 3 and 4). These results were compatible with the long-distance spread of H5N1by wild migratory ducks from South China to northern areas in South Korea, Japan and far-East Russia.
Discussion
Our synthesis showed that farming of free-grazing domestic ducks in the Poyang Lake area is intensive and synchronized with migratory movements of wild ducks. Not only are free-grazing ducks extensively raised in paddy fields when wild migratory ducks are found in the area, but the two main production cycles are synchronized with their arrival and departure periods. The production cycles lead to a pulse of juvenile domestic ducks – immunologically naïve and highly susceptible to infection – free-ranging in the rice paddy fields coincident with the time of arrival of wild migratory ducks for winter and just prior to their spring migration.
In the fall, synchrony between the pulse of a large number of susceptible individuals and introduction of new AIV strains from reservoir populations is likely to amplify circulation of AIV. Juvenile and migratory ducks have been identified as key drivers of AIV circulation in Europe (van Dijk et al. 2013). A study at Poyang Lake from 2003 to 2007 showed that the same LPAI subtypes were circulating in both wild ducks and domestic ducks with a seasonal peak of AIV prevalence from November to February after the arrival of migratory wild ducks (Duan et al. 2011).
In the spring, the synchrony between the second pulse of juvenile ducks in the paddy fields and the departure of migratory ducks could facilitate the transmission of AIV strains to migratory ducks and spread along migratory flyways. We identified spatiotemporal correspondence between the 2008 spring migration of wild ducks and the spread of closely related H5N1 strains isolated first in domestic poultry and the environment at Dongting Lake, then in domestic poultry in South Korea, and finally in wild ducks and domestic poultry in Russia and in wild swans in Japan (Fig. 5). Spread through legal or illegal trade was reported as unlikely in this specific case (Usui et al. 2009; Manin et al. 2010). Furthermore, strains from the 2.3.2 subclade have been isolated from wild birds in various locations where wild birds likely played a role in their dissemination, including South China (Smith et al. 2009), East China (Zhao et al. 2012), South Korea (Kim et al. 2012), Central China (Li et al. 2011), Mongolia (Kang et al. 2011), and Europe (Reid et al. 2011). Our study does not provide direct evidence of a continental spread of H5N1 through wild ducks migration; however, such direct evidence seems unlikely to ever be provided. Spread of HPAI by wild migratory birds may be the most likely route under certain conditions, especially when the alternative hypothesis, trade, is unlikely (Kilpatrick et al. 2006).
Although previous analyses have indicated that migration of wild ducks and outbreaks overlap spatially but are mismatched temporally (Takekawa et al. 2010), our synthesis indicates that when favorable conditions arise, isolated events may still allow for spread of AIV. In some areas such as the Central Asian Flyway, favorable conditions may occur with greater frequency resulting in more opportunities for spread (Newman et al. 2012). However, continuous and sustained poultry production systems and trade remain the major pathway for the spread of H5N1, especially at local and regional scales in China (Takekawa et al. 2010).
Although domestic ducks only use the harvested rice paddy fields during the day, capacity of AIV and H5N1 viruses in particular to persist even up to several months in water or feathers makes indirect contact likely (Domanska-Blicharz et al. 2010; Yamamoto et al. 2010). Temperatures in Poyang Lake during the winter (monthly average of 7.5°C in December, 5.1°C in January and 6.3°C in February) would allow H5N1 to persist in the environment throughout the entire period.
Satellite telemetry served as a powerful tool for our studies, because its high resolution data identified wild duck use of the rice paddy fields. Direct observations would have been more complicated, especially since wild ducks tend to forage at night. However, due to high cost, satellite transmitters were deployed on a limited number of individuals when compared to the total population in the area, and thus, our sample is not representative of all species and populations (Takekawa et al. 2010). Furthermore, we assumed that all ducks exhibited similar behavior regarding the use of paddy fields, and therefore results from seasonal trends should be interpreted with caution. Resident wild birds, sharing paddy fields with domestic free-grazing ducks and protected areas with migratory ducks, may play the role of a bridge species between domestic and migratory birds (Hill et al. 2012). In a previous study, six H5N1 viruses were isolated from apparently healthy wild ducks at Poyang Lake on two sampling occasions (January and March 2005) (Chen et al. 2006). Although these birds were assumed to be migratory by the authors, at least two (mallard and spot-billed duck) of the three species reported are either farmed or resident in Poyang Lake as demonstrated by our satellite tracking data. Thus, surveillance programs aimed at detecting AIV in wild and domestic ducks during the winter should incorporate environmental sampling and target resident species.
Conclusions
Recent emergence of H5N1 and LPAI H7N9 viruses in South China demonstrate the need for better surveillance of AIVs in this region and in particular, in intensive agriculture areas that serve as an interface between domestic and wild ducks. Prevention measures aimed at reducing the intensity of AIV transmission should be focused on these areas. Successful vaccination programs may be difficult to sustain in the long-term and should be complemented by other control measures (Desvaux et al. 2013). Our synthesis highlights the importance of free-grazing duck farming in increasing risks at the wild-domestic interface. Therefore, prevention measures could be undertaken based on a modification of agricultural practices such as: excluding juvenile ducks from rice paddy fields, creating a domestic duck-free zone around nature reserves, developing specific markets for ducks separate from general live bird markets, or isolating ducks from other domestic animals. Informing and involving local stakeholders in discussions could allow development of acceptable long-term solutions to minimize risk of spread. A bottom-up and multidisciplinary approach including social sciences should be implemented in these intensive agricultural areas to mitigate risks of emergence and spread of new, potentially zoonotic AIV strains.
Supplementary Material
Acknowledgments
This study was supported by a grant from National Institutes of Health (NIH R01 AI101028), a grant from the NIH Fogarty International Center (R01-TW007869) through the NSF/NIH Ecology of Infectious Diseases program, the Food and Agriculture Organization of the United Nations, the U. S. Geological Survey and a grant from National Aeronautics and Space Administration (NASA) Public Health Program (NNX11AF66G). Julien Cappelle was supported by the Gripavi project funded by the French Ministry of Foreign Affairs. We thank Martine Duportal for her help in designing the figures and Isa Woo with editorial assistance.
References
- Alexander DJ. An overview of the epidemiology of avian influenza. Vaccine. 2007;25:5637–5644. doi: 10.1016/j.vaccine.2006.10.051. [DOI] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE, Swaynet DE. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerging Infectious Diseases. 2008;14:136–142. doi: 10.3201/eid1401.070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Deng G, Li Z, Tian G, Li Y, Jiao P, et al. The evolution of H5N1 influenza viruses in ducks in southern China. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10452–10457. doi: 10.1073/pnas.0403212101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Smith GJD, Li KS, Wang J, Fan XH, Rayner JM, et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2845–2850. doi: 10.1073/pnas.0511120103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvaux S, Grobois V, Pham Thi Thanh H, Dao DT, Nguyen TD, Fenwick S, et al. Evaluation of the vaccination efficacy against H5N1 in domestic poultry in the Red River Delta in Vietnam. Epidemiology and Infection. 2013;141:776–788. doi: 10.1017/S0950268812001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanska-Blicharz K, Minta Z, Smietanka K, Marche S, van den Berg T. H5N1 High Pathogenicity Avian Influenza Virus Survival in Different Types of Water. Avian Diseases. 2010;54:734–737. doi: 10.1637/8786-040109-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Duan L, Zhu H, Wang J, Huang K, Cheung C-L, Peiris JSM, et al. Influenza virus surveillance in migratory ducks and sentinel ducks at Poyang Lake, China. Influenza and Other Respiratory Viruses. 2011;5:65–68. [PubMed] [Google Scholar]
- Gaidet N, Cappelle J, Takekawa JY, Prosser DJ, Iverson SA, Douglas DC, et al. Potential spread of highly pathogenic avian influenza H5N1 by wildfowl: dispersal ranges and rates determined from large-scale satellite telemetry. Journal of Applied Ecology. 2010;47:1147–1157. [Google Scholar]
- Gilbert M, Chaitaweesub P, Parakarnawongsa T, Premashthira S, Tiensin T, Kalpravidh W, et al. Free-grazing ducks and highly pathogenic avian influenza, Thailand. Emerging Infectious Diseases. 2006;12:227–234. doi: 10.3201/eid1202.050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Xiao XM, Pfeiffer DU, Epprecht M, Boles S, Czarnecki C, et al. Mapping H5N1 highly pathogenic avian influenza risk in Southeast Asia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4769–4774. doi: 10.1073/pnas.0710581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Peiris JSM, Lipatov AS, Ellis TM, Dyrting KC, Krauss S, et al. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:8950–8955. doi: 10.1073/pnas.132268999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NJ, Takekawa JY, Ackerman JT, Hobson KA, Herring G, Cardona CJ, et al. Migration strategy affects avian influenza dynamics in mallards (Anas platyrhynchos) Molecular Ecology. 2012;21:5986–5999. doi: 10.1111/j.1365-294X.2012.05735.x. [DOI] [PubMed] [Google Scholar]
- Hulse-Post DJ, Sturm-Ramirez KM, Humberd J, Seiler P, Govorkova EA, Krauss S, et al. Role of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in Asia. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10682–10687. doi: 10.1073/pnas.0504662102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–U994. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H-M, Batchuluun D, Kim M-C, Choi J-G, Erdene-Ochir T-O, Paek M-R, et al. Genetic analyses of H5N1 avian influenza virus in Mongolia, 2009 and its relationship with those of eastern Asia. Veterinary Microbiology. 2011;147:170–175. doi: 10.1016/j.vetmic.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Keawcharoen J, van Riel D, van Amerongen G, Bestebroer T, Beyer WE, van Lavieren R, et al. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5NI) Emerging Infectious Diseases. 2008;14:600–607. doi: 10.3201/eid1404.071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Chmura AA, Gibbons DW, Fleischer RC, Marra PP, Daszak P. Predicting the global spread of H5N1 avian influenza. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19368–19373. doi: 10.1073/pnas.0609227103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H-R, Lee Y-J, Park C-K, Oem J-K, Lee OS, Kang H-M, et al. Highly Pathogenic Avian Influenza (H5N1) Outbreaks in Wild Birds and Poultry, South Korea. Emerging Infectious Diseases. 2012;18:480–483. doi: 10.3201/eid1803.111490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Seiler P, Forrest HL, Khalenkov AM, Franks J, Kumar M, et al. Pathogenicity and Vaccine Efficacy of Different Clades of Asian H5N1 Avian Influenza A Viruses in Domestic Ducks. Journal of Virology. 2008;82:11374–11382. doi: 10.1128/JVI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TT-Y, Wang J, Shen Y, Zhou B, Duan L, Cheung C-L, et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature. 2013 doi: 10.1038/nature12515. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Feng Z, Jiang L, Liu Y, Xiao X. Changes in rice cropping systems in the Poyang Lake Region, China during 2004–2010. Journal of Geographical Sciences. 2012;22:653–668. [Google Scholar]
- Li Y, Liu L, Zhang Y, Duan Z, Tian G, Zeng X, et al. New Avian Influenza Virus (H5N1) in Wild Birds, Qinghai, China. Emerging Infectious Diseases. 2011;17:265–267. doi: 10.3201/eid1702.100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manin TB, Chvala IA, Kolosov SN, Pchelkina IP, Irza VN, Drygin VV. H5N1 Avian Influenza Outbreak in the Far East of Russia in 2008: New Introduction. Avian Diseases. 2010;54:509–512. doi: 10.1637/8732-032509-Case.1. [DOI] [PubMed] [Google Scholar]
- Martin V, Pfeiffer DU, Zhou X, Xiao X, Prosser DJ, Guo F, et al. Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China. PLoS Pathogens. 2011;7:e1001308. doi: 10.1371/journal.ppat.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Baas C, Lexmond P, Waldenström J, Wallensten A, Fransson T, et al. Spatial, Temporal, and Species Variation in Prevalence of Influenza A Viruses in Wild Migratory Birds. PLoS Pathogens. 2007;3:e61. doi: 10.1371/journal.ppat.0030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Wallensten A, Baas C, Rimmelzwaan GF, Schutten M, Olsen B, et al. Mallards and highly pathogenic avian influenza ancestral viruses, northern Europe. Emerging Infectious Diseases. 2005;11:1545–1551. doi: 10.3201/eid1110.050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth NM, Brown JD, Stallknecht DE, Howerth EW, Newman SH, Swayne DE. Experimental Infection of Bar-Headed Geese (Anser indicus) and Ruddy Shelducks (Tadorna ferruginea) With a Clade 2.3.2 H5N1 Highly Pathogenic Avian Influenza Virus. Veterinary Pathology Online. 2013 doi: 10.1177/0300985813490758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SH, Hill NJ, Spragens KA, Janies D, Voronkin IO, Prosser DJ, et al. Eco-Virological Approach for Assessing the Role of Wild Birds in the Spread of Avian Influenza H5N1 along the Central Asian Flyway. PLoS ONE. 2012:7. doi: 10.1371/journal.pone.0030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus A, Fouchier RAM. Global patterns of influenza A virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Prosser D, Hungerford L, Erwin RM, Ottinger MA, Takekawa JY, Ellis E. Mapping avian influenza transmission risk at the interface of domestic poultry and wild birds. Frontiers in Public Health. 2013:1. doi: 10.3389/fpubh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SM, Shell WM, Barboi G, Onita I, Turcitu M, Cioranu R, et al. First Reported Incursion of Highly Pathogenic Notifiable Avian Influenza A H5N1 Viruses from Clade 2.3.2 into European Poultry. Transboundary and Emerging Diseases. 2011;58:76–78. doi: 10.1111/j.1865-1682.2010.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortridge KF. Avian influenza A viruses of southern China and Hong Kong: ecological aspects and implications for man. Bulletin of the World Health Organization. 1982;60:129–135. [PMC free article] [PubMed] [Google Scholar]
- Smith GJD, Vijaykrishna D, Ellis TM, Dyrting KC, Leung YHC, Bahl J, et al. Characterization of Avian Influenza Viruses A (H5N1) from Wild Birds, Hong Kong, 2004–2008. Emerging Infectious Diseases. 2009;15:402–407. doi: 10.3201/eid1503.081190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songserm T, Jam-on R, Sae-Heng N, Meemak N, Hulse-Post DJ, Sturm-Ramirez KM, et al. Domestic ducks and H5N1 influenza epidemic, Thailand. Emerging Infectious Diseases. 2006;12:575–581. doi: 10.3201/eid1204.051614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm-Ramirez KM, Hulse-Post DJ, Govorkova EA, Humberd J, Seiler P, Puthavathana P, et al. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? Journal of Virology. 2005;79:11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford DL. S. Associates, editor. PAUP*: Phylogenetic analysis using parsimony (*and other methods) version 4.0 [computer program] Sinauer Associates; Sunderland, Massachusettes: 2003. [Google Scholar]
- Takekawa JY, Newman SH, Xiao X, Prosser DJ, Spragens KA, Palm EC, et al. Migration of Waterfowl in the East Asian Flyway and Spatial Relationship to HPAI H5N1 Outbreaks. Avian Diseases. 2010;54:466–476. doi: 10.1637/8914-043009-Reg.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbick N, Salas W, Xiao X, Ingraham P, Fearon MG, Biradar C, et al. Integrating SAR and optical imagery for regional mapping of paddy rice attributes in the Poyang Lake watershed, China. Canadian Journal of Remote Sensing. 2011;37:17–26. [Google Scholar]
- Usui T, Yamaguchi T, Ito H, Ozaki H, Murase T, Ito T. Evolutionary genetics of highly pathogenic H5N1 avian influenza viruses isolated from whooper swans in northern Japan in 2008. Virus Genes. 2009;39:319–323. doi: 10.1007/s11262-009-0403-9. [DOI] [PubMed] [Google Scholar]
- van Dijk JGB, Hoye BJ, Verhagen JH, Nolet BA, Fouchier RAM, Klaassen M. Juveniles and migrants as drivers for seasonal epizootics of avian influenza virus. Journal of Animal Ecology. 2013:n/a–n/a. doi: 10.1111/1365-2656.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and Ecology of Influenza-a Viruses. Microbiological Reviews. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG, Peiris M, Chen HL, Guan Y. H5N1 outbreaks and enzootic influenza. Emerging Infectious Diseases. 2006;12:3–8. doi: 10.3201/eid1201.051024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K, Yamada M, Mase M. Persistence of Avian Influenza Virus (H5N1) in Feathers Detached from Bodies of Infected Domestic Ducks. Applied and Environmental Microbiology. 2010;76:5496–5499. doi: 10.1128/AEM.00563-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G, Zhong L, Lu X, Hu J, Gu X, Kai Y, et al. Characterisation of a highly pathogenic H5N1 clade 2.3.2 influenza virus isolated from swans in Shanghai, China. Virus Genes. 2012;44:55–62. doi: 10.1007/s11262-011-0667-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.